Abstract
Lymphocytic neoplasm involving the heart is not common and usually presents with pericardial effusion or focal myocardial infiltration. Myocardial infarctions due to leukemic infiltration of the coronary arteries are rarely reported. We present the case of a 52-year-old Guatemalan man with a one-year history of untreated T-cell prolymphocytic leukemia. He was admitted to our hospital for chemotherapy and evaluation of a pulmonary cavitary lesion by wedge resection. During sedation, the patient experienced acute respiratory failure and hypovolemic shock, from which he could not be resuscitated.
Autopsy revealed that leukemic cells extensively infiltrated the aorta, myocardium, and coronary arteries. The lumina of the 3 major coronary artery branches showed 70% to 95% stenosis, with multifocal remote myocardial infarctions. Tumor cells were also detected in the lungs and other organs. The acute cardiorespiratory insufficiency secondary to leukemia—particularly the extensive infiltration of the coronary arteries and myocardium, and the multiple myocardial infarctions—eventually resulted in cardiac death.
Keywords: Aorta; autopsy; coronary arteries; heart neoplasms/secondary/etiology; leukemia, lymphoid/pathology; leukemia, T-cell prolymphocytic; myocardial infarction
Cardiac involvement by malignant lymphocytic neoplasms is a rare clinical phenomenon, but it has been reported in 8.7% to 37% of autopsy cases involving lymphoma or leukemia.1–4 Primary cardiac lymphoma usually has a B-cell origin.5 However, in cases of secondary cardiac involvement, T-cell lymphomas present more frequently and aggressively than do B-cell lymphomas. They are associated with a variety of cardiac manifestations.6 The most typical cardiac presentations are pericardial effusion, tumor thrombus, focal hemorrhage, and nodular myocardial infiltration.1–6
T-cell prolymphocytic leukemia (T-PLL) is rare, comprising only 2% of cases of lymphocytic leukemias in adults. It primarily affects patients over 30 years of age (median age, 65 yr) and is most often diagnosed in males. Patients typically have systemic disease at presentation, including hepatosplenomegaly, generalized lymphadenopathy, and skin infiltration. Peripheral blood often manifests lymphocytosis (>100 × 109/L), along with anemia and thrombocytopenia.7 Leukemic T cells are usually found in the peripheral blood, bone marrow, lymph nodes, spleen, liver, and skin, whereas cardiac involvement is rare. To our knowledge, this T-PLL case is the first reported with massive cardiovascular infiltrate that resulted in multiple silent myocardial infarctions and eventually in cardiac death.
Case Report
The patient was a 52-year-old Guatemalan man, 5 feet in height and 92 pounds in weight (body mass index, 18 kg/m2). He had a history of hepatitis B, hernia repair (followed by lysis of the adhesions), and small-bowel resection. He had developed T-PLL one year before presentation, but had received no routine treatment. The patient was admitted to our hospital for chemotherapy. A positron emission tomographic–computed tomographic scan revealed a cavitary lesion in the upper lobe of the right lung. We suspected an opportunistic infection or tuberculosis and scheduled wedge resection of the lung cavity for definitive diagnosis. During sedation, the patient experienced hypovolemic shock and acute respiratory failure. He could not be resuscitated.
Autopsy and subsequent microscopic examinations of the pulmonary cavity revealed Cryptococcus infection, which was confirmed by periodic acid-Schiff and Grocott-Gomori methenamine-silver nitrate staining. Disseminated leukemic cells were found in the lungs and in multiple other organs, involving the bone marrow, lymph nodes, liver, spleen, and gastrointestinal tract. Most strikingly, the cardiovascular system was extensively infiltrated.
The heart of this patient weighed 200 g. Gross examination revealed multiple white scars in the left ventricle and the interventricular septum (Fig. 1A). The coronary arteries were extremely narrowed, exhibiting up to 95% luminal occlusion (Fig. 1B). The gross picture was typical for myocardial infarctions due to atherosclerotic coronary artery disease. Surprisingly, microscopic examination revealed that the walls of the 3 major coronary artery branches were heavily infiltrated with leukemic cells (Fig. 1C). The lumina showed stenoses of 70% (right coronary artery) to 95% (left anterior descending and left circumflex coronary arteries), with no thrombosis identified. We also observed multifocal fibrotic scars and compensatory hypertrophic myocardium infiltrated by leukemic cells (Fig. 2A). Immunophenotyping confirmed the diagnosis of T-PLL through positive expression of CD2, CD3, CD7, CD4, and CD8, and negative expression of CD34, TdT, CD1a, and CD20 (Figs. 1D, 2B–D, and data not shown).
Fig. 1.
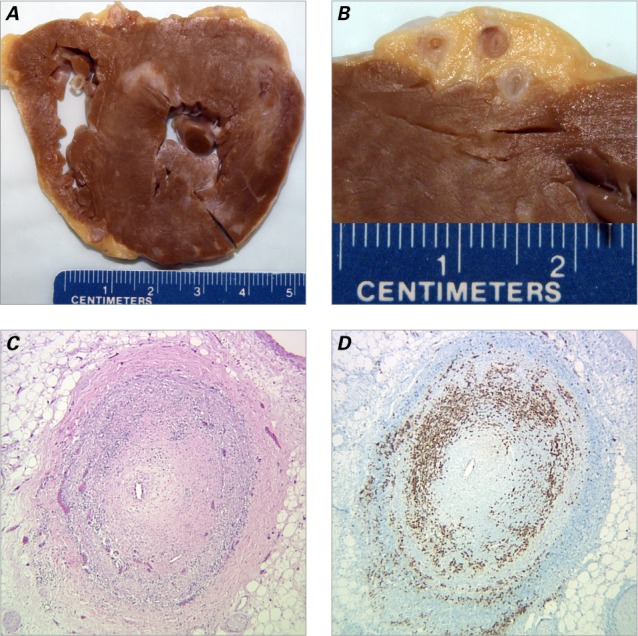
Multifocal myocardial infarction and coronary artery involvement with T-cell prolymphocytic leukemia. A) Transverse section of the heart reveals multiple remote myocardial infarctions, with fibrosis and scarring. B) Transverse section of the left anterior descending coronary artery reveals narrowed lumina and thickened vascular walls. C) Photomicrograph of one coronary artery branch reveals a pinpoint lumen. The leukemic cells have predominantly infiltrated the vascular muscular layer (H & E, orig. ×40). D) Photomicrograph reveals leukemic cells positive for CD3 (immunohistochemistry stain, orig. ×40).
Fig. 2.
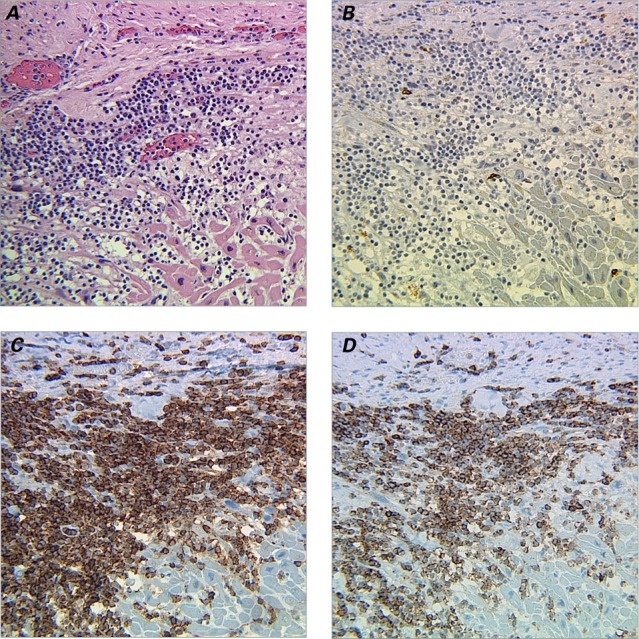
Photomicrographs show myocardium infiltrated with T-cell prolymphocytic leukocytes. A) Section shows myocardial fibrosis and compensatory hypertrophy with leukemic cell infiltration (H & E, orig. ×200). Immunohistochemical staining (orig. ×200) reveals B) leukemic cells negative for CD20, but positive for C) CD4 and D) CD8.
The aorta showed moderate calcified atherosclerosis, especially in its abdominal segment. Leukemic cell infiltration was also observed in the aortic tissue, limited chiefly to the muscular layer of the tunica media (data not shown).
Discussion
It is worth noting that atherosclerotic coronary artery disease does not appear to be the main cause of death for the patient whose case is reported here.
Our patient died of cardiac failure during sedation. His autopsy revealed stenoses—with multifocal myocardial fibrosis and scars—in up to 95% of his coronary arteries, indicating that he had experienced multiple silent myocardial infarctions before dying. Morphologic findings showed that leukemic cells infiltrated the vascular muscular layer predominately, which resulted in extensive narrowing of the vascular lumen. In the coronary arteries, we observed only mild fibrocellular plaques with scant lipids and no obvious calcification. Only a few leukemic cells infiltrated the intima.
Although the exact mechanism was not understood, the pathologic findings in this case indicated that these remote coronary artery thrombotic events might be due to a combination of tumor cell infiltration, narrowed vascular lumina, endothelial damage, hyperviscosity of leukemic cells in the blood, and previous atherosclerosis. Among these, leukemic infiltration might be the primary, rather than the secondary, event.
The leukemic cells first infiltrate the coronary arteries. The fibrous changes in the intima could be in reaction to leukemic infiltration, in addition to possibly pre-existing atherosclerosis. To compound the problem, endothelial erosion and hyperviscosity of leukemic blood could all contribute to the slowly developing thrombotic events and could eventually cause multiple remote myocardial infarctions. This case differs from most other cases of myocardial infarction, wherein atherosclerotic coronary artery disease is the primary cause.
T-cell prolymphocytic leukemia is an aggressive T-cell malignancy characterized by the proliferation of prolymphocytes of small to medium size. Myocardial and aortic tissue infiltration is rare. In our patient, the leukemic cells not only massively infiltrated the myocardium, but extensively involved the vascular system, especially the aorta and the coronary arteries. Previous investigators have suggested that T-PLL cells express cytolytic factor perforin and CD28. The leukemic cells might invade cardiovascular tissue through their interaction with the surface markers of vascular cells, including human leukocyte antigens, intercellular adhesion molecule-1, and co-stimulatory molecules B7 and B70.8,9 It is of particular interest that the leukemic infiltrates in this case were chiefly limited to the muscular layer of vessels. The intima showed only minimal leukemic cell involvement. We also observed that T-PLL cells extensively infiltrated the muscular layer of the gastrointestinal tract. These data strongly suggested that T-PLL cell infiltration follows a preferred muscular pattern. However, the exact underlying mechanisms remain unknown.
Although leukemic involvement of the heart is a well-documented phenomenon, we found no case in our review of the published literature that showed the presentation described herein. Because this patient had no cardiac symptoms other than his final cardiac failure, we could not correlate the pathologic findings with routine clinical presentations such as symptoms, blood pressure, pulse, heart murmur, electrocardiogram, enzymes, and image studies. Actually, because this patient did not receive routine treatment after his diagnosis of T-PLL, his clinical presentation might display the unique natural course of T-PLL's involvement in the cardiovascular system.
A human heart weighs between 200 and 450 g, or an average of approximately 300 g. The weight of the normal heart varies directly with the size and weight of the individual: it usually equals about 0.5% of the individual's weight. Our patient was 5 feet in height and 92 pounds in weight (with a body mass index of 18, lower than average). A 200-g heart, although at the lower end of the range for adult human hearts, might be appropriate for his size and weight. Yet heart weight can vary greatly when the heart is diseased. Hypertrophic myocardial fibers usually increase the mass of the heart. In our patient, however, the heart suffered from multifocal chronic myocardial infarctions, which replaced functional myocardiocytes with nonfunctional fibrotic tissue (scars). To compensate for lost cardiac function, the remaining myocardial fibers hypertrophied and the total mass of the heart actually decreased in response to the significant loss of myocardiocytes. Eventually, our patient died of cardiac failure.
Conclusion
When cardiac involvement is suspected in patients with malignant disease, aggressive diagnostic procedures are warranted—especially when malignant lymphomas have a T-cell phenotype. Transesophageal echocardiography, computed tomography, and magnetic resonance imaging have been sensitive in the diagnosis of cardiac involvement in patients with lymphoma.10,11 In T-PLL, CD52 is usually expressed at high density and can be used as a target of therapy.12,13 Prompt diagnosis and early systemic therapy might improve the poor prognosis of primary cardiac lymphoma.
Footnotes
From: Department of Pathology (Drs. Bhattacharyya, Butt, Cheng, Chow, de Vinck, and Yang) and John Theurer Cancer Center (Dr. Feldman), Hackensack University Medical Center, Hackensack, New Jersey 07601; and Department of Pathology and Laboratory Medicine (Dr. Cheng), University of Medicine and Dentistry of New Jersey–New Jersey Medical School, Newark, New Jersey 07103
Dr. Cheng is now at Meridian Health, Jersey Shore University Medical Center, Neptune, New Jersey. Dr. Butt is now at UT Southwestern Medical Center, Dallas, Texas.
References
- 1.Roberts WC, Bodey GP, Wertlake PT. The heart in acute leukemia. A study of 420 autopsy cases. Am J Cardiol. 1968;21(3):388–412. doi: 10.1016/0002-9149(68)90143-4. [DOI] [PubMed] [Google Scholar]
- 2.Roberts WC, Glancy DL, DeVita VT., Jr. Heart in malignant lymphoma (Hodgkin's disease, lymphosarcoma, reticulum cell sarcoma and mycosis fungoides). A study of 196 autopsy cases. Am J Cardiol. 1968;22(1):85–107. doi: 10.1016/0002-9149(68)90250-6. [DOI] [PubMed] [Google Scholar]
- 3.McDonnell PJ, Mann RB, Bulkley BH. Involvement of the heart by malignant lymphoma: a clinicopathologic study. Cancer. 1982;49(5):944–51. doi: 10.1002/1097-0142(19820301)49:5<944::aid-cncr2820490519>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.O'Mahony D, Peikarz RL, Bandettini WP, Arai AE, Wilson WH, Bates SE. Cardiac involvement with lymphoma: a review of the literature. Clin Lymphoma Myeloma. 2008;8(4):249–52. doi: 10.3816/CLM.2008.n.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nascimento AF, Winters GL, Pinkus GS. Primary cardiac lymphoma: clinical, histologic, immunophenotypic, and genotypic features of 5 cases of a rare disorder. Am J Surg Pathol. 2007;31(9):1344–50. doi: 10.1097/PAS.0b013e3180317341. [DOI] [PubMed] [Google Scholar]
- 6.Chinen K, Izumo T. Cardiac involvement by malignant lymphoma: a clinicopathologic study of 25 autopsy cases based on the WHO classification. Ann Hematol. 2005;84(8):498–505. doi: 10.1007/s00277-005-1009-5. [DOI] [PubMed] [Google Scholar]
- 7.Matutes E, Brito-Babapulle V, Swansbury J, Ellis J, Morilla R, Dearden C et al. Clinical and laboratory features of 78 cases of T-prolymphocytic leukemia. Blood. 1991;78(12):3269–74. [PubMed] [Google Scholar]
- 8.Seko Y, Azuma M, Yagita H, Okumura K, Hirai H, Nagai R, Yazaki Y. Perforin-positive leukemic cell infiltration in the heart of a patient with T-cell prolymphocytic leukemia. Intern Med. 1995;34(8):782–4. doi: 10.2169/internalmedicine.34.782. [DOI] [PubMed] [Google Scholar]
- 9.Seko Y, Azuma M, Yagita H, Okumura K, Yazaki Y. Perforin-positive leukemic cell infiltration in the aortic tissue of a patient with T-cell prolymphocytic leukemia. Int Angiol. 1996;15(3):245–8. [PubMed] [Google Scholar]
- 10.Meng Q, Lai H, Lima J, Tong W, Qian Y, Lai S. Echocardiographic and pathological characteristics of cardiac metastasis in patients with lymphoma. Oncol Rep. 2002;9(1):85–8. [PubMed] [Google Scholar]
- 11.Agrawal K, Mittal BR, Manohar K, Kashyap R, Bhattacharya A, Varma S. FDG PET/CT in detection of metastatic involvement of heart and treatment monitoring in non-Hodgkin's lymphoma. World J Nucl Med. 2012;11(1):33–4. doi: 10.4103/1450-1147.98746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pawson R, Dyer MJ, Barge R, Matutes E, Thornton PD, Emmett E et al. Treatment of T-cell prolymphocytic leukemia with human CD52 antibody. J Clin Oncol. 1997;15(7):2667–72. doi: 10.1200/JCO.1997.15.7.2667. [DOI] [PubMed] [Google Scholar]
- 13.Pangalis GA, Dimopoulou MN, Angelopoulou MK, Tsekouras C, Vassilakopoulos TP, Vaiopoulos G, Siakantaris MP. Campath-1H (anti-CD52) monoclonal antibody therapy in lymphoproliferative disorders. Med Oncol. 2001;18(2):99–107. doi: 10.1385/mo:18:2:99. [DOI] [PubMed] [Google Scholar]


