Abstract
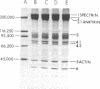
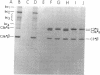
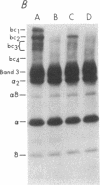
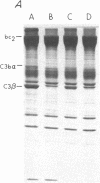
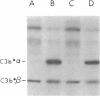
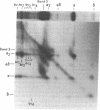
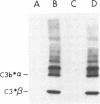
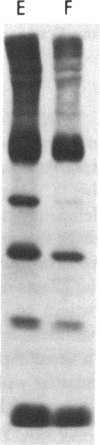
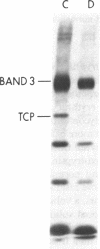
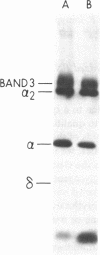
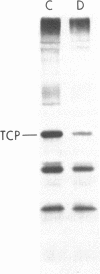
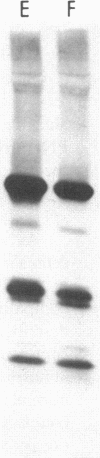
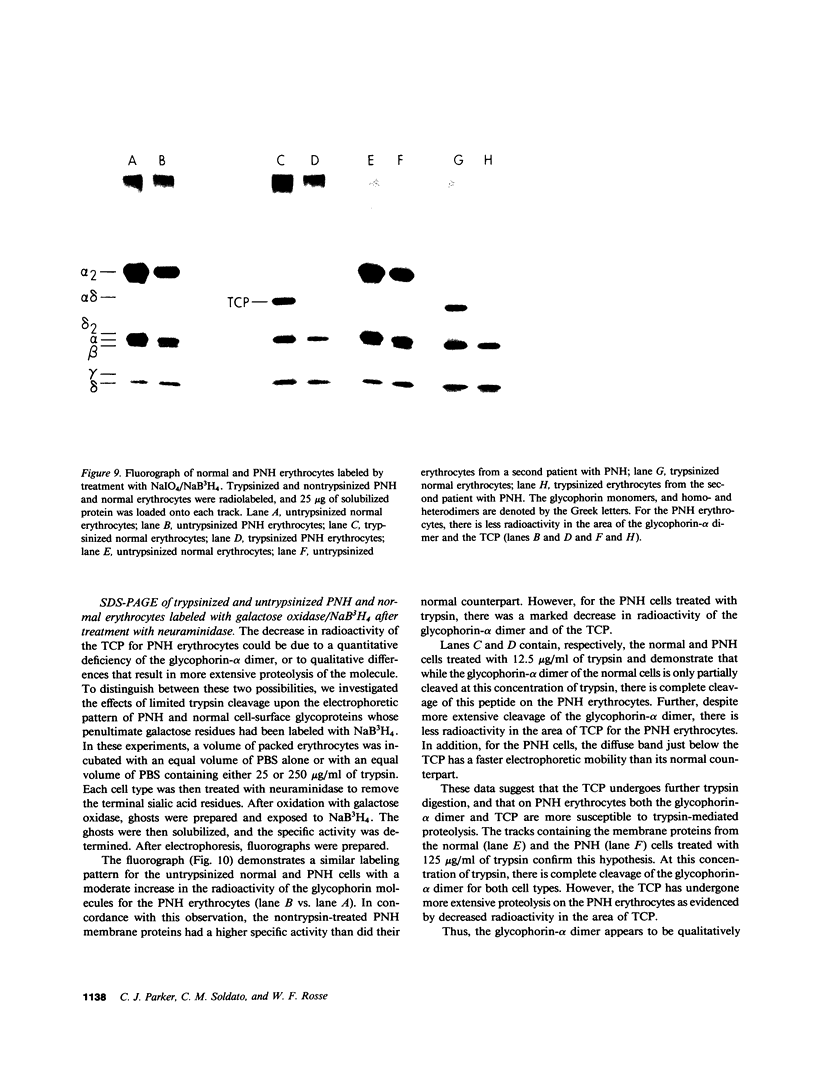
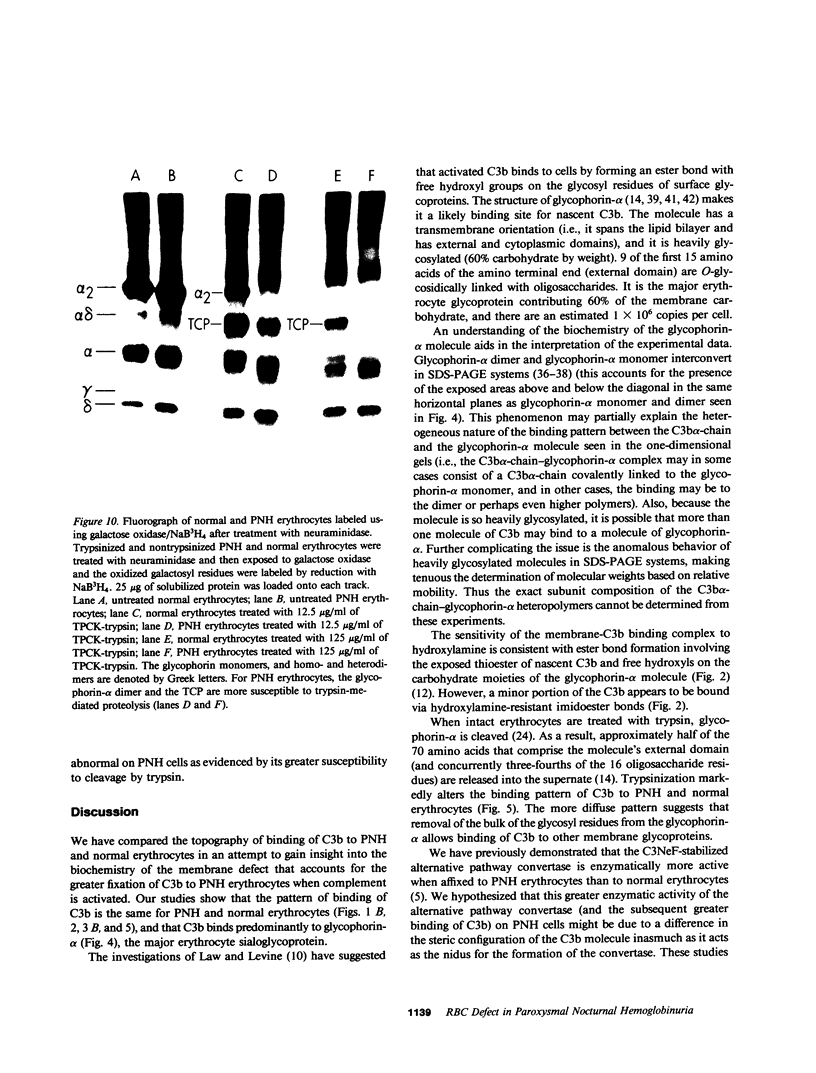
To investigate the greater enzymatic activity of the alternative pathway convertase (and the subsequent greater fixation of C3b) on paroxysmal nocturnal hemoglobinuria (PNH) erythrocytes, we have examined the topography of binding of C3b to PNH and normal erythrocytes. Using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography, the alpha-chain of C3b was found to bind via predominantly ester bonds to free hydroxyl groups on glycophorin-alpha, the major erythrocyte sialoglycoprotein. The pattern of binding of nascent C3b was the same for normal and PNH erythrocytes. Thus, although C3b binding to a different membrane constituent did not appear to account for the greater enzymatic activity of the alternative pathway convertase when affixed to PNH erythrocytes, it seemed possible that the glycoproteins to which C3b bound might be qualitatively abnormal on the PNH cells, and that structural differences in these molecules might impose modifications in the enzyme-substrate interactions of the alternative pathway convertase. Using methods for radiolabeling both protein and carbohydrate residues, we therefore compared the electrophoretic pattern of the cell-surface glycoproteins on PNH and normal erythrocytes. The glycophorin-alpha dimer was found to be qualitatively abnormal on the PNH cells as evidenced by its greater susceptibility to trypsin-mediated proteolysis. In addition, the abnormal erythrocytes from patients with PNH had fewer periodate oxidizable constituents than did normal erythrocytes, indicating a relative deficiency of cell-surface sialic acid. These investigations suggest that abnormalities in membrane glycoproteins may underlie the aberrant interactions of complement with the hematopoietic elements of PNH.
Full text
PDF


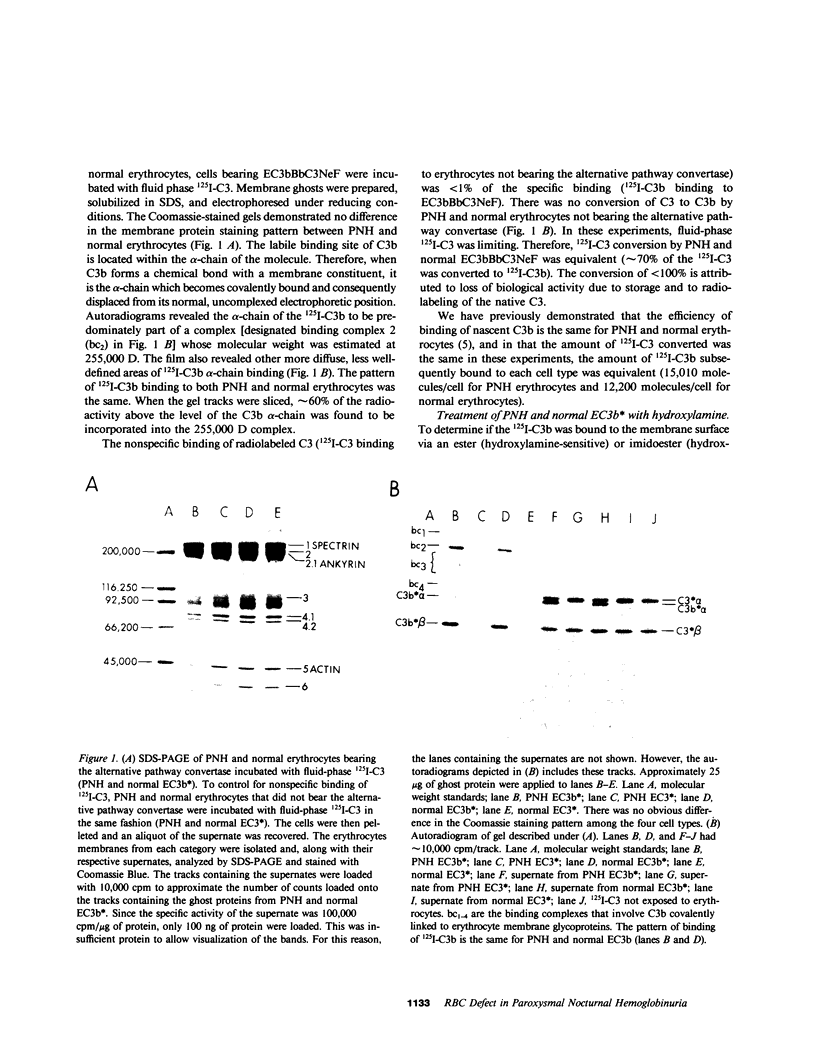
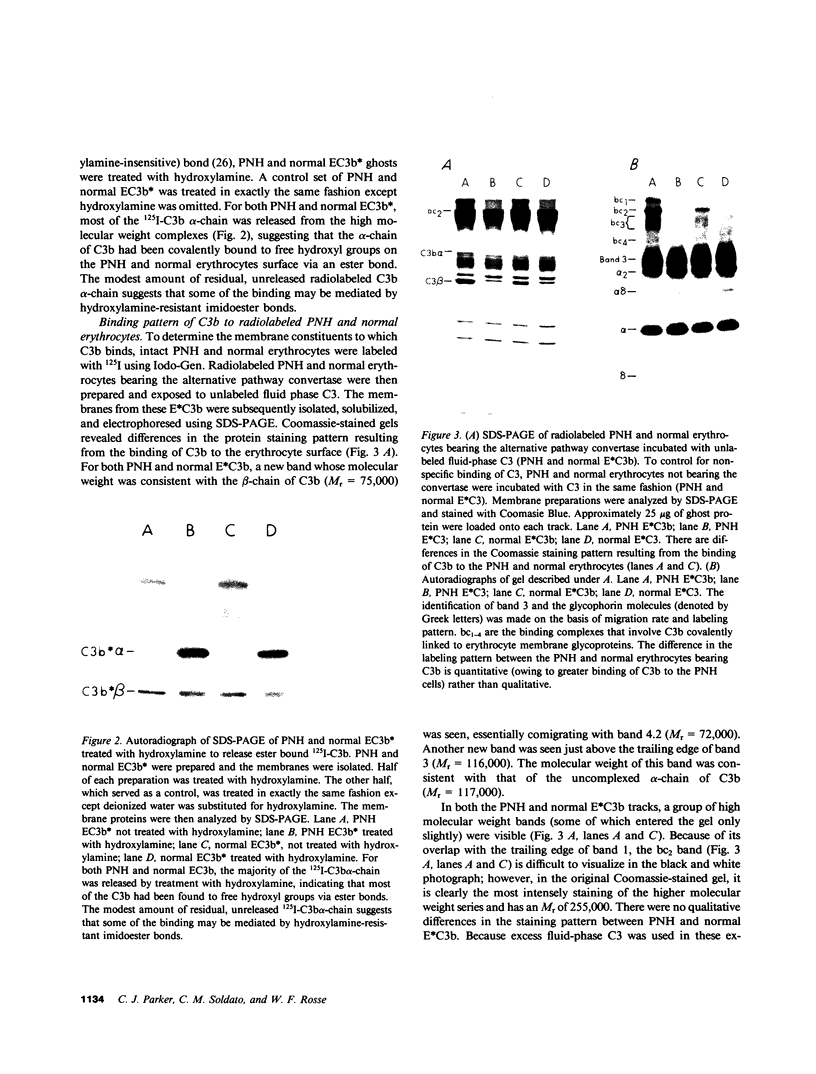
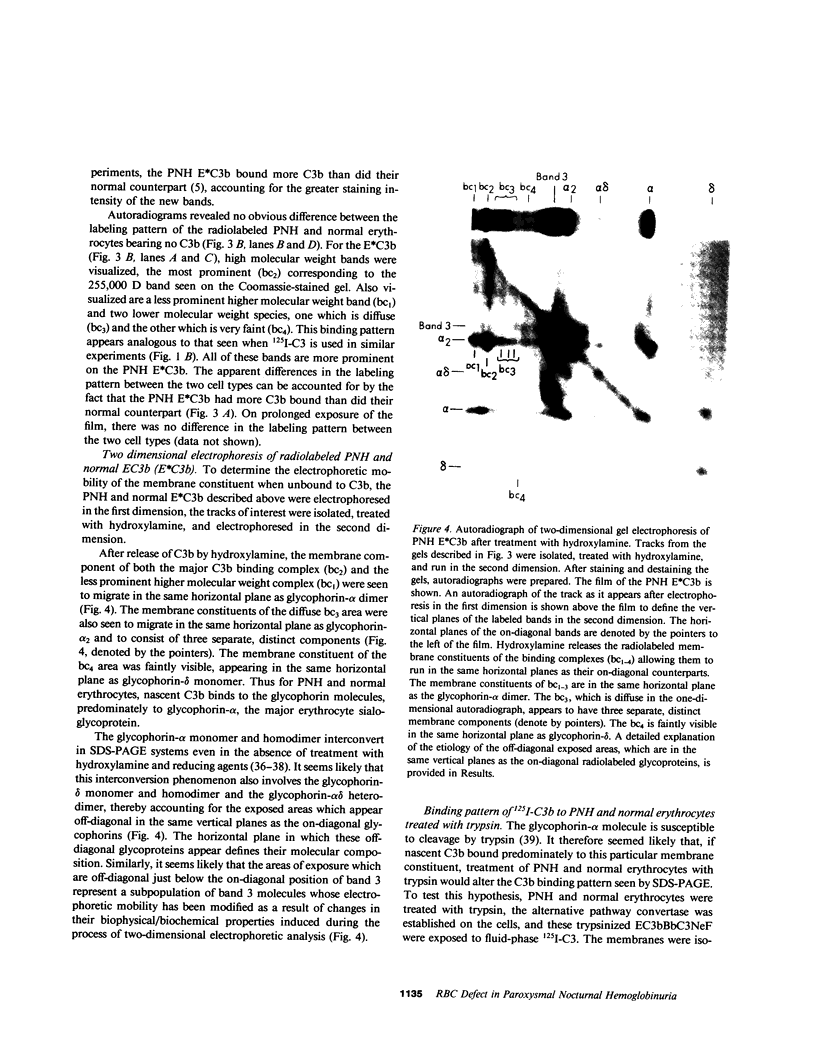
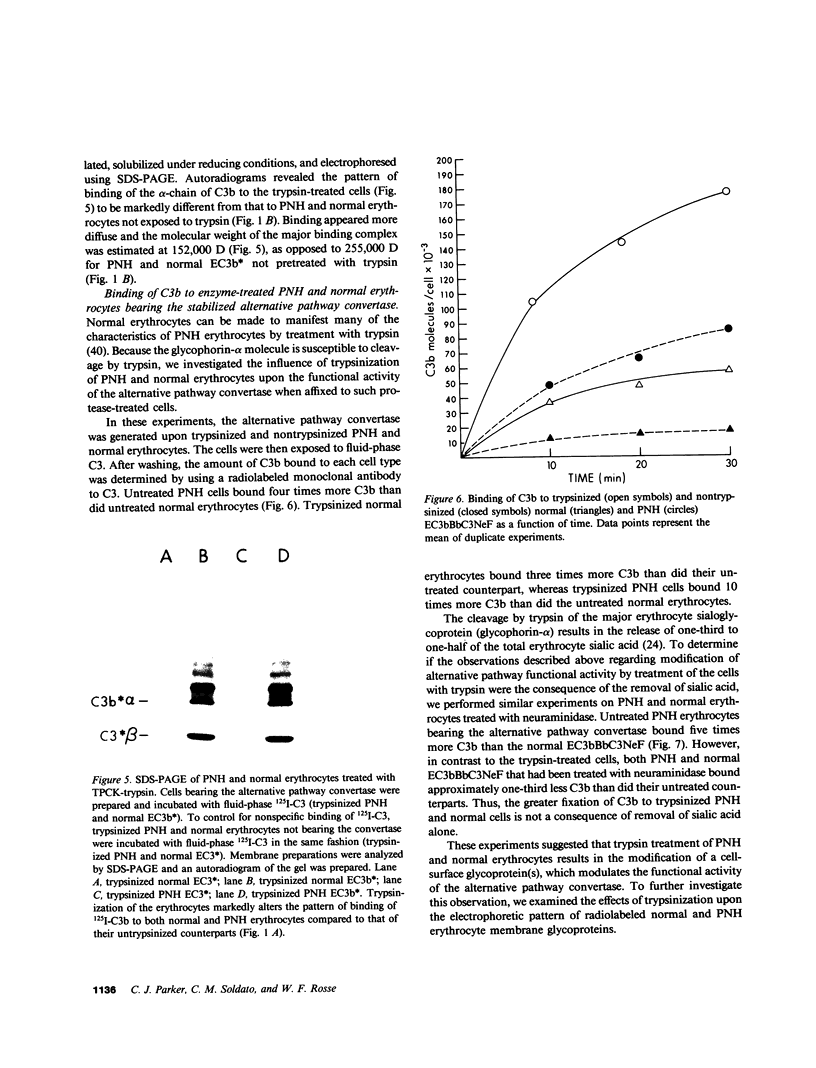
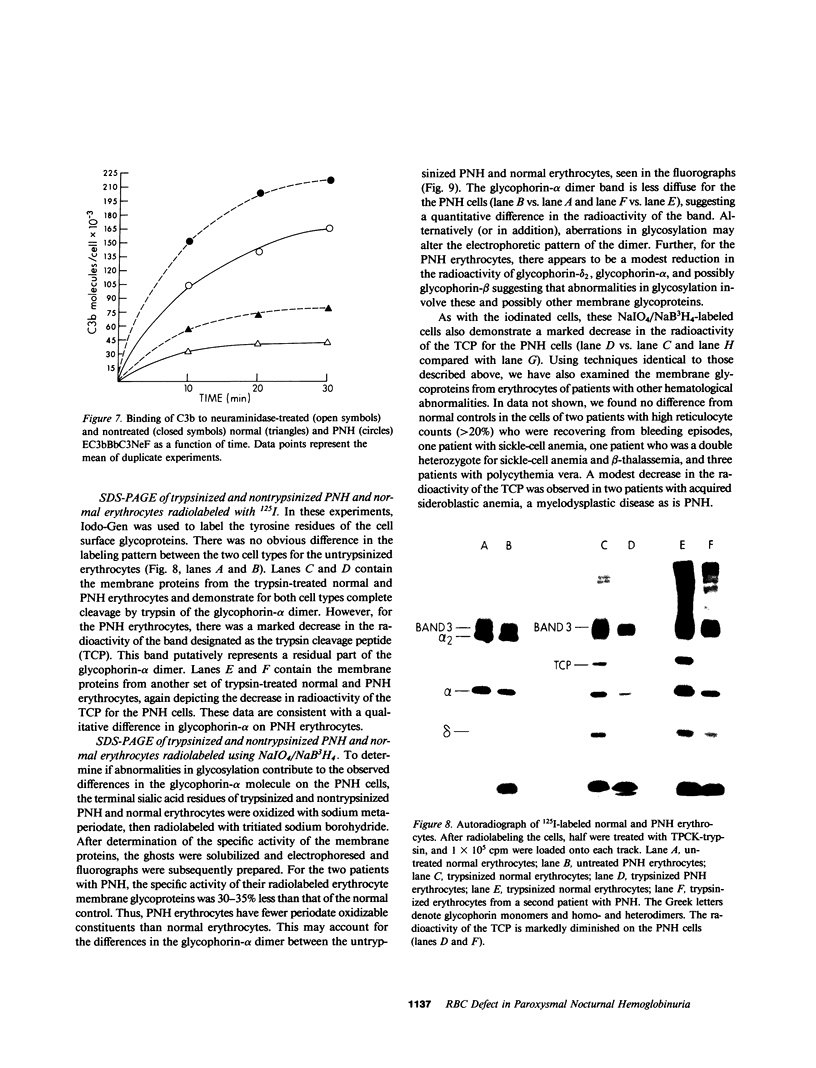




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anselstetter V., Horstmann H. J., Heimpel H. Congenital dyserythropoietic anaemia, types I and II: aberrant pattern of erythrocyte membrane proteins in CDA II, as revealed by two-dimensional polyacrylamide gel electrophoresis. Br J Haematol. 1977 Feb;35(2):209–215. doi: 10.1111/j.1365-2141.1977.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Anstee D. J. The blood group MNSs-active sialoglycoproteins. Semin Hematol. 1981 Jan;18(1):13–31. [PubMed] [Google Scholar]
- Aster R. H., Enright S. E. A platelet and granulocyte membrane defect in paroxysmal nocturnal hemoglobinuria: usefulness for the detection of platelet antibodies. J Clin Invest. 1969 Jul;48(7):1199–1210. doi: 10.1172/JCI106084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines A. J., Banga J. P., Gratzer W. B., Linch D. C., Huehns E. R. Red cell membrane protein anomalies in congenital dyserythropoietic anaemia, type II (HEMP AS). Br J Haematol. 1982 Apr;50(4):563–574. doi: 10.1111/j.1365-2141.1982.tb01956.x. [DOI] [PubMed] [Google Scholar]
- Ballow M., Cochrane C. G. Two anticomplementary factors in cobra venom: hemolysis of guinea pig erythrocytes by one of them. J Immunol. 1969 Nov;103(5):944–952. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Joiner K. A., Gaither T. A., Hammer C. H., Frank M. M. The interaction of C3b bound to pneumococci with factor H (beta 1H globulin), factor I (C3b/C4b inactivator), and properdin factor B of the human complement system. J Immunol. 1983 Jul;131(1):409–415. [PubMed] [Google Scholar]
- Brown E. J., Ramsey J., Hammer C. H., Frank M. M. Surface modulation of classical pathway activation: C2 and C3 convertase formation and regulation on sheep, guinea pig, and human erythrocytes. J Immunol. 1983 Jul;131(1):403–408. [PubMed] [Google Scholar]
- Dixon R. H., Rosse W. F. Mechanism of complement-mediated activation of human blood platelets in vitro: comparison of normal and paroxysmal nocturnal hemoglobinuria platelets. J Clin Invest. 1977 Feb;59(2):360–368. doi: 10.1172/JCI108648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Current concepts in immunology: the alternative pathway of complement--a system for host resistance to microbial infection. N Engl J Med. 1980 Jul 31;303(5):259–263. doi: 10.1056/NEJM198007313030505. [DOI] [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Furthmayr H. Glycophorins A, B, and C: a family of sialoglycoproteins. Isolation and preliminary characterization of trypsin derived peptides. J Supramol Struct. 1978;9(1):79–95. doi: 10.1002/jss.400090109. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Marchesi V. T. Subunit structure of human erythrocyte glycophorin A. Biochemistry. 1976 Mar 9;15(5):1137–1144. doi: 10.1021/bi00650a028. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977 Aug 25;252(16):5888–5894. [PubMed] [Google Scholar]
- Gahmberg C. G. External labeling of human erythrocyte glycoproteins. Studies with galactose oxidase and fluorography. J Biol Chem. 1976 Jan 25;251(2):510–515. [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Gahmberg C. G., Myllyla G., Leikola J., Pirkola A., Nordling S. Absence of the major sialoglycoprotein in the membrane of human En(a--) erythrocytes and increased glycosylation of band 3. J Biol Chem. 1976 Oct 10;251(19):6108–6116. [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. Lysis of erythrocytes by complement in the absence of antibody. J Exp Med. 1970 Nov;132(5):898–915. doi: 10.1084/jem.132.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R. C., Arnold A. B. Paroxysmal nocturnal hemoglobinuria (PNH) as a clonal disorder. Annu Rev Med. 1977;28:187–194. doi: 10.1146/annurev.me.28.020177.001155. [DOI] [PubMed] [Google Scholar]
- Hatton M. W., Regoeczi E. The proteolytic nature of commercial samples of galactose oxidase. Purification of the enzyme by a simple affinity method. Biochim Biophys Acta. 1976 Jul 8;438(2):339–346. doi: 10.1016/0005-2744(76)90251-5. [DOI] [PubMed] [Google Scholar]
- Hinz C. F., Jr Acid hemolysis revisited. Semin Hematol. 1976 Jul;13(3):201–209. [PubMed] [Google Scholar]
- Howard J. B. Methylamine reaction and denaturation-dependent fragmentation of complement component 3. Comparison with alpha2-macroglobulin. J Biol Chem. 1980 Aug 10;255(15):7082–7084. [PubMed] [Google Scholar]
- Hänsch G. M., Hammer C. H., Vanguri P., Shin M. L. Homologous species restriction in lysis of erythrocytes by terminal complement proteins. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5118–5121. doi: 10.1073/pnas.78.8.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Mornaghi R., Nussenzweig V. Complement receptor (CR1) deficiency in erythrocytes from patients with systemic lupus erythematosus. J Exp Med. 1982 May 1;155(5):1427–1438. doi: 10.1084/jem.155.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Warren K. A., Brown E. J., Swanson J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. IV. C5b-9 forms high molecular weight complexes with bacterial outer membrane constituents on serum-resistant but not on serum-sensitive Neisseria gonorrhoeae. J Immunol. 1983 Sep;131(3):1443–1451. [PubMed] [Google Scholar]
- Joiner K., Brown E., Hammer C., Warren K., Frank M. Studies on the mechanism of bacterial resistance to complement-mediated killing. III. C5b-9 deposits stably on rough and type 7 S. pneumoniae without causing bacterial killing. J Immunol. 1983 Feb;130(2):845–849. [PubMed] [Google Scholar]
- Jokinen M. Characterization of Glycophorin A and band 3 from Tn polyagglutinable erythrocytes. Scand J Haematol. 1981 Apr;26(4):272–280. doi: 10.1111/j.1600-0609.1981.tb01659.x. [DOI] [PubMed] [Google Scholar]
- Kazatchkine M. D., Fearon D. T., Austen K. F. Human alternative complement pathway: membrane-associated sialic acid regulates the competition between B and beta1 H for cell-bound C3b. J Immunol. 1979 Jan;122(1):75–81. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Law S. K., Levine R. P. Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Holcombe F. H., Levine R. P. Interaction between the labile binding sites of the fourth (C4) and fifth (C5) human complement proteins and erythrocyte cell membranes. J Immunol. 1980 Aug;125(2):634–639. [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979 Sep;123(3):1388–1394. [PubMed] [Google Scholar]
- Law S. K., Minich T. M., Levine R. P. Binding reaction between the third human complement protein and small molecules. Biochemistry. 1981 Dec 22;20(26):7457–7463. doi: 10.1021/bi00529a020. [DOI] [PubMed] [Google Scholar]
- Logue G. L., Rosse W. F., Adams J. P. Mechanisms of immune lysis of red blood cells in vitro. I. Paroxysmal nocturnal hemoglobinuria cells. J Clin Invest. 1973 May;52(5):1129–1137. doi: 10.1172/JCI107279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T. Functional proteins of the human red blood cell membrane. Semin Hematol. 1979 Jan;16(1):3–20. [PubMed] [Google Scholar]
- Marchesi V. T., Furthmayr H., Tomita M. The red cell membrane. Annu Rev Biochem. 1976;45:667–698. doi: 10.1146/annurev.bi.45.070176.003315. [DOI] [PubMed] [Google Scholar]
- Marton L. S., Garvin L. E. Subunit structure of the major human erythrocytes glycoprotein: depolymerization by heating ghosts with sodium dodecyl sulfate. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1457–1462. doi: 10.1016/0006-291x(73)90664-5. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Schreiber R. D. Molecular biology and chemistry of the alternative pathway of complement. Adv Immunol. 1980;29:1–53. doi: 10.1016/s0065-2776(08)60042-5. [DOI] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosenfeld S. I., Austen K. F. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5066–5070. doi: 10.1073/pnas.80.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurden A. T., Caen J. P. The different glycoprotein abnormalities in thrombasthenic and Bernard-Soulier platelets. Semin Hematol. 1979 Jul;16(3):234–250. [PubMed] [Google Scholar]
- Ochiai Y., Furthmayr H., Marcus D. M. Diverse specificities of five monoclonal antibodies reactive with glycophorin A of human erythrocytes. J Immunol. 1983 Aug;131(2):864–868. [PubMed] [Google Scholar]
- Packman C. H., Rosenfeld S. I., Jenkins D. E., Jr, Thiem P. A., Leddy J. P. Complement lysis of human erythrocytes. Differeing susceptibility of two types of paroxysmal nocturnal hemoglobinuria cells to C5b-9. J Clin Invest. 1979 Aug;64(2):428–433. doi: 10.1172/JCI109479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2416–2420. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Relation of putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J Exp Med. 1980 Oct 1;152(4):1102–1114. doi: 10.1084/jem.152.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5430–5434. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Trombold J. S., Müller-Eberhard H. J. Paroxysmal nocturnal hemoglobinuria: deficiency in factor H-like functions of the abnormal erythrocytes. J Exp Med. 1983 Jun 1;157(6):1971–1980. doi: 10.1084/jem.157.6.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. J., Baker P. J., Rosse W. F. Comparison of binding characteristics of factors B and H to C3b on normal and paroxysmal nocturnal hemoglobinuria erythrocytes. J Immunol. 1983 Nov;131(5):2484–2489. [PubMed] [Google Scholar]
- Parker C. J., Baker P. J., Rosse W. F. Increased enzymatic activity of the alternative pathway convertase when bound to the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1982 Feb;69(2):337–346. doi: 10.1172/JCI110457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F., Adams J. P., Thorpe A. M. The population of cells in paroxysmal nocturnal haemoglobinuria of intermediate sensitivity to complement lysis: significance and mechanism of increased immune lysis. Br J Haematol. 1974 Oct;28(2):181–190. doi: 10.1111/j.1365-2141.1974.tb06652.x. [DOI] [PubMed] [Google Scholar]
- Rosse W. F., Dacie J. V. Immune lysis of normal human and paroxysmal nocturnal hemoglobinuria (PNH) red blood cells. I. The sensitivity of PNH red cells to lysis by complement and specific antibody. J Clin Invest. 1966 May;45(5):736–748. doi: 10.1172/JCI105388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F., Logue G. L., Adams J., Crookston J. H. Mechanisms of immune lysis of the red cells in hereditary erythroblastic multinuclearity with a positive acidified serum test and paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1974 Jan;53(1):31–43. doi: 10.1172/JCI107551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F. Variations in the red cells in paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1973 Mar;24(3):327–342. doi: 10.1111/j.1365-2141.1973.tb01657.x. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Silverberg M., Furthmayr H., Marchesi V. T. The effect of carboxymethylating a single methionine residue on the subunit interactions of glycophorin A. Biochemistry. 1976 Apr 6;15(7):1448–1454. doi: 10.1021/bi00652a015. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Dawson G. Topographical distribution of complex carbohydrates in the erythrocyte membrane. J Biol Chem. 1974 Apr 10;249(7):2135–2142. [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M., Rosse W. F. Two populations of granulocytes in paroxysmal nocturnal hemoglobinuria. Blood. 1979 May;53(5):928–934. [PubMed] [Google Scholar]
- Tack B. F., Harrison R. A., Janatova J., Thomas M. L., Prahl J. W. Evidence for presence of an internal thiolester bond in third component of human complement. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5764–5768. doi: 10.1073/pnas.77.10.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M. J., Anstee D. J. The membrane change in En(a-) human erythrocytes. Absence of the major erythrocyte sialoglycoprotein. Biochem J. 1976 Feb 1;153(2):271–277. doi: 10.1042/bj1530271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M., Furthmayr H., Marchesi V. T. Primary structure of human erythrocyte glycophorin A. Isolation and characterization of peptides and complete amino acid sequence. Biochemistry. 1978 Oct 31;17(22):4756–4770. doi: 10.1021/bi00615a025. [DOI] [PubMed] [Google Scholar]
- Vainchenker W., Testa U., Deschamps J. F., Henri A., Titeux M., Breton-Gorius J., Rochant H., Lee D., Cartron J. P. Clonal expression of the Tn antigen in erythroid and granulocyte colonies and its application to determination of the clonality of the human megakaryocyte colony assay. J Clin Invest. 1982 May;69(5):1081–1091. doi: 10.1172/JCI110543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilson J. G., Wong W. W., Schur P. H., Fearon D. T. Mode of inheritance of decreased C3b receptors on erythrocytes of patients with systemic lupus erythematosus. N Engl J Med. 1982 Oct 14;307(16):981–986. doi: 10.1056/NEJM198210143071604. [DOI] [PubMed] [Google Scholar]
- Winzler R. J., Harris E. D., Pekas D. J., Johnson C. A., Weber P. Studies on glycopeptides released by trypsin from intact human erythrocytes. Biochemistry. 1967 Jul;6(7):2195–2202. doi: 10.1021/bi00859a042. [DOI] [PubMed] [Google Scholar]
- YACHNIN S., LAFORET M. T., GARDNER F. H. pH dependent hemolytic systems. I. Their relationship to paroxysmal nocturnal hemoglobinuria. Blood. 1961 Jan;17:83–96. [PubMed] [Google Scholar]