The endocrine profile of the natural cycle
Central to the modern concept of reproduction in all mammalian is the brain, from which springs the function of all the rest. It is therefore appropriate to start this part of the physiology of the reproductive system with the role of the brain.
The hypothalamus
It has long been surmised that the reproductive processes, such as the menstrual cycle or ovulation, must in some way be under nervous control, since many reproductive phenomena arise in consequence of environmental changes. For instance amenorrhoea in a woman can result from psychological stress (Bomba et al., 2007).
Within the brain, there are two major sites of action that are important for the regulation of the reproductive function: the hypothalamus and the pituitary gland (Speroff et al., 1994). The pituitary gland is divided into three regions or lobes: anterior, intermediate, and posterior. The anterior pituitary (adenohypophysis) is quite different structurally from the posterior neural pituitary (neurohypophysis), which is a direct physical extension of the hypothalamus. The adenohypophysis is derived embryologically from epidermal ectoderm from an infolding of Rathke’s pouch. Therefore, it is not composed of neural tissue, as is the posterior pituary, and does not have direct neural connections to the hypothalamus (Berek, 2002).
The elevation of the pituitary at the beginning of the 20th century put physiologists in a dilemma. No nervous connection between the brain and the anterior pituitary could be revealed. The mystery was solved by G. Harris (1970) who showed that while there was no nervous connection between the brain and the anterior pituitary, there was a direct vascular channel between the hypothalamus above and the pituitary below, which serves as a mean to convey a biological signal (neurohormones) from the nervous system to the gland.
The specific secretory cells of the anterior pituitary have been classified based on their hematoxylin- and eosin-staining pattern. The gonadotropins, LH and FSH, are secreted by basophilic cells. Acidophilic-staining cells primarily secrete GH and prolactin and, to a variable degree, ACTH (Duello and Halmi, 1979).
The neurohormone that controls gonadotrophins is called gonadotrophin-releasing hormone (GnRH) also called luteinizing hormone - releasing hormone (LHRH) (Blackwell et al., 1973). The biochemical structure of GnRH was first described by Andrew Schally and Roger Guillemin in 1971, an accomplishment, for which the authors received the Nobel Prize.
It is a decapeptide produced by neurons with cell bodies primarily in the nucleus arcuatus of the hypothalamus with a half life of 2-4 minutes (Krey et al., 1975; Plant et al., 1978; Amoss et al., 1971). The short half-life of GnRH is the result of rapid proteolytic cleavage (Soules et al., 1985; Filicori et al., 1986).
GnRH is unique among releasing hormones in that it simultaneously regulates the secretion of two hormones- FSH and LH. It also is unique among the body’s hormones because it must be secreted in a pulsatile fashion to be effective, and the pulsatile release of GnRH influences the release of the two gonadotropins (Dierschke et al., 1970; Knobil E, 1980; Belchetz et al., 1978).
GnRH is released into portal blood and regulates LH and follicle-stimulating hormone (FSH) release from the pituitary gonadotropes by binding to its specific receptors located on these cells. GnRH receptors are upregulated by pulsatile GnRH, while they are submitted to down regulation when LHRH or its analogues are administered in a non-pulsatile fashion (Melcangi, 2002).
The pulsatile secretion of GnRH varies in both frequency and amplitude throughout the menstrual cycle and is tightly regulated (Table 1, Soules et al., 1985; Filicori et al., 1986).
Table I. Menstrual cycle variation in LH pulse Frequency and Amplitude.
| Cycle Phase | Mean frequency (minutes) |
Mean Amplitude (mIU/mL) |
| Early follicular | 90 | 6.5 |
| Mid-follicular | 50 | 5 |
| Late-follicular | 60-70 | 7 |
| Early luteal | 100 | 15 |
| Mid-luteal | 150 | 12 |
| Late luteal | 200 | 8 |
From PhD thesis H.M.Fatemi, Brussels 2008.
Among many factors that integrate the activity of the GnRH neuronal system, estrogens play the most important role. Estrogens exhibit a negative feedback action on LH secretion. However, in addition to the negative feedback, E2 also exhibits a positive feedback influence upon the activity and output of GnRH neurones to generate the preovulatory LH surge and subsequent ovulation (Herbison AE, 1998). Despite the evidence supporting the essential role of estradiol in triggering the preovulatory surge of gonadotropins, there is substantial evidence that indicates an important role of progesterone (P) in inducing or in facilitating this surge (Hotchkiss et al., 1982). P appears to act at several levels, since it may exert direct regulatory effects on pituitary cells, it is also able to act at the hypothalamic level, via the modulation of GnRH synthesis and of its pulsatile release (Ramirez et al., 1985). Moreover, P appears to be required for a full pituitary responsiveness to GnRH. In fact, after ovariectomy plus adrenalectomy, E2 alone is not able to induce a preovulatory LH surge (Mahesh and Brann, 1998).
Numerous neuroactive substances have also been implicated as neurotransmitters and neuromodulators controlling GnRH release (Barraclough et al., 1984; Kalra, 1986; Terasawa, 1995). Among them NPY (Neuropeptide Y neurons), Norepinephrine (NE), GABA, glutamate, and Nitric oxide are contributors controlling pulsatile GnRH release (Terasawa, 1998). The main modulators dopamine, serotonin, opioid (mainly β-endorfin and dynorphin) decrease GnRH release from the hypothalamus (Andersen, 1987; Genazzani and Petraglia, 1989). Moreover, ovarian sex steroids can increase the secretion of central endorphins, further depressing gonadotropins (Reid et al., 1981). Endorphin levels vary significantly throughout the menstrual cycle, with peak levels in the luteal phase and a nadir during menses (Gindorff and Ferin, 1987). This inherent variability, although helping to regulate gonadotropin levels, may contribute to cycle-specific symptoms experienced by ovulatory women (Halbreich and Endicott, 1981).
Gonadotropins
The gonadotropins FSH and LH are produced by the anterior pituitary gonadotroph cells and are responsible for ovarian follicular stimulation. Structurally, there is a great similarity between FSH and LH. They are both glycoproteins that share an identical α-subunit and differ only in the structure of their β-subunit, which confers receptor specificity (Fiddes, and Talmadge, 1984). The synthesis of the β-subunit is the rate regulating step in gonadotropin biosynthesis (Lalloz et al., 1988). The α-subunit consists of 92 aminoacids stabilized by 5 disulfide bonds, while the β-subunit contains 118 amino acids and 5 sialic acid residues. Neither subunit has any intrinsic biologic activity by itself. The variation of the sialic acid component is responsible for the different half life of these hormones. Sialic acid prevents the hepatic clearance; thus, the greater the sialic acid component, the longer the half life (Morell et al., 1971). HCG, for example, with 20 sialic acid residues, has the longest half life (about 24 hours), whereas LH (1 to 2 sialic acid residues) has a very short half life (20-30 minutes) (Morell et al., 1971).
Two cells - two gonadotropins
The fundamental principle of follicular development is the two cells - two gonadotropins theory (Erickson, 1986). This theory states that there is a subdivision and compartmentalization of steroid hormone synthesis activity in the developing follicle.
According to the “Two cell two gonadotropin theory” (Kobayashi et al., 1990), both FSH and LH are necessary for ovarian follicular maturation and the synthesis of ovarian steroid hormones. LH promotes the production of androgens (dehydroepiandrosterone, androstenedione, and testosterone) from cholesterol and pregnenolone, by stimulating 17α-hydroxylase activity in the thecal cells. The androgens then diffuse to the granulosa cells where FSH stimulates the expression of the cytochrome P450 aromatase, which converts the androgens to estrogens (Erickson et al., 1985).
Rising estrogen levels have a negative feedback effect on FSH secretion. Conversely, LH undergoes biphasic regulation by circulating estrogens. At lower concentrations, estrogens inhibit LH secretion. At higher levels of estrogen (200pg/ml) for more than 48 hours, estrogen enhances the LH release (Young and Jaffe, 1976).
The local estrogen-FSH interaction in the dominant follicle induces LH receptors on the granulosa cells that results in luteinisation of the granulosa cells, production of progesterone and initiation of ovulation, that will occur in the single mature follicle 10-12 hours after the LH peak or 34-36 hours after the initial rise in mid-cycle LH (Pauerstein et al., 1978).
The mid-cycle LH surge is responsible for a dramatic increase in local concentrations of prostaglandins and proteolytic enzymes in the follicular wall (Yoshimura et al., 1987). Due to these substances the follicular wall is progressively weakened and is perforated with a slow extrusion of the oocyte through this opening (Yoshimura and Wallach., 1987).
The luteal Phase:
The luteal phase is defined as the period between ovulation and either the establishment of a pregnancy or the onset of menses 2 weeks later (Fatemi et al., 2007).
When the ovum is discharged at ovulation, it takes with it a covering of granulosa cells. The remaining granulosa cells staying behind are attached to the wall of the collapsed follicle. The exit hole of the ovum is sealed by a fibrinoid plug. From the endocrine point of view the most significant event in the early development of the corpus luteum is the fact that the capillaries of the theca interna penetrate the basal membrane in response to secretion of angiogenic factors such as the vascular endothelial growth factor (VEGF) (Anasti et al., 1998) and the granulose layer becomes vascularized. This angiogenic response allows large amounts of luteal hormones to enter the systemic circulation. The granulosa cells remaining in the follicle begin to uptake lipids causing the characteristic yellow lutein pigment. These cells are active secretory structures that produce progesterone, estrogen and inhibin A.
In women and other primates, steroid hormone production by corpora lutea depends on the presence of continued LH production (Devoto et al., 2000).
If conception and implantation occur, the developing blastocyst secretes human chorionic gonadotrophin (hCG). The role of hCG produced by the embryo is to maintain the corpus luteum and its secretions (Penzias, 2002). The estimated onset of placental steroidogenesis (the luteoplacental shift) occurs during the 5th gestational week, as calculated by the patients’ last menses (Scott et al., 1991).
Early History of the Corpus Luteum
Coiter (1573) described the presence of cavities filled with a yellow solid in the ovary, but it was de Graaf (1943) who gave the first definitive description of these structures. Malpighi (1689) provided an accurate microscopic description of these structures and was the first to apply the name corpus luteum, literally the yellow body. Beard (1897) postulated that corpora lutea were responsible for the suppression of ovulation and estrus during pregnancy, and about that time, Prenant (1898) suggested that the corpus luteum might be a gland of internal secretion directly benefiting the egg with which it appeared to be associated. It was, however, Fraenkel (1903) who demonstrated that corpora lutea were necessary for implantation and the subsequent maintenance of pregnancy in the rabbit. Corner and Allen (1929) and Allen and Corner (1930) prepared a relatively pure alcoholic extract of corpora lutea from sows and showed that this extract maintained pregnancy in ovariectomized rabbits. A few years later, the isolation of the pure crystalline hormone was reported simultaneously by four groups (Butenandt et al., 1934; Hartmann and Wettstein, 1934; Slotta et al., 1934; Wintersteiner and Allen, 1934). Slotta et al. (1934) named the compound progesterone and suggested a structural formula, and in the same year, the compound was synthesized by Butenandt and Westphal (1934).
The endometrium
The endometrium is the mucosal lining of the uterine cavity. Its basic function is the creation of a suitable environment for embryo nidation. Though implantation could occur in any human tissue, the endometrium is the only tissue, which is not receptive to embryo implantation except during a restricted frame of time called the ‘implantation window’ (Minas et al., 2005).
The endometrium can morphologically be divided into an upper two third ‘functionalis” layer and a lower one third “basalis” layer. The purpose of the functionalis layer is to prepare for implantation of the blastocyst and therefore it is the site of proliferation, secretion and degeneration. The purpose of the basalis layer is to provide the regenerative endometrium following menstrual loss of the functionalis (Speroff et al., 1994).
As the major target of sex steroid hormones, the endometrium will undergo characteristic cycles of proliferation, secretory changes and tissue shedding in response to ovarian steroid hormones (Bourgaine C., 2001/2002). The endometrial cycle is a reflection of the ovarian cycle, corresponding with two phases of cellular development, separated by ovulation.
The primary control over endometrial maturation is considered to be exerted by P and E2. Studies on pregnancy outcome suggest that an optimal balance of the two hormones is necessary for a normal progression of pregnancy (Lejeune et al., 1986).
The endometrium proliferates due to the stimulation of E2 produced by the granulosa cells in the follicular phase. The highest response is in the glands. There is first an increase in mitotic activity and secondly there is formation of a loose capillary network in the spiral vessels (Tavanioutu, 2006).
After the ovulation, there is a secretory transformation of the endometrium due to the progesterone produced by the corpus luteum.
Under the action of progesterone, endometrial proliferation ceases and glandular secretion initiates. The endometrial glands become tortoise and spiral vessels coiled. In the glandular epithelium subnuclear intracytoplasmic glycogen vacuoles appear that start to move towards the glandular lumen, followed by an active secretion of glycoproteins and peptides in the endometrial cavity. During the secretory phase, a short specific period of uterine receptivity toward embryonic implantation is designated as the ‘‘implantation window’’ (Harper, 1992). The peak of the secretory endometrial activity is around the 5-7th post-ovulatory day, coinciding with the time of the embryo implantation.
Luteal Phase defect
As early as 1949, the premature onset of menses was recognized as indicative of a luteal phase deficiency of progesterone production, which was shown to be correctable by exogenous progesterone administration (Jones, 1979). The prevalence of a luteal phase defect in natural cycles in normo-ovulatory patients with primary or secondary infertility was demonstrated to be about 8.1% (Rosenberg et al., 1980).
Pathophysiologic alterations of the complex reproductive process that lead to delayed endometrial maturation characteristic of LPD include disordered folliculogenesis, defective corpus luteum function, and abnormal luteal rescue by the early pregnancy. A variety of clinical conditions, such as hyperprolactinemia, hyperandrogenic states, weight loss, stress, and athletic training may result not in oligo- or anovulation, but rather may be manifest as LPD (Ginsburg, 1992).
The three main causes of luteal phase defect in unstimulated cycles include poor follicle production, premature demise of the corpus luteum, and failure of the uterine lining to respond to normal levels of progesterone.
Luteolysis
Basal levels of LH in the human appear to be essential to maintain the secretory function of the corpus luteum (Van de Wiele et al., 1970). In the rhesus monkey, bilateral lesions in the arcuate nucleus of the hypothalamus caused a cessation of ovarian ovulatory activity that could be restored by chronic circhoral infusions of GnRH (Knobil et al., 1980). If plasma levels of LH were reduced to undetectable levels during the midluteal phase by halting GnRH infusions in these lesioned monkeys, plasma progesterone fell to undetectable levels. However, when LH levels were restored 3 days later by resuming circhoral GnRH infusions, the corpus luteum resumed a normal pattern of progesterone secretion but regressed at the expected time (Hutchinson and Zeleznik, 1985). These studies suggest that LH acts to promote progesterone synthesis by the corpus luteum but that other factors are responsible for the loss of function and structural integrity of the primate corpus luteum during luteolysis.
It has long been considered that luteolysis might be an intraovarian event. Early studies suggested that estrogen produced by corpus luteum mediated luteolysis (Knobil, 1973). Subsequent work indicated that estrogen may act by increasing PGF2 levels in the ovary. This view was based on the finding that exogenous estrogen increased the concentration of PGF2 in ovarian venous blood (Auletta et al., 1973) and that indomethacin blocked estrogen-induced luteolysis in the rhesus monkey (Auletta et al., 1976). However, it was found that high levels of estrogen (10 µg/ml) inhibited progesterone synthesis by human luteal cells, both in the presence and absence of indomethacin (Thibier et al., 1980). Later studies suggested that the luteolytic effect of exogenous estrogen in the primate may be due to its suppression of pituitary gonadotropin secretion rather than a direct effect on the ovary (Schatz et al., 1985). Moreover, estrogen receptors are absent in all cell types of the primate corpus luteum (Hild-Petito et al., 1997). The finding that administration of either an aromatase inhibitor (Ellinwood et al., 1983) or an estrogen antagonist (Albrecht et al., 1981) does not prolong the life span of the corpus luteum in monkeys indicates that estrogen may not be a direct mediator of luteolysis in primates. However, it is possible that estrogen may have indirect actions in the ovary or the corpus luteum other than via estrogen receptors. However, the exact mechanism of luteolysis is not known and in future studies it will be required to resolve this question.
How to define a luteal phase defect?
Although LPD has been clearly described in research settings, the diagnosis remains controversial (Jordan et al., 1994). A defective luteal phase in the natural cycle was defined if the serum mid-luteal progesterone levels are less than 10 ng/ml (Jordan et al., 1994). However, mid-luteal P levels do not always reflect the endometrial maturation (Batista et al., 1994). Therefore, in the literature the most reasonable consensus of a defective luteal phase is a lag of more than two days in endometrial histological development compared to the expected day of the cycle (Jones, 1991, Dawood, 1994).
Ovarian stimulation and luteal phase defect
However, with the advent of ovarian stimulation for IVF, it has been established that the luteal phase of all stimulated IVF cycles are abnormal (Edwards et al., 1980). The etiology of luteal phase defect in stimulated IVF cycles has been debated for more than two decades. Initially, it was thought that the removal of large quantities of granulosa cells during the oocyte retrieval (OR) might diminish the most important source of progesterone synthesis by the corpora lutea, leading to a defect of the luteal phase. However, this hypothesis was disproved when it was established that the aspiration of a preovulatory oocyte in a natural cycle neither diminished the luteal phase steroid secretion nor shortened the luteal phase (Kerin et al., 1981).
Another proposal suggested that the prolonged pituitary recovery that followed the GnRH agonist co-treatment, designed to prevent spontaneous LH rise in stimulated cycles resulting in lack of support of the corpus luteum, would cause a luteal phase defect (Smitz et al., 1992). It was also suggested that the hCG administered for the final oocyte maturation in stimulated IVF cycles could potentially cause a luteal phase defect by suppressing the LH production via a short-loop feedback mechanism (Miyake et al., 1979).
However, the administration of hCG did not down-regulate the LH secretion in the luteal phase of normal, unstimulated cycles in normo-ovulatory women (Tavaniotou and Devroey, 2003).
The introduction of GnRH antagonists in IVF raised speculations that a rapid recovery of the pituitary function (Albano et al., 1996) would obviate the need for luteal phase supplementation (Elter and Nelson, 2001).
Preliminary observations in intrauterine insemination (IUI) cycles seemed to favor this contention. Ragni et al. (2001) explored the luteal phase hormone profiles in gonadotrophin stimulated cycles both with and without GnRH antagonist therapy for IUI. No deleterious effects of GnRH antagonist administration could be noted on either the luteal progesterone concentration or the duration of the luteal phase in that study.
However, various studies of GnRH antagonist co-treatment in IVF have since found different results. Luteolysis is also initiated prematurely in antagonist co-treated IVF cycles, resulting in a significant reduction in the luteal phase length and compromising the chances for pregnancy (Albano et al., 1998; Beckers et al., 2003).
Beckers et al. (2003), evaluated the non-supplemented luteal phase characteristics in patients undergoing ovarian stimulation with recombinant FSH combined with a GnRH antagonist (antide; 1mg/day). However, due to unacceptably low pregnancy rates (overall 7.5%), the decision was therefore made to cancel this study after 40 patients were included. Luteolysis also started prematurely with the administration of GnRH antagonist.
Despite the rapid recovery of the pituitary function in GnRH antagonist protocols (Dal Prato and Borini, 2005), luteal phase supplementation remains mandatory (Tarlatzis et al., 2006).
It appears that the main cause of the LPD, observed in stimulated IVF cycles, is related to the multifollicular development achieved during ovarian stimulation, which alter completely the hormonal environment. It can be postulated that one of the main causes of the luteal phase defect in stimulated IVF cycles is supra-physiological levels of steroids secreted by a high number of corpora lutea during the early luteal phase, which directly inhibit the LH release via negative feedback actions at the hypothalamic-pituary axis level (Fauser and Devroey, 2003).
Studies in human and primates have demonstrated that the corpus luteum requires a consistent LH stimulus in order to perform its physiological function (Jones, 1991). LH support during the luteal phase is entirely responsible for the maintenance and the normal steroidogenic activity of the corpus luteum (Casper and Yen, 1979). As a result, withdrawal of LH, unnecessary causes premature luteolysis (Duffy et al., 1999).
The HCG administered for final oocyte maturation covers the luteal phase for a maximum of 8 days (Fatemi et al., 2007). In normal circumstances, thereafter LH would stimulate the corpora lutea, but due to the suppressed LH levels in IVF cycles, there is no stimulus of the corpora lutea.
The luteal phase support
Progesterone
Csapo et al. (1972 and 1973) demonstrated the importance of progesterone during the first weeks of a pregnancy. In their initial study, the removal of the corpus luteum prior to seven weeks of gestation led to pregnancy loss (Csapo et al., 1972). However, the authors found that pregnancy could be maintained even after removal of the corpus luteum by external administration of progesterone (Csapo et al., 1973).
Progesterone induces a secretory transformation of the endometrium in the luteal phase (Bourgain et al.,1990). By inducing this change after adequate estrogen priming, progesterone improves endometrial receptivity (Kolibianakis and Devroey, 2002). Endometrial receptivity is a self-limited period in which the endometrial epithelium acquires a functional and transient ovarian steroid-dependent status that allows blastocyst adhesion (Martin et al., 2002). Decreased endometrial receptivity is considered largely responsible for the low implantation rates in IVF (Paulson et al., 1990).
Progesterone also promotes local vasodilatation and uterine musculature quiescence by inducing nitric oxide synthesis in the decidua (Bulletti and de Ziegler, 2005). Inadequate uterine contractility may lead to ectopic pregnancies, miscarriages, retrograde bleeding with dysmenorrhea and endometriosis (Bulletti and de Ziegler, 2005).
The uterine-relaxing properties of progesterone were supported by a study of IVF embryo transfer outcomes by Fanchin et al. (2001). This study investigated the consequences of uterine contractions (UC) as visualized by ultrasound (US) during embryo transfer. Results indicated that a high frequency of uterine contractions on the day of embryo transfer hindered transfer outcome, possibly by expelling embryos out of the uterine cavity. A negative correlation between UC frequency and progesterone concentrations was detected underlining the benefits of progesterone in IVF (Fanchin et al., 2001).
Currently available formulations of progesterone include oral, vaginal, rectal and intramuscular (i.m.) (Penzias, 2002; Chakmakjian, 1987). Progesterone administered orally is subjected to first-pass prehepatic and hepatic metabolism. This metabolic activity results in progesterone degradation to its 5α- and 5β- reduced metabolites (Penzias, 2002). Parenteral administration (vaginal, rectal and i.m.) of progesterone surpasses the metabolic consequences of orally administered progesterone (De Ziegler et al., 1995).
Oral progesterone
Oral micronized progesterone was used for luteal support in IVF with poor results until the end of 1980s (Buvat J, et al., 1990). Devroey et al. (1989) and Bourgain et al. (1990) reported an absence of the secretory transformation of the endometrium in patients with premature ovarian failure who had been treated with oral micronised progesterone when compared to patients treated with intramuscular injections or vaginal micronised progesterone. This finding suggested that oral administration reduced the hormone’s bioavailibility.
To overcome this problem, dydrogesterone (DG) was introduced to support the luteal phase of stimulated IVF cycles (Belaisch-Allart et al., 1987). DG, a retroprogesterone with good oral bioavailability, is a biologically active metabolite of progesterone and has an anti-estrogenic effect on the endometrium, achieving the desired secretory transformation (Whitehead, 1980; Chakravarty et al., 2005).
Recently, Chakravarty et al. (2005) undertook a prospective, randomized study (n = 430) that compared the efficacy, safety and tolerability of oral DG with vaginal micronised progesterone as luteal phase support after in-vitro fertilization (IVF). Both DG and P were associated with similar rates of successful pregnancies (24.1% vs. 22.8%, respectively; P = NS).
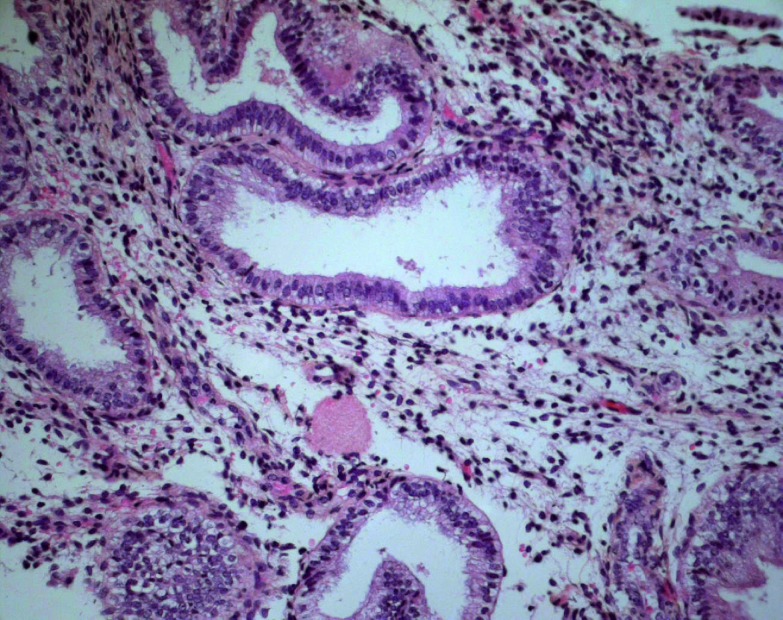
However, it has been demonstrated clearly that after sufficient estrogen endometrial priming, exogenous administered vaginal micronised progesterone is significantly more effective than oral dydrogesterone in creating an ‘in phase’ secretory endometrium. (Fig. 1, Fig. 2, Fatemi et al., 2007).
Fig. 1. Endometrial biopsy after micronized progesterone. Coiled glands with active secretion and minimal residual vacuoles. Stromal edema. Absence of mitotic activity. The maturation corresponds to day 6 of the luteal phase (HES, 200×).
Fig. 2. Endometrial biopsy after Dydrogesterone. Small glands with minimal coiling and persistant homogeneous subnuclear vacuoles and pseudostratified nuclei. No stromal edema. Focal mitotic activity. The maturation corresponds to day 2-3 of the luteal phase (HES, 200×).
The oral DG might be sufficient for luteal supplementation in IVF cycles; however more large randomized controlled trails are needed, before a conclusion can be made.
Rectal progesterone
A number of publications have evaluated the rectal use of natural progesterone in women undergoing IVF/ICSI (Chakmakijan et al., 1987; Ioannidis et al., 2005). Chakmakijan et al. (1987) studied the bioavailability of micronized progesterone (P) by measuring sequential serum P concentrations after a single bolus of 50-200 mg P given sublingually, orally (capsule and tablet), vaginally and rectally (suppositories) during the follicular phase of a group of normally menstruating women. When compared to other routes of P administration, rectal application resulted in serum concentrations during the first eight hours twice as high as other forms. However, to the best of our knowledge, there are no prospective randomized trials to compare the rectal administration of progesterone with other administration routes for IVF.
Vaginal progesterone
The intravaginal route of progesterone supplementation in IVF has gained wide application as a first choice luteal support regimen, mainly due to patient comfort and effectiveness (Levine et al., 2000). Following intravaginal administration of progesterone, high uterine progesterone concentrations with low peripheral serum values are observed, due to counter-current exchange in progesterone transport between anatomically close blood vessels (Cicinelli et al., 2000) and due to the uterine first pass effect, where liver metabolisation is absent (De Ziegler et al., 1995).
There is increasing evidence in the literature that vaginal P is at least as effective as i.m. P at providing luteal support in induced cycles (Simunic et al., 2007). In the latest meta-analysis by Nosarka et al. (2005), vaginal and intramuscular progesterone had comparable implantation and clinical pregnancy rates. In Europe, there are two different forms of intravaginal progesterone on the market, natural micronised progesterone (Utrogestan® Laboratories Besins International, Paris, France) and Crinone ®8% (Fleet Laboratories Ltd., Watford, United Kingdom), a controlled and sustained-release vaginal gel. Utrogestan ® 100 mg capsules are administered vaginally three times two capsules daily (600 mg/d) whereas Crinone 8% is administered vaginally once a day, i.e. 90 mg, (Simunic et al., 2007; Ludwig et al., 2002).
To establish the minimal effective dose of vaginal micronized progesterone, Chanson et al. (1996) conducted a small (n = 40) prospective randomized study comparing two different dose regimens (400 mg versus 600 mg each day). No differences in clinical pregnancy rates were noted. However, further prospective randomized trials are essential to define the necessary dose of vaginal micronized progesterone for luteal phase support in IVF.
In a prospective, randomized study Ludwig et al. (2002) compared vaginal Crinone 8% with vaginal Utrogestan® for luteal phase support. Clinical pregnancy rates, clinical abortion rates until 12 weeks of gestation and ongoing pregnancy rates were comparable between the two groups (Ludwig et al., 2002).
Simunic (2007) and Ludwig (2002) evaluated the tolerability and acceptability of both preparations from patients’ point of view. Crinone® 8% gel proved more tolerable than Utrogestan® vaginal capsules because of a lower number of side effects (Simunic et al., 2007; Ludwig et al., 2002).
Intra muscular (i.m.) Progesterone
I.M.. progesterone supplementation is given as an injection of natural progesterone-in-oil (Costabile et al., 2001).
In 1985, Leeton et al. first demonstrated the extension of the luteal phase of stimulated IVF cycles treated with 50 mg i.m. progesterone. The doses of i.m. progesterone used for luteal phase support vary between 25 and 100mg per day without any significant difference concerning the outcome (Pritts and Atwood, 2002).
This route of administration is often associated with a number of side effects, including painful injections and a rash (Lightman et al., 1999), causing a lack of enthusiasm for this treatment modality (Costabile et al., 2001). Injections of Progesterone in oil can also lead to inflammatory reactions and abscess formation (Propst et al., 2001).
In addition, several case reports have been published in which patients receiving i.m. progesterone for luteal supplementation have developed acute eosinophilic pneumonia (Bouckaert et al., 2004; Veysman et al., 2006). This drug-induced disease shows that the use of i.m. progesterone can also be associated with a severe morbidity in otherwise healthy young patients (Bouckaert et al., 2004).
In an open-label trial in 1184 women from 16 U.S. American centers Levine evaluated the clinical and ongoing pregnancy rates in IVF cycles involving vaginal and i.m. progesterone. Vaginal and i.m. progesterone were found to have comparable clinical (35.05% V.S. 35.2%, respectively) and ongoing pregnancy rates (30.2% and 33.64%, respectively) (Levine, 2000).
A meta-analysis published in 2002 by Pritts and Atwood included five prospective randomized trails comparing i.m. administration of progesterone with vaginal. A total of 891 cycles were evaluated in those studies. Clinical pregnancy rate and delivery rate were significantly higher when i.m. progesterone was used (RR clinical pregnancy rate/ET 1.33 (95% CI:1.02-1.75, Delivery rate 2.06 (95% CI:1.48-2.88)).
Progesterone plus estradiol
The two most important hormones produced by the corpus luteum are progesterone and estradiol (Fatemi et al., 2007). The role of progesterone for luteal support in stimulated cycles is well established (Fatemi et al., 2007). However, it has not yet been clearly demonstrated whether additional supplementation of E2 in stimulated IVF cycles may be beneficial (Fatemi et al., 2007).
In a prospective randomized study, Smitz et al. evaluated the possible benefit of adding estradiol valerate 6 mg per os daily to the vaginal micronised progesterone (600 mg daily) given as luteal supplementation in 378 women treated with a gonadotrophin releasing-hormone agonist and human menopausal gonadotrophins for in IVF (Smitz et al., 1993). The clinical pregnancy rate was similar between the two groups (29.2% with the estradiol co-treatment and 29.5% with progesterone only treatment). Also Lewin et al., (1994) in a prospectively randomized study, could not find any advantage in the addition of 2 mg estradiol valerate to Progesterone as luteal phase support of long GnRH agonist and hMG-induced IVF-ET cycles in one hundred patients (clinical pregnancy rate 26.5% versus 28% with and without estradiol co-treatment, respectively).
A meta-analysis by Pritts and Atwood (2002) suggested that addition of estrogen to progesterone might improve the implantation rates. However, the authors referred to only one study confirming the beneficial effect of estradiol in the luteal phase (Farhi et al., 2000).
Any beneficial effect of adding E2 to progesterone might depend upon its dosage. Lukaszuk et al (2005), in a prospective, randomized study, recently evaluated the effect of different E2 supplementation doses (0, 2, or 6 mg) during the luteal phase on implantation and pregnancy rates in women undergoing intracytoplasmic sperm injection (ICSI) in agonist cycles (n = 231). Significantly higher pregnancy rates (PR) were recorded in those who received low dose E2 supplementation compared with no estradiol substitution (PR 23.1% vs. 32.8%). The best pregnancy results were found in the group with high dose E2 supplementation (PR 51.3%). It was shown that the addition of a high dose of E2 to daily progesterone supplementation significantly improved the probability of pregnancy in women treated with a long GnRH analogue protocol for COH.
Farhi et al. (2000), in a prospective, randomized study, evaluated the effect of adding E2 to progestin supplementation during the luteal phase in 271 patients undergoing IVF who had E2 levels of higher than 2500 pg/dL at the day of hCG administration. All patients received progesterone supplementation at a dosage of 150 mg/d starting on the day after the oocyte retrieval (OR). Patients were randomized into two groups: those receiving 2 mg of E2 (Estrophem; Novo Nordisk, Bagsvaerd, Denmark), given orally, starting on day 7 after ET; and those receiving no exogenous E2 supplementation during the luteal phase. It was shown that for those patients who had been treated with the long GnRH agonist protocol for COH, the addition of E2 to the progestin support regimen had a beneficial effect on pregnancy and implantation rates (39.6%, and 25.6% with and without estradiol co-treatment respectively; P < .0.05). However, such an effect could not be shown for patients with a short, GnRH agonist protocol.
Different studies were conducted to examine whether the probability of pregnancy is increased by adding estrogen to progesterone for luteal phase support in patients treated by IVF. However, the currently available evidence as published in meta-analysis by Kolibianakis et al., 2008 suggests that the addition of estrogen to progesterone for luteal phase support does not increase the probability of pregnancy in IVF in both GnRH agonist and antagonist cycles.
Human Chorionic Gonadotropin (hCG)
Since it was found that the corpus luteum can be rescued by the administration of hCG, this treatment has become the standard care for luteal support since the late 1980s (52). By stimulating the corpora lutea, hCG is an indirect form of luteal support. It is known to generate an increase in estradiol and progesterone concentrations thus rescuing the failing corpora lutea in stimulated IVF cycles (Fatemi et al., 2007).
Administration of hCG has also been shown to increase the concentrations of placental protein 14, integrin and relaxin (luteal peptide hormone) which has been shown to increase at the time of implantation (Fatemi et al., 2007).
In the meta-analysis published by Pritts and Atwood in 2002, hCG was shown to be equally effective as progesterone for luteal phase support with respect to pregnancy rates.
The disadvantage of using hCG for luteal support stems from its potential for increasing hyperstimulation rates when compared with other treatments or no treatment at all. Significant increases in hyperstimulation rates have been confirmed in several studies (Fatemi et al., 2007).
With regard to ovarian hyperstimulation syndrome (OHSS), one should therefore be cautious with the administration of hCG for luteal supplementation in stimulated IVF cycles (Fatemi et al., 2007). Luteal support with hCG should be avoided if estradiol levels are above 2500-2700 pg/ml on the day of hCG administration (Fatemi et al., 2007) and if the number of follicles is above 10 (Fatemi et al., 2007).
GnRH agonist: a novel luteal-phase support?
GnRH agonist was recently suggested as a novel luteal-phase support that may act upon pituitary gonadotrophs, the endometrium and the embryo itself (Tesarik, 2006).
It has been hypothesized that GnRH agonist may support the corpus luteum by stimulating the secretion of LH by pituitary gonadotroph cells or by acting directly on the endometrium through the locally expressed GnRH receptors (Pirard et al., 2005).
In a prospective randomized study, Tesarik et al. (2006) evaluated the effect of GnRH agonist (0.1 mg triptorelin) administration in the luteal phase on outcomes in both GnRH agonist (n = 300) and GnRH antagonist (n = 300) ovarian stimulation protocols. They were randomly assigned to receive a single injection of GnRH agonist (study group) or placebo (control group) 6 days after ICSI.
The pregnancy rates were enhanced for both protocols, in long GnRH agonist protocol the clinical implantation rate were 29.8% (97/325) vs. 18.2% (60/330) respectively (P < 0.05). Ongoing pregnancy rates were 46.8% (66/141) vs. 38.0% (54/142) respectively (P = NS).
In patients treated with the GnRH antagonist protocol, clinical implantation rates were 27.1% (86/317) vs. 17.4% (57/328) respectively (P < 0.05) and ongoing pregnancy rates were 44.8% (65/145) vs. 31.9% (46/144) respectively (P < 0.05).
Luteal-phase GnRH agonist administration additionally increased the luteal-phase serum HCG, estradiol and progesterone concentrations in both ovarian stimulation regimens. It was postulated that the beneficial effect may have resulted from a combination of effects on the embryo and on the corpus luteum.
Despite these initial encouraging results, it is too early to adopt this treatment wholesale.
With regard to safety, great concern exists about possible adverse effects on oocytes and, more importantly, on embryos (Lambalk and Homburg, 2006).
To establish a potential positive role of GnRH agonist administration in the luteal phase of stimulated IVF cycles, further large prospective trials are needed.
The duration of luteal phase support
Until recently, there were no studies to either support or contest the generally accepted practice of prolonging progesterone supplementation during early pregnancy.
Schmidt et al. (2001) was the first to publish a retrospective study to compare the delivery rate with IVF or ICSI in women who received progesterone supplementation with those who did not during the first weeks of pregnancy. For three weeks following a positive hCG test, 200 pregnant women received progesterone and 200 pregnant women received none (study group). The results showed no difference in the delivery rate. Of the 200 pregnancies in the study group, 126 (63%) ended in live birth, 46 (23%) were biochemical, 5 (2.5%) were ectopic and 23 (11.5%) ended in abortion. In the control group, 128 pregnancies (64%) ended in a live birth, 35 (18%) were biochemical, 7 (3.5%) were ectopic, and 30 (15%) ended in abortion.
Subsequently, a prospective randomized controlled trial was conducted. Nyboe Andersen et al., (2002) evaluated whether the prolongation of luteal support during early pregnancy had any influences on the delivery rate after IVF. In this study, luteal phase support was administered in the form of 200 mg vaginal progesterone three times daily (600 mg/d) during 14 days from the day ET until the day of a positive HCG test. The study group (n = 150) withdrew vaginal progesterone from the day of positive HCG. The control group (n = 153) continued administration of vaginal progesterone during the next 3 weeks of pregnancy. 118 (78.7%) patients delivered in the study group given no progesterone versus 126 (82.4%) in the control group who continued with progesterone. The difference was not significant. Results indicated that prolongation of progesterone supplementation in early pregnancy had no influence on the miscarriage rate, and thus no effect on the delivery rate.
It would appear that the increase in endogenous HCG level during early pregnancy makes up for any possible lack of endogenous LH that has been caused by stimulated IVF cycles.
First trimester progesterone supplementation in IVF may support early pregnancy through 7 weeks by delaying a miscarriage but it does not improve live birth rates (Proctor et al., 2006).
Conclusions
The cause of luteal phase defect in stimulated IVF cycles seems to be related to the supra- physiologic levels of steroids.
Luteal phase support with HCG or progesterone after assisted reproduction results in an increased pregnancy rate (Fatemi, et al., 2007).
HCG is associated with a greater risk of OHSS. Luteal support with hCG should be avoided if E2 >2700pg/ml (Fatemi, et al., 2007) and if the number of follicles is >10 (Fatemi, et al., 2007).
Natural micronised progesterone is not efficient if taken orally (Fatemi, et al., 2007). Vaginal and intra muscular progesterone seem to have comparable implantation and clinical pregnancy rates and delivery rates (Fatemi, et al., 2007).
The addition of oral E2 to the progestin for luteal phase support still seems not to be beneficial (Kolibianakis et al., 2008).
The length of luteal phase support in stimulated IVF cycles does not need to exceed 14 days from the day of transfer (day 3 post OR) until the day of a positive HCG test (Nyboe Anderson et al., 2002).
In the coming years, IVF stimulation may evolve into a more physiologic process – a milder stimulation – with the significant fringe benefit of reducing or eliminating the current luteal phase defect.
Future prospects
It appears that the cause of luteal phase defect in IVF is related to the supraphysiological levels of steroids, it would be interesting to find out which is the threshold, where the luteal phase defect initiates.
Further more it should be more specified whether it is the progesterone, E2 or both causing the luteal phase defect in stimulated cycles. Therefore a progesterone antagonist could be administered in oocyte donors and the luteal endocrine profile of those patients should be evaluated. Also the combined use of an anti-estrogen, i.e. an aromatase inhibitor and a progesterone antagonist in oocyte donors should be further evaluated.
CC occupies the hypothalamic estrogen receptors for several weeks (Dickey et al., 1996). The long term receptor occupancy might lead to higher luteal LH concentrations, correcting the luteal phase defect observed in stimulated IVF cycles (Van Steirteghem et al., 1988). It would be interesting to evaluate, whether there is a luteal phase defect in cycles stimulated with clomiphene citrate/ recombinant FSH and gonadotropin-releasing hormone antagonist, despite the significantly higher LH levels measured in the luteal phase of these cycles (Tavaniotou et al., 2002).
Furthermore the administration of very low dose of HCG for luteal phase support in stimulated IVF cycles without the co-administration of P and E2 should be evaluated.
Last but not least, further genetic research of endometrium should be performed, to find out why anno 2009 still we have such a low ongoing pregnancy rates after IVF/ICSI.
References
- Al Inany HG, Abou-Setta AM, Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception. Cochrane Database Syst Rev. 2006;3(CD001750) doi: 10.1002/14651858.CD001750.pub2. [DOI] [PubMed] [Google Scholar]
- Albano C, Smitz J, Camus M, Riethmüller-Winzen H, Weigel M, Diedrich K, Van Steirteghem AC, Devroey P. Hormonal profile during the follicular phase in cycles stimulated with a combination of human menopausal gonadotrophin and gonadotrophin-releasing hormone antagonist (Cetrorelix) Hum Reprod. 1996;11:2114–2118. doi: 10.1093/oxfordjournals.humrep.a019058. [DOI] [PubMed] [Google Scholar]
- Albano C, Grimbizis G, Smitz J, Winzen H, Reissmann T, Van Steirteghem A, Devroey P. The luteal phase of nonsupplemented cycles after ovarian superovulation with human menopausal gonadotropin and the gonadotropin-releasing hormone antagonist Cetrorelix. Fertil Steril. 1998;70:357–359. doi: 10.1016/s0015-0282(98)00135-6. [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Haskins AL, Hodgen GD, Pepe GJ. Luteal function in baboons with administration of the antiestrogen ethamoxytriphetol (MER-25) throughout the luteal phase of the menstrual cycle. Biol Reprod. 1981;25:451–457. doi: 10.1095/biolreprod25.3.451. [DOI] [PubMed] [Google Scholar]
- Allen WM, Corner GW. Physiology of the corpus luteum. VII. Maintenance of pregnancy in rabbit after very early castration by corpus luteum extracts. Proc Soc Exp Biol Med. 1930;27:403–405. [Google Scholar]
- Amoss M, Burgus R, Blackwell R, Vale W, Fellows R, Guillemin R. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem Biophys Res Commun. 1971;44:205–210. doi: 10.1016/s0006-291x(71)80179-1. [DOI] [PubMed] [Google Scholar]
- Anasti JN, Kalantaridou SN, Kimzey LM, George M, Nelson LM. Human follicle fluid vascular endothelial growth factor concentrations are correlated with luteinization in spontaneously developing follicles. Hum Reprod. 1998;13:1144–1147. doi: 10.1093/humrep/13.5.1144. [DOI] [PubMed] [Google Scholar]
- Andersen AN, Hagen C, Lange P, Boesgaard S, Djursing H, Eldrup E, Micic S. Dopaminergic regulation of gonadotropin levels and pulsatility in normal women. Fertil Steril. 1987;47:391–397. [PubMed] [Google Scholar]
- Anthony FW, Smith EM, Gadd SC, Masson GM, Chard T, Perry L. Placental protein 14 secretion during in vitro fertilization cycles with and without human chorionic gonadotropin for luteal support. Fertil Steril. 1993;59:187–191. doi: 10.1016/s0015-0282(16)55637-4. [DOI] [PubMed] [Google Scholar]
- Araujo E, Jr, Bernardini L, Frederick JL, Asch RH, Balmaceda JP. Prospective randomized comparison of human chorionic gonadotropin versus intramuscular progesterone for luteal-phase support in assisted reproduction. 1994;11:74–78. doi: 10.1007/BF02215991. [DOI] [PubMed] [Google Scholar]
- Auletta FJ, Speroff L, Caldwell BV. Prostaglandin F-2 induced steroidogenesis and luteolysis in the primate corpus luteum. J Clin Endocrinol Metab. 1973;36:405–407. doi: 10.1210/jcem-36-2-405. [DOI] [PubMed] [Google Scholar]
- Auletta FJ, Caldwell BV, Speroff L. Estrogen-induced luteolysis in the rhesus monkey: reversal with indomethacin. Prostaglandins. 1976;11:745–752. doi: 10.1016/0090-6980(76)90074-5. [DOI] [PubMed] [Google Scholar]
- Balasch J, Vanrell JA, Marquez M, González-Merlo J. Dehydrogesterone versus vaginal progesterone in the treatment of the endometrial luteal phase deficiency. Fertil Steril. 1982;37:751–754. doi: 10.1016/s0015-0282(16)46333-8. [DOI] [PubMed] [Google Scholar]
- Barraclough CA, Wise PM, Selmanoff MK. A role for hypothalamic catecholamines in the regulation of gonadotropin secretion. Recent Prog Horm Res. 1984;40:487–529. doi: 10.1016/b978-0-12-571140-1.50016-5. [DOI] [PubMed] [Google Scholar]
- Baruffi R, Mauri AL, Petersen CG, Felipe V, Franco JG., Jr Effects of vaginal progesterone administration starting on the day of oocyte retrieval on pregnancy rates. J Assist Reprod Genet. 2003;20:517–520. doi: 10.1023/B:JARG.0000013653.54830.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista MC, Cartledge TP, Nieman LK, Bravo N, Loriaux DL, Merriam GR. Characterization of the normal progesterone and placental protein 14 responses to human chorionic gonadotropin stimulation in the luteal phase. Fertil Steril. 1994;61:637–644. [PubMed] [Google Scholar]
- Beard J. The Span of Gestation and the Cause of Birth: a Study of the Critical Period and Its Effects in Mammalia. Jena, Germany: Fischer; 1897. [Google Scholar]
- Beckers NG, Laven JS, Eijkemans MJ, Fauser BC. Follicular and luteal phase characteristics following early cessation of gonadotrophin-releasing hormone agonist during ovarian stimulation for in-vitro fertilization. Hum Reprod. 2000;15:43–49. doi: 10.1093/humrep/15.1.43. [DOI] [PubMed] [Google Scholar]
- Beckers NG, Macklon NS, Eijkemans MJ, Ludwig M, Felberbaum RE, Diedrich K, Bustion S, Loumaye E, Fauser BC. Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist cotreatment. J Clin Endocrinol Metab. 2003;88:4186–4192. doi: 10.1210/jc.2002-021953. [DOI] [PubMed] [Google Scholar]
- Behrman HR, Preston SL. Luteolytic actions of peroxide in rat ovarian cells. Endocrinology. 1989;124:2895–2900. doi: 10.1210/endo-124-6-2895. [DOI] [PubMed] [Google Scholar]
- Belaisch-Allart J, Testart J, Fries N, Forman RG, Frydman R. The effect of dydrogesterone supplementation in an IVF programme. Hum Reprod. 1987;2:183–185. doi: 10.1093/oxfordjournals.humrep.a136511. [DOI] [PubMed] [Google Scholar]
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202 doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- Belgisch Staatsblad. 173e Jaargang. 2003. pp. 32133–32157. [Google Scholar]
- Berek JS. Novak’s gynecology. Lippincott Williams and Wilkins; 2002. [Google Scholar]
- Blackwell R, Amoss M, Jr, Vale W, Burgus R, Rivier J, Monahan M, Ling N, Guillemin R. Concomitant release of FSH and LH induced by native and synthetic LRF. Am J Physiol. 1973;224:170–175. doi: 10.1152/ajplegacy.1973.224.1.170. [DOI] [PubMed] [Google Scholar]
- Bomba M, Gambera A, Bonini L, Peroni M, Neri F, Scagliola P, Nacinovich R. Endocrine profiles and neuropsychologic correlates of functional hypothalamic amenorrhea in adolescents. Fertil Steril. 2007;87:876–885. doi: 10.1016/j.fertnstert.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Bouckaert Y, Robert F, Englert Y, De Backer D, De Vuyst P, Delbaere A. Acute eosinophilic pneumonia associated with intramuscular administration of progesterone as luteal phase support after IVF: case report. Hum Reprod. 2004;19:1806–1810. doi: 10.1093/humrep/deh316. [DOI] [PubMed] [Google Scholar]
- Bourgain C, Devroey P, Van Waesberghe L, Smitz J, Van Steirteghem AC. Effects of natural progesterone on the morphology of the endometrium in patients with primary ovarian failure. Hum Reprod. 1990;5:537–543. doi: 10.1093/oxfordjournals.humrep.a137138. [DOI] [PubMed] [Google Scholar]
- Bourgain C, Smitz J, Camus M, Erard P, Devroey P, Van Steirteghem AC, Kloppel G. Human endometrial maturation is markedly improved after luteal supplementation of gonadotrophin-releasing hormone analogue/human menopausal gonadotrophin stimulated cycles. Hum Reprod. 1994;9:32–40. doi: 10.1093/oxfordjournals.humrep.a138316. [DOI] [PubMed] [Google Scholar]
- Bourgain C. Proefschrift tot het behalen van de graad van doctor in de Medische wetenschappen, PhD in Medical science. 2001-2002. [Google Scholar]
- Buettner GR, Jurkiewicz BA. Ascorbate free radical as a marker of oxidative stress: an EPR study. Free Radic Biol Med. 1993;14 doi: 10.1016/0891-5849(93)90508-r. [DOI] [PubMed] [Google Scholar]
- Bulletti C, de Ziegler D. Uterine contractility and embryo implantation. Curr Opin Obstet Gynecol. 2005;17:265–276. doi: 10.1097/01.gco.0000169104.85128.0e. [DOI] [PubMed] [Google Scholar]
- Butenandt A, Westphal U, Hohlweg W. Er das hormon des corpus luteum. Hoppe-Seyler’s Z Physiol Chem. 1934;227:84–98. [Google Scholar]
- Butenandt A, Westphal U. Zur isolierung und charakterisierung des corpus-luteum hormons. Berichte Deutsch Chem Geseilsch. 1934;67:1440–1442. [Google Scholar]
- Buvat J, Marcolin G, Guittard C, Dehaene JL, Herbaut JC, Louv , et al. Luteal support after administration of an LHRH analog for in vitro fertilization. Superiority of vaginal progesterone in comparison with oral progesterone. Presse Med. 1990;19:527. [PubMed] [Google Scholar]
- Casper RF, Yen SS. Induction of luteolysis in the human with a long-acting analog of luteinizing hormone-releasing factor. Science. 1979;205:408–410. doi: 10.1126/science.377491. [DOI] [PubMed] [Google Scholar]
- Casper RF. Aromatase inhibitors in ovarian stimulation. J Steroid Biochem Mol Biol. 2007;106:71–75. doi: 10.1016/j.jsbmb.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Casper RF. Letrozole versus clomiphene citrate: which is better for ovulation induction? Fertil Steril. 2007 doi: 10.1016/j.fertnstert.2007.03.094. [DOI] [PubMed] [Google Scholar]
- Chakmakjian ZH, Zachariah NY. Bioavailability of progesterone with different modes of administration. J Reprod Med. 1987;32:443–448. [PubMed] [Google Scholar]
- Chakravarty BN, Shirazee HH, Dam P, Goswami SK, Chatterjee R, Ghosh S. Oral dydrogesterone versus intravaginal micronised progesterone as luteal phase support in assisted reproductive technology (ART) cycles: results of a randomised study. J Steroid Biochem Mol Biol. 2005;97:416–420. doi: 10.1016/j.jsbmb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Chanson A, Germond K, Lagnaux Y. Comparison of two progesterone dose regimens for luteal phase support after embryo transfer: a prospective randomized study. Human reproduction. 1996;11:170. [Google Scholar]
- Chappel SC, Resko JA, Norman RL, Spies HG. Studies in rhesus monkeys on the site where estrogen inhibits gonadotropins: delivery of 17 beta-estradiol to the hypothalamus and pituitary gland. J Clin Endocrinol Metab. 1981;52:1–8. doi: 10.1210/jcem-52-1-1. [DOI] [PubMed] [Google Scholar]
- Cicinelli E, de Ziegler D, Bulletti C, Matteo MG, Schonauer LM, Galantino P. Direct transport of progesterone from vagina to uterus. Obstet Gynecol. 2000;95:403–406. doi: 10.1016/s0029-7844(99)00542-6. [DOI] [PubMed] [Google Scholar]
- Claman P, Domingo M, Leader A. Luteal phase support in in-vitro fertilization using gonadotrophin releasing hormone analogue before ovarian stimulation: a prospective randomized study of human chorionic gonadotrophin versus intramuscular progesterone. Hum Reprod. 1992;7:487–489. doi: 10.1093/oxfordjournals.humrep.a137676. [DOI] [PubMed] [Google Scholar]
- Clayton RN, Catt KJ. Receptor-binding affinity of gonadotropin-releasing hormone analogs: analysis by radioligand-receptor assay. Endocrinology. 1980;106:1154–1159. doi: 10.1210/endo-106-4-1154. [DOI] [PubMed] [Google Scholar]
- Coiter V. In Officina Theodorici Gerlatzeni. Nuremberg, Germany: 1573 Externarum et internarum principalium humani corporis partium tabulae microform: atque exercitationes observationesque, novis, diversis, ac artificosissimus figeris illustratae, philosophis, medicis, in primis autem anatomico studio addictis summe utiles. [Google Scholar]
- Corner GW, Allen WM. Normal growth and implantation of embryos after very early ablation of the ovaries, under the influence of extracts of the corpus luteum. Am J Physiol. 1929;88:340–346. [Google Scholar]
- Costabile L, Gerli S, Manna C, Rossetti D, Di Renzo GC, Unfer V. A prospective randomized study comparing intramuscular progesterone and 17alpha-hydroxyprogesterone caproate in patients undergoing in vitro fertilization-embryo transfer cycles. Fertil Steril. 2001;76:394–396. doi: 10.1016/s0015-0282(01)01901-x. [DOI] [PubMed] [Google Scholar]
- Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, Legro RS, McGovern PG, Schlaff WD, Carr BR, Steinkampf MT, Silva S, Vogel DL, Leppert PC. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril. 2004;82:1264–1272. doi: 10.1016/j.fertnstert.2004.03.069. [DOI] [PubMed] [Google Scholar]
- Couzinet B, Brailly S, Bouchard P, Schaison G. Progesterone stimulates luteinizing hormone secretion by acting directly on the pituitary. J Clin Endocrinol Metab. 1992;74:374–378. doi: 10.1210/jcem.74.2.1730816. [DOI] [PubMed] [Google Scholar]
- Couzinet B, Schaison G. The control of gonadotrophin secretion by ovarian steroids. Hum Reprod. 1993;8(Suppl 2):97–101. doi: 10.1093/humrep/8.suppl_2.97. [DOI] [PubMed] [Google Scholar]
- Csapo AI, Pulkkinen MO, Ruttner B, Sauvage JP, Wiest WG. The significance of the human corpus luteum in pregnancy maintenance. I. Preliminary studies. Am J Obstet Gynecol. 1972;112:1061–1067. doi: 10.1016/0002-9378(72)90181-0. [DOI] [PubMed] [Google Scholar]
- Csapo AI, Pulkkinen MO, Wiest WG. Effects of luteectomy and progesterone replacement therapy in early pregnant patients. Am J Obstet Gynecol. 1973;115:759–765. doi: 10.1016/0002-9378(73)90517-6. [DOI] [PubMed] [Google Scholar]
- Dal Prato L, Borini A. Use of antagonists in ovarian stimulation protocols. Reprod Biomed Online. 2005;10:330–338. doi: 10.1016/s1472-6483(10)61792-0. [DOI] [PubMed] [Google Scholar]
- Dallenbach-Hellweg G. The endometrium of infertility. A review. Pathol Res Pract. 1984;178:527–537. doi: 10.1016/S0344-0338(84)80084-9. [DOI] [PubMed] [Google Scholar]
- Dawood MY. Corpus luteal insufficiency. Curr Opin Obstet Gynecol. 1994;6:121–127. [PubMed] [Google Scholar]
- De Graaf . G. W. Corner. In: Essays in Biology. Berkeley, CA: Univ. of California Press; 1943. Mullierum Organis Generationi Inservientibus. Leyden, 1672. [Google Scholar]
- De Ziegler D, Bergeron C, Cornel C, Medalie DA, Massai MR, Milgrom E, Frydman R, Bouchard P. Effects of luteal estradiol on the secretory transformation of human endometrium and plasma gonadotropins. J Clin Endocrinol Metab. 1992;74:322–331. doi: 10.1210/jcem.74.2.1730810. [DOI] [PubMed] [Google Scholar]
- De Ziegler D, Seidler L, Scharer E, Bouchard P. Non-oral administration of progesterone: experiences and possibilities of the transvaginal route. Schweiz Rundsch Med Prax. 1995;84:127–133. [PubMed] [Google Scholar]
- Devoto L, Vega M, Kohen P, Castro A, Castro O, Christenson LK, Carvallo P, Strauss JF., 3rd Endocrine and paracrine-autocrine regulation of the human corpus luteum during the mid-luteal phase. J Reprod Fertil. 2000;55(Suppl):13–20. [PubMed] [Google Scholar]
- Devroey P, Palermo G, Bourgain C, Van Waesberghe L, Smitz J, Van Steirteghem AC. Progesterone administration in patients with absent ovaries. Int J Fertil. 1989;34:188–193. [PubMed] [Google Scholar]
- Devroey P, Van Steirteghem A. A review of ten years experience of ICSI. Hum Reprod Update. 2004;10:19–28. doi: 10.1093/humupd/dmh004. [DOI] [PubMed] [Google Scholar]
- Dickey RP, Taylor SN, Curole DN, Rye PH, Pyrzak R. Incidence of spontaneous abortion in clomiphene pregnancies. Hum Reprod. 1996;11:2623–2628. doi: 10.1093/oxfordjournals.humrep.a019182. [DOI] [PubMed] [Google Scholar]
- Dierschke DJ, Bhattacharya AN, Atkinson LE, Knobil E. Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology. 1970;87:850–853. doi: 10.1210/endo-87-5-850. [DOI] [PubMed] [Google Scholar]
- Dlugi AM, Laufer N, Polan ML, DeCherney AH, Tarlatzis BC, MacLusky NJ, Behrman HR. 17 beta-estradiol and progesterone production by human granulosa-luteal cells isolated from human menopausal gonadotropin-stimulated cycles for in vitro fertilization. J Clin Endocrinol Metab. 1984;59:986–992. doi: 10.1210/jcem-59-5-986. [DOI] [PubMed] [Google Scholar]
- Drouva SV, Laplante E, Kordon C. Effects of ovarian steroids on in vitro release of LHRH from mediobasal hypothalamus. Neuroendocrinology. 1983;37:336–341. doi: 10.1159/000123572. [DOI] [PubMed] [Google Scholar]
- Duello TM, Halmi NS. Ultrastructural-immunocytochemical localization of growth hormone and prolactin in human pituitaries. J Clin Endocrinol Metab. 1979;49:189–196. doi: 10.1210/jcem-49-2-189. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stewart DR, Stouffer RL. Titrating luteinizing hormone replacement to sustain the structure and function of the corpus luteum after gonadotropin-releasing hormone antagonist treatment in rhesus monkeys. J Clin Endocrinol Metab. 1999;84:342–349. doi: 10.1210/jcem.84.1.5362. [DOI] [PubMed] [Google Scholar]
- Edwards RG, Steptoe PC, Purdy JM. Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynaecol. 1980;87:737–756. doi: 10.1111/j.1471-0528.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- Ellinwood WE, Resko JA. Effect of inhibition of estrogen synthesis during the luteal phase on function of the corpus luteum in rhesus monkeys. Biol Reprod. 1983;28:636–644. doi: 10.1095/biolreprod28.3.636. [DOI] [PubMed] [Google Scholar]
- Elter K, Nelson LR. Use of third generation gonadotropin-releasing hormone antagonists in in vitro fertilization-embryo transfer: a review. Obstet Gynecol Surv. 2001;56:576–588. doi: 10.1097/00006254-200109000-00024. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Magoffin DA, Dyer CA, Hofeditz C. The ovarian androgen producing cells: a review of structure/function relationships. Endocr Rev. 1985;6:371–399. doi: 10.1210/edrv-6-3-371. [DOI] [PubMed] [Google Scholar]
- Falcone , Hurd . Mosby, Elsevier; 2007. Clinical Reproductive Medicine and Surgery; p. 20. [Google Scholar]
- Fanchin R, Righini C, de Ziegler D, Olivennes F, Ledée N, Frydman R. Effects of vaginal progesterone administration on uterine contractility at the time of embryo transfer. Fertil Steril. 2001;75:1136–1140. doi: 10.1016/s0015-0282(01)01787-3. [DOI] [PubMed] [Google Scholar]
- Farhi J, Weissman A, Steinfeld Z, Shorer M, Nahum H, Levran D. Estradiol supplementation during the luteal phase may improve the pregnancy rate in patients undergoing in vitro fertilization-embryo transfer cycles. Fertil Steril. 2000;73:761–766. doi: 10.1016/s0015-0282(99)00632-9. [DOI] [PubMed] [Google Scholar]
- Fatemi HM, Platteau P, Albano C, Van Steirteghem A, Devroey P. Rescue IVF and coasting with the use of a GnRH antagonist after ovulation induction. Reprod Biomed Online. 2002;5:273–275. doi: 10.1016/s1472-6483(10)61832-9. [DOI] [PubMed] [Google Scholar]
- Fatemi HM, Kolibianakis E, Tournaye H, Camus M, Van Steirteghem AC, Devroey P. Clomiphene citrate versus letrozole for ovarian stimulation: a pilot study. Reprod Biomed Online. 2003;7:543–546. doi: 10.1016/s1472-6483(10)62070-6. [DOI] [PubMed] [Google Scholar]
- Fatemi HM, Kolibianakis EM, Camus M, Tournaye H, Donoso P, Papanikolaou E, Devroey P, et al. Addition of estradiol to progesterone for luteal supplementation in patients stimulated with GnRH antagonist/rFSH for IVF: a randomized controlled trial. Hum Reprod. 2006;21:2628–2632. doi: 10.1093/humrep/del117. [DOI] [PubMed] [Google Scholar]
- Fatemi HM, Popovic-Todorovic B, Papanikolaou E, Donoso P, Devroey P. An update of luteal phase support in stimulated IVF cycles. Hum Reprod Update. 2007;13:581–590. doi: 10.1093/humupd/dmm021. [DOI] [PubMed] [Google Scholar]
- Fauser BC, Devroey P. Reproductive biology and IVF: ovarian stimulation and luteal phase consequences. Trends Endocrinol Metab. 2003;14:236–242. doi: 10.1016/s1043-2760(03)00075-4. [DOI] [PubMed] [Google Scholar]
- Fiddes JC, Talmadge K. Structure, expression, and evolution of the genes for the human glycoprotein hormones. Recent Prog Horm Res. 1984;40:43–78. doi: 10.1016/b978-0-12-571140-1.50006-2. [DOI] [PubMed] [Google Scholar]
- Filicori M, Santoro N, Merriam GR, Crowley WF., Jr Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab. 1986;62:1136–1144. doi: 10.1210/jcem-62-6-1136. [DOI] [PubMed] [Google Scholar]
- Fisher SA, Reid RL, Van Vugt DA, Casper RF. A randomized double-blind comparison of the effects of clomiphene citrate and the aromatase inhibitor letrozole on ovulatory function in normal women. Fertil Steril. 2002;78:280–285. doi: 10.1016/s0015-0282(02)03241-7. [DOI] [PubMed] [Google Scholar]
- Fraenkel L. Die Funktion des Corpus luteum. Archiv Gynaekol (Munich) 1903;68:438–443. [Google Scholar]
- Genazzani AR, Petraglia F. Opioid control of luteinizing hormone secretion in humans. J Steroid Biochem. 1989;33:751–755. doi: 10.1016/0022-4731(89)90487-1. [DOI] [PubMed] [Google Scholar]
- Geva E, Amit A, Lerner-Geva L, Yaron Y, Daniel Y, Schwartz T, Azem F, Yovel I, Lessing JB. Prednisone and aspirin improve pregnancy rate in patients with reproductive failure and autoimmune antibodies: a prospective study. Am J Reprod Immunol. 2000;43:36–40. doi: 10.1111/j.8755-8920.2000.430107.x. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Stewart DR, Nayak NR, Lasley BL, Overstreet JW, Hendrickx AG, Sengupta J. Serum concentrations of oestradiol-17beta, progesterone, relaxin and chorionic gonadotrophin during blastocyst implantation in natural pregnancy cycle and in embryo transfer cycle in the rhesus monkey. Hum Reprod. 1997;12:914–920. doi: 10.1093/humrep/12.5.914. [DOI] [PubMed] [Google Scholar]
- Gindoff PR, Ferin M. Endogenous opioid peptides modulate the effect of corticotropin-releasing factor on gonadotropin release in the primate. Endocrinology. 1987;121:837–842. doi: 10.1210/endo-121-3-837. [DOI] [PubMed] [Google Scholar]
- Ginsburg KA. Luteal phase defect. Etiology, diagnosis, and management. Endocrinol Metab Clin North Am. 1992;21:85–104. [PubMed] [Google Scholar]
- Griesinger G, Franke K, Kinast C, Kutzelnigg A, Riedinger S, Kulin S, Kaali SG, Feichtinger W, et al. Ascorbic acid supplement during luteal phase in IVF. J Assist Reprod Genet. 2002;19:164–168. doi: 10.1023/A:1014837811353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesinger G, Diedrich K, Devroey P, Kolibianakis EM. GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol: a systematic review and meta-analysis. Hum Reprod Update. 2006;12:159–168. doi: 10.1093/humupd/dmi045. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Endicott J. Possible involvement of endorphin withdrawal or imbalance in specific premenstrual syndromes and postpartum depression. Med Hypotheses. 1981;7:1045–1058. doi: 10.1016/0306-9877(81)90100-6. [DOI] [PubMed] [Google Scholar]
- Harper MJ. The implantation window. Baillieres Clin Obstet Gynaecol. 1992;6:351–371. doi: 10.1016/s0950-3552(05)80092-6. [DOI] [PubMed] [Google Scholar]
- Harris GW, Naftolin F. The hypothalamus and control of ovulation. Br Med Bull. 1970;26:3–9. doi: 10.1093/oxfordjournals.bmb.a070739. [DOI] [PubMed] [Google Scholar]
- Hartmann H, Wettstein A. Ein krystallisiertes hormon aus corpus luteum. Helv Chim Acta. 1934;17:878–882. [Google Scholar]
- Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- Herman A, Ron-El R, Golan A, Raziel A, Soffer Y, Caspi E. Pregnancy rate and ovarian hyperstimulation after luteal human chorionic gonadotropin in in vitro fertilization stimulated with gonadotropin-releasing hormone analog and menotropins. Fertil Steril. 1990;53:92–96. doi: 10.1016/s0015-0282(16)53222-1. [DOI] [PubMed] [Google Scholar]
- Hild-Petito S, Fazleabas AT. Expression of steroid receptors and steroidogenic enzymes in the baboon (Papio anubis) corpus luteum during the menstrual cycle and early pregnancy. J Clin Endocrinol Metab. 1997;82:955–962. doi: 10.1210/jcem.82.3.3813. [DOI] [PubMed] [Google Scholar]
- Hoing LM, Devroey P, Van Steirteghem AC. Treatment of infertility because of oligoasthenoteratospermia by transcervical intrauterine insemination of motile spermatozoa. Fertil Steril. 1986;45:388–391. doi: 10.1016/s0015-0282(16)49222-8. [DOI] [PubMed] [Google Scholar]
- Honda T, Fujiwara H, Yamada S, Fujita K, Nakamura K, Nakayama T, Higuchi T, Ueda M, Maeda M, Mori T. Integrin alpha5 is expressed on human luteinizing granulosa cells during corpus luteum formation, and its expression is enhanced by human chorionic gonadotrophin in vitro. Mol Hum Reprod. 1997;3:979–984. doi: 10.1093/molehr/3.11.979. [DOI] [PubMed] [Google Scholar]
- Hotchkiss J, Dierschke DJ, Butler WR, Fritz GR, Knobil E. Relation between levels of circulating ovarian steroids and pituitary gonadotropin content during the menstrual cycle of the rhesus monkey. Biol Reprod. 1982;26:241–248. doi: 10.1095/biolreprod26.2.241. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Fedorkow DM, Daya S, Sagle MA, Van de Koppel P, Collins JA. The routine use of gonadotropin-releasing hormone agonists prior to in vitro fertilization and gamete intrafallopian transfer: a meta-analysis of randomized controlled trials. Fertil Steril. 58:888–896. doi: 10.1016/s0015-0282(16)55430-2. [DOI] [PubMed] [Google Scholar]
- Hurst BS, Bhojwani JT, Marshburn PB, Papadakis MA, Loeb TA, Matthews ML. Low-dose aspirin does not improve ovarian stimulation, endometrial response, or pregnancy rates for in vitro fertilization. J Exp Clin Assist Reprod. 2005:8. doi: 10.1186/1743-1050-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson-Williams KA, Lunenfeld B, Diamond MP, Lavy G, Boyers SP, DeCherney AH. Human chorionic gonadotropin, estradiol, and progesterone profiles in conception and nonconception cycles in an in vitro fertilization program. Fertil Steril. 1989;52:441–445. doi: 10.1016/s0015-0282(16)60915-9. [DOI] [PubMed] [Google Scholar]
- Hutchinson-Williams KA, DeCherney AH, Lavy G, Diamond MP, Naftolin F, Lunenfeld B. Luteal rescue in in vitro fertilization-embryo transfer. Fertil Steril. 1990;53:495–501. [PubMed] [Google Scholar]
- Hutchinson JS, Zelesnik AJ. The corpus luteum of the primate menstrual cycle is capable of recovering from a transient withdrawal of pituitary gonadotropin support. Endocrinology. 1985;117:1043–1049. doi: 10.1210/endo-117-3-1043. [DOI] [PubMed] [Google Scholar]
- Hutchison JS, Kubik CJ, Nelson PB, Zeleznik AJ. Estrogen induces premature luteal regression in rhesus monkeys during spontaneous menstrual cycles, but not in cycles driven by exogenous gonadotropin-releasing hormone. Endocrinology. 1987;121:466–474. doi: 10.1210/endo-121-2-466. [DOI] [PubMed] [Google Scholar]
- Ioannidis G, Sacks G, Reddy N, Seyani L, Margara R, Lavery S, Trew G. Day 14 maternal serum progesterone levels predict pregnancy outcome in IVF/ICSI treatment cycles: a prospective study. Hum Reprod. 2005;20:741–746. doi: 10.1093/humrep/deh644. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Abbas AA, Irvine R, Riddle AF, Norman-Taylor JQ, Grudzinskas JG, Collins WP, Nicolaides KH. Regulation of corpus luteum function. Hum Reprod. 1994;9:41–48. doi: 10.1093/oxfordjournals.humrep.a138317. [DOI] [PubMed] [Google Scholar]
- Jones GS. Luteal phase defect: a review of pathophysiology. Curr Opin Obstet Gynecol. 1991;3:641–648. [PubMed] [Google Scholar]
- Jones GES. Some new aspects of management of infertility. JAMA. 1979;141:1123. doi: 10.1001/jama.1949.02910160013004. [DOI] [PubMed] [Google Scholar]
- Jordan J, Craig K, Clifton DK. Luteal phase defect: the sensitivity and specificity of diagnostic methods in common clinical use. Fertil Steril. 1994;62:54–62. doi: 10.1016/s0015-0282(16)56815-0. [DOI] [PubMed] [Google Scholar]
- Kalra PS, Kalra SP. Steroidal modulation of the regulatory neuropeptides: luteinizing hormone releasing hormone, neuropeptide Y and endogenous opioid peptides. J Steroid Biochem. 1986;25:733–740. doi: 10.1016/0022-4731(86)90302-x. [DOI] [PubMed] [Google Scholar]
- Karten MJ, Rivier JE. Gonadotropin-releasing hormone analog design. Structure-function studies toward the development of agonists and antagonists: rationale and perspective. Endocr Rev. 1986;7:44–66. doi: 10.1210/edrv-7-1-44. [DOI] [PubMed] [Google Scholar]
- Kerin JF, Broom TJ, Ralph MM, Edmonds DK, Warnes GM, Jeffrey R, Crocker JM, Godfrey B, Cox LW, Seamark RF, Matthews CD. Human luteal phase function following oocyte aspiration from the immediately preovular graafian follicle of spontaneous ovular cycles. Br J Obstet Gynaecol. 1981;88:1021–1028. doi: 10.1111/j.1471-0528.1981.tb01691.x. [DOI] [PubMed] [Google Scholar]
- Kiesel L, Runnebaum B. Gonadotropin releasing hormone and analogs. Physiology and pharmacology. Gynakol Geburtshilfliche Rundsch. 1992;32:22–30. doi: 10.1159/000271829. [DOI] [PubMed] [Google Scholar]
- King RJ, Whitehead MI. Assessment of the potency of orally administered progestins in women. Fertil Steril. 1986;46:1062–1066. doi: 10.1016/s0015-0282(16)49880-8. [DOI] [PubMed] [Google Scholar]
- Klingmuller D, Schepke M, Enzweiler C, Bidlingmaier F. Hormonal responses to the new potent GnRH antagonist Cetrorelix. Acta Endocrinol (Copenh) 1993;128:15–18. doi: 10.1530/acta.0.1280015. [DOI] [PubMed] [Google Scholar]
- Knobil E. On the regulation of the primate corpus luteum. Biol Reprod. 1973;8:246–248. [Google Scholar]
- Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. doi: 10.1016/b978-0-12-571136-4.50008-5. [DOI] [PubMed] [Google Scholar]
- Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G. Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science. 1980;207:1371–1373. doi: 10.1126/science.6766566. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Nakano R, Ooshima A. Immunohistochemical localization of pituitary gonadotrophins and gonadal steroids confirms the ‘two-cell, two-gonadotrophin’ hypothesis of steroidogenesis in the human ovary. J Endocrinol. 1990;126:483–488. doi: 10.1677/joe.0.1260483. [DOI] [PubMed] [Google Scholar]
- Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Van Steirteghem A, Devroey P. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil Steril. 2002;78 doi: 10.1016/s0015-0282(02)03323-x. [DOI] [PubMed] [Google Scholar]
- Kolibianakis EM, Devroey P. The luteal phase after ovarian stimulation. Reprod.Biomed.Online. 2002;5(Suppl 1):26–35. doi: 10.1016/s1472-6483(11)60214-9. [DOI] [PubMed] [Google Scholar]
- Kolibianakis EM, Albano C, Camus M, Tournaye H, Van Steirteghem AC, Devroey P. Prolongation of the follicular phase in in vitro fertilization results in a lower ongoing pregnancy rate in cycles stimulated with recombinant follicle-stimulating hormone and gonadotropin-releasing hormone antagonists. Fertil Steril. 2004;82:102–107. doi: 10.1016/j.fertnstert.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Krey LC, Lu KH, Bulter WR, Hotchkiss J, Piva F, Knobil E. Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. II. GH and cortisol secretion. Endocrinology. 1975;96:1088–1093. doi: 10.1210/endo-96-5-1088. [DOI] [PubMed] [Google Scholar]
- Lalloz MR, Detta A, Clayton RN. Gonadotropin-releasing hormone desensitization preferentially inhibits expression of the luteinizing hormone beta-subunit gene in vivo. Endocrinology. 1988;122:1689–1694. doi: 10.1210/endo-122-4-1689. [DOI] [PubMed] [Google Scholar]
- Lambalk CB, Homburg R. GnRH agonist for luteal support in IVF? Setting the balance between enthusiasm and caution. Hum Reprod. 2006;21:2580–2582. doi: 10.1093/humrep/del321. [DOI] [PubMed] [Google Scholar]
- Lee KA, Koo JJ, Yoon TK. Immunosuppression by corticosteroid has no effect on the pregnancy rate in routine in-vitro fertilization/embryo transfer patients. Hum Reprod. 1994;9:1832–1835. doi: 10.1093/oxfordjournals.humrep.a138343. [DOI] [PubMed] [Google Scholar]
- Leeton J, Trounson A, Jessup D. Support of the luteal phase in in vitro fertilization programs: results of a controlled trial with intramuscular Proluton. J In Vitro Fert Embryo Transf. 1985;2:166–169. doi: 10.1007/BF01131506. [DOI] [PubMed] [Google Scholar]
- Lejeune B, Camus M, Deschacht J, Leroy F. Differences in the luteal phases after failed or successful in vitro fertilization and embryo replacement. J In Vitro Fert Embryo Transf. 1986;3:358–365. doi: 10.1007/BF01133248. [DOI] [PubMed] [Google Scholar]
- Levine H. Luteal support in IVF using the novel vaginal progesterone gel Crinone 8%: results of an open-label trial in 1184 women from 16 US centers. Fertil Steril. 2000;74:836–837. doi: 10.1016/s0015-0282(00)01497-7. [DOI] [PubMed] [Google Scholar]
- Lewin A, Benshushan A, Mezker E, Yanai N, Schenker JG, Goshen R. The role of estrogen support during the luteal phase of in vitro fertilization-embryo transplant cycles: a comparative study between progesterone alone and estrogen and progesterone support. Fertil Steril. 1994;62:121–125. doi: 10.1016/s0015-0282(16)56826-5. [DOI] [PubMed] [Google Scholar]
- Lightman A, Kol S, Eldor J. A prospective randomized study comparing intramuscular with intravaginal natural progesterone in programmed thaw cycles. Hum Reprod. 1999;14:2596–2599. doi: 10.1093/humrep/14.10.2596. [DOI] [PubMed] [Google Scholar]
- Lindsay PC, Shaw RW, Bennink HJ, Kicovic P. The effect of add-back treatment with tibolone (Livial) on patients treated with the gonadotropin-releasing hormone agonist triptorelin (Decapeptyl) Fertil Steril. 1996;65:342–348. doi: 10.1016/s0015-0282(16)58096-0. [DOI] [PubMed] [Google Scholar]
- Loumaye E. The control of endogenous secretion of LH by gonadotrophin-releasing hormone agonists during ovarian hyperstimulation for in-vitro fertilization and embryo transfer. Hum Reprod. 1990;5:357–376. doi: 10.1093/oxfordjournals.humrep.a137105. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Finas A, Katalinic A, Kowalcek I, Schwartz P, Felberbaum R, Küpker W, Schöpper B, Al Hasani S, Diedrich K. Prospective, randomized study to evaluate the success rates using hCG, vaginal progesterone or a combination of both for luteal phase support. Acta Obstet Gynecol Scand. 2001;80 [PubMed] [Google Scholar]
- Ludwig M, Diedrich K. Evaluation of an optimal luteal phase support protocol in IVF. Acta Obstet Gynecol Scand. 2001;80:452–466. doi: 10.1034/j.1600-0412.2001.080005452.x. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Schwartz P, Babahan B, Katalinic A, Weiss JM, Felberbaum R, Hasani S, Diedrich K. Luteal phase support using either Crinone 8% or Utrogest: results of a prospective, randomized study. Eur J Obstet Gynecol Reprod Biol. 2002;103:48–52. doi: 10.1016/s0301-2115(02)00010-6. [DOI] [PubMed] [Google Scholar]
- Lukaszuk K, Liss J, Lukaszuk M, Maj B, et al. Optimization of estradiol supplementation during the luteal phase improves the pregnancy rate in women undergoing in vitro fertilization-embryo transfer cycles. Fertil Steril. 2005;83:1372–1376. doi: 10.1016/j.fertnstert.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Macklon NS, Fauser BC. Impact of ovarian hyperstimulation on the luteal phase. J Reprod Fertil Suppl. 2000;55:101–108. [PubMed] [Google Scholar]
- Mahesh VB, Brann DW. Regulation of the preovulatory gonadotropin surge by endogenous steroids. Steroids. 1998;63:616–629. doi: 10.1016/s0039-128x(98)00075-0. [DOI] [PubMed] [Google Scholar]
- Malpighi M. De Structura Glandarum Conglobatarum. London: Apud Richardum Chiswell; 1689. [Google Scholar]
- Margolin Y, Aten RF, Behrman HR. Antigonadotropic and antisteroidogenic actions of peroxide in rat granulosa cells. Endocrinology. 1990;127:245–250. doi: 10.1210/endo-127-1-245. [DOI] [PubMed] [Google Scholar]
- Martin J, Dominguez F, Avila S. Human endometrial receptivity: gene regulation. J Reprod Immunol. 2002;55:131–139. doi: 10.1016/s0165-0378(01)00140-1. [DOI] [PubMed] [Google Scholar]
- Maslar IA, Ansbacher R. Effect of short-duration progesterone treatment on decidual prolactin production by cultures of proliferative human endometrium. Fertil Steril. 1988;50:250–254. doi: 10.1016/s0015-0282(16)60068-7. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Martini L, Galbiati M. Growth factors and steroid hormones: a complex interplay in the hypothalamic control of reproductive functions. Prog Neurobiol. 2002;67:421–449. doi: 10.1016/s0301-0082(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Messinis IE. Ovarian feedback, mechanism of action and possible clinical implications. Hum Reprod Update. 2006;12:557–571. doi: 10.1093/humupd/dml020. [DOI] [PubMed] [Google Scholar]
- Millar J. Vitamin C – the primate fertility factor? Med Hypotheses. 1992;38:292–295. doi: 10.1016/0306-9877(92)90019-9. [DOI] [PubMed] [Google Scholar]
- Minas V, Loutradis D, Makrigiannakis A. Factors controlling blastocyst implantation. Reprod Biomed Online. 2005;10:205–216. doi: 10.1016/s1472-6483(10)60942-x. [DOI] [PubMed] [Google Scholar]
- Mitwally MF, Casper RF. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. 2001;75:305–309. doi: 10.1016/s0015-0282(00)01705-2. [DOI] [PubMed] [Google Scholar]
- Miyake A, Aono T, Kinugasa T, Tanizawa O, Kurachi K. Suppression of serum levels of luteinizing hormone by short- and long-loop negative feedback in ovariectomized women. J Endocrinol. 1979;80:353–356. doi: 10.1677/joe.0.0800353. [DOI] [PubMed] [Google Scholar]
- Mochtar MH, Hogerzeil HV, Mol BW. Progesterone alone versus progesterone combined with HCG as luteal support in GnRHa/HMG induced IVF cycles: a randomized clinical trial. Hum Reprod. 1996;11:1602–1605. doi: 10.1093/oxfordjournals.humrep.a019453. [DOI] [PubMed] [Google Scholar]
- Mochtar MH, Van Wely M, Van der Veen F. Timing luteal phase support in GnRH agonist down-regulated IVF/embryo transfer cycles. Hum Reprod. 2006;21:905–908. doi: 10.1093/humrep/dei437. [DOI] [PubMed] [Google Scholar]
- Moffitt D, Queenan JT, Jr, Schoolcraft W, Miller CE, Muasher SJ. Low-dose glucocorticoids after in vitro fertilization and embryo transfer have no significant effect on pregnancy rate. Fertil.Steril. 1995;63:571–577. doi: 10.1016/s0015-0282(16)57428-7. [DOI] [PubMed] [Google Scholar]
- Morell AG, Gregoriadis G, Scheinberg IH, Hickman J, Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971;246:1461–1467. [PubMed] [Google Scholar]
- Nagahara Y, Miyake A., Tasaka K., Kawamura Y, Aono T, Tanizawa O. Possible site of negative and positive feedback action of oestrogen on gonadotrophin secretion in normal women. Acta Endocrinol (Copenh) 1985;108:440–444. doi: 10.1530/acta.0.1080440. [DOI] [PubMed] [Google Scholar]
- Nippoldt TB, Reame NE, Kelch RP, Marshall JC. The roles of estradiol and progesterone in decreasing luteinizing hormone pulse frequency in the luteal phase of the menstrual cycle. J Clin Endocrinol Metab. 1989;69:67–76. doi: 10.1210/jcem-69-1-67. [DOI] [PubMed] [Google Scholar]
- Nosarka S, Kruger T, Siebert I, Grové D. Luteal phase support in in vitro fertilization: meta-analysis of randomized trials. Gynecol Obstet Invest. 2005;60:67–74. doi: 10.1159/000084546. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- Nyboe AA, Popovic-Todorovic B, Schmidt KT, et al. Progesterone supplementation during early gestations after IVF or ICSI has no effect on the delivery rates: a randomized controlled trial. Hum Reprod. 2002;17:357–361. doi: 10.1093/humrep/17.2.357. [DOI] [PubMed] [Google Scholar]
- Okuda K, Miyamoto Y, Skarzynski DJ. Regulation of endometrial prostaglandin F(2alpha) synthesis during luteolysis and early pregnancy in cattle. Domest Anim Endocrinol. 2002;23:255–264. doi: 10.1016/s0739-7240(02)00161-3. [DOI] [PubMed] [Google Scholar]
- Olivennes F, Belaisch-Allart J, Emperaire JC, Dechaud H, Alvarez S, Moreau L, Nicollet B, Zorn JR, Bouchard P, Frydman R. Prospective, randomized, controlled study of in vitro fertilization-embryo transfer with a single dose of a luteinizing hormone-releasing hormone (LH-RH) antagonist (cetrorelix) or a depot formula of an LH-RH agonist (triptorelin) Fertil Steril. 2000;73:314–320. doi: 10.1016/s0015-0282(99)00524-5. [DOI] [PubMed] [Google Scholar]
- Paeschke KD. Participation of ascorbic acid in ovulation. Arch Gynakol. 1969;207:54–55. doi: 10.1007/BF00683030. [DOI] [PubMed] [Google Scholar]
- Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–18. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- Pauerstein CJ, Eddy CA, Croxatto HD, Hess R, Siler-Khodr TM, Croxatto HB. Temporal relationships of estrogen, progesterone, and luteinizing hormone levels to ovulation in women and infrahuman primates. Am J Obstet Gynecol. 1978;130:876–886. doi: 10.1016/0002-9378(78)90264-8. [DOI] [PubMed] [Google Scholar]
- Paulson RJ, Sauer MV, Lobo RA. Factors affecting embryo implantation after human in vitro fertilization: a hypothesis. Am J Obstet Gynecol. 1990;163:2020–2023. doi: 10.1016/0002-9378(90)90790-e. [DOI] [PubMed] [Google Scholar]
- Pellicer A, Matallin P, Miro F, Rivera J, Bonilla-Musoles FM. Progesterone versus dehydrogesterone as replacement therapy in women with premature ovarian failure. Hum Reprod. 1989;4:777–781. doi: 10.1093/oxfordjournals.humrep.a136984. [DOI] [PubMed] [Google Scholar]
- Penzias AS. Luteal phase support. Fertil Steril. 2002;77:318–323. doi: 10.1016/s0015-0282(01)02961-2. [DOI] [PubMed] [Google Scholar]
- Pirard C, Donnez J, Loumaye E. GnRH agonist as novel luteal support: results of a randomized, parallel group, feasibility study using intranasal administration of buserelin. Hum Reprod. 2005;20:1798–1804. doi: 10.1093/humrep/deh830. [DOI] [PubMed] [Google Scholar]
- Plant TM, Krey LC, Moossy J, McCormack JT, Hess DL, Knobil E. The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta) Endocrinology. 1978;102:52–62. doi: 10.1210/endo-102-1-52. [DOI] [PubMed] [Google Scholar]
- Polak G, Koziol-Montewka M, Tarkowski R, Kotarski J. Peritoneal fluid and plasma 4-hydroxynonenal and malonyldialdehyde concentrations in infertile women. Ginekol Pol. 2001;72:1316–1320. [PubMed] [Google Scholar]
- Prenant LA. La valeur morphologique du corps jaune. Son action physiologique et theripeutique possible (Abstract) Rev Gen Sci Pures Appl. 1898;9:646. [Google Scholar]
- Pritts EA, Atwood AK. Luteal phase support in infertility treatment: a meta-analysis of the randomized trials. Hum Reprod. 2002;17:2287–2299. doi: 10.1093/humrep/17.9.2287. [DOI] [PubMed] [Google Scholar]
- Proctor A, Hurst BS, Marshburn PB, Matthews ML, et al. Effect of progesterone supplementation in early pregnancy on the pregnancy outcome after in vitro fertilization. Fertil Steril. 2006;85:1550–1552. doi: 10.1016/j.fertnstert.2005.10.060. [DOI] [PubMed] [Google Scholar]
- Propst AM, Hill JA, Ginsburg ES, et al. A randomized study comparing Crinone 8% and intramuscular progesterone supplementation in in vitro fertilization-embryo transfer cycles. Fertil Steril. 2001;76:1144–1149. doi: 10.1016/s0015-0282(01)02872-2. [DOI] [PubMed] [Google Scholar]
- Ragni G, Vegetti W, Baroni E, Colombo M, Arnoldi M, Lombroso G, Crosignani PG. Comparison of luteal phase profile in gonadotrophin stimulated cycles with or without a gonadotrophin-releasing hormone antagonist. Hum Reprod. 2001;16:2258–2262. doi: 10.1093/humrep/16.11.2258. [DOI] [PubMed] [Google Scholar]
- Ramirez VD, Kim K, Dluzen D. Progesterone action on the LHRH and the nigrostriatal dopamine neuronal systems: in vitro and in vivo studies. Recent Prog Horm Res. 1985;41:421–472. doi: 10.1016/b978-0-12-571141-8.50014-7. [DOI] [PubMed] [Google Scholar]
- Reid RL, Hoff JD, Yen SS, Li CH. Effects of exogenous beta h-endorphin on pituitary hormone secretion and its disappearance rate in normal human subjects. J Clin Endocrinol Metab. 1981;52:1179–1184. doi: 10.1210/jcem-52-6-1179. [DOI] [PubMed] [Google Scholar]
- Rosenberg SM, Luciano AA, Riddick DH. The luteal phase defect: the relative frequency of, and encouraging response to, treatment with vaginal progesterone. Fertil Steril. 1980;34:17–20. doi: 10.1016/s0015-0282(16)44831-4. [DOI] [PubMed] [Google Scholar]
- Rossmanith WG, Monn M, Benz R. Effects of chronic opioid antagonism on gonadotrophin and ovarian sex steroid secretion during the luteal phase. Clin Endocrinol (Oxf) 1998;49:343–351. doi: 10.1046/j.1365-2265.1998.00474.x. [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Roy D, Angelini NL, Belsham DD. Estrogen directly respresses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-alpha (ERalpha)- and ERbeta-expressing GT1-7 GnRH neurons. Endocrinology. 1999;140:5045–5053. doi: 10.1210/endo.140.11.7117. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Marazzi A, Polak DF. Low-dose aspirin treatment improves ovarian responsiveness, uterine and ovarian blood flow velocity, implantation, and pregnancy rates in patients undergoing in vitro fertilization: a prospective, randomized, double-blind placebo-controlled assay. Fertil Steril. 1999;71:825–829. doi: 10.1016/s0015-0282(99)00088-6. [DOI] [PubMed] [Google Scholar]
- Schatz F, Markiewicz L, Barg P, Gurpide E. In vitro effects of ovarian steroids on prostaglandin F2 alpha output by human endometrium and endometrial epithelial cells. J Clin Endocrinol Metab. 1985;61:361–367. doi: 10.1210/jcem-61-2-361. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Ziebe S, Popovic B, Lindhard A, Loft A, Andersen AN. Progesterone supplementation during early gestation after in vitro fertilization has no effect on the delivery rate. Fertil Steril. 2001;75:337–341. doi: 10.1016/s0015-0282(00)01709-x. [DOI] [PubMed] [Google Scholar]
- Scott R, Navot D, Liu HC, Rosenwaks Z. A human in vivo model for the luteoplacental shift. Fertil Steril. 1991;56:481–484. doi: 10.1016/s0015-0282(16)54544-0. [DOI] [PubMed] [Google Scholar]
- Shetty G, Krishnamurthy H, Krishnamurthy HN, Bhatnagar S, Moudgal RN. Effect of estrogen deprivation on the reproductive physiology of male and female primates. J Steroid Biochem Mol Biol. 1997;61:157–166. [PubMed] [Google Scholar]
- Simunic V, Tomic V, Tomic J, Nizic D, et al. Comparative study of the efficacy and tolerability of two vaginal progesterone formulations, Crinone 8% gel and Utrogestan capsules, used for luteal support. Fertil Steril. 2007;87:83–87. doi: 10.1016/j.fertnstert.2006.05.067. [DOI] [PubMed] [Google Scholar]
- Sinha S, Kaseta J, Santner SJ, Demers LM, Bremmer WJ, Santen RJ. Effect of CGS 20267 on ovarian aromatase and gonadotropin levels in the rat. Breast Cancer ResTreat. 1998;48:45–51. doi: 10.1023/a:1005937900788. [DOI] [PubMed] [Google Scholar]
- Slotta KH, Ruschig H, Fels E. Reindarstellung der hormone aus dem corpus luteum. Berich Dtsch Chem Geselischaft. 1934;67:1270. [Google Scholar]
- Smitz J, Erard P, Camus M, Devroey P, Tournaye H, Wisanto A, Van Steirteghem AC. Pituitary gonadotrophin secretory capacity during the luteal phase in superovulation using GnRH-agonists and HMG in a desensitization or flare-up protocol. Hum Reprod. 1992;7:1225–1229. doi: 10.1093/oxfordjournals.humrep.a137831. [DOI] [PubMed] [Google Scholar]
- Smitz J, Bourgain C, Van Waesberghe L, Camus M, Devroey P, Van Steirteghem AC. A prospective randomized study on oestradiol valerate supplementation in addition to intravaginal micronized progesterone in buserelin and HMG induced superovulation. Hum Reprod. 1993;8:40–45. doi: 10.1093/oxfordjournals.humrep.a137871. [DOI] [PubMed] [Google Scholar]
- Soules MR, Steiner RA, Clifton DK, Bremner WJ. Abnormal patterns of pulsatile luteinizing hormone in women with luteal phase deficiency. Obstet Gynecol. 1984;63:626–629. [PubMed] [Google Scholar]
- Soules MR, Steiner RA, Clifton DK, Cohen NL, Aksel S, Bremner WJ. Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. J Clin Endocrinol Metab. 1984;58:378–383. doi: 10.1210/jcem-58-2-378. [DOI] [PubMed] [Google Scholar]
- Soules MR, Steiner RA, Cohen NL, Bremner WJ, Clifton DK. Nocturnal slowing of pulsatile luteinizing hormone secretion in women during the follicular phase of the menstrual cycle. J Clin Endocrinol Metab. 1985;61:43–49. doi: 10.1210/jcem-61-1-43. [DOI] [PubMed] [Google Scholar]
- Speroff L, Glass HR, Kase N. Clinical Endocrinology and Infertility. 5th Ed. Baltimore, USA: Williams and Wilkins Publ; 1994. [Google Scholar]
- Struthers RS, Xie Q, Sullivan SK, Reinhart GJ, Kohout TA, Zhu YF, Chen C, Liu XJ, Ling N, Yang W, Maki RA, Bonneville AK, Chen TK, Bozigian HP. Pharmacological characterization of a novel nonpeptide antagonist of the human gonadotropin-releasing hormone receptor, NBI-42902. Endocrinology. 2007;148:857–867. doi: 10.1210/en.2006-1213. [DOI] [PubMed] [Google Scholar]
- Tarlatzis BC, Kolibianakis EM. GnRH agonists vs antagonists. Best Pract Res Clin Obstet Gynaecol. 2007;21:57–65. doi: 10.1016/j.bpobgyn.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Tavaniotou A, Albano C, Smitz J, Devroey P. Impact of ovarian stimulation on corpus luteum function and embryonic implantation. J Reprod Immunol. 2002;55:123–130. doi: 10.1016/s0165-0378(01)00134-6. [DOI] [PubMed] [Google Scholar]
- Tavaniotou A, Devroey P. Effect of human chorionic gonadotropin on luteal luteinizing hormone concentrations in natural cycles. Fertil Steril. 2003;80:654–655. doi: 10.1016/s0015-0282(03)00789-1. [DOI] [PubMed] [Google Scholar]
- Tavaniotou A, Devroey P. Luteal hormonal profile of oocyte donors stimulated with a GnRH antagonist compared with natural cycles. Reprod Biomed Online. 2006;13:326–330. doi: 10.1016/s1472-6483(10)61435-6. [DOI] [PubMed] [Google Scholar]
- Tavanioutu A. The effect of ovarian stimulation on luteal phase endocrinology-correlations in natural cycles, Thesis for degree of doctor in medical science. 2006. [Google Scholar]
- Terasawa E. Control of luteinizing hormone-releasing hormone pulse generation in nonhuman primates. Cell Mol Neurobiol. 1995;15:141–164. doi: 10.1007/BF02069563. [DOI] [PubMed] [Google Scholar]
- Terasawa E. Cellular mechanism of pulsatile LHRH release. Gen Comp Endocrinol. 1998;112:283–295. doi: 10.1006/gcen.1998.7155. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Hazout A, Mendoza-Tesarik R, Mendoza N, Mendoza C. Beneficial effect of luteal-phase GnRH agonist administration on embryo implantation after ICSI in both GnRH agonist- and antagonist-treated ovarian stimulation cycles. Hum Reprod. 2006;21:2572–2579. doi: 10.1093/humrep/del173. [DOI] [PubMed] [Google Scholar]
- Thibier M, el Hassan N, Clark MR, LeMaire WJ, Marsh JM. Inhibition by estradiol of human chorionic gonadotropin-induced progesterone accumulation in isolated human luteal cells: lack of mediation by prostaglandin F. J Clin Endocrinol Metab. 1980;50:590–592. doi: 10.1210/jcem-50-3-590. [DOI] [PubMed] [Google Scholar]
- Ubaldi F, Rienzi L, Ferrero S, Anniballo R, Iacobelli M, Cobellis L, Greco E. Low dose prednisolone administration in routine ICSI patients does not improve pregnancy and implantation rates. Hum Reprod. 17:1544–1547. doi: 10.1093/humrep/17.6.1544. [DOI] [PubMed] [Google Scholar]
- Urman B, Mercan R, Alatas C, Balaban B, Isiklar A, Nuhoglu A. Low-dose aspirin does not increase implantation rates in patients undergoing intracytoplasmic sperm injection: a prospective randomized study. J Assist Reprod Genet. 2000;17:586–590. doi: 10.1023/A:1026491426423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Landuyt L, De Vos A, Joris H, Verheyen G, Devroey P, Van Steirteghem A. Blastocyst formation in in vitro fertilization versus intracytoplasmic sperm injection cycles: influence of the fertilization procedure. Fertil Steril. 2005;83:1397–1403. doi: 10.1016/j.fertnstert.2004.10.054. [DOI] [PubMed] [Google Scholar]
- Van Steirteghem AC, Smitz J, Camus M, Van Waesberghe L, Deschacht J, Khan I, Staessen C, Wisanto A, Bourgain C, Devroey P. The luteal phase after in-vitro fertilization and related procedures. Hum W Reprod. 1988;3:161–164. doi: 10.1093/oxfordjournals.humrep.a136667. [DOI] [PubMed] [Google Scholar]
- Vande Wiele RL, Bogumil J, Dyrenfurth I, Ferin M, Jewelewicz R, Warren M, Rizkallah T, Mikhail G, et al. Mechanisms regulating the menstrual cycle in women. Recent Prog Horm Res. 1970;26:63–103. doi: 10.1016/b978-0-12-571126-5.50006-6. [DOI] [PubMed] [Google Scholar]
- Vane JR, Flower RJ, Botting RM. History of aspirin and its mechanism of action. Stroke. 1990;21:IV12–IV23. [PubMed] [Google Scholar]
- Veysman B, Vlahos I, Oshva L. Pneumonitis and eosinophilia after in vitro fertilization treatment. Ann Emerg Med. 2006;47:472–475. doi: 10.1016/j.annemergmed.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Wada I, Hsu CC, Williams G, Macnamee MC, Brinsden PR. The benefits of low-dose aspirin therapy in women with impaired uterine perfusion during assisted conception. Hum Reprod. 1994;9:1954–1957. doi: 10.1093/oxfordjournals.humrep.a138366. [DOI] [PubMed] [Google Scholar]
- Weckstein LN, Jacobson A, Galen D, Hampton K, Hammel J. Low-dose aspirin for oocyte donation recipients with a thin endometrium: prospective, randomized study. Fertil Steril. 1997;68:927–930. doi: 10.1016/s0015-0282(97)00330-0. [DOI] [PubMed] [Google Scholar]
- Westergaard LG, Erb K, Laursen SB, Rex S, Rasmussen PE. Human menopausal gonadotropin versus recombinant follicle-stimulating hormone in normogonadotropic women down-regulated with a gonadotropin-releasing hormone agonist who were undergoing in vitro fertilization and intracytoplasmic sperm injection: a prospective randomized study. Fertil Steril. 2001;76:543–549. doi: 10.1016/s0015-0282(01)01973-2. [DOI] [PubMed] [Google Scholar]
- Whelan JG III, Vlahos NF. The ovarian hyperstimulation syndrome. Fertil Steril. 2000;73:883–896. doi: 10.1016/s0015-0282(00)00491-x. [DOI] [PubMed] [Google Scholar]
- Whitehead MI, Townsend PT, Gill DK, Collins WP, Campbell S. Absorption and metabolism of oral progesterone. Br Med J. 1980;280:825–827. doi: 10.1136/bmj.280.6217.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SC, Oehninger S, Gibbons WE, Van Cleave WC, Muasher SJ. Delaying the initiation of progesterone supplementation results in decreased pregnancy rates after in vitro fertilization: a randomized, prospective study. Fertil Steril. 2001;76:1140–1143. doi: 10.1016/s0015-0282(01)02914-4. [DOI] [PubMed] [Google Scholar]
- Wintersteiner O, Allen WM. Crystalline progestin. J Biol Chem. 1934;107:321–336. doi: 10.1126/science.80.2069.190-a. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Santulli R, Atlas SJ, Fujii S, Wallach EE. The effects of proteolytic enzymes on in vitro ovulation in the rabbit. Am J Obstet Gynecol. 1987;157:468–475. doi: 10.1016/s0002-9378(87)80197-7. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Wallach EE. Studies of the mechanism(s) of mammalian ovulation. Fertil Steril. 1987;47:22–34. [PubMed] [Google Scholar]
- Young JR, Jaffe RB. Strength-duration characteristics of estrogen effects on gonadotropin response to gonadotropin-releasing hormone in women. II. Effects of varying concentrations of estradiol. J Clin Endocrinol Metab. 1976;42:432–442. doi: 10.1210/jcem-42-3-432. [DOI] [PubMed] [Google Scholar]
- Younis JS, Ezra Y, Sherman Y, Simon A, Schenker JG, Laufer N. The effect of estradiol depletion during the luteal phase on endometrial development. Fertil Steril. 1994;62:103–105. doi: 10.1016/s0015-0282(16)56823-x. [DOI] [PubMed] [Google Scholar]