Abstract
Aim: To evaluate the performance of subjective evaluation of ultrasound findings (pattern recognition) to discriminate endometriomas from other types of adnexal masses and to compare the demographic and ultrasound characteristics of the true positive cases with those cases that were presumed to be an endometrioma but proved to have a different histology (false positive cases) and the endometriomas missed by pattern recognition (false negative cases).
Methods: All patients in the International Ovarian Tumor Analysis (IOTA ) studies were included for analysis. In the IOTA studies, patients with an adnexal mass that were preoperatively examined by expert sonologists following the same standardized ultrasound protocol were prospectively included in 21 international centres.
Sensitivity and specificity to discriminate endometriomas from other types of adnexal masses using pattern recognition were calculated.
Ultrasound and some demographic variables of the masses presumed to be an endometrioma were analysed (true positives and false positives) and compared with the variables of the endometriomas missed by pattern recognition (false negatives) as well as the true negatives.
Results: IOTA phase 1, 1b and 2 included 3511 patients of which 2560 were benign (73%) and 951 malignant (27%). The dataset included 713 endometriomas. Sensitivity and specificity for pattern recognition were 81% (577/713) and 97% (2723/2798). The true positives were more often unilocular with ground glass echogenicity than the masses in any other category. Among the 75 false positive cases, 66 were benign but 9 were malignant (5 borderline tumours, 1 rare primary invasive tumour and 3 endometrioid adenocarcinomas). The presumed diagnosis suggested by the sonologist in case of a missed endometrioma was mostly functional cyst or cystadenoma.
Conclusion: Expert sonologists can quite accurately discriminate endometriomas from other types of adnexal masses, but in this dataset 1% of the masses that were classified as endometrioma by pattern recognition proved to be malignancies.
Keywords: Ultrasonography, endometriosis, endometrioma, adnexal tumours, pattern recognition, subjective evaluation
Introduction
The main benefit of an accurate preoperative classification of an adnexal mass as benign or malignant is that patients can be offered the best treatment strategy for their pathology, whether this involves expectant management, laparoscopy or debulking surgery by a gynaecologic oncologist in case of malignancy. To date, the best method described to discriminate between benign and malignant adnexal masses is the use of pattern recognition by an expert sonologist (Valentin et al., 1999, 2001, 2004; Timmerman et al., 2004). Pattern recognition is the subjective evaluation of the morphology and vascularity of the mass during an ultrasound examination (Valentin 2004). An increasing number of gynaecology centres have a specialised multidisciplinary team for endometriosis patients in order to give the patient the best treatment options. In particular, patients with severe and/or deep endometriosis may benefit from this strategy as every suboptimal attempt to excise endometriosis will create more fibrosis and adhesions making further surgery more complicated (Langgebrekke et al., 2006; Mereu et al., 2007). Accordingly it is crucial that the preoperative assessment of an adnexal mass not only discriminates between the benign and malignant nature of a mass, but also correctly identifies the presence of endometriomas and the severity of endometriosis elsewhere in the pelvis (for example in the case of deep rectovaginal or vesico-uterine endometriotic nodules or a frozen pelvis) (Okaro et al., 2006).
The aim of this study was to evaluate the diagnostic performance of pattern recognition to discriminate between endometriomas and other types of adnexal masses. A secondary aim was to describe the ultrasound characteristics of adnexal masses that 1° were presumed to be an endometrioma and on final histology proved to be an endometrioma (true positives) or 2° proved to have a different histology (false positives) as well as 3° the endometriomas that were missed by pattern recognition (false negatives).
Background
The underlying pathophysiology of endometriosis is not totally clear, but there is evidence that genetic factors are involved as well as molecular changes leading to an overproduction of oestrogen, prostaglandins and cytokines (Bulun et al., 2009; Bischoff et al., 2000; Campbell et al., 2001; Kennedy et al., 2003; Thomas et al., 2000) . However, there is controversy regarding the cellular origin of endometriosis (Bulun et al., 2009). Spread of endometrial tissue by retrograde menstruation (“the implantation theory”) (Schenken et al., 1989; Olive et al., 1987) is one of the hypotheses. The endometrial deposits would subsequently invaginate in the ovarian cortex resulting in endometriotic cysts or endometriomas. Another hypothesis is dissemination through lymphatic and blood vessels or metaplastic differentiation of the peritoneum (Dmowski et al., 1994; Witz et al., 2000).
Three forms of endometriosis are described: peritoneal endometriosis consisting of small implants on the peritoneal surface or external surface of the ovary, ovarian endometriotic cyst or endometrioma, and deep endometriosis affecting the ureters, bladder or rectovaginal wall and consisting of a conglomerate of endometriotic tissue, adipose tissue and fibrosis (Bulun et al., 2009; Giudice et al., 2004).
Epidemiology
Endometriosis occurs mostly in premenopausal women in the third decade of life. Estimating the true prevalence of endometriosis is difficult since most studies report on endometriosis in women that have undergone surgery and so had a confirmed histological diagnosis. The reported prevalence in fertile women is up to 20%, for patients undergoing a laparoscopy for pelvic pain it is between 20% and 50% and in the overall population it is estimated to be between 1 and 10% (Sangi-Haghpeykar et al., 1995; Chatman et al., 1982; Missmer et al., 2004; Eskenazi et al., 1997).
Symptoms
Almost 75% of the symptomatic patients report pelvic pain and dysmenorrhoea caused by active bleeding of the endometriotic tissue, production of cytokines and secondary development of adhesions (Sinaii et al., 2008). Other symptoms are dyspareunia, abnormal bleeding and in case of deep rectovaginal or bladder nodes premenstrual dyschezia or mictalgia is reported (Sinaii et al. 2008; Kennedy 2005).
Tumor markers
Serum CA 125 is often elevated in patients with endometriosis and rises with increasing extensiveness of the disease (Cheng et al., 2002; Mol et al., 1998; Van Calster et al., 2007). Increased CA-125 levels often cause anxiety, because because of their association with malignancies. CA-125 cannot be used as marker to identify endometriosis as the sensitivity and specificity are too low (Yang et al., 2004).
Prognosis
The prognosis is usually good but the morbidity caused by the disease depends on the severity and the degree of extraovarian spread affecting other organs. Adhesions formed by endometriotic tissue may result in reduced mobility of some organs such as the ovaries. Adhesions may also block the tubes and this may negatively affect fertility. Typically, patients with a history of infertility and a presumed sonographic diagnosis of adhesions or frozen pelvis are referred for surgery. The impact on fertility and the surgical treatment to improve fertility in patients with mild endometriosis, e.g. small peritoneal implants or a solitary endometrioma remains a matter of discussion although some reports indicate that laparoscopic resection or ablation of minimal and mild endometriosis enhances fecundity in infertile women (Marcoux et al., 1997; Jacobson et al., 2002; Lin et al., 2005). Most experts believe that severe endometriosis in infertility patients should be surgically treated. Therefore, most patients undergo surgery when the endometrioma is more than 4 cm in diameter (Beretta et al., 1998; Somigliana et al., 2006). Unfortunately, surgery may also harm the vascularisation of the ovary or damage part of the ovarian cortex resulting in a decreased ovarian reserve. A further issue is that several studies highlight the potential risk of developing an endometrioid or clear cell carcinoma inside an endometrioma (Van Gorp et al., 2004; Fukunaga et al., 1997; Sampson 1925).
Macroscopy
Macroscopically, pelvic endometriosis is seen as small bluish nodules often with surrounding fibrosis. In the ovaries, lesions are commonly of a considerable size, are cystic and contain altered blood. Because of the dark colour of the cyst content, they are often referred to as ‘chocolate cysts’. Ovarian endometriosis is frequently associated with dense adhesions, the ovaries being bound down to the broad ligament or bowel (Muir’s textbook of pathology).
Microscopy
The chocolate-like appearance of the cyst fluid that gives an endometrioma its typical ground glass echogenicity is produced by endometrial shedding associated with cyst wall exudation, congested wall vessels and inflammation around intracystic endometrial foci (Brosens and Brosens 2000; Brosens and Puttemans 1994). Initially there is no real cyst wall, and the endometrioma is delineated by a very thin layer of endometriotic tissue. Later on the cyst wall becomes thickened and irregular due to fibrosis, acute and chronic inflammation, oedema of the cyst wall, necrosis and in some exceptional cases even decidualisation. Decidualisation during pregnancy can stimulate the growth of solid tissue and increase the vascularisation making the differential diagnosis with ovarian cancer extremely difficult (Kawaguchi et al., 2008).
Methods
The patients included in this study are all 3511 patients with validated data in the International Ovarian Tumour Analysis (IOTA) database (Timmerman et al., 2000; 2005; Van Holsbeke et al., 2009). The IOTA studies (IOTA phase 1 (Timmerman et al. 2005), IOTA phase 1b (Van Holsbeke et al., 2009), and IOTA phase 2 are large multicentre studies that prospectively collected patients with an adnexal mass. The patients were recruited in 21 different ultrasound centres in nine countries. They were all scanned transvaginally by an expert sonologist following a strict research protocol (Timmerman et al., 2000). In addition to collecting information on more than 40 ultrasound variables and a few clinical variables, at the end of the ultrasound examination the sonologist classified the adnexal mass as benign or malignant using pattern recognition (subjective evaluation of ultrasound findings). Moreover, he/she reported the level of diagnostic confidence with which the prediction of benignity/malignancy was made and suggested a specific histological diagnosis. During the IOTA phase 1 study, the ultrasound examiner could suggest any diagnosis, but during IOTA phase 1b and 2 the examiner had to choose a specific histological diagnosis from a predefined list of 15 diagnoses (endometrioma, teratoma, (serous or mucinous) cystadenoma, simple cyst/para-ovarian cyst, functional cyst, hydro/pyosalpinx, peritoneal pseudocyst, abscess, fibro(thecoma), rare benign tumour, (serous or mucinous) borderline tumour, ovarian cancer, rare malignant tumour, metastatic ovarian cancer or not possible). Whenever the ultrasound examiner suggested more than one presumed diagnosis or said it was impossible to suggest a diagnosis, the suggested diagnosis was classified as inconclusive. A presumed diagnosis of “adnexal cyst”, “complex cyst” or “benign ovarian cyst” was also regarded as inconclusive because this was not specific enough for the analysis of this study.
The gold standard was the histological diagnosis of the surgically removed adnexal mass. Only patients who had the adnexal mass surgically removed within 120 days after the ultrasound examination were included. More information on the IOTA studies and the ultrasound protocol can be found in published IOTA studies (Timmerman et al., 2000, 2005; Van Holsbeke et al. 2009).
Sensitivity and specificity for pattern recognition to discriminate endometriomas from other adnexal pathology was calculated, together with 95% confidence intervals based on Wilson’s score interval method (Newcombe 1998). The true histologies of the false positive cases are reported (i.e. tumors wrongly characterised as endometriomas), as well as the presumed diagnoses of false negatives (i.e. endometriomas missed by pattern recognition).
Descriptive statistics for the ultrasound variables, some demographic variables and the serum CA-125 level were computed for true positives (i.e. endometriomas correctly presumed to be an endometrioma), false positives (benign and malignant), false negatives, and true negatives (i.e. tumours correctly presumed not to be endometriomas).
Results
During the IOTA phase 1, 1b and 2, a total of 3511 patients with an adnexal mass were prospectively included in 21 ultrasound centres from 9 countries. Of these, 2560 masses were benign (73%) and 951 malignant (27%). The dataset included 713 (20%) endometriomas (Table I). An ultrasound diagnosis of endometrioma was made in 652 cases of which 577 (88.5%) proved to be an endometrioma on final histology. Thus, pattern recognition distinguished endometriomas from other types of adnexal masses with a sensitivity of 80.9% (95% CI 77.9-83.6) (577/713) and a specificity of 97.3% (95% CI 96.7-97.9) (2723/2798).
Table I. Demographic and ultrasound data of all patients (n = 3511).
| Mean age, y (range) | 45 | 9-94 |
| Postmenopausal, n. (%) | 1377 | 39% |
| Histological diagnosis, (%) | ||
|---|---|---|
| All benign tumors | 2560 | 72.9% |
| Endometrioma | 713 | 0.3% |
| Dermoid / teratoma | 402 | 11.4% |
| Serous cystadenoma | 420 | 12.0% |
| Simple cyst/parasalpingeal cyst | 281 | 8.0% |
| Mucinous cystadenoma | 270 | 7.7% |
| Fibroma | 152 | 4.3% |
| Functional cyst | 116 | 3.3% |
| Hydrosalpinx/Salpingitis | 100 | 2.8% |
| Abscess | 42 | 1.2% |
| Rare benign tumor* | 43 | 1.2% |
| Peritoneal pseudocyst | 21 | 0.6% |
| All malignant tumors | 951 | 27.1% |
| Common primary invasive | 575 | 16.4% |
| Rare primary invasive† | 70 | 2.0% |
| Stage I | 168 | 4.8% |
| Stage II | 50 | 1.4% |
| Stage III | 351 | 10.0% |
| Stage IV | 66 | 1.9% |
| Stage unknown | 10 | 0.3% |
| Borderline | 186 | 5.3% |
| Metastatic | 120 | 3.4% |
* For example: Brenner tumor, Struma ovarii, Leydig cell tumor; † For example: granulosa cell tumors, dysgerminoma, immature teratoma.
Of the 652 presumed endometriomas, 75 (12%) proved to be something else on final histology. Sixty-six of these 75 false positive cases were benign, the most frequent histological types being functional cysts and cystadenomas (Table II) (Fig. 1 and 2). However, in nine cases the presumed endometrioma turned out to be a malignancy (five borderline tumours, three endometrioid adenocarcinomas, and one case presumed to be an adnexal mass proved to be a uterine clear cell adenocarcinoma) (Table II) (Fig. 3 and 4).
Table II. Final histology of the false positive cases and presumed diagnosis using pattern recognition of the false negative cases.
| False positives, Benign (n = 66) | False positives, Malignant (n = 9) | False negatives (n = 136) | ||||||
|---|---|---|---|---|---|---|---|---|
| Final histology, n (%) | Final histology, n (%) | Presumed diagnosis using pattern recognition, n (%) | ||||||
| Functional cyst | 25 | (38%) | Borderline | Cystadenoma | 27 | (20%) | ||
| Mucinous cystadenoma | 12 | (18%) | All | 5 | (56%) | Simple/functional cyst | 18 | (13%) |
| Serous cystadenoma | 8 | (12%) | Serous type | 3 (33%) | Hydrosalpinx | 13 | (10%) | |
| Simple cysts | 6 | (9%) | Mucinous type | 2 | (22%) | Abscess | 7 | (5%) |
| Hydrosalpinx | 6 | (9%) | Common primary invasive | Teratoma | 7 | (5%) | ||
| Abscess | 5 | (8%) | All* | 3 | (33%) | Peritoneal pseudocyst | 5 | (4%) |
| Dermoid cysts | 4 | (6%) | Stage I | 2 | (22%) | Fibroma | 2 | (1%) |
| Stage II-IV | 1 | (11%) | Rare benign | 2 | (1%) | |||
| Rare primary invasive | Borderline tumor | 8 | (6%) | |||||
| All** | 1 | (11%) | Primary ovarian cancer | 7 | (5%) | |||
| Inconclusive/not possible | 40 | (29%) | ||||||
*: all 3 masses were endometrioid adenocarcinomas; **: uterine clear cell carcinoma.
Fig. 1. Unilocular cyst with ground glass echogenicity in a 39-year-old patient with a serum CA-125 level of 250 kU/l that was presumed to be an endometrioma but proved to be a functional cyst on final histology.
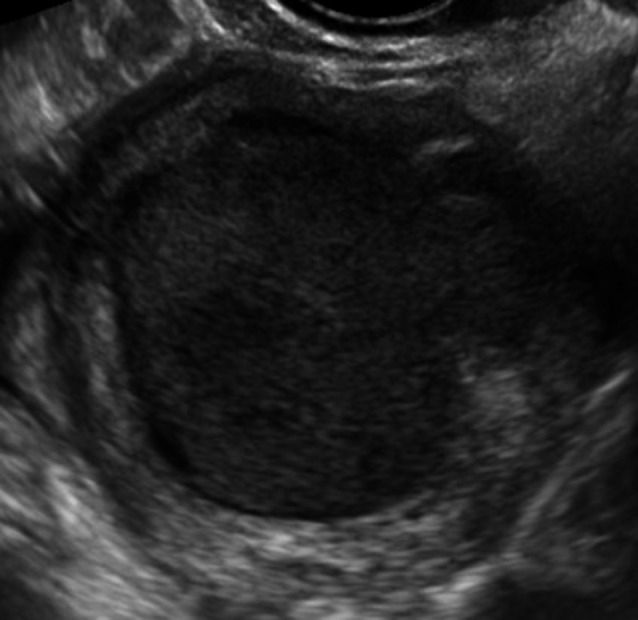
Fig. 2. Unilocular cyst with ground glass echogenicity in a 64-year-old patient that was presumed to be an endometrioma but proved to be a mucinous cystadenoma on final histology.
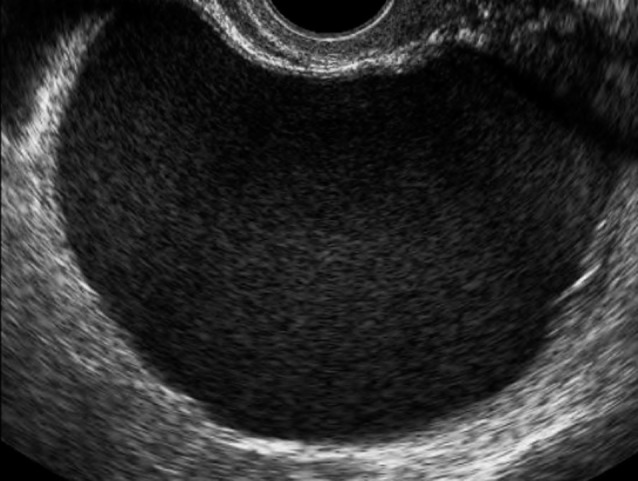
Fig. 3. Multilocular-solid mass with ground glass echogenicity of the cyst fluid and with a largest diameter of 108 mm in a 33-year-old patient that proved to be a mucinous borderline tumor of the endocervical type.
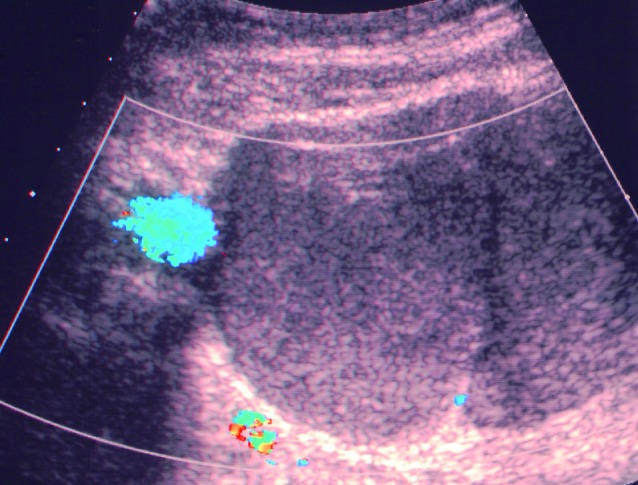
Fig. 4. Multilocular cyst with two locules, ground glass echogenicity of the cyst fluid and a largest diameter of 22 mm in a 75-year-old patient undergoing the ultrasound examination for preoperative staging of endometrial cancer. The adnexal cyst proved to be a serous borderline tumor.
Nineteen percent of the endometriomas (136/713) were missed by pattern recognition. The specific histologies that were most often suggested in these cases were functional cysts and cystadenomas (Table II).
Clinical and ultrasound features of true positives, false positives, false negatives and true negatives with regard to endometrioma using pattern recognition are presented in Table III and IV.
Table III. Description of ultrasound variables of all true positive, false positive, false negative and true negative cases.
| Clinical or demographic variable | True positives, (n = 577) | False positives, benign (n = 66) | False positives, malignant (n = 9) | False negatives (n = 136) | True negatives, benign (n = 1781) | True negatives, malignant (n = 942) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n, (%) | n, (%) | n, (%) | n, (%) | n, (%) | n, (%) | |||||||
| Type of tumor | ||||||||||||
| Unilocular | 418 | (72.4%) | 35 | (53.0%) | 3 | (33.3%) | 45 | (33.1%) | 638 | (35.8%) | 9 | (1.0%) |
| Unilocular-solid | 39 | (6.7%) | 8 | (12.1%) | 0 | (0%) | 21 | (15.4%) | 207 | (11.6%) | 157 | (16.7%) |
| Multilocular | 96 | (16.6%) | 17 | (25.7%) | 3 | (33.3%) | 34 | (25.0%) | 419 | (23.5%) | 54 | (5.7%) |
| Multilocular-solid | 21 | (3.6%) | 5 | (7.5%) | 3 | (33.3%) | 29 | (21.3%) | 309 | (17.3%) | 381 | (40.4%) |
| Solid | 3 | (0.5%) | 1 | (1.5%) | 0 | (0%) | 7 | (5.1%) | 199 | (11.2%) | 341 | (36.2%) |
| unclassifiable | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 6 | (0.3%) | 0 | (0%) |
| Echogenicity of cyst fluid | ||||||||||||
| anechoic | 9 | (1.6%) | 1 | (1.5%) | 0 | (0%) | 25 | (18.4%) | 139 | (7.8%) | 250 | (26.5%) |
| low level | 58 | (10.1%) | 15 | (22.7%) | 0 | (0%) | 37 | (27.2%) | 398 | (22.3%) | 224 | (23.8%) |
| ground glass | 486 | (84.2%) | 40 | (60.6%) | 9 | (100%) | 34 | (25.0%) | 28 | (1.6%) | 51 | (5.4%) |
| haemorrhagic | 7 | (1.2%) | 4 | (6.1%) | 0 | (0%) | 6 | (4.4%) | 70 | (3.9%) | 7 | (0.7%) |
| mixed | 14 | (2.4%) | 5 | (7.6%) | 0 | (0%) | 27 | (19.9%) | 321 | (18.0%) | 110 | (11.7%) |
| no cyst fluid | 1 | (0.2%) | 1 | (1.5%) | 0 | (0%) | 7 | (5.1%) | 785 | (44.1%) | 245 | (26.0%) |
| Largest diameter of lesion, (median), (mm (range)) | 51.0 mm (15-175) |
54.0 mm (10-139) |
64.5 mm (22-129) |
63.0 mm (15-180) |
65.0 mm (8-760) |
93.0 mm (8-410) |
||||||
| Largest diameter of solid component, (median); (mm (range)) | 14.0 mm (4-50) |
20.0 mm (6-50) |
37.0 mm (13-38) |
24.5 mm (4-50) |
25.0 mm (3-230) |
50.0 mm (4-50) |
||||||
| Presence of papillations | 45 | (7.8%) | 5 | (7.6%) | 1 | (11.1%) | 28 | (20.6%) | 299 | (16.8%) | 378 | (40.1%) |
| Number of papillations* | ||||||||||||
| 1 | 33/45 | (73.3%) | 3/5 | (60.0%) | 0 | 18/28 | (64.2%) | 184/299 | (61.5%) | 110/378 | (29.1%) | |
| 2 | 7/45 | (15.6%) | 0 | 0 | 1/28 | (3.6%) | 41/299 | (13.7%) | 37/378 | (9.8%) | ||
| 3 | 3/45 | (6.7%) | 0 | 1 | (100%) | 3/28 | (10.7%) | 34/299 | (11.4%) | 41/378 | (10.8%) | |
| > 3 | 2/45 | (4.4%) | 2/5 | (40.0%) | 0 | 6/28 | (21.4%) | 40/299 | (13.4%) | 190/378 | (50.3%) | |
| Irregular surface of papillation* | 15 | (2.6%) | 2 | (3.0%) | 1 | (11.1%) | 16 | (11.8%) | 164 | (9.2%) | 313 | (33.2%) |
| Flow inside papillation* | 9 | (1.6%) | 2 | (3.0%) | 1 | (11.1%) | 9 | (6.6%) | 74 | (4.2%) | 286 | (30.4%) |
| Irregular cyst wall | 131 | (22.7%) | 18 | (27.2%) | 4 | (44.4%) | 57 | (41.9%) | 540 | (30.3%) | 670 | (71.1%) |
| Diagnostic level of confidence | ||||||||||||
| Certainly benign | 476 | (82.5%) | 39 | (59.0%) | 1 | (11.1%) | 69 | (50.7%) | 1066 | (59.9%) | 23 | (2.4%) |
| Probably benign | 98 | (17.0%) | 26 | (39.3%) | 6 | (66.7%) | 37 | (27.2%) | 481 | (27.0%) | 46 | (4.9%) |
| Uncertain | 3 | (0.5%) | 1 | (0.7%) | 2 | (22.2%) | 19 | (14.0%) | 148 | (8.3%) | 71 | (7.5%) |
| Probably malignant | 8 | (5.9%) | 70 | (3.9%) | 276 | (29.3%) | ||||||
| Certainly malignant | 3 | (2.2%) | 16 | (0.9%) | 526 | (55.8%) | ||||||
*; concerns only those masses with a papillary projection (true positives: n = 45; false negatives: n = 28; false positives benign: n = 47; false positives malignant: n = ; true negatives benign: n = 299.
Table IV. Description of demographic, clinical variables and serum CA 125.
| Clinical or demographic variable | True positives, (n = 577) | False positives, benign (n = 66) | False positives, malignant (n = 9) | False negatives (n = 136) | True negatives, benign (n = 1781) | True negatives, malignant (n = 942) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n, (%) | n, (%) | n, (%) | n, (%) | n, (%) | n, (%) | |||||||
| Age, median (years,(range)) | 33 | (19-76) | 39 | (16-74) | 50 | (32-75) | 40 | (12-63) | 46 | (9-90) | 56 | (15-94) |
| Postmenopausal | 11 | (1.9%) | 10 | (15.1%) | 5 | (55.6%) | 19 | (14.0%) | 711 | (40.0%) | 621 | (65.9%) |
| Personal history of ovarian cancer | 1 | (0.2%) | 3 | (5%) | 1 | (11.1%) | 2 | (1.4%) | 14 | (0.8%) | 33 | (3.5%) |
| Pain during the ultrasound examination | 216 | (37.4%) | 20 | (30.3%) | 1 | (11.1%) | 37 | (27.2%) | 330 | (18.5%) | 15 | (15.4%) |
| Median CA 125# (kU/l) | 46 | (4-3500) | 22 | (2-800) | 24 | (11-78) | 38 | (2-9556) | 15 | (1-40140) | 174 | (2-38161) |
#: concerns only those masses for which the serum CA 125 level was available (true positives: n = 364, false positives benign: n = 47, false positives malignant: n = 8, false negatives: n = 92, true negatives benign: n = 1293, true negatives malignant: n = 854).
The true positive cases were most often unilocular (72.4% vs. 53% false positives benign and 33.3% false positives malignant, 33.1% false negatives, 35.8% true negatives benign, and 1.0% true negatives malignant. Ground glass echogenicity was seen in 84.2% of the true positive cases vs. in 60.6% of false positives benign cases, and in 25.0% of the false negatives, in 1.6% of the benign true negatives and in 5.4% of the malignant true negatives. However, all of the false positive malignant cases (n = 9) had ground glass echogenicity. Papillary projections were as common in the true positive endometriomas as in the false positive benign cases (7.8% and 7.6%) but less common than in all the other categories (11.1% in false positive malignant cases, 20.6% in the false negative cases, 16.8% in the true negative benign cases, and 40.1% in true negative malignant cases). Flow inside the papillary projections was rare in the true positive cases of endometrioma (1.6% vs. 3% - 11.1% - 6.6% - 4.2% - 30.4%). Within the groups of masses with papillary projections the true positive endometriomas most often contained only one papillation instead of several papillations (73.3% vs. 60.0% - 0% - 64.2% - 61.5% - 29.1%). The patients in the group of true positives were the youngest (median age 33 years vs. 39-50-40-46-56 years) and the least often postmenopausal (1.9% vs. 15.1% - 55.6% - 14% - 40% - 65.9%). When compared to the other groups, the sonologists were most often convinced about the benign character of the mass in the group of true positives (82.5% vs. 59.0% false positive benign, 11.1% false positive malignant, 50.7% false negative, 59.9% true negative benign).
The median serum CA 125 level was often above the suggested cut-off of 30 kU/ml to indicate malignancy in the group of the malignant true negatives (median 174 kU/l, (range 2-38161)) but also in the group of the true positives (46 kU/l, (range 4-3500)) and false negatives (38 kU/l, (range 2-9556)) (Table IV).
In the group of false positive malignant cases (n = 9), six cases were unilocular (n = 3) or multilocular (n = 3) and all had ground glass echogenicity. Only one case presented with papillary projections. The surface of the papillary projection was irregular and colour Doppler examination demonstrated flow inside the papillation. Most of these patients (5/9, 56%) were postmenopausal with a median age of 50 years. In one of the nine cases the sonologist was convinced that the mass was benign, in six cases the sonologist assumed it was probably benign and in two cases he or she was completely uncertain about the benign or malignant character. The serum CA 125 level was available for eight of the nine patients with a median CA 125 level of 24 kU/l.
The group of the false negative cases or “missed” endometriomas (136/713, 19%) presented more often with atypical endometrioma features than the group of the true positives with only 9% (12/136) unilocular cysts with ground glass echogenicity. Twenty six % were multilocular-solid or solid compared to only 9% of the true positives. Only 25% had ground glass echogenicity of the cyst fluid compared to 84% of the true positives. The sonologists were also less confident about the benign character of the mass and in 18 cases (13%) even suspected a malignancy (Table III) (Fig. 5).
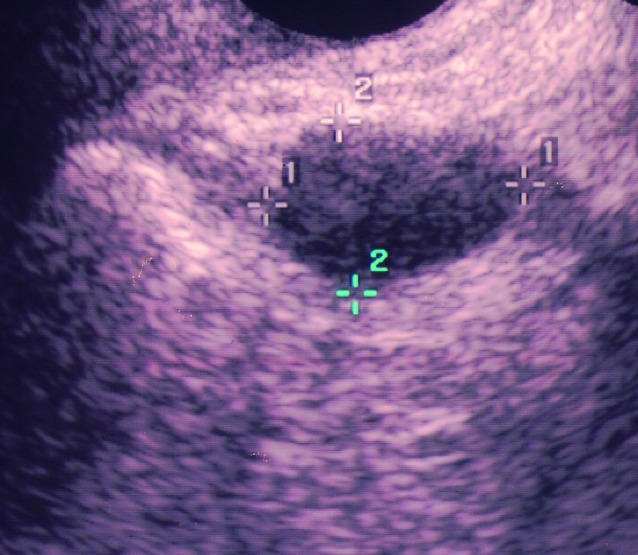
Fig. 5. Endometrioma that was missed by pattern recognition. Multilocular-solid mass with ground glass echogenicity in a 24-year-old patient. The ultrasound diagnosis suggested was borderline tumor.
Discussion
As far as we know the present study is the largest study examining not only the performance of pattern recognition to discriminate endometriomas from other types of adnexal masses but also examining the differences in morphology of the false positive and false negative cases, with the purpose of explaining the misclassifications. This study demonstrated that an expert sonologist can distinguish endometriomas from other masses, but that 1% of the masses presumed to be an endometrioma may be malignant.
In the past several authors reported that the ultrasound morphology of endometriomas is so characteristic that pattern recognition has excellent sensitivity and specificity with regard to endometrioma (Guerriero et al., 1995, 1996, 1997; Mais et al., 1993 (2), Patel et al., 1999; Van Holsbeke et al., 2009; Jermy et al., 2001; Sampson 1921, Asch and Levine 2007; Alcazar et al., 1997). In unpublished work (Van Holsbeke et al., 2009) we have demonstrated that the diagnostic performance of pattern recognition to discriminate between images of endometriomas and images of other types of adnexal masses is highly influenced by the level of ultrasound experience. The sensitivity and specificity of expert sonologists was very good (88% and 99%, respectively). However, for senior trainees in gynaecology, the sensitivity was only 56 and 69% and specificity 93 and 94%, and for junior trainees in gynaecology the sensitivity ranged between 6 and 69% and specificity between 91% and 97% (Van Holsbeke et al., 2009). In this study the expert sonologists achieved a sensitivity of 81% and a specificity of 97% to discriminate endometriomas from other adnexal pathology. This is in line with previous studies that have evaluated the performance of pattern recognition in the diagnosis of endometriomas (sensitivity varying between 81 and 86% and specificity between 89 and 97% (Valentin 2004; Guerriero et al., 1996, 1997; Mais et al., 1993 (2); Jermy et al., 2001; Guerriero et al., 1995; Sampson 1921; Asch and Levine 2007; Alcazar et al., 1997). The first prospective study that evaluated the ability of transvaginal ultrasound to discriminate endometriomas from other types of adnexal masses was performed by Guerriero et al. on a dataset of 93 adnexal masses that included 24 endometriomas. Pattern recognition gave a sensitivity of 83% and a specificity of 89% (Guerriero et al., 1995). The false positive cases in their study had the same ultrasound characteristics as the true positive cases which is in contrast to our study where the endometriomas that were missed and the false positive cases had different ultrasound morphology. False negative cases or endometriomas missed by pattern recognition were most commonly thought to be cystadenomas, functional cysts, hydrosalpinges and abscesses or even ovarian cancer when using pattern recognition. We found that false positive cases occurred most commonly in functional cysts and cystadenomas. It is not surprising that these types of histologies were misclassified as endometriomas because the echogenicity of the cyst content of these masses (blood, pus or mucus) may appear as ground glass. Among these false positive functional cysts and cystadenomas the rate of ground glass echogenicity was 52% (13/25) and 60% (12/20), respectively. This is less than what we found in another unpublished study that described the ultrasound characteristics of 713 endometriomas. In the study cited 73% (520/713) of the endometriomas had ground glass echogenicity of the cyst content (unpublished data, Van Holsbeke et al., 2009) instead of only 6% (109/1847) of the benign non-endometrioma cases (Van Holsbeke et al., 2009). The whole IOTA database contained 116 functional cysts, 690 cystadenomas and 42 abscesses of which only 15 (13%), 36 (5%) and 11 (26%) had ground glass echogenicity. Of these masses with ground glass echogenicity 10/15 (67%), 11/36 (31%) and 5/11 (45%) were classified as endometriomas when using pattern recognition, most probably because of the ground glass echogenicity.
Amongst the false positive cases there were also nine cases of ovarian cancer. It is a concern that among these false positive malignant cases only three demonstrated overt features suspicious for a malignancy. Contrary to most other studies our dataset included both pre- and postmenopausal patients. The prevalence of cancer in this group of postmenopausal patients with a presumed diagnosis of an endometrioma was 19% (5/26) whereas in the premenopausal group it was 0.6% (4/626). It is clear from this that there are risks associated with making a presumed diagnosis of an endometrioma in postmenopausal women.
The personal history of an ovarian cancer should also be taken into account because the recurrence of a borderline tumour or invasive ovarian carcinoma can initially also present as a small mass with ground glass echogenicity due to the haemorrhagic or necrotic cyst content. This was the case in one of the nine malignant masses misclassified as endometrioma.
In a previous study we demonstrated the poor value of CA-125 in the preoperative assessment of an adnexal mass (Van Calster et al., 2007). Also in this study we found no benefit of measuring the serum CA 125 level for correct classification of the masses. The median CA-125 level was lower in the false positive malignant masses (median 24kU/l) than in the true positive cases (median 46 kU/l).
We should also stress that within the IOTA studies the ultrasound examinations were performed by expert sonologists. This may have lead to an overestimation of the diagnostic performance of pattern recognition. Moreover, only patients who were operated on within 4 months were included. This means that a significant number of endometriomas that had overt features of an endometrioma on ultrasound are likely not to have been included, because of long waiting lists for benign surgery in some centres or because of conservative management. If these “easy” cases would have been included, the prevalence of cancer within the group of masses presumed to be an endometrioma would have been smaller.
We can conclude that expert sonologists are able to discriminate between endometriomas and other types of adnexal masses in most cases. The number of misclassifications and especially misclassified cancers could be significantly reduced by taking great care when making a presumed diagnosis of an endometrioma whenever the mass does not demonstrate ground glass echogenicity or if it is found in a postmenopausal patient.
Acknowledgments
Research supported by Research Council KUL: GOAAMBioRICS, CoE EF/05/006 Optimization in Engineering (OPTEC); FWO: G.0407.02 (support vector machines), G.0302.07 (SVM), G.0341.07 (Data fusion), research communities (ICCoS, ANMMM); IWT-TBM 070706 (IOTA); Belgian Federal Science Policy Office: IUAP P6/04 (DYSCO); EU: BIOPATTERN (FP6-2002-IST 508803); Ben Van Calster is a postdoctoral research from the Research Foundation – Flanders (FWO).
References
- Alcázar JL, Laparte C, Jurado M, López-García G. The role of transvaginal ultrasonography combined with color velocity imaging and pulsed Doppler in the diagnosis of endometrioma. Fertil Steril. 1997;67:487–491. doi: 10.1016/s0015-0282(97)80074-x. [DOI] [PubMed] [Google Scholar]
- Asch E, Levine D. Variations in appearance of endometriomas. J Ultrasound Med. 2007;26:993–1002. doi: 10.7863/jum.2007.26.8.993. [DOI] [PubMed] [Google Scholar]
- Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas:cystectomy versus drainage and coagulation. Fertil Steril. 1998;70:1176–1180. doi: 10.1016/s0015-0282(98)00385-9. [DOI] [PubMed] [Google Scholar]
- Bischoff F, Simpson J. Heritability and molecular genetic studies of endometriosis. Hum Reprod Update. 2000;6:37–44. doi: 10.1093/humupd/6.1.37. [DOI] [PubMed] [Google Scholar]
- Brosens I, Putttemans P. Kurjak KA, editor: Ultrasound and the ovary. Carnforth: Parthenon Publishing; 1994. Endometriosis and the ovary; 163 pp. [Google Scholar]
- Brosens I, Brosens J. Endometriosis. Eur J Obstet Gynecol Reprod Biol. 2000;90:159–164. doi: 10.1016/s0301-2115(00)00265-7. [DOI] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis. NEJM. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- Campbell I, Thomas E. Endometriosis:candidate genes. Hum Reprod Update. 2001;7:15–20. doi: 10.1093/humupd/7.1.15. [DOI] [PubMed] [Google Scholar]
- Chatman D, Ward A. Endometriosis in adolescents. J Reprod Med. 1982;27:156–160. [PubMed] [Google Scholar]
- Cheng Y, Wang S, Chou C. Serum CA 125 in preoperative patients at high risk for endometriosis. Obstet Gynecol. 2002;99:375–380. doi: 10.1016/s0029-7844(01)01731-8. [DOI] [PubMed] [Google Scholar]
- Dmowski W, Gebel H, Braun D. The role of cell-mediated immunity in pathogenesis of endometriosis. Acta Obstet Gynecol Scand Suppl. 1994;159:7–14. [PubMed] [Google Scholar]
- Eskenazi A, Warner M. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Nomura K, Ishikawa E, Ushigome S. Ovarian atypical endometriosis:its close association with malignant epithelial tumors. Histop. 1997;30:249–255. doi: 10.1046/j.1365-2559.1997.d01-592.x. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- Guerriero S, Mais V, Ajossa S, Paoletti AM, Angiolucci M, Labate F, Melis GB. The role of endovaginal ultrasound in differentiating endometriomas from other ovarian cysts. Clin Exp Obstet Gynecol. 1995;22:20–22. [PubMed] [Google Scholar]
- Guerriero S, Mais V, Ajossa S, Paoletti AM, Angiolucci M, Benedetto-Melis G. Transvaginal ultrasonography combined with CA-125 plasma levels in the diagnosis of endometrioma. Fertil Steril. 1996;65:293–299. doi: 10.1016/s0015-0282(16)58088-1. [DOI] [PubMed] [Google Scholar]
- Guerriero S, Mallarini G, Ajossa S, Risalvato A, Satta R, Mais V, Angiolucci M, Benedetto-Melis G. Transvaginal ultrasound and computed tomography combined with clinical parameters and CA-125 determinations in the differential diagnosis of persistent ovarian cysts in premenopausal women. Ultrasound Obstet Gynecol. 1997;9:339–343. doi: 10.1046/j.1469-0705.1997.09050339.x. [DOI] [PubMed] [Google Scholar]
- Jacobson TZ, Barlow DH, Koninckx PR, Olive D, Farquhar C. Laparoscopic surgery for subfertility associated with endometriosis. Cochrane Database Syst Rev. 2002;(CD001398) doi: 10.1002/14651858.CD001398. [DOI] [PubMed] [Google Scholar]
- Jermy K, Luise C, Bourne T. The characterization of common ovarian cysts in premenopausal women. Ultrasound Obstet Gynecol. 2001;17:140–144. doi: 10.1046/j.1469-0705.2001.00330.x. [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Tsuji Y, Haruta S, Kanayama S, Sakata M, Yamada Y, Fujita H, Saito H, Tsuneto K, Kobayashi H. Clinicopathologic features of ovarian cancer in patients with ovarian endometrioma. J Obstet Gynaecol Res. 2008;34 doi: 10.1111/j.1447-0756.2008.00849.x. [DOI] [PubMed] [Google Scholar]
- Kennedy S. Genetics of endometriosis. Semin Reprod Med . 2003;21:111–118. doi: 10.1055/s-2003-41317. [DOI] [PubMed] [Google Scholar]
- Kennedy S, Bergqvist A, Chapron C, Hooghe T, Dunselman G, Greb R, Hummelshoj L, Prentice A, Saridogan E. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20:2698–2704. doi: 10.1093/humrep/dei135. [DOI] [PubMed] [Google Scholar]
- Langgebrekke A, Istre O, Busund B, Johannessen HO, Qvigstad E. Endoscopic treatment of deep infiltrating endometriosis (DIE) involving the bladder and rectosigmoid colon. Acta Obstet Gynecol Scand. 2006;85:712–715. doi: 10.1080/00016340500449907. [DOI] [PubMed] [Google Scholar]
- Lin JF, Sun CX, Hua KQ, Xue XH, Li Y. Clinical study of effect of laparoscopic diagnosis and treatment on pelvic endometriosis-associated infertility. Zhonghua Fu Chan Ke Za Zhi. 2005;40:9–12. [PubMed] [Google Scholar]
- Mais V, Guerriero S, Ajossa S, Angiolucci M, Paoletti AM, Benedetto , Melis G. The efficiency of transvaginal ultrasonography in the diagnosis of endometrioma. Fertil Steril. 1993;60:776–780. doi: 10.1016/s0015-0282(16)56275-x. [DOI] [PubMed] [Google Scholar]
- Marcoux S, Maheux R, Bérubé S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis. . N Engl J Med. 1997;337:217–222. doi: 10.1056/NEJM199707243370401. [DOI] [PubMed] [Google Scholar]
- Mereu L, Ruffo G, Landi S, Barbieri F, Zaccoletti R, Fiaccavento A, Stepniewska A, Pontrelli G, Minelli L. Laparoscopic treatment of deep endometriosis with segmental colorectal resection:short term morbidity. J Minim Invasive Gynecol. 2007;14:463–469. doi: 10.1016/j.jmig.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Missmer S, Hankinson S, Spiegelman D, Barbieri R, Marshall L, Hunter D. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160:784–796. doi: 10.1093/aje/kwh275. [DOI] [PubMed] [Google Scholar]
- Mol BW, Bayram N, Lijmer J, Wiegerinck M, Bongers M, van der Veen F, Bossuyt P. The performance of CA 125 measurement in the detection of endometriosis:a meta-analysis. Fertil Steril. 1998;70:1101–1108. doi: 10.1016/s0015-0282(98)00355-0. [DOI] [PubMed] [Google Scholar]
- Mac Sween RNM, Whaley K. Muir’s textbook of pathology. Thirteenth edition. [Google Scholar]
- Newcombe R. Two-sided confidence intervals for the single proportion:comparison of seven methods. Statistics in Medicine. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Okaro E, Condous G, Khalid A, Timmerman D, Ameye L, Van Huffel S, Bourne T. The use of ultrasound-based “soft markers” for the prediction of pelvic pathology in women with chronic pelvic pain, can we reduce the need for laparoscopy? . BJOG. 2006;113:251–256. doi: 10.1111/j.1471-0528.2006.00849.x. [DOI] [PubMed] [Google Scholar]
- Olive D, Henderson D. Endometriosis and Mullerian anomalies. Obstet Gynecol. 1987;69:412–415. [PubMed] [Google Scholar]
- Patel M, Feldstein V, Chen D, Lipson S, Filly R. Endometriomas:Diagnostic performance of US. Radiology. 1999;210:739–745. doi: 10.1148/radiology.210.3.r99fe61739. [DOI] [PubMed] [Google Scholar]
- Sangi Haghpeykar H, Poindexter AN., 3rd Epidemiology of endometriosis among parous women. Obstet Gynecol. 1995;85:983–992. doi: 10.1016/0029-7844(95)00074-2. [DOI] [PubMed] [Google Scholar]
- Samson JA. Endometrial carcinoma of the ovary arising in endometrial tissue in that organ. Arch Surg. 1925;10:1–72. [Google Scholar]
- Samson JA. Perforating hemorrhagic (chocolate) cysts of the ovary. Arch Surg. 1921;2:245–323. [Google Scholar]
- Schenken R. Endometriosis:Contemporary concepts in clinical management, Schenken RS (Ed) Philadelphia: JB Lippincott Company; 1989. Pathogensis; p. 1. [Google Scholar]
- Sinaii N, Plumb K, Cotton L, Lambert A, Kennedy S, Zondervan K, Stratton P. Differences in characteristics among 1,000 women with endometriosis based on extent of disease. Fertil Steril. 2008;89:538–545. doi: 10.1016/j.fertnstert.2007.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somigliana E, Vercellini P, Vigano P, Ragni G, Crosignani PG. Should endometriomas be treated before IVF-ICSI cycles? . Hum Reprod Update. 2006;12:57–64. doi: 10.1093/humupd/dmi035. [DOI] [PubMed] [Google Scholar]
- Thomas E, Campbell I. Molecular genetic defects in endometriosis. Gynecol Obstet Invest. 2000;50:44–50. doi: 10.1159/000052878. [DOI] [PubMed] [Google Scholar]
- Timmerman D, Valentin L, Bourne T, Collins WP, Verrelst H, Vergote I. International Ovarian Tumor Analysis (IOTA) Group. Terms, definitions and measurements to describe the sonographic features of adnexal tumors:a consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet Gynecol. 2000;16 doi: 10.1046/j.1469-0705.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- Timmerman D. The use of mathematical models to evaluate pelvic masses;can they beat an expert operator? Best Pract Res Clin Obstet Gynaecol. 2004;18:91–104. doi: 10.1016/j.bpobgyn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Timmerman D, Testa AC, Bourne T, Ferrazzi E, Ameye L, Konstantinovic ML. International Ovarian Tumor Analysis Group. Logistic regression model to distinguish between the benign and malignant adnexal mass before surgery:a multicenter study by the International Ovarian Tumor Analysis Group. J Clin Oncol. 2005;23:8794–8801. doi: 10.1200/JCO.2005.01.7632. [DOI] [PubMed] [Google Scholar]
- Valentin L. Use of morphology to characterize and manage common adnexal masses. Best Pract Res Clin Obstet Gynaecol. 2004;18:71–89. doi: 10.1016/j.bpobgyn.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Valentin L. Prospective cross-validation of Doppler ultrasound examination and gray-scale ultrasound imaging for discrimination of benign and malignant pelvic masses. Ultrasound Obstet Gynecol. 1999;14:273–283. doi: 10.1046/j.1469-0705.1999.14040273.x. [DOI] [PubMed] [Google Scholar]
- Valentin L, Hagen B, Tingulstad S, Eik-Nes S. Comparison of ‘pattern recognition’ and logistic regression models for discrimination between benign and malignant pelvic masses:a prospective cross validation. Ultrasound Obstet Gynecol. 2001;18:357–365. doi: 10.1046/j.0960-7692.2001.00500.x. [DOI] [PubMed] [Google Scholar]
- Van Calster B, Timmerman D, Bourne T, Testa A, Van Holsbeke C, Domali E, Jurkovic D, Neven P, Van Huffel S, Valentin L. Discrimination between benign and malignant adnexal masses by specialist ultrasound examination versus serum CA-125. J Natl Cancer Inst. 2007;99:1706–1714. doi: 10.1093/jnci/djm199. [DOI] [PubMed] [Google Scholar]
- Van Gorp T, Amant F, Neven P, Vergote I, Moerman P. Endometriosis and the development of malignant tumours of the pelvis. A review of literature. Best Pract Res Clin Obstet Gynaecol. 2004;18:349–371. doi: 10.1016/j.bpobgyn.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Van Holsbeke C, Van Calster B, Testa AC, Domali E, Lu C, Van Huffel S, Valentin L, Timmerman D. Prospective internal validation of mathematical models to predict malignancy in adnexal masses:results from the nternational Ovarian Tumor Analysis (IOTA) study. Clin Cancer Res. 2009;15:684–691. doi: 10.1158/1078-0432.CCR-08-0113. [DOI] [PubMed] [Google Scholar]
- Van Holsbeke C, Daemen A, Yazbek J, Holland TK, Bourne T, Mesens T, Lannoo L, Boes AS, Joos A, Van De Vijver A, Roggen N, de Moor B, de Jonge E, Testa AC, Valentin L, Jurkovic D, Timmerman D. The diagnostic performance of pattern recognition for the preoperative classification of adnexal masses when used by sonologists with different levels of ultrasound experience. Ultrasound in Obstetrics and Gynecology. (Unpublished data) [Google Scholar]
- Van Holsbeke C, Van Calster B, Guerriero S, Savelli L, Paladini D, Lissoni AA, Czekierdowski A, Fischerova D, Jinghzang , Mestdagh G, Testa AC, Bourne T, Valentin L, Timmerman D. Endometriomas:their ultrasound characteristics. Ultrasound Obstet Gyneco. 2009 doi: 10.1002/uog.7668. [DOI] [PubMed] [Google Scholar]
- Witz C. Interleukin-6: another piece of the endometriosis-cytokine puzzle. Fertil Steril. 2000;73:212–214. doi: 10.1016/s0015-0282(99)00556-7. [DOI] [PubMed] [Google Scholar]
- Yang WC, Chen HW, Au HK, Chang CW, Huang CT, Yen YH, Tzeng CR. Serum and endometrial markers. Best Pract Res Clin Obstet Gynaecol. 2004;18:305–318. doi: 10.1016/j.bpobgyn.2004.03.003. [DOI] [PubMed] [Google Scholar]


