Introduction
The past decades have seen enormous progress in the ability to visualize the developing fetal and embryonic central nervous system (CNS) with ultrasound (US) and MRI (magnetic resonance imaging). The purpose of this article is to provide a review of the available literature, and to detail some recent research into the topic.
US imaging started in the 1960’s with A-mode US (Pasto and Kurtz, 1986). This yields a one-dimensional display of a graph representing the distance between structures producing echoes and the amplitude of these echoes. Imaging of the fetal CNS with this was limited to the measurement of the fetal biparietal diameter and visualization of the cranial midline. Today, A-mode US is used mainly in ophthalmology where precise measurements are required. The development of B-mode US made it possible to visualize fetal structures in two dimensional (2D) cross-sectional planes with a two-dimensional display. This led to an explosion in knowledge of US of embryonic and fetal development to such an extent that it is impossible to imagine practicing obstetrics or fetal medicine without the support of US imaging. Initially, axial images of the fetal brain were used for screening and diagnosis. Coronal and sagittal views are increasingly used, and essential for a complete fetal neursonographical examination (Malinger et al., 2007). With the arrival of three dimensional (3D) US, three orthogonal planes could be displayed simultaneously, planes which might otherwise have been inaccessible could be visualized, and volumes could be measured more accurately (Kalache et al., 2006).
During the same time, magnetic resonance imaging (MRI) developed from a technique which could provide “tissue characterization information that complements the superior anatomic detail of US (McCarthy et al., 1985) in the mid eighties, to arguably “the optimal method for depicting the specific abnormalities that characterize each type of malformation of the brain in the fetus” (Raybaud et al., 2003). It is certainly being argued with great heat whether US or MRI is the optimal method of fetal brain imaging (Malinger et al., 2004). Theoretically, the two modalities should be synergistic, as the physics behind image generation is completely different. US generates images by means of the US waves reflected at the interface between areas with different US conductivity, and MRI contrast depends on the relative fat, water and proton content of tissues (Bitar et al., 2006). In practice, it is difficult to determine the relative merit of either technique from the literature which is often clouded by an implicit or explicit bias for either modality. Also, imaging does not equate diagnosis, and several publications confirm the value of a multidisciplinary approach to the diagnosis of fetal central nervous system abnormalities (Hagmann et al, 2008; Malinger et al., 2004). Both techniques are operator-dependent (Brugger et al., 2006), and both techniques are deemed to be safe in pregnancy (Abramowicz, 2007; Kanal, 1994).
Physiology
First trimester
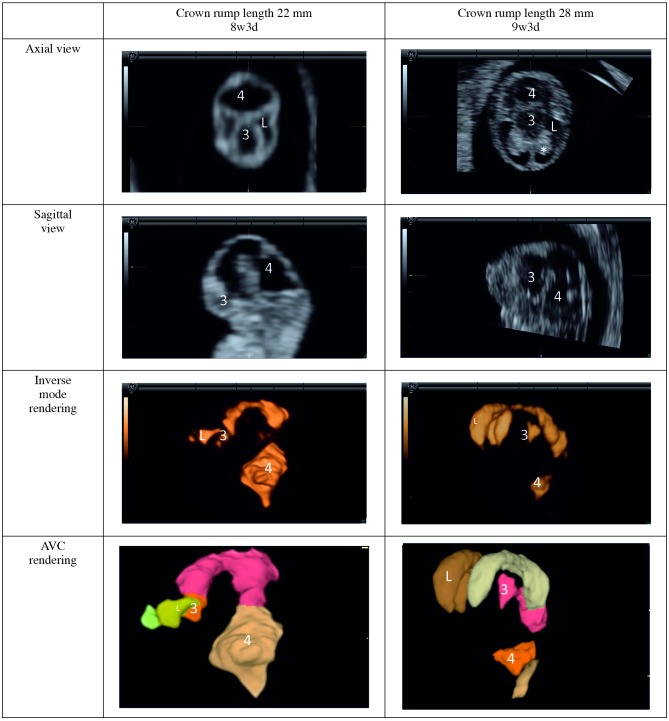
MRI has very limited applicability in the first trimester – not so much because of safety concerns as the lack of resolution. On the other hand, it has been possible to visualize the structure of the embryonic central nervous system with US more than two decades ago (Timor-Tritsch et al., 1988). A decade later, the first three dimensional (3D) reconstructions were done with experimental transducers (Blaas et al., 1998). It is now possible to obtain two dimensional (2D) and 3D images of the embryonic ventricular system from eight weeks of (postmenstrual) gestational age. These can be visualized in orthogonal planes or with 3D rendering techniques. With inverse mode rendering, hypo-echogenic structures (such as ventricles) are rendered, and with automated volume calculation (AVC) the volume of these ventricles can be calculated automatically (Fig. 1). The single three-dimensional sweep used to obtain these images as well as images which could be used for crown rump length measurement and further anatomical evaluation could also minimize the exposure of the rapidly developing embryo to US energy (Pistorius et al., 2009). The applicability for screening remains to be evaluated in greater detail, but several reports have appeared of holoprosencephaly diagnosed during the first trimester with these techniques (Kim et al., 2008; Timor-Tritsch et al., 2008).
Fig. 1. Embryonic brain ventricles.
L = lateral ventricle; 3 = third ventricle; 4 = fourth ventricle; * = choroid plexus (note appearance at 9 postmenstrual weeks).
Ventricle size and symmetry: second and trimester
Screening for ventriculomegaly later in pregnancy is well established. A simple cut-off of 10mm for the ventricular atrium as measured on an axial view has been used as a screening test for ventriculomegaly throughout the second half of pregnancy. This cut-off had been developed from cross-sectional data more than twenty years ago (Cardoza et al., 1988), but has stood the test of time (Malinger et al., 2007). On the other hand, there is little information on the prenatal prevalence of physiological asymmetry of the lateral cerebral ventricles. Postnatally, there is significant asymmetry in more than 40% of infants (Shen and Huang, 1989), but it has been reported in only 0.2-0.4% of fetuses (Achiron et al., 1997). This might be a true difference, or might reflect an ascertainment bias, as the proximal hemisphere is often obscured with reverberation artefacts during antenatal US imaging (Monteagudo, 1998). Evaluation of both left and right lateral cerebral ventricles can be improved by obtaining the midcoronal view on 2D US and using volume contrast imaging (a modality of 3D US). Despite adequate visualization of both lateral ventricles, we demonstrated asymmetry of the lateral ventricles in only 4% of examinations in a longitudinal study of 28 fetuses (unpublished observations). It would be interesting to examine the difference between antenatal and postnatal asymmetry prospectively a group of infants before and after delivery.
Cortical development
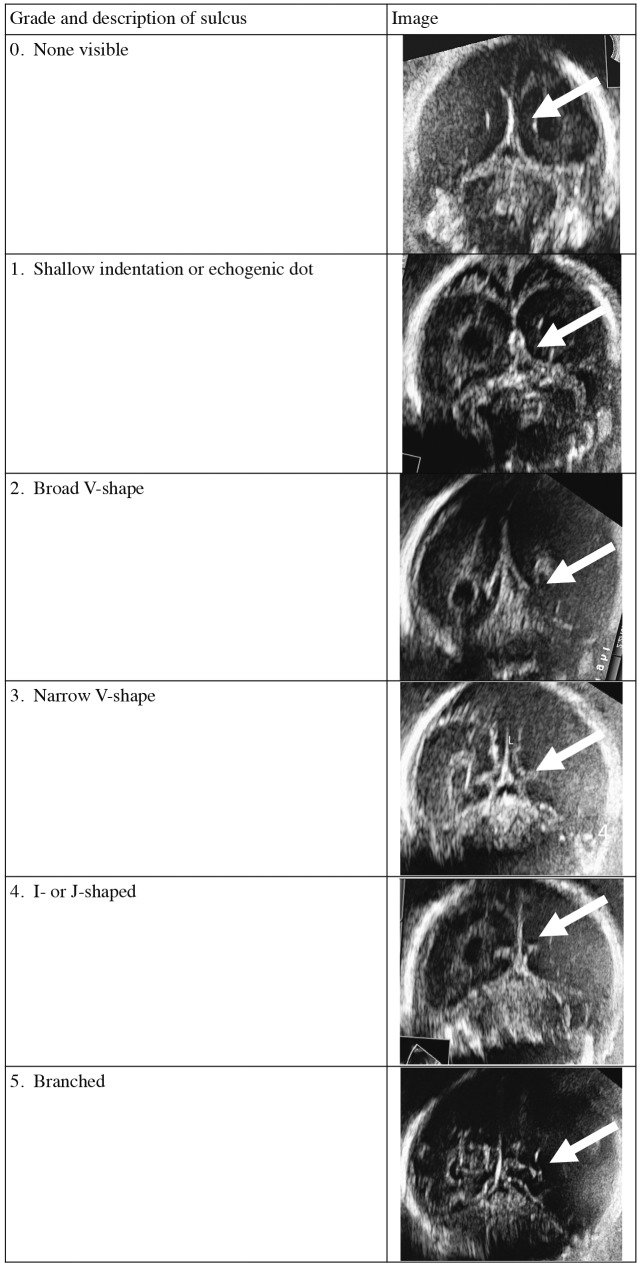
The prenatal development of the cerebral cortex has been described on anatomical (Chi, 1977) and MRI studies (Garel, 2004; Levine and Barnes, 1999). However, these were all cross-sectional, rather than longitudinal, studies, and fetuses were examined for a variety of maternal and fetal indications. Despite popular belief that it is difficult to ascertain fetal cortical development with US, several publications have demonstrated clearly that fetal cortical development can be demonstrated well with US (Cohen-Sacher et al., 2006; Monteagudo and Timor-Tritsch, 1997; Toi et al., 2004). These include well-designed, longitudinal studies, but it is still difficult to translate these findings into practice. Typically, the first appearance of sulci and gyri were typically described, rather than the development over time, and with little attention to interindividual variation. Another limitation of the available literature was the lack of a systematical description of physiological asymmetry. It is possible to modify a scoring system used in MRI imaging (van der Knaap et al., 1996) to score the progression of cortical folding by US (Fig. 2). In a prospective study of 28 fetuses between 20 and 40 weeks, a good intra- and inter-observer agreement was achieved when grading cortical development with this simple scoring system. Asymmetrical cortical development was seen in a third of fetuses between 24 and 28 gestational weeks (unpublished data). This scoring system now needs to be evaluated prospectively to determine whether it is useful to detect fetuses with malformations of cortical development.
Fig. 2. Cortical grading (calcarine sulcus used as example).
Cerebellar volume
Asymmetry of the fetal cerebellar volume was also found in our longitudinal study of cerebellar volume measured with antenatal US (Rutten et al., 2009), especially before 32 weeks, when the left cerebellar volume can be up to 54% greater than the right and the right cerebellar volume can be up to 6% greater than the left. Both multiplanar and VOCAL™ techniques had a good intra- and inter-observer variability, and yielded similar results when calculating the cerebellar volume. As is the case with the cortical scoring system, it remains to be evaluated whether measurement of the cerebellar volume, rather than a one-dimensional measurement such as the transverse cerebellar diameter, is more sensitive and specific to detect syndromic (McCann et al., 2005) or acquired (Johnsen et al., 2005) cerebellar abnormalities.
Pathology
Spina bifida
To determine whether the parameters visible on prenatal imaging could predict future prognosis, we followed up infants who were antenatally diagnosed with spina bifida until demise or five years of age. Multivariate regression analysis in this group of 41 infants showed that a higher lesion level and head circumference at or above the 90th percentile were independent predictors of demise. None of the US features were associated with motor or mental functioning at five years of age. The accuracy of the prenatal US prediction of the anatomical lesion level within one level of the postnatal findings was 50% performed between 1997 and 2002, and 89% between 2006 and 2007 (p < 0.01). This improvement in the accuracy of US is offset by a difference between the functional level of neurological deficit and the anatomical lesion level which still limits the ability of US to predict motor function (Vossen et al., 2009).
Hydrocephalus and middle cerebral artery Doppler US
Doppler indices of cerebral blood flow are used postnatally to diagnose raised intracranial pressure in infants with hydrocephalus (Hanlo et al., 1995). This raises the question whether measuring middle cerebral artery (Fong et al., 2004) flow is of prognostic value in a fetus with ventriculomegaly, and we investigated the relationship between the mca pulsatility index (PI) and outcome in a retrospective study. A rise in mca PI was seen in four out of 29 fetuses with ventriculomegaly. Two of these four fetuses suffered a perinatal death, and the other two had subsequent severe neurodevelopmental abnormalities (unpublished observations). An abnormal mca PI therefore seems too insensitive to serve as a useful parameter to aid the timing of delivery in fetuses with ventriculomegaly.
Parvovirus B19 and malformations of cortical migration
It is well known that prenatal infection with Parvovirus B19 can lead to fetal anemia due to a transient aplastic crisis (Anderson et al., 1985). If severe and untreated, this could in turn lead to the development of hydrops and fetal demise. Until recently, it was assumed that successful treatment with intra-uterine transfusion was associated with normal neurodevelopmental outcome (Dembinski et al., 2002). We described a case of a fetus that developed malformations of cortical development after requiring intra-uterine transfusion for a severe Parvovirus B19 infection. Antenatal MRI demonstrated mild unilateral ventriculomegaly. Polymicrogyria and heterotopia were confirmed on postnatal MRI (Pistorius et al., 2008). Recent literature also reports mild to severe neurodevelopmental delay in five out of sixteen Parvovirus B19 hydrops survivors (Nagel et al., 2007).
Comparison between US and MRI
Although there are many articles comparing the diagnostic capabilities of prenatal US and MRI, many are hampered by an obvious bias by comparing a diagnosis made in primary care on US with a tertiary diagnosis by MRI, with only few trying to compare like with like (Malinger et al., 2004; Nagel et al., 2007; Levine et al., 2003). It is also difficult to distinguish between the effect of MRI and that of a multidisciplinary discussion (Malinger et al., 2002). This is important, as a multidisciplinary discussion has been shown to be important in improving the accuracy of diagnosis of fetal central nervous system abnormalities (Hagmann et al., 2008).
A previous study in our unit demonstrated additional value of MRI in the diagnosis of fetal (CNS) abnormalities where US examination yielded uncertain or limited results (Gerards et al., 2001). We therefore decided to analyze our subsequent results to compare the accuracy of US, MRI and the additional value of a multidisciplinary discussion with the postnatal diagnosis in a retrospective cohort of patients where an MRI of the fetal central nervous system was performed between 2000 and 2008. We also compared the accuracy of standard US examinations with that of neurosonograms, and found that the diagnosis made during the multidisciplinary discussion was accurate in 62% before and 73% of patients after MRI. The diagnosis remained unchanged in 23 out of 28 patients. The accuracy of the diagnosis improved in four patients and decreased in one patient after MRI. Standard US examinations were accurate in 42% of patients, and neurosonograms in 63% of patients. The highest accuracy of prenatal diagnosis of fetal CNS lesions (78%) was obtained with a multidisciplinary discussion after neurosonography and MRI (unpublished observations). No improvement in accuracy after MRI was seen in patients with neural tube lesions, and the biggest improvement was seen in patients with an US diagnosis of ventriculomegaly.
A review of the available literature on US and MRI imaging of the fetal CNS indicated that MRI does occasionally provide additional information which is not available on detailed neurosonography.
US imaging of the fetal central nervous system seems preferable for screening or repeated examinations, examinations before 20 weeks of gestation, fetal movement assessment in the first and second trimester, evaluation of cerebral blood flow, evaluation of associated (extracranial) abnormalities and where MRI is contra-indicated or has failed.
MRI seems preferable in cases of a difficult US, the assessment of posterior fossa abnormalities, schizencephaly, acute fetal asphyxia or severe microcephaly, for evaluating fetal movements in the late third trimester, detecting and determining the age of intracranial bleeding, detecting intracranial tuberous sclerosis and for postmortem brain imaging.
A synergistic effect between US and MRI can be expected in fetuses suspected of cerebellar telangiectasis, cytomegaloviral infection, intracranial tumors or trauma, vein of Galen abnormalities, germinal matrix and intraventricular bleeding, hemimegalencephaly and septo-optic dysplasia.
In the second half of pregnancy, either modality could be used to diagnose suspected holoprosencephaly, abnormalities of the corpus callosum, ventriculomegaly or craniosynostosis.
Acknowledgments
I would like to thank Gerard Visser and Linda de Vries for promoting this doctoral thesis on which this article is based, all the other contributors to the thesis (in the order in which their names appear in the thesis), namely: Petra Hellmann, Gustavo Malinger, Daniela Prayer, Philip Stoutenbeek. Floris Groenendaal, Wendy Manten, Edu Mulder, Marjet Rutten, Sylvia Kuc, Manon Benders, Sanne van der Vossen, Marc Platenkamp, Rob Gooskens, Jaime Smal, Lieve Page-Christiaens, Malgosia Verboon-Maciolek, Dick Oepkes, Helen Torrance and Ron Voorbij; and all volunteers who participated in the prospective study.
References
- Abramowicz JS. Prenatal exposure to ultrasound waves: is there a risk? Ultrasound Obstet Gynecol. 2007;29:363–367. doi: 10.1002/uog.3983. [DOI] [PubMed] [Google Scholar]
- Achiron R, Yagel S, Rotstein Z, Inbar O, Mashiach S, Lipitz S. Cerebral lateral ventricular asymmetry: is this a normal ultrasonographic finding in the fetal brain? Obstet Gynecol. 1997;89:233–237. doi: 10.1016/S0029-7844(96)00506-6. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Higgins PG, Davis LR, Willman JS, Jones SE, Kidd IM, Pattison JR, Tyrrell DA. Experimental parvoviral infection in humans. J Infect Dis. 1985;152:257–265. doi: 10.1093/infdis/152.2.257. [DOI] [PubMed] [Google Scholar]
- Bitar R, Leung G, Perng R, Tadros S, Moody AR, Sarrazin J, et al. MR pulse sequences: what every radiologist wants to know but is afraid to ask. Radiographics. 2006;26:513–537. doi: 10.1148/rg.262055063. [DOI] [PubMed] [Google Scholar]
- Blaas HG, Nes SH, Berg S, Torp H. In-vivo three-dimensional ultrasound reconstructions of embryos and early fetuses. Lancet. 1998;352:1182–1186. doi: 10.1016/S0140-6736(98)03227-9. [DOI] [PubMed] [Google Scholar]
- Brugger PC, Stuhr F, Lindner C, Prayer D. Methods of fetal MR: beyond T2-weighted imaging. Eur J Radiol. 2006;57:172–181. doi: 10.1016/j.ejrad.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Cardoza JD, Goldstein RB, Filly RA. Exclusion of fetal ventriculomegaly with a single measurement: the width of the lateral ventricular atrium. Radiology. 1988;169:711–714. doi: 10.1148/radiology.169.3.3055034. [DOI] [PubMed] [Google Scholar]
- Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. 1977;1:86–93. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- Cohen-Sacher B, Lerman-Sagie T, Lev D, Malinger G. Sonographic developmental milestones of the fetal cerebral cortex: a longitudinal study. Ultrasound Obstet Gynecol. 2006;27:494–502. doi: 10.1002/uog.2757. [DOI] [PubMed] [Google Scholar]
- Dembinski J, Haverkamp F, Maara H, Hansmann M, Eis-Hubinger AM, Bartmann P. Neurodevelopmental outcome after intrauterine red cell transfusion for parvovirus B19-induced fetal hydrops. BJOG. 2002;109:1232–1234. doi: 10.1046/j.1471-0528.2002.02118.x. [DOI] [PubMed] [Google Scholar]
- Fong KW, Toi A, Salem S, Hornberger LK, Chitayat D, Keating SJ, et al. Detection of fetal structural abnormalities with US during early pregnancy. Radiographics. 2004;24:157–174. doi: 10.1148/rg.241035027. [DOI] [PubMed] [Google Scholar]
- Garel C. The role of MRI in the evaluation of the fetal brain with an emphasis on biometry, gyration and parenchyma. Pediatr Radiol. 2004;34:694–699. doi: 10.1007/s00247-004-1249-x. [DOI] [PubMed] [Google Scholar]
- Hagmann CF, Robertson NJ, Leung WC, Chong KW, Chitty LS. Foetal brain imaging: ultrasound or MRI. A comparison between magnetic resonance imaging and a dedicated multidisciplinary neurosonographic opinion. Acta Paediatr. 2008;97:414–419. doi: 10.1111/j.1651-2227.2008.00689.x. [DOI] [PubMed] [Google Scholar]
- Hanlo PW, Gooskens RH, Nijhuis IJ, Faber JA, Peters RJ, van Huffelen AC, et al. Value of transcranial Doppler indices in predicting raised ICP in infantile hydrocephalus. A study with review of the literature. Childs Nerv Syst. 1995;11:595–603. doi: 10.1007/BF00300999. [DOI] [PubMed] [Google Scholar]
- Johnsen SD, Bodensteiner JB, Lotze TE. Frequency and nature of cerebellar injury in the extremely premature survivor with cerebral palsy. J Child Neurol. 2005;20:60–64. doi: 10.1177/08830738050200011001. [DOI] [PubMed] [Google Scholar]
- Kalache KD, Eder K, Esser T, Proquitte H, Stoltenburg-Didinger G, Hartung JP, et al. Three-dimensional ultrasonographic reslicing of the fetal brain to assist prenatal diagnosis of central nervous system anomalies. J Ultrasound Med. 2006;25:509–514. doi: 10.7863/jum.2006.25.4.509. [DOI] [PubMed] [Google Scholar]
- Kanal E. Pregnancy and the safety of magnetic resonance imaging. Magn Reson Imaging Clin N Am. 1994;2:309–317. [PubMed] [Google Scholar]
- Kim MS, Jeanty P, Turner C, Benoit B. Three-dimensional sonographic evaluations of embryonic brain development. J Ultrasound Med. 2008;27:119–124. doi: 10.7863/jum.2008.27.1.119. [DOI] [PubMed] [Google Scholar]
- Levine D, Barnes PD, Robertson RR, Wong G, Mehta TS. Fast MR imaging of fetal central nervous system abnormalities. Radiology. 2003;229:51–61. doi: 10.1148/radiol.2291020770. [DOI] [PubMed] [Google Scholar]
- Levine D, Barnes PD. Cortical maturation in normal and abnormal fetuses as assessed with prenatal MR imaging. Radiology. 1999;210:751–758. doi: 10.1148/radiology.210.3.r99mr47751. [DOI] [PubMed] [Google Scholar]
- Malinger G, Ben-Sira L, Lev D, Ben-Aroya Z, Kidron D, Lerman-Sagie T. Fetal brain imaging: a comparison between magnetic resonance imaging and dedicated neurosonography. Ultrasound Obstet Gynecol. 2004;23:333–340. doi: 10.1002/uog.1016. [DOI] [PubMed] [Google Scholar]
- Malinger G, Lev D, Lerman-Sagie T. Fetal central nervous system: MR imaging versus dedicated US – need for prospective, blind, comparative studies. Radiology. 2004;232:306–307. doi: 10.1148/radiol.2321032051. [DOI] [PubMed] [Google Scholar]
- Malinger G, Lev D, Lerman-Sagie T. Is fetal magnetic resonance imaging superior to neurosonography for detection of brain anomalies? Ultrasound Obstet Gynecol. 2002;20:317–321. doi: 10.1046/j.1469-0705.2002.00825.x. [DOI] [PubMed] [Google Scholar]
- Malinger G, Monteagudo A, Pilu G, Timor-Tritsch IE, Toi A. Sonographic examination of the fetal central nervous system: guidelines for performing the ‘basic examination’ and the ‘fetal neurosonogram’. Ultrasound Obstet Gynecol. 2007;29:109–116. doi: 10.1002/uog.3909. [DOI] [PubMed] [Google Scholar]
- McCann E, Pilling D, Hesseling M, Roberts D, Subhedar N, Sweeney E. Pontomedullary disconnection: fetal and neonatal considerations. Pediatr Radiol. 2005;35:812–814. doi: 10.1007/s00247-005-1455-1. [DOI] [PubMed] [Google Scholar]
- McCarthy SM, Filly RA, Stark DD, Hricak H, Brant-Zawadzki MN, Callen PW, et al. Obstetrical magnetic resonance imaging: fetal anatomy. Radiology. 1985;154:427–432. doi: 10.1148/radiology.154.2.3966129. [DOI] [PubMed] [Google Scholar]
- Monteagudo A, Timor-Tritsch IE. Development of fetal gyri, sulci and fissures: a transvaginal sonographic study. Ultrasound Obstet Gynecol. 1997;9:222–228. doi: 10.1046/j.1469-0705.1997.09040222.x. [DOI] [PubMed] [Google Scholar]
- Monteagudo A. Fetal neurosonography: should it be routine? Should it be detailed? Ultrasound Obstet Gynecol. 1998;12:1–5. doi: 10.1046/j.1469-0705.1998.12010001.x. [DOI] [PubMed] [Google Scholar]
- Nagel HT, de Haan TR, Vandenbussche FP, Oepkes D, Walther FJ. Long-term outcome after fetal transfusion for hydrops associated with parvovirus B19 infection. Obstet Gynecol. 2007;109:42–47. doi: 10.1097/01.AOG.0000249611.67873.94. [DOI] [PubMed] [Google Scholar]
- Pasto ME, Kurtz AB. Ultrasonography of the normal fetal brain. Neuroradiology. 1986;28:380–385. doi: 10.1007/BF00344093. [DOI] [PubMed] [Google Scholar]
- Pistorius LR, Smal J, de Haan TR, Page-Christiaens GC, Verboon-Maciolek M, Oepkes D, et al. Disturbance of cerebral neuronal migration following congenital parvovirus B19 infection. Fetal Diagn Ther. 2008;24:491–494. doi: 10.1159/000180119. [DOI] [PubMed] [Google Scholar]
- Pistorius LR, Stoutenbeek P, Visser GH. First trimester neurosonoembryology with automated follicle tracking: Preliminary findings. J Matern Fetal Neonatal Med. 2009;22:949–951. doi: 10.1080/14767050902929388. [DOI] [PubMed] [Google Scholar]
- Raybaud C, Levrier O, Brunel H, Girard N, Farnarier P. MR imaging of fetal brain malformations. Childs Nerv Syst. 2003;19:455–470. doi: 10.1007/s00381-003-0769-2. [DOI] [PubMed] [Google Scholar]
- Rutten MJ, Pistorius LR, Mulder EJ, Stoutenbeek P, de Vries LS, Visser GH. Fetal cerebellar volume and symmetry on three-dimensional ultrasound:Volume measurement with multiplanar and VOCAL techniques. Ultrasound Med Biol. 2009;35:1284–1289. doi: 10.1016/j.ultrasmedbio.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Shen EY, Huang FY. Sonographic finding of ventricular asymmetry in neonatal brain. Arch Dis Child. 1989;64:730–732. doi: 10.1136/adc.64.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timor-Tritsch IE, Farine D, Rosen MG. A close look at early embryonic development with the high-frequency transvaginal transducer. Am J Obstet Gynecol. 1988;159:676–681. doi: 10.1016/s0002-9378(88)80033-4. [DOI] [PubMed] [Google Scholar]
- Timor-Tritsch IE, Monteagudo A, Santos R. Three-dimensional inversion rendering in the first- and early second-trimester fetal brain: its use in holoprosencephaly. Ultrasound Obstet Gynecol. 2008;32:744–750. doi: 10.1002/uog.6245. [DOI] [PubMed] [Google Scholar]
- Toi A, Lister WS, Fong KW. How early are fetal cerebral sulci visible at prenatal ultrasound and what is the normal pattern of early fetal sulcal development? Ultrasound Obstet Gynecol. 2004;24:706–715. doi: 10.1002/uog.1802. [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, Wezel-Meijler G, Barth PG, Barkhof F, Ader HJ, Valk J. Normal gyration and sulcation in preterm and term neonates: appearance on MR images. Radiology. 1996;200:389–396. doi: 10.1148/radiology.200.2.8685331. [DOI] [PubMed] [Google Scholar]
- Vossen S, Pistorius LR, Mulder EJ, Platenkamp M, Stoutenbeek P, Visser GH, et al. The role of prenatal ultrasound in predicting survival and mental and motor functioning in children with spina bifida. Ultrasound Obstet Gynecol. 2009;34:253–258. doi: 10.1002/uog.6423. [DOI] [PubMed] [Google Scholar]