Abstract
Pheochromocytoma should be considered in young patients who have acute cardiac decompensation, even if they have no history of hypertension. Atrioventricular node ablation and pacemaker placement should be considered for stabilizing pheochromocytoma patients with cardiogenic shock due to atrial tachyarrhythmias.
A 38-year-old black woman presented with cardiogenic shock (left ventricular ejection fraction, <0.15) that did not respond to the placement of an intra-aortic balloon pump. A TandemHeart® Percutaneous Ventricular Assist Device was inserted emergently. After atrioventricular node ablation and placement of a temporary pacemaker, the TandemHeart was removed. Computed tomography of the abdomen revealed a pheochromocytoma. After placement of a permanent pacemaker, the patient underwent a right adrenalectomy.
This is, to our knowledge, the first reported case of pheochromocytoma-induced atrial tachyarrhythmia that led to cardiogenic shock and cardiac arrest unresolved by the placement of 2 different ventricular assist devices, but that was completely reversed by radiofrequency ablation of the atrioventricular node and the placement of a temporary pacemaker. We present the patient's clinical, laboratory, and imaging findings, and we review the relevant literature.
Keywords: Adrenal gland neoplasms/complications/diagnosis/genetics; catecholamines; pheochromocytoma/diagnosis/secretion; shock, cardiogenic/etiology; tachycardia, atrial/etiology; ventricular dysfunction, left/etiology
Pheochromocytomas can cause numerous cardiovascular abnormalities, including life-threatening ventricular arrhythmias, conduction disturbances, cardiogenic shock, and hypertension emergencies.1 Of pheochromocytoma patients, 9% to 12% present with cardiac complications2,3 that are usually reversible with adrenergic blockade, circulatory mechanical support, or tumor resection.2,4–13
Case Report
In January 2011, a 38-year-old black woman presented at another hospital with cardiogenic shock (left ventricular ejection fraction [LVEF], <0.15) that did not respond to the placement of an intra-aortic balloon pump. She was transferred by helicopter to our cardiac center for further care. The patient then experienced 2 cardiac arrests and needed cardiopulmonary resuscitation for 5 minutes. A TandemHeart® Percutaneous Ventricular Assist Device (CardiacAssist, Inc.; Pittsburgh, Pa) was inserted emergently. A 12-lead electrocardiogram confirmed atrial tachycardia (Fig. 1A). A trial of intravenous metoprolol caused a complete cessation of blood flow from the TandemHeart device. The patient responded to glucagon and intravenous fluids. Comprehensive electrophysiologic studies revealed multifocal atrial tachycardia and junctional ectopic tachycardia, which were treated with radiofrequency ablation of the atrioventricular (AV) node and the placement of a temporary pacemaker. This treatment dramatically stabilized the patient's hemodynamic status and within a few hours improved her LVEF to 0.25–0.30. Consequently, the patient was weaned off vasopressors, and the TandemHeart was removed. The patient subsequently developed a junctional escape rhythm at 78 beats/min and AV dissociation (Fig. 1B). Her blood pressure was maintained in the normal range after tapering of the vasopressors.
Fig. 1.
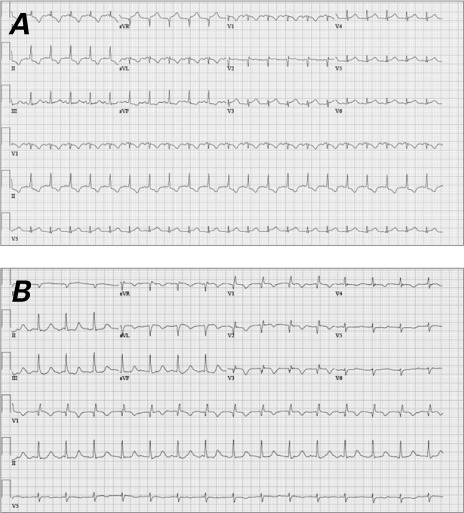
Electrocardiograms A) at the patient's presentation to our institution revealed atrial tachycardia and B) after radiofrequency ablation of the atrioventricular node revealed accelerated junctional tachycardia at a rate of 93 beats/min and atrioventricular dissociation.
At presentation, the patient's plasma brain-type natriuretic peptide level was 881 pg/mL (normal level, <100 pg/mL). Her serum levels were troponin I, 11.6 ng/mL (normal, <0.1 ng/mL); thyroid-stimulating hormone, 0.24 mIU/mL (a 2nd test one week later yielded a level of 1.9 mIU/mL; normal range, 0.35–4.5 mIU/mL); creatinine, 1.63 mg/dL (normal range, 0.5–1.1 mg/dL); aspartate aminotransferase, 240 U/L (normal, <40 U/L); alanine aminotransferase, 275 U/L (normal, <36 U/L); sodium, 151 mEq/L (normal range, 135–145 mEq/L); potassium, 4.6 mEq/L (normal range, 3.5–5 mEq/L); and lactic acid concentration, 53 mmol/L (normal, <2 mmol/L). Serum and urine drug screens were negative. Chest radiographs revealed bilateral interstitial infiltrates. The preliminary diagnosis was acute myocarditis as the cause of arrhythmia and acute heart failure.
One week after the patient's admission, another echocardiographic study showed an LVEF of >0.70. Cardiac pacing was not required, because the junctional rhythm persisted at a rate of 70 to 85 beats/min. In response to bilateral acute deep vein thrombosis in the patient's lower extremities, an inferior vena cava filter was placed. Follow-up chest radiographs revealed substantial pneumoperitoneum. Subsequent computed tomograms of the abdomen and pelvis incidentally revealed a 4.2 × 4.8-cm right adrenal mass with a pre-contrast density of 14.8 Hounsfield units (Fig. 2). The findings of biochemical analysis were consistent with pheochromocytoma (Table I). Metaiodobenzylguanidine scintigraphy revealed increased uptake in the adrenal mass only (Fig. 3).
Fig. 2.
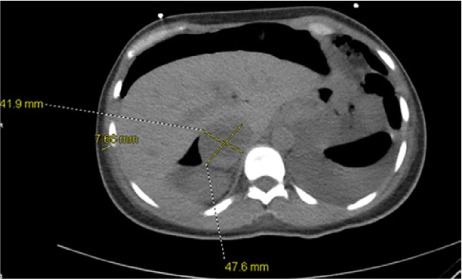
Axial abdominal computed tomography without intravenous contrast shows a 4.2 × 4.8-cm right adrenal mass with a density of 14.8 Hounsfield units.
TABLE I.
Plasma-Free Fractionated Metanephrine Levels and 24-Hour Urine Fractionated Metanephrine and Catecholamine Levels
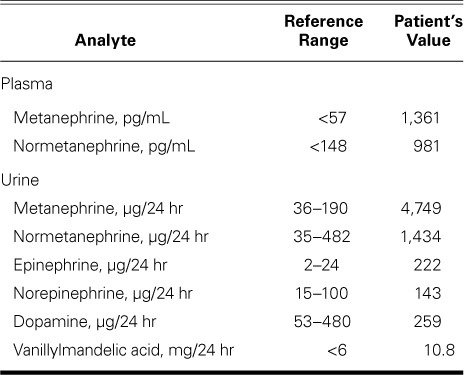
Fig. 3.
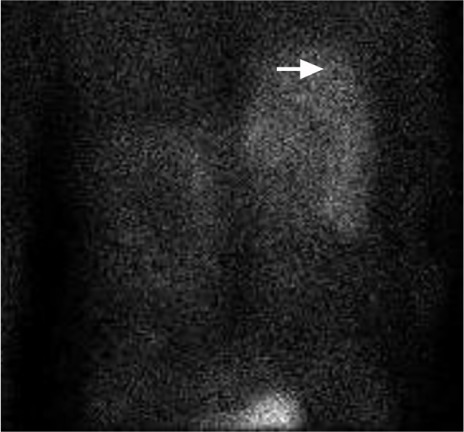
Metaiodobenzylguanidine scintigraphy (posterior view) shows a right complex adrenal mass (arrow) with a photopenic defect, which was consistent with central necrosis.
In retrospect, the patient had reported no history of hypertension. However, she did report episodes—experienced over the preceding few months—of palpitations, diaphoresis, shortness of breath, and headache. The last such episode occurred the day of her presentation at the other hospital. She had sought medical attention from a cardiologist for the above episodes. She was found to have paroxysmal supraventricular tachycardia, which, according to the patient, did not necessitate treatment. She reported no family history of hypertension, sudden death during sedation for surgical procedures, or adrenal tumors. Two weeks after her admission, her supine blood pressure was 120/63 mmHg at a heart rate of 67 beats/min; after she stood for 2 min, it was 110/61 mmHg at a heart rate of 77 beats/min. Physical examination was remarkable only for few crackles in the lung bases, diffuse muscular weakness, and a fine tremor.
Intake of salt and oral fluids was encouraged, and she was given prazosin and subsequently carvedilol over 12 days, in preparation for surgery. Doses were titrated up gradually, without significant side effects. A biventricular pacemaker was implanted, and she underwent an open right adrenalectomy. Intraoperatively, her systolic blood pressure increased to 210 mmHg, requiring the transient administration of nitroprusside and phentolamine. Histologic examination confirmed the diagnosis of pheochromocytoma, which tumor measured 7.5 cm in its greatest dimension and did not exhibit lymphovascular invasion.
Follow-Up. The patient's blood pressure remained normal after the surgery. Four weeks after surgery, her 24-hour urinary fractionated metanephrine levels fell within the normal range. Complete resolution of the atrial and junctional tachycardia was documented, and her heart rhythm became completely amenable to pacing. She had no residual neurologic deficit. Her generalized weakness improved with physical therapy. She was referred to genetic consultants to screen for inherited causes of pheochromocytoma.
Discussion
We report the first case (known to us) of pheochromocytoma-induced atrial tachyarrhythmia that led to cardiogenic shock and cardiac arrest unresolved by the placement of 2 different ventricular assist devices—but that was completely reversed by radiofrequency ablation of the AV node and the placement of a temporary pacemaker.
Pheochromocytoma is not often considered in the differential diagnosis of patients who present with cardiac abnormalities.2 Although 9% to 12% of pheochromocytoma patients initially present with cardiac complications,2,3 cardiogenic shock and cardiac arrest in these patients are rare. For unclear reasons, most pheochromocytoma patients who present with cardiac complications are normotensive and appear to be in excellent physical condition,2 as did our patient. In addition, they usually have larger tumors and much higher catecholamine levels than do other pheochromocytoma patients.2
The cause of cardiomyopathy in pheochromocytoma patients is thought to be chronic tachycardiomyopathy and coronary vasospasm.14 Elevated catecholamine levels intensify peroxidative and lipoperoxidative metabolism in the cell membrane and increase the generation of free radicals, thereby causing potentially irreversible myocardial injury.15 In addition, β-adrenergic receptor genetic polymorphisms have been reported to cause severe left ventricular dysfunction by increasing catecholamine sensitivity or by elevating synaptic norepinephrine levels via loss of negative feedback.16
Cardiomyopathy is usually transient and reversible with treatment17,18; for example, Agarwal and colleagues12 found that both medical therapy with α-blockade and the resection of pheochromocytoma led to a significant improvement in left ventricular function that was sustained for at least 6 months. However, the pathogenesis of cardiac complications and myocardial stunning in pheochromocytoma patients might not be related to elevated catecholamine levels alone. Many authors have reported that these cardiac complications in pheochromocytoma patients were completely reversed by mechanical circulatory support techniques such as ventricular assist devices and extracorporeal life support—without adrenergic blockade or even tumor resection.2,7–11,13 Our experience supports this claim: AV node ablation and pacemaker placement stabilized our patient's hemodynamic status within a few hours and normalized her LVEF within a week—well before we entertained the diagnosis of pheochromocytoma.
The necessity of preparing normotensive pheochromocytoma patients preoperatively with α-blockade is controversial. Most experts recommend that these patients be prepared for surgery with hydration and cautious α-adrenergic blockade.19 However, other authors have suggested that such treatment does not help in maintaining intraoperative hemodynamic stability and increases the need for vasoactive drugs and colloid infusion (this last during surgery).20 In the present case, we elected to prepare our patient medically for surgery because she reported having spells of headache, palpitations, and diaphoresis, and because echocardiography revealed moderate concentric left ventricular hypertrophy probably suggestive of paroxysmal hypertension.
Our experience suggests that AV node ablation and pacemaker placement should be considered for pheochromocytoma patients who present with cardiogenic shock due to atrial tachyarrhythmias. In addition, pheochromocytoma should be included in the differential diagnosis when young patients present with acute cardiac decompensation, even if they are normotensive at baseline.
Footnotes
From: Department of Diabetes, Endocrinology, and Metabolism, Baylor College of Medicine and CHI St. Luke's Health – Baylor St. Luke's Medical Center, Houston, Texas 77030
References
- 1.Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A. Mortality associated with phaeochromocytoma. Horm Metab Res. 2013;45(2):154–8. doi: 10.1055/s-0032-1331217. [DOI] [PubMed] [Google Scholar]
- 2.Yu R, Nissen NN, Bannykh SI. Cardiac complications as initial manifestation of pheochromocytoma: frequency, outcome, and predictors. Endocr Pract. 2012;18(4):483–92. doi: 10.4158/EP11327.OR. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein RE, O'Neill JA, Jr, Holcomb GW, 3rd, Morgan WM, 3rd, Neblett WW, 3rd, Oates JA et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229(6):755–66. doi: 10.1097/00000658-199906000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A. Cardiovascular manifestations of phaeochromocytoma. J Hypertens. 2011;29(11):2049–60. doi: 10.1097/HJH.0b013e32834a4ce9. [DOI] [PubMed] [Google Scholar]
- 5.Yu R, Furmark L, Wong C. Cardiac abnormalities associated with pheochromocytoma and other adrenal tumors. Endocr Pract. 2009;15(1):10–6. doi: 10.4158/EP.15.1.10. [DOI] [PubMed] [Google Scholar]
- 6.Galetta F, Franzoni F, Bernini G, Poupak F, Carpi A, Cini G et al. Cardiovascular complications in patients with pheochromocytoma: a mini-review. Biomed Pharmacother. 2010;64(7):505–9. doi: 10.1016/j.biopha.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Chao A, Yeh YC, Yen TS, Chen YS. Phaeochromocytoma crisis--a rare indication for extracorporeal membrane oxygenation. Anaesthesia. 2008;63(1):86–8. doi: 10.1111/j.1365-2044.2007.05251.x. [DOI] [PubMed] [Google Scholar]
- 8.Grinda JM, Bricourt MO, Salvi S, Carlier M, Grossenbacher F, Brasselet C, Fabiani JN. Unusual cardiogenic shock due to pheochromocytoma: recovery after bridge-to-bridge (extra-corporeal life support and DeBakey ventricular assist device) and right surrenalectomy. J Thorac Cardiovasc Surg. 2006;131(4):913–4. doi: 10.1016/j.jtcvs.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Sojod G, Diana M, Wall J, D'Agostino J, Mutter D, Marescaux J. Successful extracorporeal membrane oxygenation treatment for pheochromocytoma-induced acute cardiac failure. Am J Emerg Med. 2012;30(6):1017.e1–3. doi: 10.1016/j.ajem.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Banfi C, Juthier F, Ennezat PV, de Saint Denis T, Carnaille B, Leteurtre E et al. Central extracorporeal life support in pheochromocytoma crisis. Ann Thorac Surg. 2012;93(4):1303–5. doi: 10.1016/j.athoracsur.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Huang JH, Huang SC, Chou NK, Ko WJ, Chen YS, Wang SS. Extracorporeal membrane oxygenation rescue for cardiopulmonary collapse secondary to pheochromocytoma: report of three cases. Intensive Care Med. 2008;34(8):1551–2. doi: 10.1007/s00134-008-1117-5. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal G, Sadacharan D, Kapoor A, Batra A, Dabadghao P, Chand G et al. Cardiovascular dysfunction and catecholamine cardiomyopathy in pheochromocytoma patients and their reversal following surgical cure: results of a prospective case-control study. Surgery. 2011;150(6):1202–11. doi: 10.1016/j.surg.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Wu GY, Doshi AA, Haas GJ. Pheochromocytoma induced cardiogenic shock with rapid recovery of ventricular function. Eur J Heart Fail. 2007;9(2):212–4. doi: 10.1016/j.ejheart.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Redfield MM, Kay GN, Jenkins LS, Mianulli M, Jensen DN, Ellenbogen KA. Tachycardia-related cardiomyopathy: a common cause of ventricular dysfunction in patients with atrial fibrillation referred for atrioventricular ablation. Mayo Clin Proc. 2000;75(8):790–5. doi: 10.4065/75.8.790. [DOI] [PubMed] [Google Scholar]
- 15.Singal PK, Khaper N, Palace V, Kumar D. The role of oxidative stress in the genesis of heart disease. Cardiovasc Res. 1998;40(3):426–32. doi: 10.1016/s0008-6363(98)00244-2. [DOI] [PubMed] [Google Scholar]
- 16.Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med. 2002;347(15):1135–42. doi: 10.1056/NEJMoa020803. [DOI] [PubMed] [Google Scholar]
- 17.Wood R, Commerford PJ, Rose AG, Tooke A. Reversible catecholamine-induced cardiomyopathy. Am Heart J. 1991;121(2 Pt 1):610–3. doi: 10.1016/0002-8703(91)90740-9. [DOI] [PubMed] [Google Scholar]
- 18.Bybee KA, Prasad A. Stress-related cardiomyopathy syndromes. Circulation. 2008;118(4):397–409. doi: 10.1161/CIRCULATIONAHA.106.677625. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Pantaleon AD, Simon B. Nonclassic presentation of pheochromocytoma: difficulties in diagnosis and management of the normotensive patient. Endocr Pract. 2008;14(4):470–3. doi: 10.4158/EP.14.4.470. [DOI] [PubMed] [Google Scholar]
- 20.Shao Y, Chen R, Shen ZJ, Teng Y, Huang P, Rui WB et al. Preoperative alpha blockade for normotensive pheochromocytoma: is it necessary? J Hypertens. 2011;29(12):2429–32. doi: 10.1097/HJH.0b013e32834d24d9. [DOI] [PubMed] [Google Scholar]


