Abstract
Introduction
To assess the ability of the shock index (SI) to predict 28-day mortality in traumatic hemorrhagic shock patients treated in the diaspirin cross-linked hemoglobin (DCLHb) resuscitation clinical trials.
Methods
We used data from two parallel DCLHb traumatic hemorrhagic shock efficacy trials, one in U.S. emergency departments, and one in the European Union prehospital setting to assess the relationship between SI values and 28-day mortality.
Results
In the 219 patients, the mean age was 37 years, 64% sustained a blunt injury, 48% received DCLHb, 36% died, and 88% had an SI≥1.0 at study entry. The percentage of patients with an SI≥1.0 dropped by 57% (88 to 38%) from the time of study entry to 120 minutes after study resuscitation (p<0.001). Patients with a SI≥1.0, 1.4, and 1.8 at any time point were 2.3, 2.7, and 3.1 times, respectively, more likely to die by 28 days than were patients with SI values below these cutoffs (p<0.001). Similarly, after 120 minutes of resuscitation, patients with a SI≥1.0 were 3.9× times more likely to die by 28 days (40 vs. 15%, p<0.001). Although the distribution of SI values differed based on treatment group, the receiver operator characeristics data showed no difference in SI predictive ability for 28-day mortality in patients treated with DCLHb.
Conclusion
In these traumatic hemorrhagic shock patients, the shock index correlates with 28-day mortality, with higher SI values indicating greater mortality risk. Although DCLHb treatment did alter the distribution of SI values, it did not influence the ability of the SI to predict 28-day mortality.
INTRODUCTION
Despite efforts to optimize the resuscitation of traumatic hemorrhagic shock patients, significant mortality is still associated with this clinical condition.1–3 The ability to accurately assess the degree of hemorrhage and the volume of resuscitation required to achieve a state of compensated shock is essential to maximizing patient outcomes.4,5 The exact fluid resuscitation volume that achieves a compensated shock state is still not clearly defined, nor are the vital signs that define adequate compensation.6 Even when vital signs are normalized, uncompensated traumatic hemorrhagic shock may persist and remain unnoticed by clinicians unless detected by other means, such as serial serum lactate measurements.7–9
The use of a standardized tool for detecting shock compensation in traumatic hemorrhagic shock patients may optimize their resuscitation and assure adequate perfusion without accelerating hemorrhage.10,11 The shock index (SI) has been proposed as an easy and clinically effective method of detecting uncompensated shock through determining the ratio of heart rate (HR) to systolic blood pressure (SBP) [SI = HR/SBP].12 Published studies to date suggest that a SI > 1 (HR>SBP) generally indicates an uncompensated shock state that may require further resuscitation.13–23
Diaspirin cross-linked hemoglobin (DCLHb), a hemoglobin-based oxygen carrier (HBOC), was studied as a traumatic hemorrhagic shock resuscitation agent in part because of a proposed beneficial pressor effect related to its tetrameric structure23–25 Two parallel efficacy trials in U.S. emergency departments (ED) and in the European Union (EU) pre-hospital setting studied DCLHb not only as an oxygen carrier, but also as a therapeutic agent that could increase critical organ tissue perfusion during hemorrhagic shock.23, 26–29 Although a previous analysis of these studies failed to demonstrate consistent blood pressure changes with DCLHb use, there is still a theoretical concern that any DCLHb pressor effect could alter HR and SBP, causing the resuscitation of traumatic hemorrhagic shock patients to be inadequate, leading to an uncompensated shock state.30 Prior study of these DCLHb data found that a SI ≥ 1 at the time of ED disposition better predicted trauma mortality than did HR or SBP alone, suggesting that an ED resuscitation that achieves HR that is lower than the SBP (SI < 1) may predict an improved survival likelihood for traumatic hemorrhagic shock patients.31
This study analyzed the ability of the SI to predict 28-day mortality in patients resuscitated in the DCLHb clinical trials. The findings from this study may elucidate the use of the SI as a guide to emergent trauma patient resuscitation both in clinical practice and in future HBOC clinical trials.
METHODS
We obtained data from two parallel, multi-center, randomized, single-blinded, normal saline (NS) controlled, efficacy studies of DCLHb in the treatment of severe traumatic hemorrhagic shock patients, which enrolled 98 patients from 17 U.S. trauma centers between February 1997 and January 1998, and 121 patients from 32 EU trauma centers from July 1997 to May 1998.28,29 We pooled the data because of the similarity of the traumatic hemorrhagic shock patients in these studies.
Inclusion criteria required that patients have hemorrhage and proven hypoperfusion as demonstrated by SBP < 90 mm Hg and HR > 120 beats/min, SBP < 90 mm Hg and HR < 60 beats/min, or a base deficit > 15 mEq/L. We excluded from the studies the following patients: those with demonstrated traumatic brain injury; patients with suggested imminent death, patients whose injury occurred more than four hours prior to infusion, patients less than 18 years of age, and pregnant women.
HR and SBP data were obtained for each patient in the U.S. trial at enrollment (Entry, 0 minutes), 30, 60, 90, and 120 minutes as well as after 2, 3, and 4 units of resuscitation fluid infusion, which corresponded to mean times of 46, 62, and 66 minutes, respectively. In the EU trial, values were obtained at enrollment, 15, 30, 45, 60, 90, and 120 minutes. The combined dataset contains data from 219 patients at these 10 collection time points. We used all SI values in the prediction of 28-day mortality, based on the supposition that any abnormal SI value from any time point may suggest uncompensated shock. All heart rates are expressed in beats per minute (beats/min), and all systolic blood pressures are expressed in mmHg.
We determined the SI using the definition of SI = HR/SBP.12 The statistical analysis included the following: 1. mean and standard deviation comparison by two sample t-tests; 2. chi-square testing of demographics; 3. comparisons of the distribution of patients who had elevated SI values of ≥ 1.8, ≥ 1.4, and ≥ 1 in the different treatment, outcome, and mechanism of injury (MOI) groups via 2xN and chi-square testing; and 4. area under the curve (AUC) analysis of SI predictive power using receiver operator characteristic (ROC) curves. We used a logistic regression to model 28-day mortality based on SI on study entry, SI at 120 minutes, maximum SI during resuscitation, MOI, age, and study site (U.S. vs. EU). (IBM SPSS Statistics v20.0, Epi Info StatCalc v3.5.1, Microsoft Excel 2003) The SI cutoff values in this study were chosen based on their potential clinical impact as noted in the discussion. Final patient survival status (lived vs. died) was based on all-cause 28-day mortality.
The database for the current analysis came from the original datasets that were collected by Baxter Healthcare for the U.S. and EU studies. The protocols used in the U.S. and EU clinical trials were approved by the institutional review board (IRB) of each participating institution prior to the enrollment of any subjects. The U.S. study was conducted under federal regulations governing emergency research with an exception to informed consent (21CFR 50.24). We conducted the current analysis of the data with IRB approval from the local institutional review committee.
RESULTS
There were a total of 219 patients studied in the combined dataset, with 55% coming from the EU study (Table 1). The mean age was 37 ± 17 years, 64% of the patients sustained a blunt injury, 48% received DCLHb resuscitation, 73% were male, and the mean injury severity score (ISS) was 30 ± 18. In the 193 patients for whom a study entry SI was available, 88% had an SI ≥ 1. There were no differences in the baseline demographic and clinical variables, as well as predicted mortality, based on treatment group or study site. Actual mortality was 1.5× higher in patients treated with DCLHb as compared to those treated with NS (44 vs. 29%, 95% CI=1.1–3.6, p<0.02).
Table 1.
Patient demographics and clinical variables in the United States (U.S.) and European Union (EU) DCLHb.
| Total | DCLHb | NS | p-value | |
|---|---|---|---|---|
| N (%) | 219 (100%) | 106 (48.4%) | 113 (51.6%) | -- |
| Age (years) | 37.3 ± 17.2 | 36.4 ± 17.6 | 38.1 ± 16.8 | ns |
| Gender | ||||
| Male | 159 (72.6%) | 81 (76.4%) | 78 (69.0%) | ns |
| Female | 60 (27.4%) | 25 (23.6%) | 35 (31.0%) | |
| Study setting | ||||
| US | 98 (44.7%) | 52 (49.1%) | 46 (40.7%) | |
| EU | 121 (55.3%) | 54 (50.9%) | 67 (59.3%) | |
| Mechanism of injury | ||||
| Blunt | 139 (63.5%) | 64 (60.4%) | 75 (66.4%) | ns |
| Penetrating | 80 (36.5%) | 42 (39.6%) | 38 (33.6%) | |
| Blunt injury type | ||||
| MVC | 94 (67.6%) | 43 (67.2%) | 51 (68.0%) | |
| Fall | 31 (22.3%) | 14 (21.9%) | 17 (22.7%) | |
| Other | 14 (10.1%) | 7 (10.9%) | 7 (9.3%) | |
| Penetrating injury type | ||||
| GSW | 35 (43.8%) | 18 (42.8%) | 17 (44.7%) | 0.20 |
| Stab wound | 27 (33.7%) | 13 (31.0%) | 14 (36.8%) | |
| Other | 11 (13.7%) | 9 (21.4%) | 2 (5.3%) | |
| MVC | 6 (7.5%) | 2 (4.8%) | 4 (10.6%) | |
| Fall | 1 (1.3%) | 0 (0.0%) | 1 (2.6%) | |
| Baseline SI | ||||
| SI≥1 | 169 (87.6%) | 83 (87.4%) | 86 (87.8%) | ns |
| SI<1 | 24 (12.4%) | 12 (12.6%) | 12 (12.2%) | |
| ISS | 30.4 ± 18.1% | 31.3 ± 18.8% | 69.6 ± 32.6% | 0.13 |
| TRISS-predicted survival rate | ||||
| Mortality | ||||
| Predicted | 33.8% | 38.0% | 30.4% | |
| Actual | 36.5% (80/219) | 44.3%(47/106) | 29.2%(33/113) | 0.02 |
DCLHb, diaspirin cross-linked hemoglobin; NS, normal saline; MVC, motor vehicle crash; GSW, gun shot wound; TRISS, trauma and injury severity score; ISS, injury severity score; SI, shock index
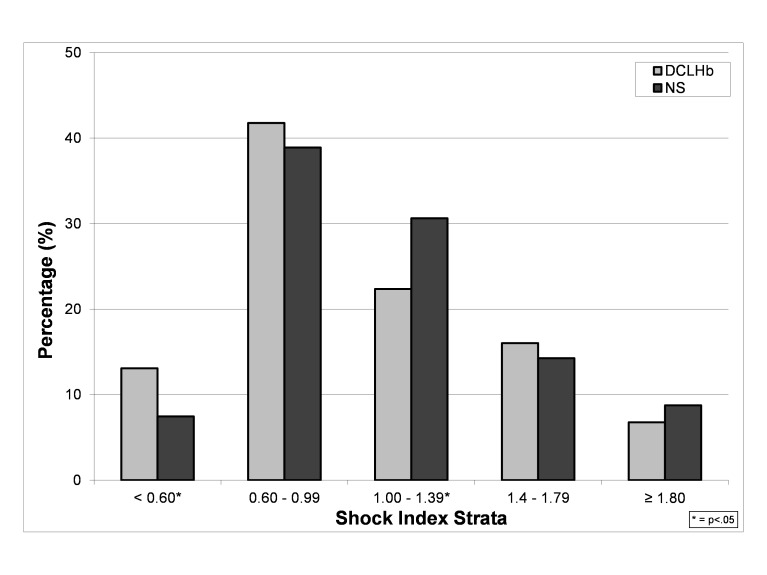
The distribution of the 1297 SI values from all of the time points differed based on treatment group (Figure 1). The incidence of SI < 0.6 values was 75% higher in DCLHb treated patients as compared to NS treated patients (15.2 vs. 8.7%, OR=1.9, 95% CI=1.3–2.8, p<0.001). The incidence of SI values between 1.00 and 1.39 was 46% higher in NS treated patients as compared to DCLHb treated patients (31.2 vs. 21.4%, OR=1.7, 95% CI=1.3–2.2, p<0.001) The incidence of SI ≥ 1.0 values was 19% higher in NS treated patients as compared to DCLHb patients (54 vs. 45%, OR=1.4, 95%CI=1.1–1.8, p<0.003) (Table 2). There were no differences based on treatment group in the incidences of SI values ≥ 1.4 and ≥ 1.8. Higher SI ≥ 1.0 values in NS treated patients (but not at the SI ≥ 1.4 and 1.8 cutoffs) were also observed within each study site subgroup and 28-day outcome subgroup.
Figure 1.
Distribution of shock index values in the United States and European Union DCLHb clinical trials at all time points by treatment.
DCLHb, diasprin crosslinked hemoglobin; NS, normal saline
Table 2.
Elevated shock index frequencies based on clinical trial group and treatment group from the DCLHb traumatic hemorrhagic shock clinical trials.
| Study | SI ≥ 1.8 | p-value | SI ≥ 1.4 | p-value | SI ≥ 1.0 | p-value |
|---|---|---|---|---|---|---|
| Combined | ||||||
| DCLHb | 46/680 (6.8%) | ns | 155/680 (22.7%) | ns | 307/680 (45.1%) | 0.003 |
| NS | 54/617 (8.8%) | 142/617 (23.0%) | 331/617 (53.6%) | |||
| US trial | ||||||
| DCLHb | 18/313 (5.8%) | ns | 69/313 (22.0%) | ns | 136/313 (43.5%) | 0.005 |
| NS | 23/274 (8.4%) | 69/274 (25.2%) | 142/274 (51.8%) | |||
| EU trial | ||||||
| DCLHb | 28/367 (7.6%) | ns | 86/367 (23.4%) | ns | 171/367 (46.6%) | 0.028 |
| NS | 31/343 (9.0%) | 73/343 (21.3%) | 189/343 (55.1%) | |||
| All patients who lived | ||||||
| DCLHb | 12/392 (3.1%) | ns | 60/392 (15.3%) | ns | 139/392 (35.5%) | 0.001 |
| NS | 29/462 (6.3%) | 81/462 (17.5%) | 220/462 (47.6%) | |||
| All patients who died | ||||||
| DCLHb | 34/288(11.8%) | ns | 95/288 (33.0%) | ns | 168/288 (58.3%) | 0.008 |
| NS | 25/155(16.1%) | 61/155 (39.4%) | 111/155 (71.6%) | |||
DCLHb, diaspirin cross-linked hemoglobin; SI, shock index; NS, normal saline; US, United States; EU, European Union
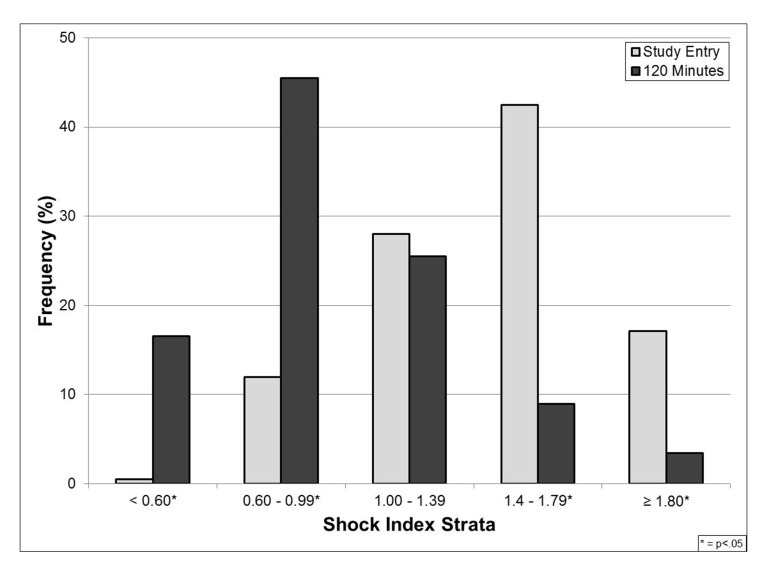
There was no difference in the distribution of SI values based on treatment group at the time of study entry or after 120 minutes of resuscitation. However, the overall distribution of SI values at these two time points did differ as a result of the emergent resuscitation, with a 57% decrease in the number of trauma patients with a SI ≥ 1.0 after 120 minutes of resuscitation (38 vs. 88%, OR=11.5, 95% CI=6.5–20.6, p<0.001) (Figure 2).
Figure 2.
Shock index distribution at study entry and after 120 minutes of resuscitation.
At each study site and in both treatment groups, patients who had an elevated SI value above all three cutoff values were more likely to expire by 28 days (Table 3). Overall, patients with an SI ≥ 1.0, ≥ 1.4, and ≥ 1.8 at any time point had a 2.3× (95% CI=1.85–3.03), 2.7× (95% CI=2.1–3.6), and 3.1× (95% CI=2.0–4.7), respectively, greater odds of dying from the traumatic hemorrhagic shock than patients with all SI values below these cutoffs (p<0.001). At the 120 minutes resuscitation time point, patients with an SI≥1.0 had a 3.9× (95% CI=1.7–8.7) greater odds of dying from the traumatic hemorrhagic shock than patients with an SI value below this cutoff (28-day mortality 40 vs. 15%, p<0.001). At this same 120-minute time point, patients with an SI≥1.4 also had a greater odds of 28-day mortality (61 vs. 20%, OR= 6.4, 95% CI=2.3–18.3, p<.001) and those with an SI≥1.8 trended to have a greater odds of 28-day mortality (60 vs. 23%, OR=5.1, p < .09).
Table 3.
Relationship between elevated shock index values and 28-day mortality from the DCLHb traumatic hemorrhagic shock clinical trials.
| Study | SI ≥ 1.8 | p-value | SI ≥ 1.4 | p-value | SI ≥ 1.0 | p-value |
|---|---|---|---|---|---|---|
| Combined | ||||||
| Lived | 41/854 (4.8%) | 0.001 | 141/854 (16.5%) | 0.001 | 359/854 (42.0%) | 0.001 |
| Died | 59/443(13.3%) | 156/443 (35.2%) | 279/443 (63.0%) | |||
| US trial | ||||||
| Lived | 17/396 (4.3%) | 0.001 | 73/396 (18.4%) | 0.001 | 166/396 (41.9%) | 0.001 |
| Died | 24/191(12.6%) | 65/191 (34.0%) | 112/191 (58.6%) | |||
| EU trial | ||||||
| Lived | 24/458 (5.2%) | 0.001 | 68/458 (14.8%) | 0.001 | 193/458 (42.1%) | 0.001 |
| Died | 35/252(13.9%) | 91/252 (36.1%) | 167/252 (66.3%) | |||
| All DCLHb patients | ||||||
| Lived | 12/392 (3.1%) | 0.001 | 60/392 (15.3%) | 0.001 | 139/392 (35.5%) | 0.001 |
| Died | 34/288(11.8%) | 95/288 (33.0%) | 168/288 (58.3%) | |||
| All normal saline patients | ||||||
| Lived | 29/462 (6.3%) | 0.001 | 81/462 (17.5%) | 0.001 | 220/462 (47.6%) | 0.001 |
| Died | 25/155(16.1%) | 61/155 (39.4%) | 111/155 (71.6%) | |||
DCLHb, diaspirin cross-linked hemoglobin; SI, shock index; NS, normal saline; US, United States; EU, European Union
Patients who suffered a blunt trauma injury had a 1.5× (95% CI=1.2–1.9) and 1.4× (95% CI=1.1–2.0) greater odds of having an SI ≥ 1.0 and an SI ≥ 1.4, respectively, at any time point as compared to patients with a penetrating injury (p<0.001).
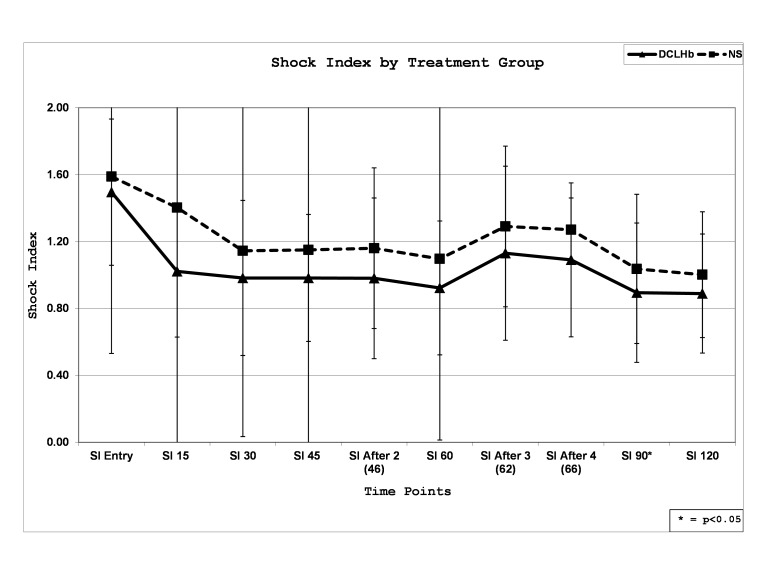
Mean SI values in DCLHb treated patients were comparable during the 120-minute resuscitation period except for the 90-minute time point, which had a higher mean SI value in NS treated patients (p<0.05) (Figure 3). Patients who expired as compared to those who survived had a higher mean SI at all but the “After 4 Units Infused” time point during the 120-minute resuscitation (p<0.05).
Figure 3.
Mean shock index over time by treatment group in the United States and European Union diaspirin cross-linked hemoglobinclinical trials.
SI, shock index; DCLHb, diasprin crosslinked hemoglobin; NS, normal saline
Because of the higher observed mortality in DCLHb treated patients only in the U.S. study, we analyzed mean SI values based on 28-day mortality and treatment group separately in the U.S. and EU studies. Similar to the aggregate analysis, higher mean SI values were more often seen in patients who expired as compared to those who survived in both the U.S. and EU studies. There was no difference in mean SI values over time based on treatment in the EU study.
To determine if the ability of the SI to predict 28-day mortality differed with DCLHb use, we generated ROC curves by treatment group. Although SI was not predictive of outcome at study entry, SI was equally predictive at all of the subsequent time points with an AUC value of 0.71 for patients in both treatment groups. (See supplemental digital content.)
We used logistic regression to analyze the predictive value of relevant clinical variables and SI in determining 28-day mortality. The SI at 120 min (t=12.1 p=0.001) and the trauma MOI (t=9.1, p=0.003) were both significant predictors of 28-day mortality. Logistic regression was also used to analyze the risk of the trauma patients sustaining an elevated SI. Blunt trauma injury was a significant predictor of a patient experiencing an SI ≥ 1.4 (t=10.5, p<0.001), and an SI ≥ 1.8 (t=13.1, p<0.001). Treatment group (DCLHb vs. NS) was not predictive in either logistic regression model.
DISCUSSION
Clinicians are required to assess whether or not traumatic hemorrhagic shock patients are adequately resuscitated in order to assure that tissue perfusion is supported and that critical organ failure does not occur.2,4,5 Although strategies such as “permissive hypotension” are proposed in order to balance the need for adequate tissue oxygenation with the risk of accelerated hemorrhage, there are no published clinical guidelines that state either what is an adequate fluid resuscitation volume or what clinical variables (besides lactate levels and clearance) are best used as endpoints that reliably suggest that adequate resuscitation has occurred.32 As such, it is often difficult to clinically assess the effectiveness of the emergent resuscitation of trauma patients with suspected uncompensated hemorrhagic shock.
The shock index is important clinically as it pairs two readily obtainable vital signs in creating an easily interpretable measure of shock compensation. Both HR and SBP are repeatedly measured during hemorrhagic shock resuscitation in the pre-hospital and in-hospital settings. Multiple SI readings give a clinical assessment of a patient’s shock state both at any given moment and over time. Most importantly, these two values can be assessed by any emergency care provider, including the initial field EMS responder, paramedic, field medic, nurse, mid-level provider, or physician.
Prior analyses of the SI have found that critically ill patients who have relatively normal vital signs can be identified by a SI elevated beyond the normal range of 0.5–0.7. Rady found that a SI > 0.9 was a strong predictor of illness requiring ED resuscitation and admission to an intensive care unit.33 In a pulmonary embolism patient study by Toosi, it was found that a SI ≥ 1 in conjunction with a pulmonary arterial pressure of > 50 mm Hg correlated strongly with increased in-hospital mortality.16 Otero demonstrated that the SI provides high sensitivity in predicting 30-day mortality, and that the independent reading of a SBP < 90 mm Hg provides greater specificity in mortality prediction.17 Zarzaur determined that a SI > 0.83 as a strong predictor of serious shock in patients age ≤ 55.22 In analyzing ectopic pregnancy patients, Birkhahn found that a SI > 0.85 gave a 15× greater chance for adverse events.19
No studies have specifically examined the utility of the shock index in treating traumatic hemorrhagic shock patients, nor was the SI used as a clinical adjunct in the DCLHb or PolyHeme blood substitute clinical trials.28,29,34 In a traumatic hemorrhagic shock patient with a SBP of 90 mm Hg, the SI equals 1.0 when the HR is 90. With this same SBP of 90 mm Hg, the SI equals 1.4 when the HR is 126, and the SI equal 1.8 when the HR is 162, suggesting a significant lack of shock compensation. These cutoffs were empirically chosen for their potential clinical significance in severely traumatized patients with hemorrhagic shock.
Hemoglobin-based oxygen carriers (HBOCs) have been suggested to have a pressor effect that could alter both HR and SBP values in traumatic hemorrhagic shock patients.23 Despite the belief that DCLHb could be associated with the greatest pressor effect because of its tetrameric structure, analysis of the two DCLHb clinical trials did not demonstrate a consistent blood pressure effect with DCLHb infusion.30 Regardless of the absence of a measured pressor effect in the clinical setting with use of DCLHb, concern still exists that the changes in HR and SBP that could be seen with the infusion of HBOCs may lead clinicians to underutilize resuscitation fluids because patients could appear to be adequately resuscitated.25 In the development of the HBOC-201 pre-hospital traumatic hemorrhagic shock clinical trial, this specific concern was made known to the trial developers by the Food and Drug Administration scientists.
The patient population of these paired clinical trials was typical of class III–IV hemorrhage patients, with most patients exhibiting a SI ≥ 1 at study entry and a majority sustaining a blunt mechanism of injury. Patients in the EU study received pre-hospital resuscitation with DCLHb, while U.S. study patients received in-hospital resuscitation. These two clinical trials (U.S. and EU) were similar with respect to all demographics, mechanism of injury, all baseline vital signs, and TRISS-predicted survival. These similarities allowed for a single aggregate analysis of the clinical trials data from these two separate studies, even though the SI values in the two clinical trials were not recorded at exactly the same times after the onset of the shock resuscitation.
The distribution of elevated SI values differed based on resuscitation treatment group (NS vs. DCLHb). NS treated patients more often had SI values in the 1.00 to 1.39 range, indicative of mild uncompensated shock. DCLHb treated patients more often had SI values < 0.6, which was consistent with adequate shock resuscitation. This association could have occurred because of the purported pressor effect which may have raised the SBP in these DCLHb-treated patients. Despite these differences, the relationship between SI and 28-day mortality was not influenced by treatment with DCLHb. Additionally, the finding that there was no difference in the distribution of SI values at the higher cutoffs (SI ≥ 1.4 and SI ≥ 1.8) based on treatment group also suggests that any DCLHb or other HBOC pressor effects may only minimally influence SI values for the most critically ill trauma patients, in whom SI values and 28-day mortality are expected to be the highest.
The ROC prediction curves for DCLHb- and NS-treated patients were the same, suggesting no quantifiable DCLHb treatment effect on the ability of the SI to predict uncompensated shock that caused higher 28-day mortality in these clinical trials.
There was a significant reduction in the number of patients with elevated SI values at the 120- minute time point, suggesting a positive resuscitation effect. The preliminary analysis of the data from these two DCLHb clinical trials found that the SI at the time of ED disposition (after the resuscitation period) was the most predictive of short- and long-term patient outcome.31 Elevated SI values in this present analysis were found to be predictive of higher 28-day mortality at all of the cutoff values during the two hours of resuscitation regardless of MOI or treatment subgroup. Also, patients with SI value ≥ 1 and ≥ 1.4 at 120 minutes had a higher 28-day mortality than those with SI values < 1, regardless of the study or treatment group. These observations suggest that the SI could be used in predicting a persistent uncompensated shock state during or at the end of the acute resuscitation phase.
The higher mortality in the U.S. study with DCLHb infusion was not associated with a significantly different SI value distribution, nor did it influence the relationship between SI values and 28-day mortality. The distribution of SI values differed based on MOI, a finding that could be related to the higher mortality seen with DCLHb use in penetrating trauma patients from the U.S. study, or due to the different quality and severity of injury seen with blunt trauma patients. Overall, mechanism of injury should continue to be examined as a covariate in any future study of SI in the resuscitation of traumatic hemorrhagic shock patients.
LIMITATIONS
This study establishes the potential ability of the SI to predict mortality and the need for further resuscitation in hypovolemic traumatic hemorrhagic shock patients, regardless of treatment with an HBOC product or other resuscitation drugs or devices. These observations are limited by a relatively small patient population, such that further examination of SI effects with HBOC use in traumatic hemorrhagic shock clinical trials be conducted, perhaps using the larger data set available from the PolyHeme study.34 Additionally, there is the possibility for introduction of confounding variables when aggregating data from two study sites, even though study site did not appear to influence the mortality predictive ability of the SI. Lastly, the age of this data is of concern, as resuscitation methods may have changed in a manner significant enough to cause a difference in the relationship between SI and 28-day mortality in current traumatic hemorrhagic shock patients.
Future traumatic hemorrhagic shock clinical trials might assess the value of the SI and other clinically useful tools, such as serial serum lactate levels, in assessing adequate shock compensation and resuscitation. A more current data set from trauma patients treated using current shock resuscitation protocols will address the potential confounding effect of changing treatment methods on the predictive value of the shock index. Also, a data set that does not involve a study intervention may allow the SI to be evaluated without the potential confounding effect of a resuscitation therapy such as DCLHb. Future studies might also determine what minimal fluid requirements are necessary to provide adequate resuscitation, as the SI is of potential utility because it may detect patients who require further fluid resuscitation.35
CONCLUSION
In conclusion, we found a modest effect of DCLHb on the distribution of SI values from these two clinical trials. Regardless of this effect, elevated SI values still correlated strongly with the 28-day mortality of the traumatic hemorrhagic shock patients from these two DCLHb trauma clinical trials, especially at the time point 120 minutes following resuscitation.
Footnotes
Supervising Section Editor: John Sarko, MD
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The US clinical study was originally supported in part by a contract between Baxter and the University of Illinois at Chicago. There were no subsequent grants or contracts to support this particular research or manuscript.
REFERENCES
- 1.Duranteau J, Harrois A. [Hemorrhagic shock]. Rev Prat. 2006;56:849–57. [PubMed] [Google Scholar]
- 2.Kauvar DS, Wade CE. The epidemiology and modern management of traumatic hemorrhage: US and international perspectives. Crit Care. 2005;9(Suppl 5):S1–9. doi: 10.1186/cc3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Rossaint R, Cerny V, Coats TJ, et al. Key issues in advanced bleeding care in trauma. Shock. 2006;26:322–31. doi: 10.1097/01.shk.0000225403.15722.e9. [DOI] [PubMed] [Google Scholar]
- 5.Krausz MM. Initial resuscitation of hemorrhagic shock. World J Emerg Surg. 2006;1:14. doi: 10.1186/1749-7922-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krausz MM. Controversies in shock research: hypertonic resuscitation--pros and cons. Shock. 1995;3:69–72. [PubMed] [Google Scholar]
- 7.Wo CC, Shoemaker WC, Appel PL, et al. Unreliability of blood pressure and heart rate to evaluate cardiac output in emergency resuscitation and critical illness. Crit Care Med. 1993;21:218–23. doi: 10.1097/00003246-199302000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Little RA, Kirkman E, Driscoll P, et al. Preventable deaths after injury: why are the traditional ‘vital’ signs poor indicators of blood loss? J Accid Emerg Med. 1995;12:1–14. doi: 10.1136/emj.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanson JM, Van Hoeyweghen R, Kirkman E, et al. Use of stroke distance in the early detection of simulated blood loss. J Trauma. 1998;44:128–34. doi: 10.1097/00005373-199801000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Dutton RP. Current concepts in hemorrhagic shock. Anesthesiol Clin. 2007;25:23–34. viii. doi: 10.1016/j.atc.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Deitch EA, Dayal SD. Intensive care unit management of the trauma patient. Crit Care Med. 2006;34:2294–301. doi: 10.1097/01.CCM.0000233857.94604.73. [DOI] [PubMed] [Google Scholar]
- 12.Rady MY, Nightingale P, Little RA, et al. Shock index: a re-evaluation in acute circulatory failure. Resuscitation. 1992;23:227–34. doi: 10.1016/0300-9572(92)90006-x. [DOI] [PubMed] [Google Scholar]
- 13.King RW, Plewa MC, Buderer NM, et al. Shock index as a marker for significant injury in trauma patients. Acad Emerg Med. 1996;3:1041–5. doi: 10.1111/j.1553-2712.1996.tb03351.x. [DOI] [PubMed] [Google Scholar]
- 14.Nakasone Y, Ikeda O, Yamashita Y, et al. Shock index correlates with extravasation on angiographs of gastrointestinal hemorrhage: a logistics regression analysis. Cardiovasc Intervent Radiol. 2007;30:861–5. doi: 10.1007/s00270-007-9131-5. [DOI] [PubMed] [Google Scholar]
- 15.Liao CC, Hsu YP, Chen CT, et al. Transarterial embolization for intractable oronasal hemorrhage associated with craniofacial trauma: evaluation of prognostic factors. J Trauma. 2007;63:827–30. doi: 10.1097/TA.0b013e31814b9466. [DOI] [PubMed] [Google Scholar]
- 16.Toosi MS, Merlino JD, Leeper KV. Prognostic value of the shock index along with transthoracic echocardiography in risk stratification of patients with acute pulmonary embolism. Am J Cardiol. 2008;101:700–5. doi: 10.1016/j.amjcard.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 17.Otero R, Trujillo-Santos J, Cayuela A, et al. Haemodynamically unstable pulmonary embolism in the RIETE Registry: systolic blood pressure or shock index? Eur Respir J. 2007;30:1111–6. doi: 10.1183/09031936.00071007. [DOI] [PubMed] [Google Scholar]
- 18.Birkhahn RH, Gaeta TJ, Van Deusen SK, et al. The ability of traditional vital signs and shock index to identify ruptured ectopic pregnancy. Am J Obstet Gynecol. 2003;189:1293–6. doi: 10.1067/s0002-9378(03)00663-x. [DOI] [PubMed] [Google Scholar]
- 19.Birkhahn RH, Gaeta TJ, Bei R, et al. Shock index in the first trimester of pregnancy and its relationship to ruptured ectopic pregnancy. Acad Emerg Med. 2002;9:115–9. doi: 10.1111/j.1553-2712.2002.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 20.Onah HE, Oguanuo TC, Mgbor SO. An evaluation of the shock index in predicting ruptured ectopic pregnancy. J Obstet Gynaecol. 2006;26:445–7. doi: 10.1080/01443610600747314. [DOI] [PubMed] [Google Scholar]
- 21.Kahyaoglu S, Turgay I, Gocmen M, et al. A new predictive scoring system including shock index for unruptured tubal pregnancy patients. Eur J Obstet Gynecol Reprod Biol. 2006;126:99–103. doi: 10.1016/j.ejogrb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Zarzaur BL, Croce MA, Fischer PE, et al. New vitals after injury: shock index for the young and age × shock index for the old. J Surg Res. 2008;147:229–36. doi: 10.1016/j.jss.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 23.Reah G, Bodenham AR, Mallick A, et al. Initial evaluation of diaspirin cross-linked hemoglobin (DCLHb) as a vasopressor in critically ill patients. Crit Care Med. 1997;25:1480–8. doi: 10.1097/00003246-199709000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Przybelski RJ, Daily EK, Micheels J, et al. A safety assessment of diaspirin cross-linked hemoglobin (DCLHb) in the treatment of hemorrhagic, hypovolemic shock. Prehosp Disaster Med. 1999;14:251–64. [PubMed] [Google Scholar]
- 25.Alayash AI, D’Agnillo F, Buehler PW. First-generation blood substitutes: what have we learned? Biochemical and physiological perspectives. Expert Opin Biol Ther. 2007;7:665–75. doi: 10.1517/14712598.7.5.665. [DOI] [PubMed] [Google Scholar]
- 26.Przybelski RJ, Daily EK, Kisicki JC, et al. Phase I study of the safety and pharmacologic effects of diaspirin cross-linked hemoglobin solution. Crit Care Med. 1996;24:1993–2000. doi: 10.1097/00003246-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Baron JF. Blood substitutes. Haemoglobin therapeutics in clinical practice. Crit Care. 1999;3:R99–102. doi: 10.1186/cc365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sloan EP, Koenigsberg M, Gens D, et al. Diaspirin cross-linked hemoglobin (DCLHb) in the treatment of severe traumatic hemorrhagic shock: a randomized controlled efficacy trial. Jama. 1999;282:1857–64. doi: 10.1001/jama.282.19.1857. [DOI] [PubMed] [Google Scholar]
- 29.Kerner T, Ahlers O, Veit S, et al. DCL-Hb for trauma patients with severe hemorrhagic shock: the European “On-Scene” multicenter study. Intensive Care Med. 2003;29:378–85. doi: 10.1007/s00134-002-1622-x. [DOI] [PubMed] [Google Scholar]
- 30.Sloan EP, Philbin NB, Koenigsberg MD, et al. The lack of consistent diaspirin cross-linked hemoglobin infusion blood pressure effects in the US and EU traumatic hemorrhagic shock clinical trials. Shock. 2010;33:123–33. doi: 10.1097/shk.0b013e3181ac482b. [DOI] [PubMed] [Google Scholar]
- 31.Sloan EP, Weir W, Filbin NB, et al. Shock Index Does Not Differ Following the Infusion of DCLHb in Two Clinical Trials of Traumatic Hemorrhagic Shock, Such That Its Clinical Use as a Measure of Compensated Shock is Valid [abstract] Annals of Emergency Medicine. 2008;52:S109. [Google Scholar]
- 32.Beekley AC. Damage control resuscitation: a sensible approach to the exsanguinating surgical patient. Crit Care Med. 2008;36:S267–74. doi: 10.1097/CCM.0b013e31817da7dc. [DOI] [PubMed] [Google Scholar]
- 33.Rady MY, Smithline HA, Blake H, et al. A comparison of the shock index and conventional vital signs to identify acute, critical illness in the emergency department. Ann Emerg Med. 1994;24:685–90. doi: 10.1016/s0196-0644(94)70279-9. [DOI] [PubMed] [Google Scholar]
- 34.Moore EE, Moore FA, Fabian TC, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 2009;208:1–13. doi: 10.1016/j.jamcollsurg.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Bernard AC, Moore EE, Moore FA, et al. Postinjury resuscitation with human polymerized hemoglobin prolongs early survival: a post hoc analysis. J Trauma. 2011;70:S34–7. doi: 10.1097/TA.0b013e31821a586e. [DOI] [PubMed] [Google Scholar]