Abstract
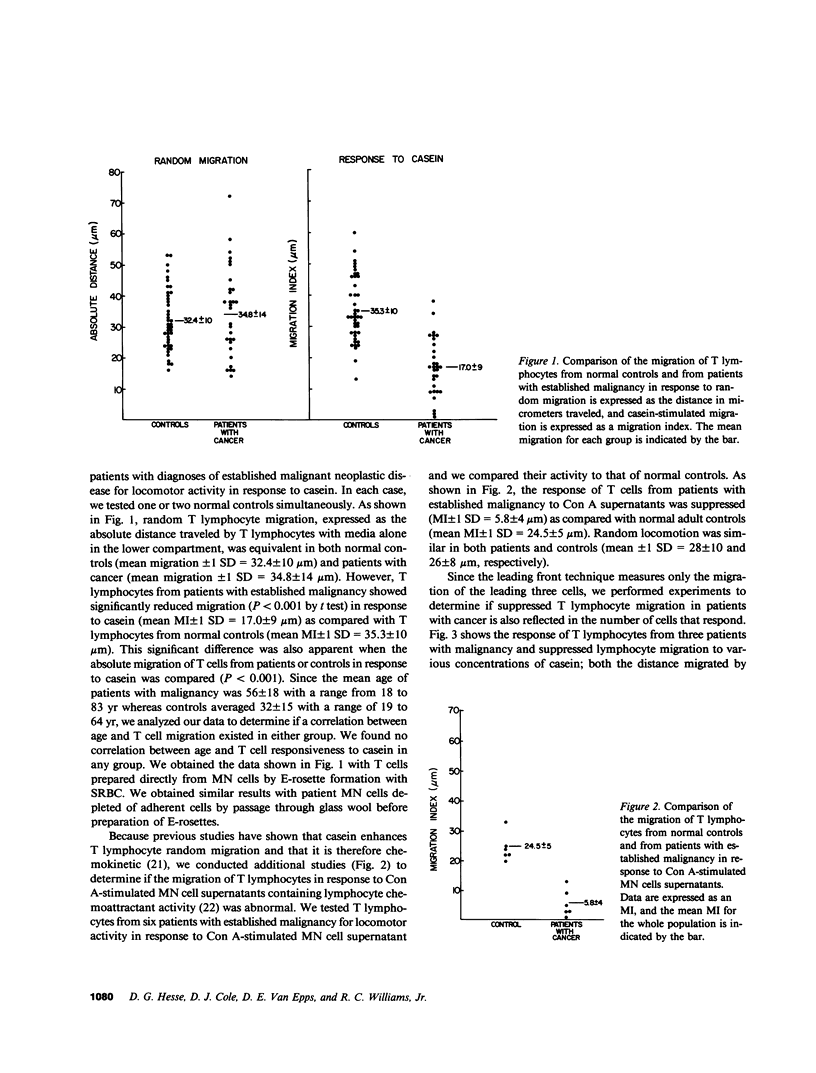
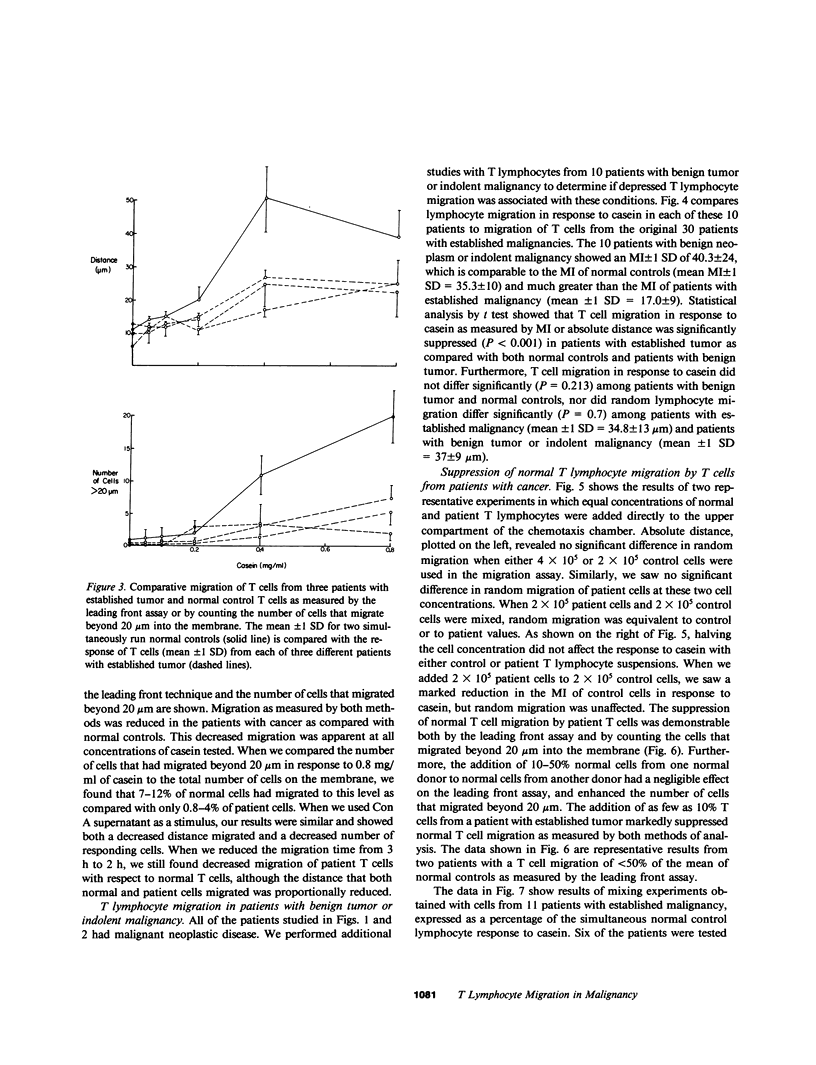
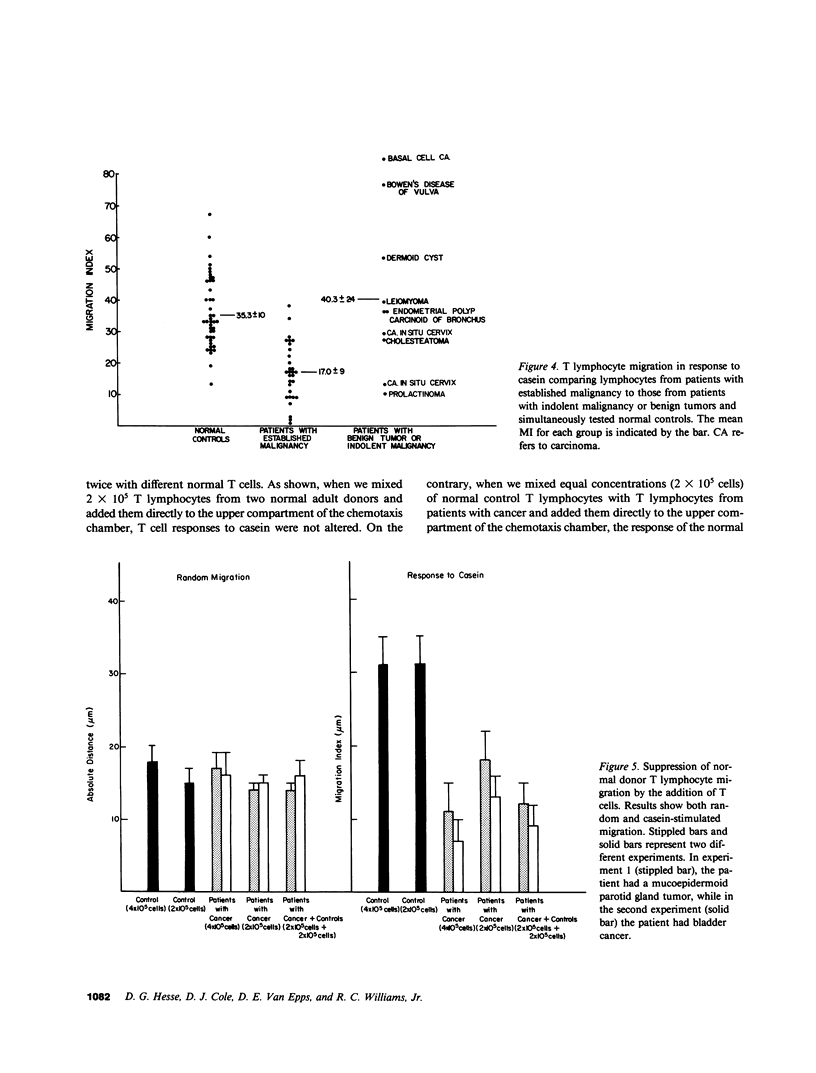
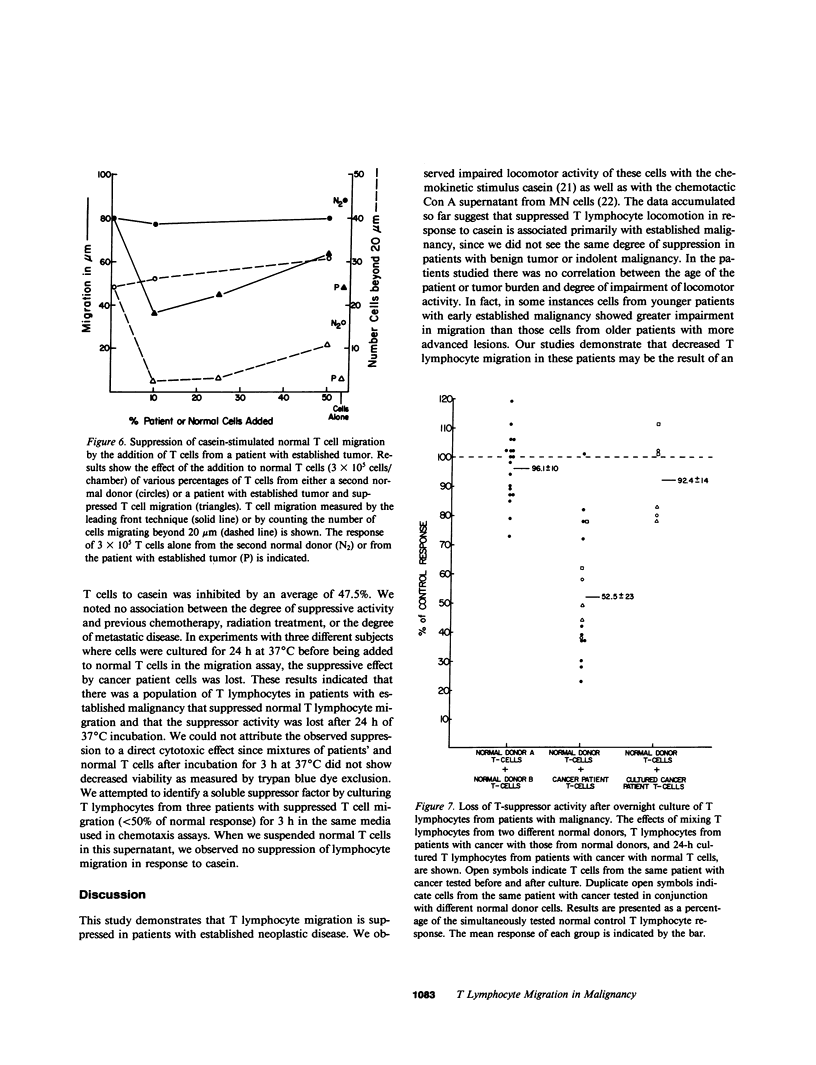
The migration and concentration of lymphocytes at sites of antigenic challenge are an integral part of the expression of delayed cutaneous hypersensitivity, as well as of tumor and graft rejection. In this study, we have analyzed the migration of T lymphocytes from patients with malignancy. We used casein and concanavalin A (Con A)-stimulated mononuclear cell supernatants to stimulate T cell locomotion. Peripheral blood T lymphocytes from 30 patients with established malignancy, 10 patients with indolent malignancy or benign tumor, and 42 normal adult controls were tested. Data are expressed as a migration index (MI), which represents the difference in micrometers between the distance migrated in response to a stimulus and the distance migrated in response to media alone. We observed a marked depression in casein-stimulated T lymphocyte migration in patients with established malignancy (mean MI +/- 1 SD = 17.0 +/- 9 microns) as compared with normal adult controls (mean MI +/- 1 SD = 35.3 +/- 10 microns). Similar results were observed with migration in response to Con A supernatants. T cells from patients with established malignancy had a mean MI of 5.8 +/- 4 microns to Con A supernatants as compared with 24.5 +/- 5 for controls. This depressed migration was apparent both in the distance that cells migrated and in the number of cells that migrated into the membrane. Of 10 patients with indolent malignancy or benign tumor, T cell migration in 8 was not significantly decreased as compared with controls. When we mixed equal concentrations of normal control T lymphocytes with T lymphocytes from patients with cancer and added the mixture directly to the upper compartment of the chemotaxis chamber, the response of the normal T cells to casein was inhibited by an average of 48%. We observed inhibition of this migration of normal cells when we added as little as 10% of patient cells to normal cells. When we mixed normal control T lymphocytes from different donors and added them directly to the upper compartment of the chemotaxis chamber, T lymphocyte migration in response to casein was not significantly altered. If T cells from patients with cancer were cultured overnight, the suppressive effect on lymphocyte locomotion was lost. Our results indicate that there is a population of T lymphocytes in patients with cancer that suppress normal T lymphocyte migration. This suppressor activity may partially explain the subversion of immunosurveillance in established neoplastic states, as well as the defective inflammatory reaction to intradermal injection of antigen observed in many patients with malignancy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler A., Stein J. A., Ben-Efraim S. Immunocompetence, immunosuppression, and human breast cancer. II. Further evidence of initial immune impairment by integrated assessment effect of nodal involvement (N) and of primary tumor size (T). Cancer. 1980 Apr 15;45(8):2061–2073. doi: 10.1002/1097-0142(19800415)45:8<2061::aid-cncr2820450813>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Boetcher D. A., Leonard E. J. Abnormal monocyte chemotactic response in cancer patients. J Natl Cancer Inst. 1974 Apr;52(4):1091–1099. doi: 10.1093/jnci/52.4.1091. [DOI] [PubMed] [Google Scholar]
- Bresnihan B., Jasin H. E. Suppressor function of peripheral blood mononuclear cells in normal individuals and in patients with systemic lupus erythematosus. J Clin Invest. 1977 Jan;59(1):106–116. doi: 10.1172/JCI108607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- Catalona W. J., Sample W. F., Chretien P. B. Lymphocyte reactivity in cancer patients: correlation with tumor histology and clinical stage. Cancer. 1973 Jan;31(1):65–71. doi: 10.1002/1097-0142(197301)31:1<65::aid-cncr2820310109>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Cianciolo G., Hunter J., Silva J., Haskill J. S., Snyderman R. Inhibitors of monocyte responses to chemotaxins are present in human cancerous effusions and react with monoclonal antibodies to the P15(E) structural protein of retroviruses. J Clin Invest. 1981 Oct;68(4):831–844. doi: 10.1172/JCI110338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers A. E., Wunderlich J. R. Suppressor cells in tumor-bearing mice capable of nonspecific blocking of in vitro immunization against transplant antigens. J Immunol. 1975 May;114(5):1554–1556. [PubMed] [Google Scholar]
- Eilber F. R., Morton D. L. Impaired immunologic reactivity and recurrence following cancer surgery. Cancer. 1970 Feb;25(2):362–367. doi: 10.1002/1097-0142(197002)25:2<362::aid-cncr2820250213>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- El-Naggar A. K., Van Epps D. E., Williams R. C., Jr Human-B and T-lymphocyte locomotion in response to casein, C5a, and f-met-leu-phe. Cell Immunol. 1980 Dec;56(2):365–373. doi: 10.1016/0008-8749(80)90112-4. [DOI] [PubMed] [Google Scholar]
- El-Naggar A., Van Epps D. E., Williams R. C., Jr Effect of culturing on the human lymphocyte locomotion response to casein, C5a, and fMet-Leu-Phe. Cell Immunol. 1981 May 1;60(1):43–49. doi: 10.1016/0008-8749(81)90246-x. [DOI] [PubMed] [Google Scholar]
- Fischer A., Durandy A., Griscelli C. Role of prostaglandin E2 in the induction of nonspecific T lymphocyte suppressor activity. J Immunol. 1981 Apr;126(4):1452–1455. [PubMed] [Google Scholar]
- GARDNER R. J., HART J. T., SUTTON W. R., PRESTON F. W. Survival of skin homografts in terminal cancer patients. Surg Forum. 1961;12:167–168. [PubMed] [Google Scholar]
- GARDNER R. J., PRESTON F. W. Prolonged skin homograft survival in advanced cancer and cirrhosis of the liver. Surg Gynecol Obstet. 1962 Oct;115:399–402. [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Peake G. T. Prostaglandin suppression of mitogen-stimulated lymphocytes in vitro. Changes with mitogen dose and preincubation. J Clin Invest. 1978 Oct;62(4):753–760. doi: 10.1172/JCI109186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. Subpopulations of human T lymphocytes. XVI. Maldistribution of T cell subsets associated with abnormal locomotion of T cells in untreated adult patients with Hodgkin's disease. Clin Exp Immunol. 1980 Oct;42(1):186–195. [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Tan C. Subpopulations of human T lymphocytes. XIV. Abnormality of T-cell locomotion and of distribution of subpopulations of T and B lymphocytes in peripheral blood and spleen from children with untreated Hodgkin's disease. Clin Immunol Immunopathol. 1980 Feb;15(2):133–143. doi: 10.1016/0090-1229(80)90026-4. [DOI] [PubMed] [Google Scholar]
- Hillinger S. M., Herzig G. P. Impaired cell-mediated immunity in Hodgkin's disease mediated by suppressor lymphocytes and monocytes. J Clin Invest. 1978 Jun;61(6):1620–1627. doi: 10.1172/JCI109082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Kuperman O., Fortner G. W., Lucas Z. J. Immune response to a syngeneic mammary adenocarcinoma. III. Development of memory and suppressor functions modulating cellular cytotoxicity. J Immunol. 1975 Nov;115(5):1282–1287. [PubMed] [Google Scholar]
- Maderazo E. G., Anton T. F., Ward P. A. Serum-associated inhibition of leukotaxis in humans with cancer. Clin Immunol Immunopathol. 1978 Feb;9(2):166–176. doi: 10.1016/0090-1229(78)90068-5. [DOI] [PubMed] [Google Scholar]
- Nelson H. S. Delayed hypersensitivity in cancer patients: cutaneous and in vitro lymphocyte response to specific antigens. J Natl Cancer Inst. 1969 May;42(5):765–770. [PubMed] [Google Scholar]
- O'Neill G. J., Parrott D. M. Locomotion of human lymphoid cells. I. Effect of culture and con A on T and non-T lymphocytes. Cell Immunol. 1977 Oct;33(2):257–267. doi: 10.1016/0008-8749(77)90156-3. [DOI] [PubMed] [Google Scholar]
- Parrott D. M., Good R. A., O'Neill G. J., Gupta S. Heterogeneity of locomotion in human T cell subsets. Proc Natl Acad Sci U S A. 1978 May;75(5):2392–2395. doi: 10.1073/pnas.75.5.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler W. J., Lum L., Broder S. Fc-receptors on human T lymphocytes. I. Transition of Tgamma to Tmu cells. J Immunol. 1978 Oct;121(4):1540–1548. [PubMed] [Google Scholar]
- Roberts M. M., Jones-Williams W. The delayed hypersensitivity reaction in breast cancer. Br J Surg. 1974 Jul;61(7):549–552. doi: 10.1002/bjs.1800610713. [DOI] [PubMed] [Google Scholar]
- Sibbitt W. L., Jr, Bankhurst A. D., Williams R. C., Jr Studies of cell subpopulations mediating mitogen hyporesponsiveness in patients with Hodgkin's disease. J Clin Invest. 1978 Jan;61(1):55–63. doi: 10.1172/JCI108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C. An inhibitor of macrophage chemotaxis produced by neoplasms. Science. 1976 Apr 23;192(4237):370–372. doi: 10.1126/science.946556. [DOI] [PubMed] [Google Scholar]
- Treves A. J., Cohen I. R., Feldman M. A syngeneic metastatic tumor model in mice: the natural immune response of the host and its manipulation. Isr J Med Sci. 1976 Apr-May;12(4-5):369–384. [PubMed] [Google Scholar]
- Van Epps D. E., Durant D. A., Potter J. W. Migration of human helper/inducer T cells in response to supernatants from Con A-stimulated suppressor/cytotoxic T cells. J Immunol. 1983 Aug;131(2):697–700. [PubMed] [Google Scholar]
- Van Epps D. E. Suppression of human lymphocyte migration by PGE2. Inflammation. 1981 Mar;5(1):81–87. doi: 10.1007/BF00910782. [DOI] [PubMed] [Google Scholar]
- Wanebo H. J., Thaler H. T., Hansen J. A., Rosen P. P., Robbins G. F., Urban J. A., Oettgen H. F., Good R. A. Immunologic reactivity in patients with primary operable breast cancer. Cancer. 1978 Jan;41(1):84–94. doi: 10.1002/1097-0142(197801)41:1<84::aid-cncr2820410113>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Sarraf M., Sardesai S., Vaitkevicius V. K. Clinical immunologic responsiveness in malignant disease. II. In vitro lymphocyte response to phytohemagglutinin and the effect of cytotoxic drugs. Oncology. 1972;26(4):357–368. doi: 10.1159/000224687. [DOI] [PubMed] [Google Scholar]



