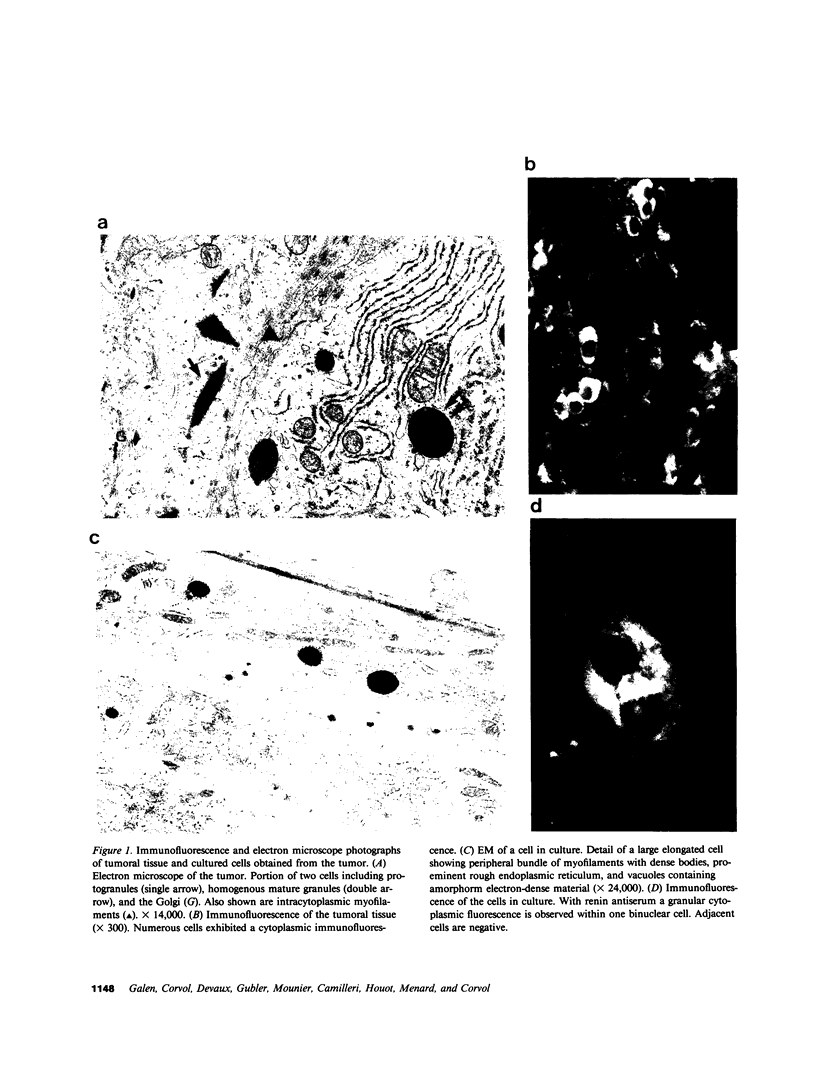
Abstract
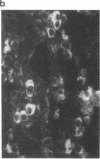
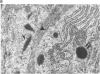
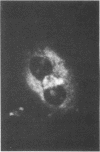
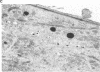
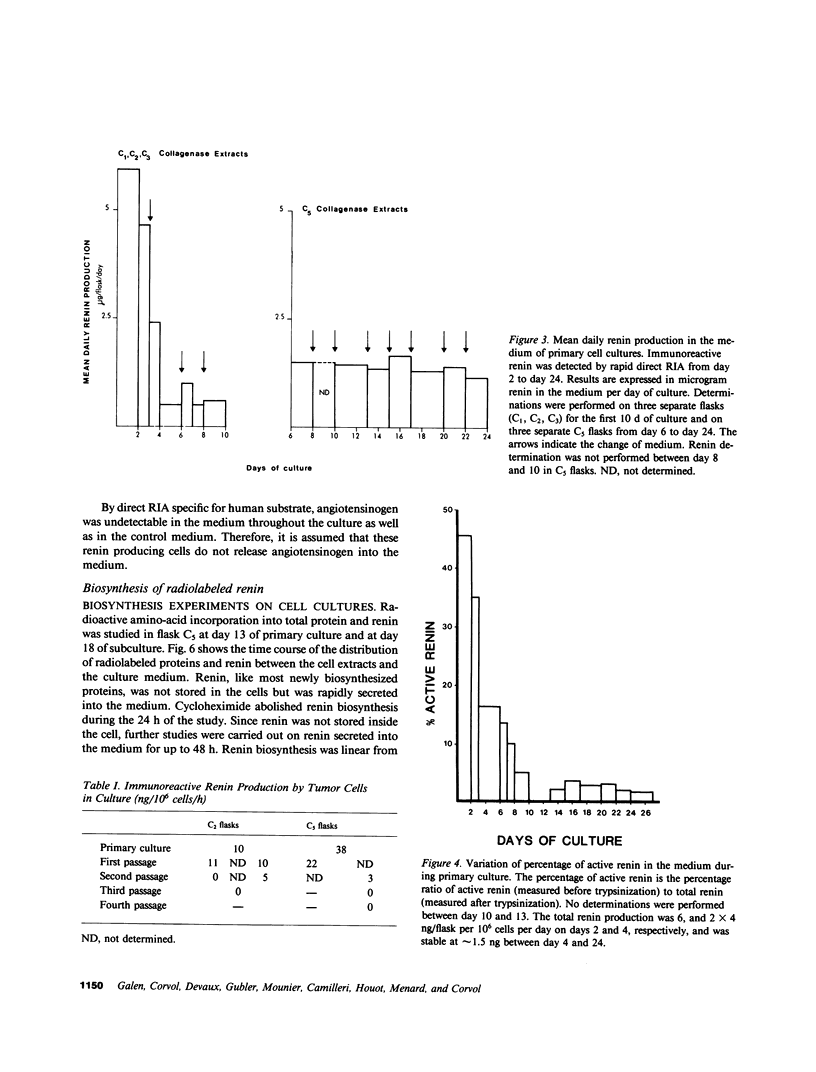
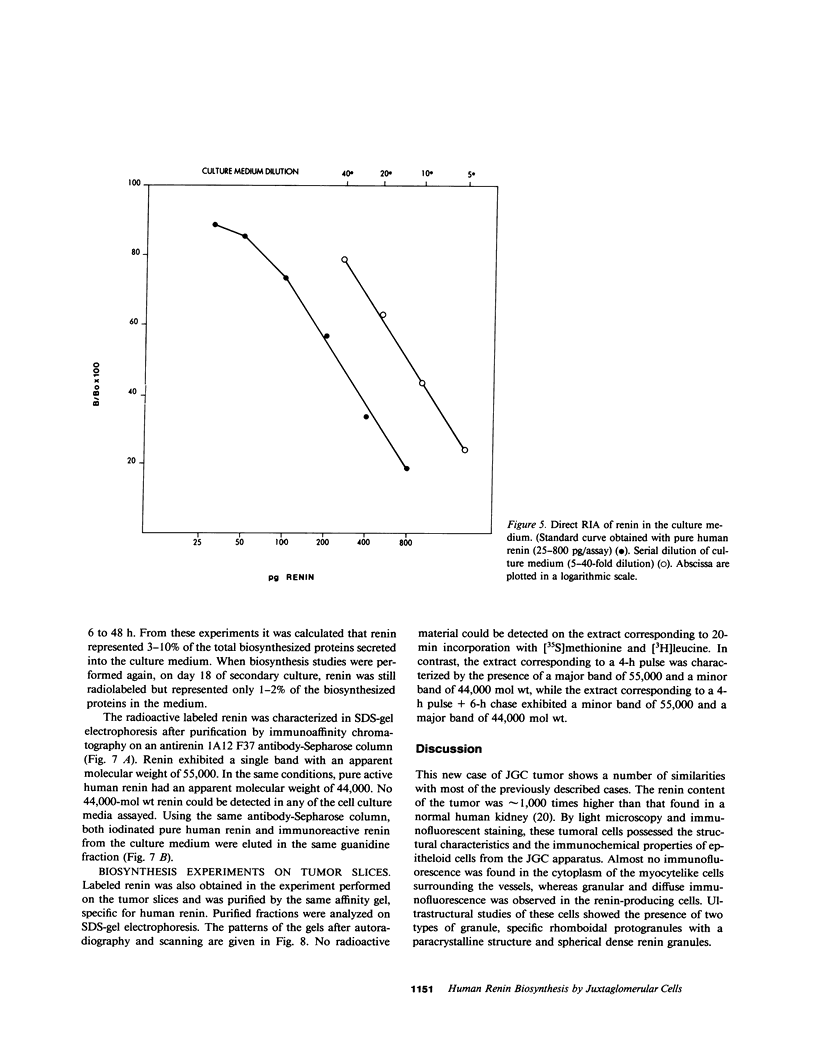
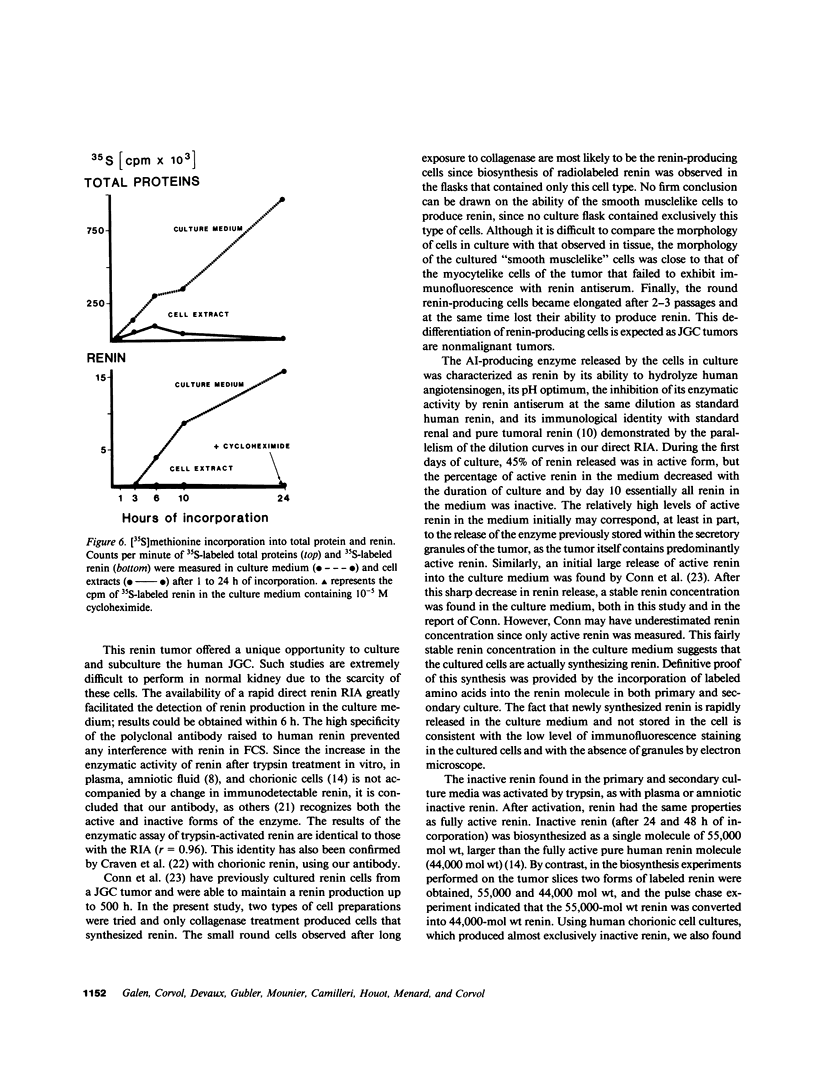
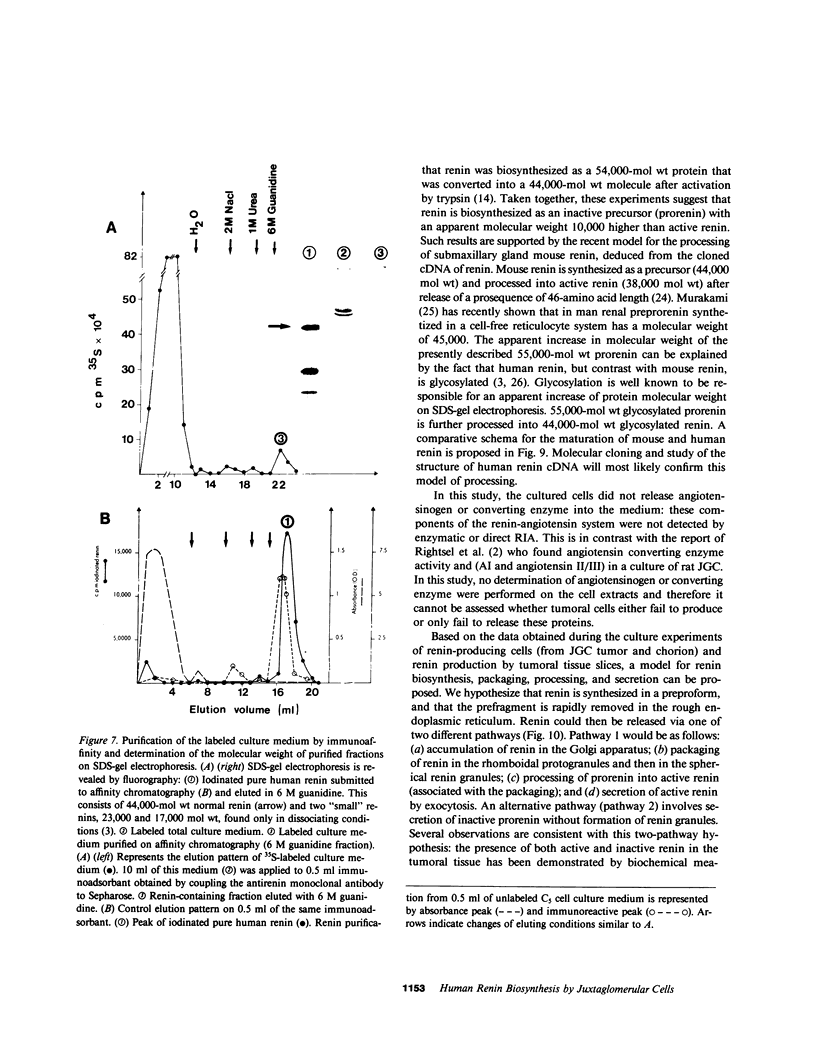
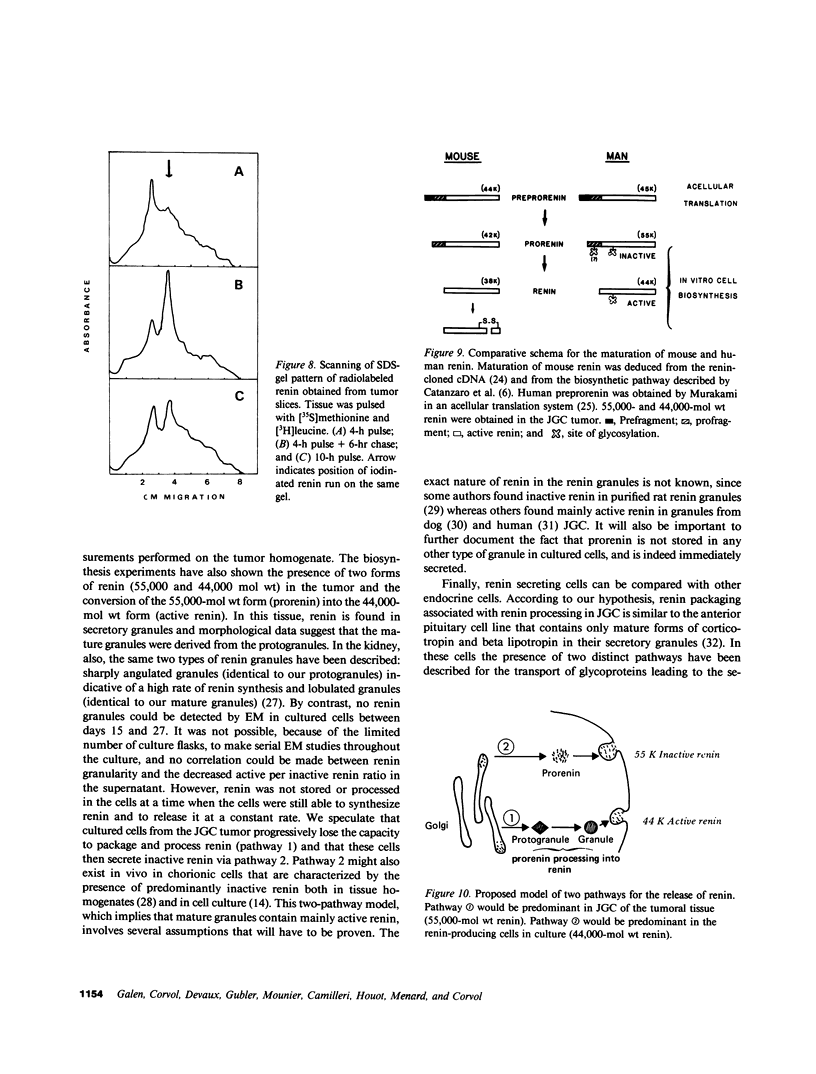
Renin biosynthesis was studied in a juxtaglomerular cell tumor. The tumoral tissue had a high renin content (180 Goldblatt Units/g of tissue), was heavily stained by immunofluorescence using human renin antiserum, and exhibited numerous characteristic secretory granules by electron microscopy. In one series of experiments, renin biosynthesis was studied in tissue slices, by following the incorporation of radiolabeled amino acids into specific immunoprecipitable renin. Time course studies showed that renin was first synthesized in a high molecular weight form, 55,000 mol wt, i.e., 10,000 mol wt higher than that of active renin, and was then converted into a 44,000-mol wt form. In a second series of experiments renin tumoral cells were cultured. Small, round, birefringent cells obtained after collagenase digestion produced renin in both primary culture and subculture media. After 5 d most of the renin found in the culture medium was inactive, but could be activated by trypsin treatment. The tumoral tissue exhibited a strong renin immunofluorescence and numerous secretory granules were observed by electron microscopy. In contrast, the renin-producing cells isolated from this tumor and grown in culture showed little renin immunofluorescence and no secretory granule could be observed. The renin-producing cells in primary culture and subculture were pulsed with radiolabeled amino acids, and immunoprecipitable radiolabeled renin was found in the culture media, thus demonstrating the actual biosynthesis of the enzyme. This renin was not stored inside cultured cells but was rapidly released into the medium and had a molecular weight of 55,000. No conversion of this inactive high molecular weight renin into the active, 44,000 mol wt form of renin was observed. We postulate the existence of two pathways for the processing, packaging, and secretion of renin in the tumoral cells: in juxtaglomerular cells of tumoral tissue renin is synthesized as a preprorenin and rapidly converted into prorenin (55,000 mol wt), which is in turn packaged in secretory granules where it is processed into active renin (44,000 mol wt) and finally secreted; in the cultured tumoral cells renin is still biosynthesized as a preprorenin molecule and then converted into prorenin, but is neither stored as granules nor processed into active renin. In this case the renin is released in an inactive form.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker G. M., Galen F. X., Devaux C., Foote S., Papernik E., Pesty A., Menard J., Corvol P. Human chorionic cells in primary culture: a model for renin biosynthesis. J Clin Endocrinol Metab. 1982 Nov;55(5):902–909. doi: 10.1210/jcem-55-5-902. [DOI] [PubMed] [Google Scholar]
- Camilleri J. P., Phat V. N., Bariety J., Corvol P., Menard J. Use of a specific antiserum for renin detection in human kidney. J Histochem Cytochem. 1980 Dec;28(12):1343–1346. doi: 10.1177/28.12.7014714. [DOI] [PubMed] [Google Scholar]
- Catanzaro D. F., Mullins J. J., Morris B. J. The biosynthetic pathway of renin in mouse submandibular gland. J Biol Chem. 1983 Jun 25;258(12):7364–7368. [PubMed] [Google Scholar]
- Conn J. W., Cohen E. L., Lucas C. P., McDonald W. J., Mayor G. H., Blough W. M., Jr, Eveland W. C., Bookstein J. J., Lapides J. Primary reninism. Hypertension, hyperreninemia, and secondary aldosteronism due to renin-producing juxtaglomerular cell tumors. Arch Intern Med. 1972 Nov;130(5):682–696. doi: 10.1001/archinte.130.5.682. [DOI] [PubMed] [Google Scholar]
- Cushman D. W., Cheung H. S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971 Jul;20(7):1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- Galen F. X., Devaux C., Guyenne T., Menard J., Corvol P. Multiple forms of human renin. Purification and characterization. J Biol Chem. 1979 Jun 10;254(11):4848–4855. [PubMed] [Google Scholar]
- Galen F. X., Guyenne T. T., Devaux C., Auzan C., Corvol P., Menard J. Direct radioimmunoassay of human renin. J Clin Endocrinol Metab. 1979 Jun;48(6):1041–1043. doi: 10.1210/jcem-48-6-1041. [DOI] [PubMed] [Google Scholar]
- Genain C., Aldigier J. C., Guyenne T. T., Corvol P., Ménard J. Direct radioimmunoassays of renin and renin substrate during converting-enzyme inhibition. Clin Exp Hypertens A. 1982;4(11-12):2193–2202. doi: 10.3109/10641968209062383. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Kelly R. B. Secretory granules of an anterior pituitary cell line, AtT-20, contain only mature forms of corticotropin and beta-lipotropin. Proc Natl Acad Sci U S A. 1981 Jan;78(1):318–322. doi: 10.1073/pnas.78.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B., Kelly R. B. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982 Jan;28(1):51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- Guyene T. T., Galen F. X., Devaux C., Corvol P., Menard J. Direct radioimmunoassay of human renin: comparison with renin activity in plasma and amniotic fluid. Hypertension. 1980 Jul-Aug;2(4):465–470. doi: 10.1161/01.hyp.2.4.465. [DOI] [PubMed] [Google Scholar]
- Haas E., Goldblatt H., Gipson E. C., Lewis L. Extraction, purification, and assay of human renin free of angiotensinase. Circ Res. 1966 Oct;19(4):739–749. doi: 10.1161/01.res.19.4.739. [DOI] [PubMed] [Google Scholar]
- Imai T., Miyazaki H., Hirose S., Murakami K. Cell-free translation of human renin mRNA. Clin Exp Hypertens A. 1983;5(7-8):961–968. doi: 10.3109/10641968309048834. [DOI] [PubMed] [Google Scholar]
- Kawamura M., Akabane S., Ito K., Ogino K., Yutani C., Go S., Ikeda M. The storage form of human renal renin. Hypertension. 1982 Mar-Apr;4(2):211–218. doi: 10.1161/01.hyp.4.2.211. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Menard J., Catt K. J. Measurement of renin activity, concentration and substrate in rat plasma by radioimmunoassay of angiotensin I. Endocrinology. 1972 Feb;90(2):422–430. doi: 10.1210/endo-90-2-422. [DOI] [PubMed] [Google Scholar]
- Morris B. J., Johnston C. I. Isolation of renin granules from rat kidney cortex and evidence for an inactive form of renin (prorenin) in granules and plasma. Endocrinology. 1976 Jun;98(6):1466–1474. doi: 10.1210/endo-98-6-1466. [DOI] [PubMed] [Google Scholar]
- Murakami K., Inagami T. Isolation of pure and stable renin from hog kidney. Biochem Biophys Res Commun. 1975 Feb 3;62(3):757–763. doi: 10.1016/0006-291x(75)90464-7. [DOI] [PubMed] [Google Scholar]
- Panthier J. J., Foote S., Chambraud B., Strosberg A. D., Corvol P., Rougeon F. Complete amino acid sequence and maturation of the mouse submaxillary gland renin precursor. Nature. 1982 Jul 1;298(5869):90–92. doi: 10.1038/298090a0. [DOI] [PubMed] [Google Scholar]
- Re R., Fallon J. T., Dzau V., Quay S. C., Haber E. Renin synthesis by canine aortic smooth muscle cells in culture. Life Sci. 1982 Jan 4;30(1):99–106. doi: 10.1016/0024-3205(82)90641-5. [DOI] [PubMed] [Google Scholar]
- Rightsel W. A., Okamura T., Inagami T., Pitcock J. A., Takii Y., Brooks B., Brown P., Muirhead E. E. Juxtaglomerular cells grown as monolayer cell culture contain renin, angiotensin I-converting enzyme, and angiotensin I and II/III. Circ Res. 1982 Jun;50(6):822–829. doi: 10.1161/01.res.50.6.822. [DOI] [PubMed] [Google Scholar]
- Simon D., Galen F. X., Devaux C., Soubrier F., Pau B., Menard J., Corvol P. Monoclonal antibody against human renin. J Clin Endocrinol Metab. 1981 Aug;53(2):453–455. doi: 10.1210/jcem-53-2-453. [DOI] [PubMed] [Google Scholar]
- Soubrier F., Devaux C., Galen F. X., Skinner S. L., Aurell M., Genest J., Menard J., Corvol P. Biochemical and immunological characterization of ectopic tumoral renin. J Clin Endocrinol Metab. 1982 Jan;54(1):139–144. doi: 10.1210/jcem-54-1-139. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Cunningham D., Spigelman L., Aten B. Insulin biosynthesis: evidence for a precursor. Science. 1967 Aug 11;157(3789):697–700. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- Takaori K., Funakawa S., Ikemoto F., Yamamoto K. Low molecular weight renin as a storage form in renin granules of the dog. Biochem Biophys Res Commun. 1979 Nov 14;91(1):214–221. doi: 10.1016/0006-291x(79)90605-3. [DOI] [PubMed] [Google Scholar]
- Warren A. Y., Craven D. J., Symonds E. M. Production of active and inactive renin by cultured explants from the human female genital tract. Br J Obstet Gynaecol. 1982 Aug;89(8):628–632. doi: 10.1111/j.1471-0528.1982.tb04717.x. [DOI] [PubMed] [Google Scholar]
- Yokosawa H., Holladay L. A., Inagami T., Haas E., Murakami K. Human renal renin. Complete purification and characterization. J Biol Chem. 1980 Apr 25;255(8):3498–3502. [PubMed] [Google Scholar]
- Yokosawa H., Yokosawa N., Inagami T. Specific antibody to human renal renin and its cross-reactivity with inactive human plasma prorenin. Proc Soc Exp Biol Med. 1980 Sep;164(4):466–470. doi: 10.3181/00379727-164-40897. [DOI] [PubMed] [Google Scholar]
- Zavagli G., Aleotti A., Farinelli A. Human renin granules: ultrastructural aspects. Nephron. 1983;33(1):29–33. doi: 10.1159/000182900. [DOI] [PubMed] [Google Scholar]