Abstract
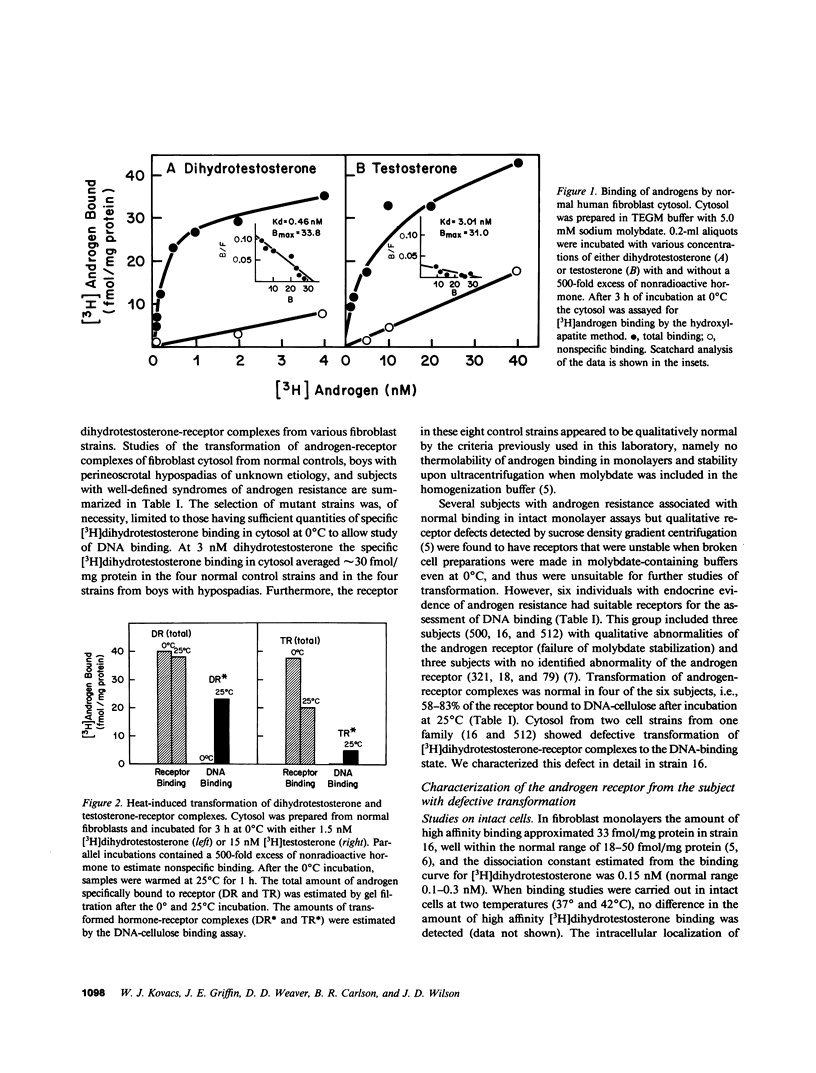
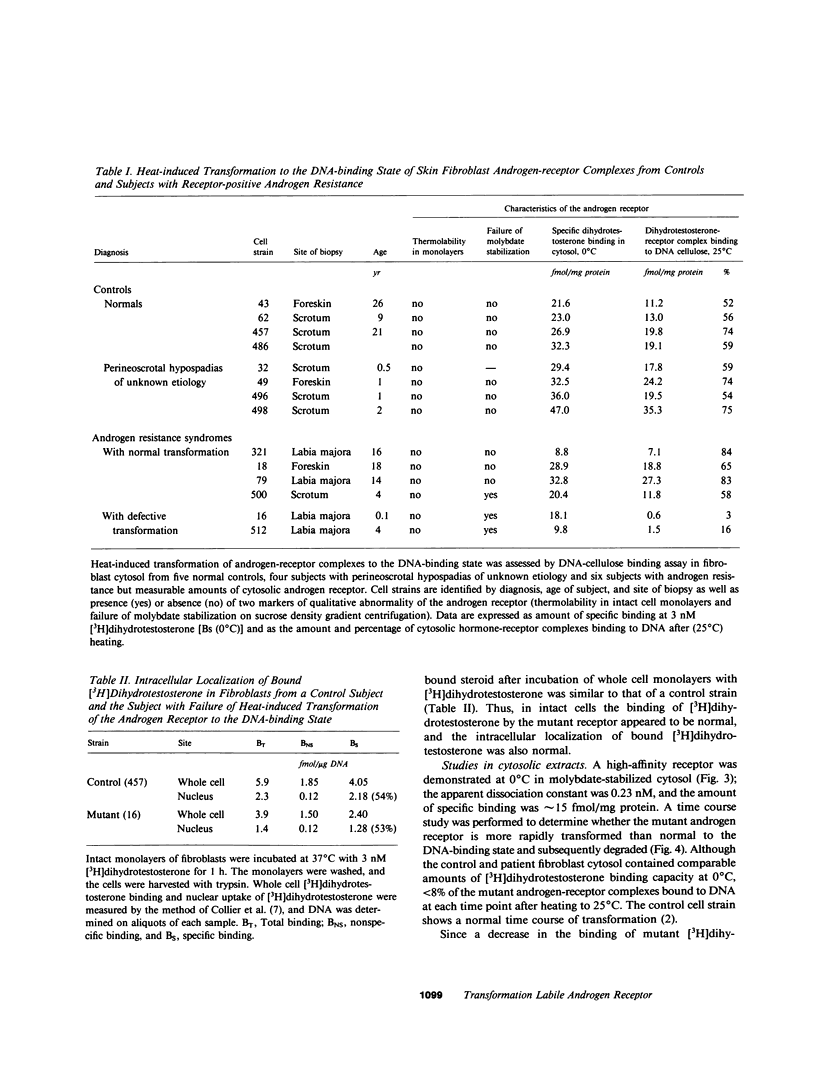
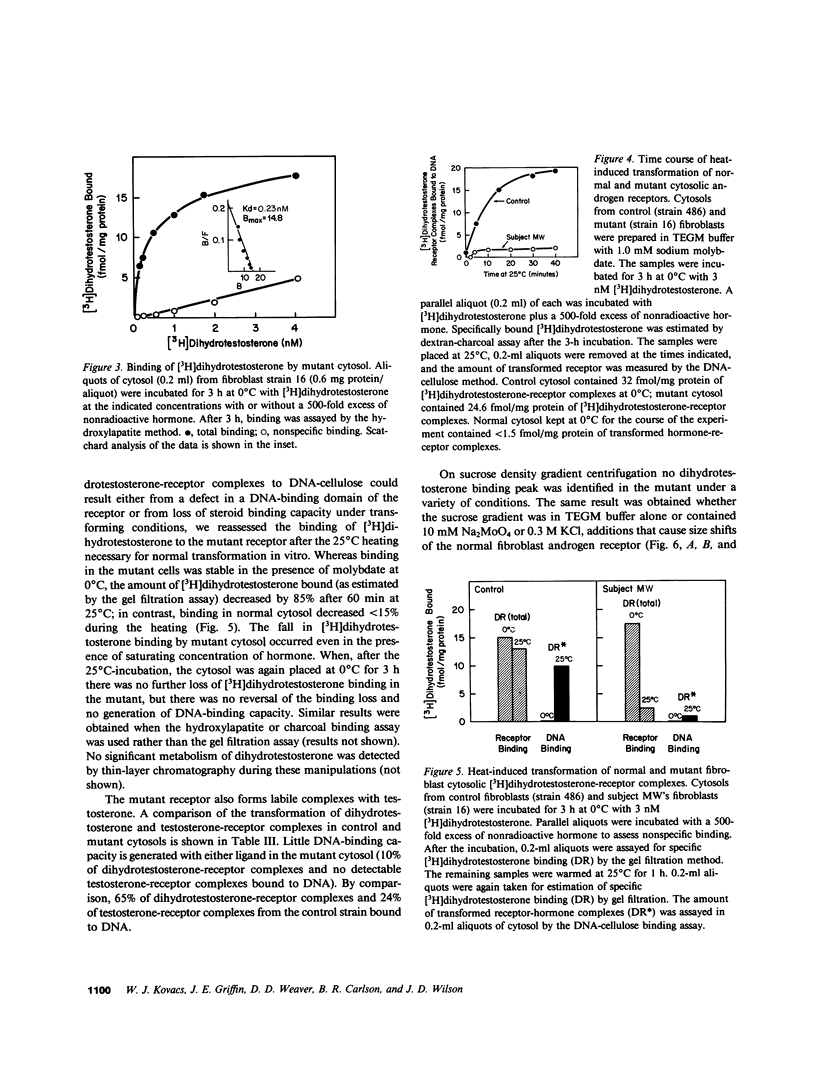
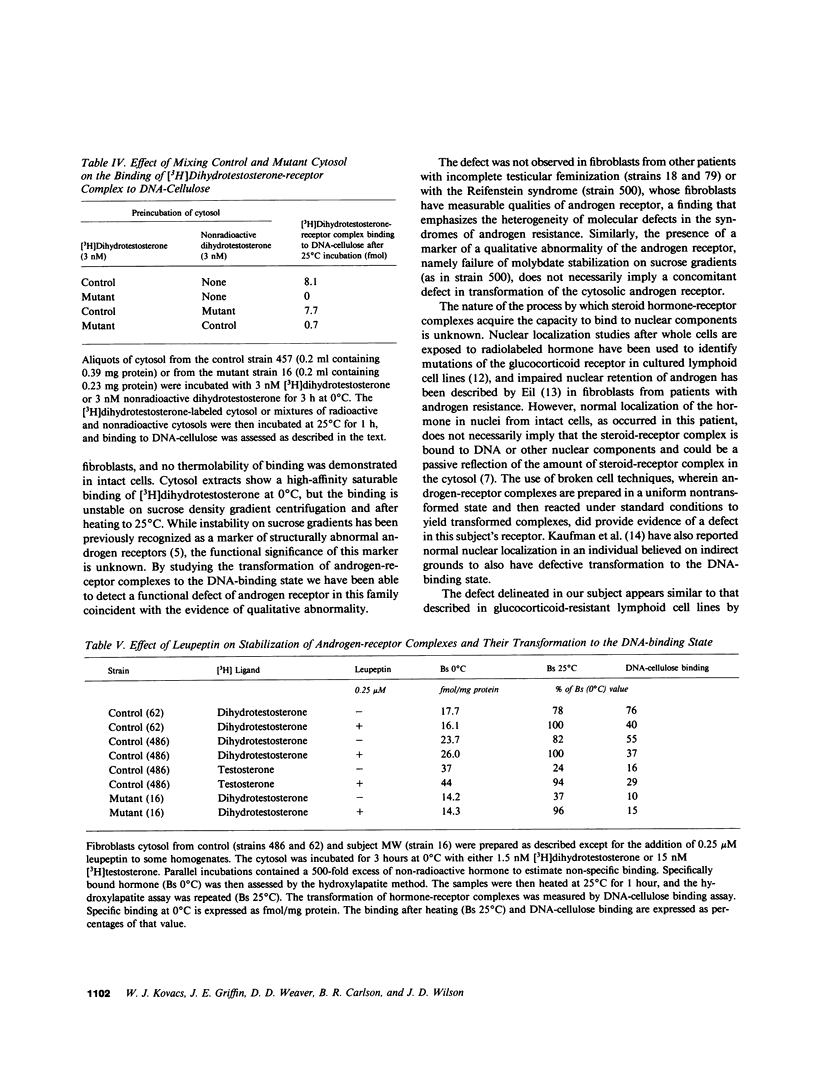
Dihydrotestosterone-receptor complexes formed in human fibroblast cytosol prepared at 0 degrees C in the presence of sodium molybdate can be readily transformed to the DNA-binding state by heating at 25 degrees C. Under these conditions 50-70% of dihydrotestosterone-receptor complexes bind to DNA. We describe here studies of the transformation process in cytosols derived from normal cells and from fibroblasts propagated from subjects with syndromes of androgen resistance. In contrast to the situation with dihydrotestosterone, normal testosterone-receptor complexes are unstable under in vitro transforming conditions. Although equal amounts of hormone-receptor complex are formed at 0 degrees C, only 15% of testosterone-receptor complexes remain stable and acquire DNA-binding capacity after warming. This instability is not reversible upon lowering the temperature and is corrected by low concentrations (0.25 microM) of the protease inhibitor leupeptin. We have also identified two cousins with androgen resistance whose androgen-receptor complexes exhibit similar in vitro transformation lability with both dihydrotestosterone and testosterone. Phenotypic evidence in these subjects indicates that dihydrotestosterone-mediated processes are more completely impaired than are testosterone-mediated events. These findings suggest that dihydrotestosterone may amplify the androgenic signal at its targets not only by its higher affinity for the receptor but also by its more efficient conversion to the DNA-binding state and that such amplification may be less critical in target tissues in which testosterone suffices for androgenic effect. This offers one possible explanation of how a mutation that affects a single receptor protein may differentially impair the actions of two binding ligands of the receptor.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collier M. E., Griffin J. E., Wilson J. D. Intranuclear binding of [3H]dihydrotestosterone by cultured human fibroblasts. Endocrinology. 1978 Oct;103(4):1499–1505. doi: 10.1210/endo-103-4-1499. [DOI] [PubMed] [Google Scholar]
- Eil C. Familial incomplete male pseudohermaphroditism associated with impaired nuclear androgen retention. Studies in cultured skin fibroblasts. J Clin Invest. 1983 Apr;71(4):850–858. doi: 10.1172/JCI110839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. E., Durrant J. L. Qualitative receptor defects in families with androgen resistance: failure of stabilization of the fibroblast cytosol androgen receptor. J Clin Endocrinol Metab. 1982 Sep;55(3):465–474. doi: 10.1210/jcem-55-3-465. [DOI] [PubMed] [Google Scholar]
- Griffin J. E., Punyashthiti K., Wilson J. D. Dihydrotestosterone binding by cultured human fibroblasts. Comparison of cells from control subjects and from patients with hereditary male pseudohermaphroditism due to androgen resistance. J Clin Invest. 1976 May;57(5):1342–1351. doi: 10.1172/JCI108402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. E. Testicular feminization associated with a thermolabile androgen receptor in culutred human fibroblasts. J Clin Invest. 1979 Dec;64(6):1624–1631. doi: 10.1172/JCI109624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. E., Wilson J. D. The syndromes of androgen resistance. N Engl J Med. 1980 Jan 24;302(4):198–209. doi: 10.1056/NEJM198001243020404. [DOI] [PubMed] [Google Scholar]
- Grody W. W., Schrader W. T., O'Malley B. W. Activation, transformation, and subunit structure of steroid hormone receptors. Endocr Rev. 1982 Spring;3(2):141–163. doi: 10.1210/edrv-3-2-141. [DOI] [PubMed] [Google Scholar]
- Hughes M. R., Compton J. G., Schrader W. T., O'Malley B. W. Interaction of the chick oviduct progesterone receptor with deoxyribonucleic acid. Biochemistry. 1981 Apr 28;20(9):2481–2491. doi: 10.1021/bi00512a019. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kaufman M., Pinsky L., Simard L., Wong S. C. Defective activation of androgen-receptor complexes: a marker of androgen insensitivity. Mol Cell Endocrinol. 1982 Feb;25(2):151–162. doi: 10.1016/0303-7207(82)90048-x. [DOI] [PubMed] [Google Scholar]
- Kovacs W. J., Griffin J. E., Wilson J. D. Transformation of human androgen receptors to the deoxyribonucleic acid-binding state. Endocrinology. 1983 Nov;113(5):1574–1581. doi: 10.1210/endo-113-5-1574. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maes M., Sultan C., Zerhouni N., Rothwell S. W., Migeon C. J. Role of testosterone binding to the androgen receptor in male sexual differentiation of patients with 5 alpha-reductase deficiency. J Steroid Biochem. 1979 Oct;11(4):1385–1392. doi: 10.1016/0022-4731(79)90110-9. [DOI] [PubMed] [Google Scholar]
- Montelaro R. C., Rueckert R. R. Radiolabeling of proteins and viruses in vitro by acetylation with radioactive acetic anhydride. J Biol Chem. 1975 Feb 25;250(4):1413–1421. [PubMed] [Google Scholar]
- Payvar F., Wrange O., Carlstedt-Duke J., Okret S., Gustafsson J. A., Yamamoto K. R. Purified glucocorticoid receptors bind selectively in vitro to a cloned DNA fragment whose transcription is regulated by glucocorticoids in vivo. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6628–6632. doi: 10.1073/pnas.78.11.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfahl M. Specific binding of the glucocorticoid-receptor complex to the mouse mammary tumor proviral promoter region. Cell. 1982 Dec;31(2 Pt 1):475–482. doi: 10.1016/0092-8674(82)90140-4. [DOI] [PubMed] [Google Scholar]
- Schmidt T. J., Harmon J. M., Thompson E. B. 'Activation-labile' glucocorticoid-receptor complexes of a steroid-resistant variant of CEM-C7 human lymphoid cells. Nature. 1980 Jul 31;286(5772):507–510. doi: 10.1038/286507a0. [DOI] [PubMed] [Google Scholar]
- Sherman M. R., Tuazon F. B., Miller L. K. Estrogen receptor cleavage and plasminogen activation by enzymes in human breast tumor cytosol. Endocrinology. 1980 Jun;106(6):1715–1727. doi: 10.1210/endo-106-6-1715. [DOI] [PubMed] [Google Scholar]
- Wilbert D. M., Griffin J. E., Wilson J. D. Characterization of the cytosol androgen receptor of the human prostate. J Clin Endocrinol Metab. 1983 Jan;56(1):113–120. doi: 10.1210/jcem-56-1-113. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Stampfer M. R., Tomkins G. M. Receptors from glucocorticoid-sensitive lymphoma cells and two clases of insensitive clones: physical and DNA-binding properties. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3901–3905. doi: 10.1073/pnas.71.10.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]



