Abstract
Purpose
Tasquinimod (Active Biotech) is an oral immunomodulatory, anti-angiogenic, and anti-metastatic agent that delayed metastatic disease progression in a randomized placebo-controlled phase II trial in men with metastatic castration-resistant prostate cancer (mCRPC). Here, we report long-term survival with biomarker correlates from this trial.
Experimental Design
Two hundred and one (134 tasquinimod and 67 placebo) men with mCRPC were evaluated. Forty-one men randomized to placebo crossed over to tasquinimod. Survival data were collected with a median follow-up time of 37 months. Exploratory biomarker studies at baseline and over time were collected to evaluate potential mechanism-based correlates with tasquinimod efficacy including progression-free survival (PFS) and overall survival (OS).
Results
With 111 mortality events, median OS was 33.4 months for tasquinimod versus 30.4 months for placebo overall, and 34.2 versus 27.1 months in men with bone metastases (n = 136), respectively. Multivariable analysis demonstrated an adjusted HR of 0.52 [95% confidence interval (CI), 0.35–0.78; P = 0.001] for PFS and 0.64 (95% CI, 0.42–0.97; P = 0.034) for OS, favoring tasquinimod. Time-to-symptomatic progression was improved with tasquinimod (P = 0.039, HR = 0.42). Toxicities tended to be mild in nature and improved over time. Biomarker analyses suggested a favorable impact on bone alkaline phosphatase and lactate dehydrogenase (LDH) over time and a transient induction of inflammatory biomarkers, VEGF-A, and thrombospondin-1 levels with tasquinimod. Baseline levels of thrombos-pondin-1 less than the median were predictive of treatment benefit.
Conclusions
The survival observed in this trial of men with minimally symptomatic mCRPC suggests that the prolongation in PFS with tasquinimod may lead to a survival advantage in this setting, particularly among men with skeletal metastases, and has a favorable risk:benefit ratio.
Introduction
The number of treatment options for metastatic castration-resistant prostate cancer (mCRPC) has recently increased, including immunotherapy (sipuleucel-T), chemotherapeutics (docetaxel and cabazitaxel), hormonal therapies (abiraterone acetate and enzalutamide), and bone-targeted therapies (denosumab and radium-223; refs. 1–6). Despite these advances, median overall survival (OS) improvements remain modest at 3 to 5 months for active agents, and novel approaches that result in further improvements in survival while minimizing toxicity are needed.
Tasquinimod is a quinoline-3-carboxamide derivative with antiangiogenic, immunomodulatory, and antimetastatic properties (7–10). A molecular target for tasquinimod is S100A9 (MRP-14), an immunomodulatory protein expressed on myeloid-derived suppressor cells (MDSC; ref. 11) that is a ligand for RAGE (renaturing agarose gel electrophoresis) and TLR-4 (Toll-like receptor 4). MDSCs are present in the tumor microenvironment and stimulate angiogenesis and immune tolerance (12). Tumor growth is impaired in S100A9 knockout mice, suggesting S100A9 to be a suitable therapeutic target in oncology (11). Preclinical studies have shown an impact on TSP-1 (13) and TGF-β1 levels (10), and based on the potential impact of these molecules on VEGF-C (14, 15), tasquinimod appears to have unique properties when compared with other anti-cancer agents in development. In vitro studies have also described histone deacetylase 4 (HDAC4) to be a potential target, which needs to be further addressed. Tasquinimod binding to HDAC4 may result in reductions in stress-mediated hypoxia signaling and angiogenesis induction in the tumor microenvironment (16). Overall, these data suggest a multifaceted and novel mechanism for tasquinimod efficacy in prostate cancer that is distinct from other agents that have been evaluated to date.
A phase I dose-escalation trial of oral tasquinimod from 0.25 mg/d up to 1.0 mg/d over 4 weeks in men with CRPC has been performed (17, 18). The most common side effects were grade 1 to 2 gastrointestinal events and muscle or joint pains. In a randomized double-blinded international phase II trial (17), in 201 (134 tasquinimod, 67 placebo) men with mCRPC, the primary endpoint of the 6-month progression-free proportion as well as the overall progression-free survival (PFS) was improved in the tasquinimod group (69% vs. 37%, P = 0.0001; 7.6 vs. 3.3 months, P = 0.0042, respectively). In this article, we report long-term follow-up of OS, symptomatic progression, safety, and correlative biomarker studies to determine the overall risk/benefit with this agent in the pre-docetaxel mCRPC state as well as potential predictors of tasquinimod efficacy in the clinic. The exploratory analysis of biomarkers is considered hypothesis generating, and serum biomarkers were selected from those identified in preclinical tasquinimod mechanistic studies or those that have a known prognostic role in the progression of mCRPC.
Materials and Methods
Eligibility criteria and treatment
Eligibility criteria and treatment as well as intervals for laboratory and imaging tests in this study has been published previously (17); men with mCRPC who were asymptomatic to minimally symptomatic were eligible. Based on individual tolerability, both groups received once-daily oral dosing of up to 1.0 mg tasquinimod or placebo after initial titration (0.25 mg/d for 2 weeks, then 0.5 mg/d for 2 weeks) and up to 6 months double-blind treatment at their individually tolerated dose level. Asymptomatic patients in the placebo group with disease-progression during the first 6 months or without progression at 6 months were offered tasquinimod open-label treatment (with titration of dose) until disease progression. Patients on tasquinimod with no disease progression at 6 months were offered open-label treatment until progression.
The primary endpoint was number of patients without disease progression (radiologic or symptomatic) after 6 months, with OS being a secondary endpoint. Survival data were collected once per year starting in 2011 through a separate long-term follow-up protocol at participating sites, with mortality data collected through chart review, patient contact, or review of death indices. For patients with no additional information received through the separate long-term follow up protocol, the OS information obtained in the original protocol was used.
This study (ClinicalTrials.gov identifier NCT00560482) was approved by institutional review boards or independent ethics committees, and all subjects provided written informed consent.
Efficacy outcome measures
Symptomatic disease progression was defined as at least one of the following: (i) pain criteria, including regular consumption of narcotic analgesics (single intravenous narcotic medication administration or >10 out of 14 days of oral narcotic use) or radiotherapy for control of tumor-related pain, a visual analog scale (VAS) pain rating greater than 4 due to cancer pain on two consecutive ratings; (ii) need for radiotherapy or surgery for pathologic fracture or spinal cord compression. PFS was defined as the earliest of symptomatic progression, radiologic progression [using RECIST or Prostate Cancer Working Group 2 (PCWG2) bone scan progression guidelines], or death (17, 19). Increased prostate-specific antigen (PSA) was not a criterion for progression. Toxicity was evaluated using NCI (National Cancer Institute) CTC version 3.0 criteria. Survival data was collected up to database lock May 9, 2012.
Biomarker analysis
Systemic levels of C-reactive protein (CRP) and lactate dehydrogenase (LDH) were carried out at a central laboratory using commercially available standard assays. Blood samples for these analyses were shipped to the central laboratory (ACM Global) directly after collection. For the remaining biomarkers, serum or plasma was prepared at the sites, then directly frozen to −70°C and shipped for retrospective central analysis at Active Biotech. Systemic levels of thrombospondin-1 (TSP-1), TGF-β1, VEGF-A, and VEGF-C were measured in plasma using the respective Human Quantikine ELISA kit (R&D Systems. Serum levels of bone alkaline phosphatase (BAP) were analyzed with the Ostease BAP kit (IDS Ltd) and serum levels of PSA with the CanAg PSA EIA kits (Fujirebio Diagnostics Inc.). All kits were used according to the manufacturer’s instructions, and all samples were analyzed in duplicates. The median value was used if the coefficient of variation ranged from 70% to 130%; otherwise, the samples were reanalyzed in duplicates with the same criteria. The biomarkers were analyzed in samples taken before start of therapy (baseline) and after 4, 8, 12, and 24 weeks in all patients attending these visits.
Statistical considerations
The statistics and assumptions for the trial around the primary endpoint have previously been described (17). For the secondary endpoint of OS, time-to-event variables were analyzed using Kaplan–Meier (KM) methods, and patients without date of death were censored at last date known to be alive. Treatment differences were tested using the log-rank test. HRs and 95% confidence intervals (CI) were estimated with the Cox Proportional Hazard Model using SAS version 9.3. Protocol prespecified OS analyses included the intent-to-treat (ITT) population as well as subgroups based on localization of metastases as defined by PCWG2 (19). Additional exploratory multivariate analyses were performed using previously defined prognostic factors (20, 21), including the Gleason score, Karnofsky score, alkaline phosphatase, hemoglobin, LDH, PSA, PSA-slope, visceral metastases, and pain, with laboratory values that were log-transformed. The multivariate analysis on the prognostic factors used backward selection where factors with P values greater than 5% were removed (PROC PHREG; SAS). Additional exploratory studies of biomarker levels based on the median levels for a given biomarker were performed at baseline and posttreatment time points to describe the association between biomarker levels with OS and PFS, and correlations of biomarker changes over time with these outcomes. These analyses were descriptive in nature and hypothesis generating for future validation studies.
Results
Patient characteristics
Randomization of 201 (134 tasquinimod and 67 placebo) men in the study occurred between December 2007 and June 2009 at 40 centers in the United States, Canada, and Sweden; 41 patients crossed over from placebo to tasquinimod after a mean duration of approximately 5 months whereas 34 patients who received tasquinimod continued into the open-label phase 6 months after start of therapy. Long-term survival data were obtained on 190 patients from 38 centers up to April 2012, with 111 mortality events (55%) recorded during a median follow-up time of 37 months for patients still alive and database lock of May 9, 2012, with only 5% missing data and in which 55% of patients have died (111 events, 71 tasquinimod and 40 placebo). Notably, poor prognosis factors at baseline such as tumor pain (28% vs. 11%) or visceral metastases (24% vs. 15%) were more common in the tasquinimod group, which also had a higher median baseline PSA level (29 vs. 19) and shorter median PSA doubling time (4.2 vs. 5.1 months; Supplementary Table S1).
Efficacy
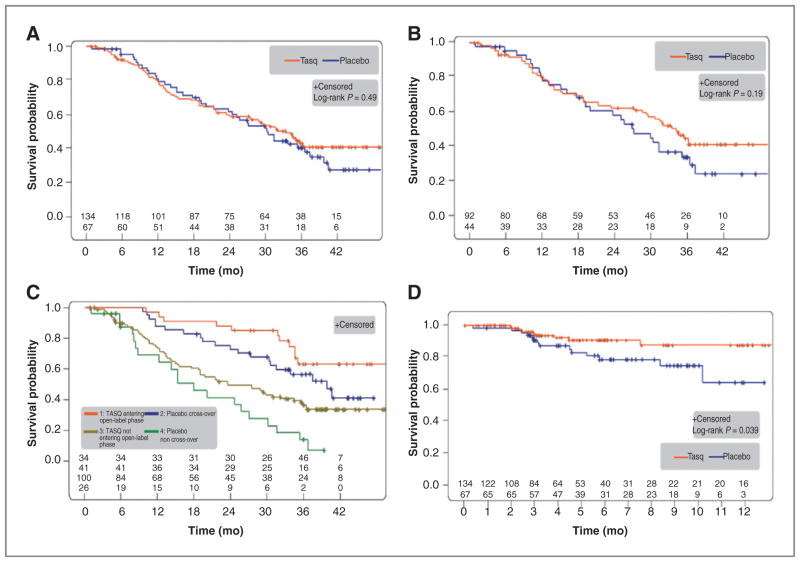
As previously reported (17), the median PFS was 7.6 months versus 3.3 months [tasquinimod/placebo; P = 0.0042; HR, 0.57 (0.39–0.85)] in the ITT population and 8.8 versus 3.4 months [P = 0.019, HR, 0.56 (0.34–0.92)] for men with bone metastases with or without nodal metastases. Median time to death in the ITT population was 33.4 versus 30.4 months [HR, 0.87 (0.59–1.29); Fig. 1A]. Median time to death in the PCWG2 bone-metastatic subgroup (n = 92/44) was 34.2 versus 27.1 months [HR, 0.73 (0.46–1.17); Fig. 1B]. At the end of open-label treatment, men that crossed over to tasquinimod had more favorable prognostic characteristics (Supplementary Table S1) and had approximately 22 months longer median survival than those that did not cross over (Fig. 1C). Men who crossed over from placebo to tasquinimod had more favorable prognostic characteristics (supplementary Table 2), indicating an element of selection bias during cross-over. Men that did not cross over had a more rapid deterioration in pain, prognostic laboratory markers, and performance status during the double-blind phase prior to cross-over. Time to symptomatic progression was longer in tasquinimod-treated patients (P = 0.039, HR, 0.42; Fig. 1D) although the median was not reached. The number of symptomatic events was low and included pain (8 vs. 8 events) or skeletal-related events requiring surgery/radiotherapy (1 vs. 1 events). The tasquinimod-treated men had a delay in the onset of these events.
Figure 1.
OS and time to symptomatic progression. A, overall survival in the ITT population with median OS 33.4 versus 30.4 months (P = 0.49; HR, 0.87; 95% CI, 0.59–1.29; n = 201; tasquinimod/placebo n = 134/67; events = 71/40). B, OS in the bone-metastatic disease subgroup identified by PCWG2 with median OS 34.2 versus 27.1 months (P = 0.19; HR, 0.73; 95% CI, 0.46–1.17; n = 136; tasquinimod/placebo n = 92/44; events = 47/28). C, OS in subgroups based on open-label treatment, that is, tasquinimod entering open-label/placebo cross-over/tasquinimod not entering open-label/placebo non–cross-over n = 34/41/100/26. Events = 11/20/60/20; median OS = not reached/39.6/23.4/17.8 months, n = 201. D, time to symptomatic progression in the ITT population. Number of events tasquinimod/placebo n = 9/14; HR, 0.42; 95% CI, 0.18–0.98; P = 0.039.
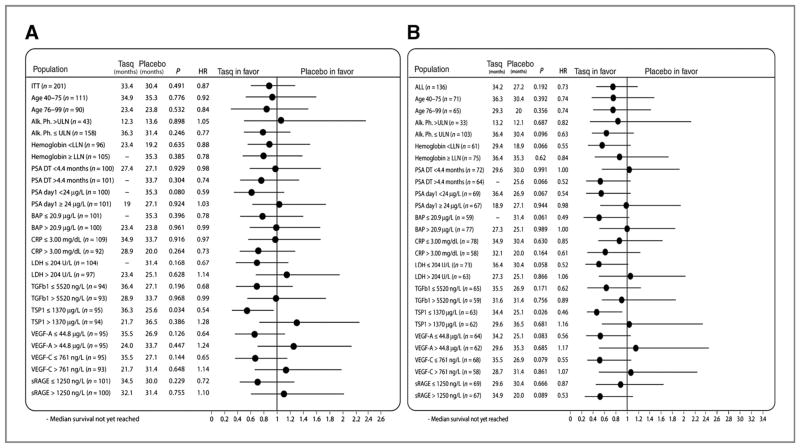
Forest plot analyses for OS were performed both in the ITT population (Fig. 2A) and in the bone-metastatic subgroup (Fig. 2B). In most subgroups, the point estimate for tasquinimod efficacy suggested a treatment benefit for both PFS (17) and OS, with a more pronounced effect in men with baseline bone metastases. These data are consistent with what has been demonstrated previously for PFS (17). However, the 95% CIs for OS estimates were broad, and firm conclusions on survival endpoints cannot be drawn at this time without a larger sample size.
Figure 2.
HRs for the risk of death in subgroups based in the intent-to-treat population (A) and the PCWG2-defined subgroup of men with bone-metastatic disease (B). The dashes indicate that median survival has not been reached in that subgroup. Horizontal bars, 95% CIs.
Multivariate analysis
Given the imbalances in baseline clinical characteristics due to randomization that appeared to favor the placebo arm, we conducted an exploratory multivariable analysis using known CRPC prognostic factors along with the treatment arm to assess their independent role on PFS and OS. Both univariable and multivariable analyses were conducted, and only significant factors were retained in the final multivariable model as per the methods. These factors included PSA, LDH, PSA doubling time, and hemoglobin (20, 21). In this final model, tasquinimod demonstrated an adjusted HR for PFS of 0.52 (95% CI, 0.35–0.78; P = 0.001) and OS of 0.64 (95% CI, 0.42–0.97; P = 0.034) in the total ITT population (Table 1). In the univariable analysis, besides treatment arm in the PFS analysis, baseline PSA was shown to have the strongest prognostic value for both PFS and OS. Using the same parameters in the bone-metastatic subgroup, the adjusted multivariable HR for PFS and OS was 0.51 (95% CI, 0.31–0.85; P = 0.009) and 0.61 (95% CI, 0.38–1.01; P = 0.053; Table 1).
Table 1.
Multivariate analysis of progression-free survival and overall survival in the intent-to-treat population and the PCWG2 bone-metastatic subgroup
| Factorb | Intent-to-treat population (n = 201)
|
Bone-metastatic disease populationa (n = 136)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate
|
Multivariate
|
Univariate
|
Multivariate
|
|||||
| P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | |
| OS | ||||||||
| Tasquinimod arm | 0.491 | 0.87 (0.59–1.29) | 0.034 | 0.64 (0.42–0.97) | 0.194 | 0.73 (0.46–1.17) | 0.053 | 0.61 (0.38–1.01) |
| Hemoglobinc | <0.001 | 0.13 (0.05–0.35) | 0.004 | 0.20 (0.07–0.59) | 0.013 | 0.22 (0.07–0.73) | 0.031 | 0.24 (0.07–0.88) |
| LDHc | <0.001 | 2.68 (1.72–4.18) | 0.023 | 1.75 (1.08–2.82) | 0.006 | 2.06 (1.23–3.44) | 0.065 | 1.65 (0.97–2.81) |
| PSAc | <0.001 | 1.25 (1.15–1.35) | <0.001 | 1.18 (1.08–1.30) | 0.001 | 1.18 (1.07–1.31) | 0.051 | 1.13 (1.00–1.27) |
| Tumor pain (VAS) | 0.013 | 1.27 (1.05–1.53) | 0.026 | 1.24 (1.03–1.50) | 0.010 | 1.32 (1.07–1.64) | 0.010 | 1.33 (1.07–1.64) |
| PFS | ||||||||
| Tasquinimod arm | 0.005 | 0.57 (0.39–0.85) | 0.001 | 0.52 (0.35–0.78) | 0.022 | 0.56 (0.34–0.92) | 0.009 | 0.51 (0.31–0.85) |
| LDHc | 0.288 | 0.75 (0.43–1.28) | 0.011 | 0.48 (0.27–0.85) | 0.275 | 0.70 (0.36–1.33) | 0.018 | 0.44 (0.22–0.87) |
| PSAc | 0.028 | 1.09 (1.01–1.18) | 0.010 | 1.12 (1.03–1.22) | 0.112 | 1.09 (0.98–1.21) | 0.059 | 1.12 (1.00–1.26) |
| Tumor pain (VAS) | 0.039 | 1.25 (1.01–1.56) | 0.003 | 1.39 (1.12–1.73) | 0.003 | 1.44 (1.14–1.83) | <0.001 | 1.56 (1.23–1.99) |
NOTE: For OS and PFS, the following parameters were evaluated: treatment group, PCWG2 visceral metastases, Gleason sum at diagnosis, Karnofsky Performance Status, VAS pain score, PSA slope (velocity), alkaline phosphatase, hemoglobin, lactate dehydrogenase (LDH), and prostate-specific antigen level at baseline (PSA). Only parameters found statistically significant in the multivariate model are shown.
Prostate Cancer Working Group 2 classification.
Remaining variables after backward selection in the intent-to-treat population.
log2 transformed.
Biomarker analysis
During the trial, baseline and on-treatment levels were analyzed for a range of known mCRPC prognostic biomarkers (LDH, BAP, PSA) as well as those relevant for the proposed mechanism of action of tasquinimod on angiogenesis and immune modulation, with a focus on biomarkers identified preclinically or involved in the S100A9 axis, including soluble RAGE (sRAGE; ref. 22), angiogenesis including thrombospondin-1 (13) and VEGF levels (VEGF-A and C; 23), and immune response including TGF-β1 (10). These analyses are considered exploratory and hypothesis generating only. A general biomarker of immune activation, CRP, was also evaluated over time.
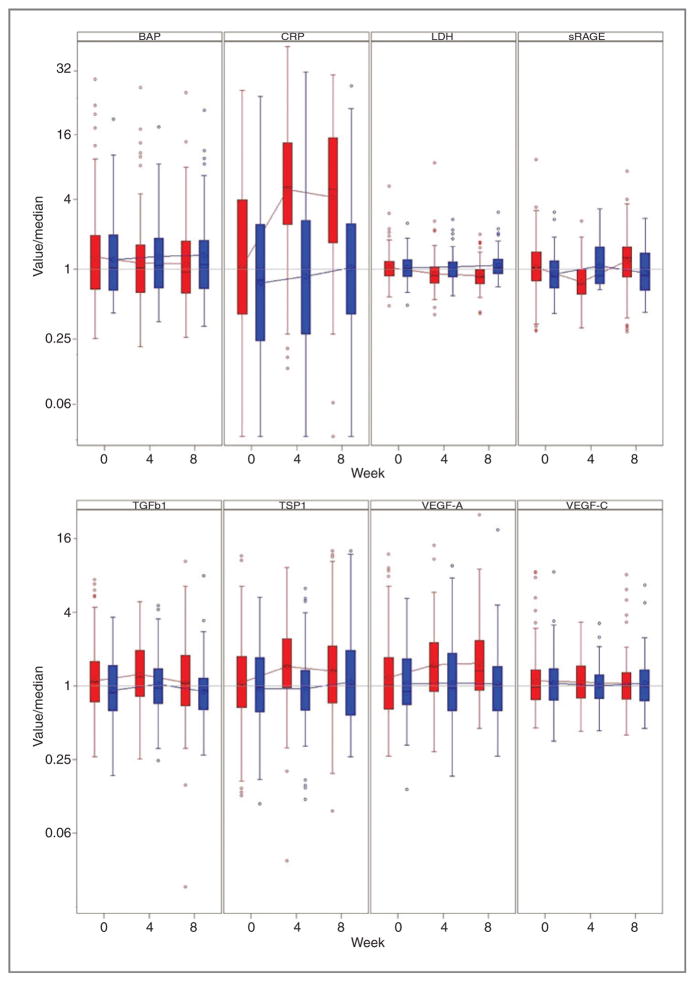
During the treatment with tasquinimod, a transient increase was observed in levels of TSP-1, VEGF-A, and sRAGE, whereas a sustained increase in CRP levels was observed. However, serum levels of TGF-β1, VEGF-C, were not significantly altered (Fig. 3). Baseline imbalances in TGF-β1 levels between the treatment arms as well as crossover effects and dropouts due to progression or toxicities made the assessments of longitudinal changes difficult. Levels of BAP and LDH stabilized in tasquinimod-treated men, but increased in men who received placebo (Fig. 3). Baseline levels of TSP-1 below median were predictive of a survival advantage versus placebo (Fig. 2), whereas no significant predictive survival benefit was observed using the baseline levels of the other biomarkers studied. However, baseline levels below their respective medians for the majority of the biomarkers evaluated including PSA, BAP, LDH, TSP-1, TGF-β1, and VEGF-C levels were associated with improved OS with tasquinimod as compared with placebo (Fig. 2). Similar trends were observed in the PCWG-2 bone-metastatic subpopulation as shown in the forest plots in Fig. 2.
Figure 3.
Normalized box-plot analyses of the biomarkers bone alkaline phosphatase (BAP), C-reactive protein (CRP), LDH, TGFβ-1, TSP-1, VEGF-A, VEGF-C, and sRAGE over time of the double-blind part of the study (baseline, week 4, and week 8). The median baseline values for the biomarkers were (tasquinimod/placebo): BAP (20.9/21.3 μg/L), CRP (3.0/2.4 mg/dL), LDH (202/206 U/L), TGF-β1 (5940/4820 ng/L), TSP-1 (1410/1330 μg/L), VEGF-A (45.3/40.2 μg/L), VEGF-C (740/799 ng/L), and sRAGE (1280/1060 ng/L). Red refers to tasquinimod treatment whereas blue refers to placebo.
Safety
Safety during the double-blind phase, as originally reported (17), demonstrated a mild-to-moderate side-effect profile for tasquinimod with gastrointestinal events, muscle and joint pain, or fatigue (Table 2). The majority of AEs were of grades 1 and 2, but treatment was discontinued in 22% of subjects with 16% stopping therapy already during the first 9 weeks of therapy. In most cases, termination due to an adverse event was caused by grades 1 to 2 toxicity. Among the 34 men who continued tasquinimod therapy in the open-label phase and, thereby, received more than 6 months treatment, three (9%) discontinued during the open-label phase; one due to grade 3 anemia and two for grade 2 adverse events.
Table 2.
Most common adverse events and important grade 3 and 4 toxicities reported during the double-blind phase of the trial as well as during the first 6 months of open-label treatment with tasquinimod
| TASQ double-blind (0–6 months) n = 134
|
TASQ open-label (7–12 months) n = 34
|
Placebo double-blind (0–6 months) n = 67
|
Cross-over patients n = 41
|
|||||
|---|---|---|---|---|---|---|---|---|
| Grades 1–4 | Grades 3 and 4 | Grades 1–4 | Grades 3 and 4 | Grades 1–4 | Grades 3 and 4 | Grades 1–4 | Grades 3 and 4 | |
| Fatigue | 39 (29%) | 1 (1%) | 5 (15%) | 12 (18%) | 5 (12%) | 1(2%) | ||
| Nausea | 36 (27%) | 2 (1%) | 2 (6%) | 11 (16%) | 9 (22%) | 1(2%) | ||
| Constipation | 34 (25%) | 2 (1%) | 3 (9%) | 11 (16%) | 3 (7%) | |||
| Back pain | 32 (24%) | 2 (1%) | 4 (12%) | 1(3%) | 7 (10%) | 2(3%) | 6 (15%) | 2(5%) |
| Decreased appetite | 27 (20%) | 1 (1%) | 1 (3%) | 5 (7%) | 2 (5%) | |||
| Pain in extremity | 25 (19%) | 1 (1%) | 5 (15%) | 4 (6%) | 4 (10%) | 2(5%) | ||
| Flatulence | 22 (16%) | 7 (10%) | 1 (2%) | |||||
| Arthralgia | 21 (16%) | 2 (1%) | 4 (12%) | 5 (7%) | 2 (5%) | |||
| Anemia | 16 (12%) | 4 (3%) | 2 (6%) | 2(6%) | 4 (6%) | 1(1%) | 1 (2%) | 1(2%) |
| Diarrhea | 16 (12%) | 1 (1%) | 2 (6%) | 9 (13%) | 3 (7%) | |||
| Insomnia | 16 (12%) | 4 (6%) | 1 (2%) | |||||
| Weight decreased | 16 (12%) | 1 (1%) | 3 (7%) | 1(2%) | ||||
| Abdominal pain | 14 (10%) | 1 (3%) | 4 (6%) | 1 (2%) | ||||
| Vomiting | 14 (10%) | 5 (7%) | 4 (10%) | 1(2%) | ||||
| Blood amylase increased | 13 (10%) | 1 (1%) | 1 (3%) | 1 (2%) | ||||
| Lipase increased | 13 (10%) | 7 (5%) | 2 (6%) | 1(3%) | ||||
| Myalgia | 13 (10%) | 1 (1%) | 2 (6%) | 3 (4%) | 2 (5%) | |||
| Edema peripheral | 13 (10%) | 2 (6%) | 4 (6%) | 4 (10%) | 1(2%) | |||
| Musculoskeletal pain | 12 (9%) | 2 (6%) | 5 (7%) | 2 (5%) | ||||
| Cough | 11 (8%) | 2 (6%) | 3 (4%) | 1 (2%) | ||||
| Dyspnea | 11 (8%) | 2 (1%) | 2 (3%) | 2 (5%) | ||||
| Headache | 11 (8%) | 3 (9%) | 7 (10%) | 2 (5%) | ||||
| Urinary tract infection | 10 (7%) | 2 (1%) | 1 (3%) | 4 (6%) | ||||
| Deep vein thrombosis | 5 (4%) | 4 (3%) | 1 (2%) | |||||
| Myocardial infarction | 1 (1%) | 1 (1%) | 1 (2%) | 1(2%) | ||||
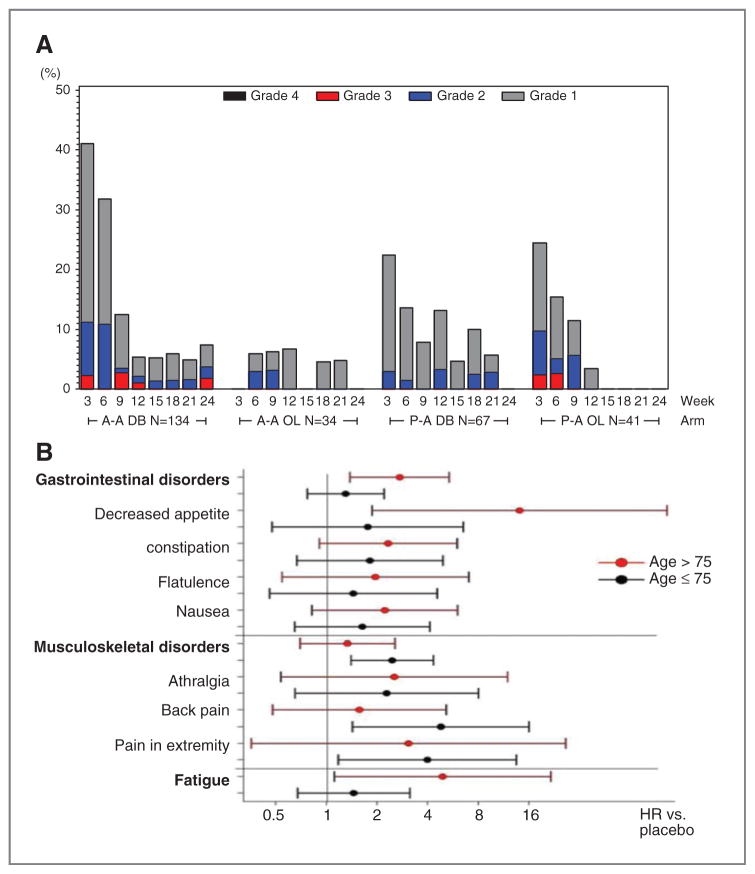
The most common AEs in the tasquinimod arm were fatigue (29%), nausea (27%), and constipation (25%), whereas the most common grades 3 and 4 adverse events were increased lipase (5%) and muscular weakness (4%). Placebo patients crossing over to tasquinimod had a similar side effect profile after starting tasquinimod (Table 2, Fig. 4), although at slightly lower numbers. Nausea (22%) and back pain (15%) were the most common adverse events in this group. Gastrointestinal adverse events were already observed during the dose-escalation phase at both 0.25-and 0.5-mg dose levels. Notably, these events were of transient nature and less commonly observed after 9 weeks of therapy (Fig. 4). Patients continuing on tasquinimod in the open-label part (months 7–12) of the study had a lower number of AEs, and toxicities of grade 3 or higher were infrequent. Here, the most common AEs were pain in extremity (15%), fatigue (15%), arthralgia (12%), and back pain (12%) with lower incidence of gastrointestinal disorders. Only one event, an asymptomatic lipase increase, was grade 4. In general, the incidence of gastrointestinal disorders was more pronounced during the first 9 weeks of treatment in both arms as well as after cross-over (Fig. 4). The mean dose of tasquinimod was higher between months 7 and 12 than during the double-blind phase (0.74 vs. 0.60 mg/d).
Figure 4.
A, number and grade of gastrointestinal adverse events over treatment time. B, forest plot showing HRs for various adverse events in patients younger or older than 75 years. A-A indicates the tasquinimod treatment group whereas P-A indicates the placebo treatment group. A-A OL indicates tasquinimod-treated men who continued on tasquinimod open-label after 6 months, whereas P-A OL indicated the placebo-treated men who crossed over to tasquinimod at 6 months or progression.
At least partly due to a lower drug clearance, the risk for side effects with tasquinimod treatment increase with age, and men older than 75 years were more likely to develop AEs (Fig. 4). As a consequence, dose reductions or terminations during the first 9 weeks were more common among men older than 75 (68%) as compared with men up to 75 years of age (45%). Men older than 75 years received a mean dose of 0.52 mg/d over 106 days, whereas men who were 75 years and younger received a mean dose of 0.66 mg/d over 120 days. Notably, a more detailed analysis indicated that the older group of men was at higher risk only for some classes of AEs (e.g., gastrointestinal disorders such as decreased appetite; Fig. 4) and fatigue, whereas patients younger than 75 years were at higher risk for musculoskeletal disorders.
Discussion
We previously reported that in this multicenter, double-blinded, randomized phase II trial, tasquinimod therapy resulted in a prolonged composite PFS (radiographic and symptomatic criteria) as compared with placebo in men with asymptomatic to mildly symptomatic mCRPC (17). Based on these positive results, tasquinimod is now being evaluated in a phase III international placebo-controlled trial (clinicaltrials.gov number NCT01234311) in men with bone mCRPC. The current survival and long-term safety analysis extends these previous findings, suggesting a survival benefit in a multivariable analysis with a favorable risk/benefit ratio in this disease state.
Delaying radiographic and symptomatic progression, and thereby the need for chemotherapy is of high clinical importance for this group of men. However, it is currently unclear if a delay in PFS leads to improvement in OS. For example, although immune therapies have reported improvements in OS without a noticeable impact on PFS (2, 24), antiangiogenic therapies have reported significantly improved PFS without an increase in OS (25). In addition to its antimetastatic properties (17, 26), tasquinimod has both immunomodulatory (10) and VEGF-independent antiangiogenic properties (7); therefore, the relationship of PFS improvement with OS for tasquinimod at present is not clear and requires adequately powered studies to address.
The median survival of 33.4 months in this study is 8 to 12 months longer than previously published in this patient population (2, 24, 27) and similar to that recently reported for abiraterone acetate. For example, the randomized phase II and III trials of sipuleucel-T, prostvac, and zibotentan showed a survival of 24.5 to 25.8 months in the treatment arms (2, 24, 28), and abiraterone acetate on top of corticosteroids showed 30.1 months in the control arm versus 35.3 months in the abiraterone arm (5, 29). Similar to abiraterone, the tasquinimod phase II trial enrolled a more favorable chemonaive group of men with mCRPC, suggesting that the OS in these men is likely to be nearly 3 years in the current era. This improved natural history in these selected men with minimal symptoms must be considered in planning for larger definitive trials designed to detect a survival advantage. No patients in the tasquinimod trial had received sipuleucel-T, abiraterone, or enzalutamide prior to entry; however, it is likely that some patients received recently approved second-line therapies or investigational drugs that could have contributed to the prolonged survival observed. Thus, over time, the natural history and OS of these men may continue to improve. The prolonged OS seen in each treatment arm in this trial could not only reflect this enrollment of a favorable prognostic group as well as improved treatment options over the lifetime of these men, but also suggests a potential differential and positive direct effect of tasquinimod on OS.
The improvement in PFS and suggestion of improved OS in the bone-metastatic mCRPC subgroup supports the focus on bone-metastatic patients in the phase III trial. There are several potential reasons for this observation, which was not expected a priori. One possibility is that all subgroups benefited, as was reported previously (17), but the bone-metastatic subgroup had the greatest power/sample size to more conclusively demonstrate this benefit. Another possibility is that bone-metastatic disease may have a different biology that leads to a greater benefit with tasquinimod. Preclinical models of PC treated with tasquinimod have demonstrated favorable effects in reducing or delaying both bone and visceral metastatic disease (26). As S100A9 is expressed in bone, and deficient mice have defective osteoclast activity, one hypothesis is that modulating the myeloid components surrounding a bone metastasis with tasquinimod may reduce pathologic bone resorption and skeletal events over time (30, 31). Given that metastases in men with CRPC commonly spreads to bone and results in a high rate of bone pain and fractures (32) over time, tasquinimod may result in an overall favorable outcome in this group of men. Biomarker studies in this trial suggested favorable impacts on bone biology and tumor biology through measurements of BAP and the metabolic enzyme LDH, suggesting a delay in the progression of tumor in bone with tasquinimod. These changes were not accompanied by major effects on PSA (33), suggesting that tasquinimod’s effect may be directed at the tumor microenvironment or non–PSA-producing prostate cancer stem cells in the bone (34).
Exploratory biomarker analyses performed in this study indicate that tasquinimod treatment had a more pronounced effect on OS in men with low levels of biomarkers including LDH, BAP, TSP-1, PSA, and VEGF levels. This may reflect chance but may also indicate a greater impact in men with a lower disease burden, similar to the observations for other immunotherapies such as sipuleucel-T (35). We observed a survival benefit with tasquinimod versus placebo in men with systemic TSP-1 levels below the median at baseline. Notably, the systemic levels of TSP-1 increased transiently with tasquinimod therapy. The role of TSP-1 in prostate cancer progression is controversial but recent data suggests that upregulation of TSP-1 in bone marrow-derived myeloid cells can create a metastasis-resistant environment in prostate cancer (13, 36, 37). However, more clarity on systemic versus tumor levels needs to be obtained before the role of TSP-1 during tasquinimod therapy can be fully understood. These biomarker studies serve as hypothesis generating exercises for validation in the phase III trial.
The current analysis did not show a clear statistically significant improvement in survival in the unadjusted ITT analysis. Several limitations make it difficult to fully estimate the impact of tasquinimod on the OS results observed. First, 41 patients (61%) crossed over from placebo to tasquinimod after 3 to 6 months of treatment at a mean time of approximately 5 months. Men with a more favorable prognostic profile (lack of strong pain, long PSA doubling time) crossed-over, reducing the probability of observing an OS benefit if truly present. A second limitation is that due to chance imbalances at randomization, patients with visceral disease were overrepresented on tasquinimod. This may have resulted in differences in PFS, OS, and biomarker results as a result of chance due to the relatively small sample size. In addition, patients in the placebo arm had a favorable prognosis with lower baseline PSA, less pain, and longer PSA doubling time (Supplementary Table S1). Thus, multivariable analysis can be helpful in correcting for these imbalances. In addition, in an exploratory analysis, we evaluated patients in the more balanced bone-metastatic subgroup. Here, a clear trend for a survival benefit was observed with a 7.1-month difference, corresponding to an approximately 30% lower risk of death in the tasquinimod arm. Notably, patients with bone metastases also had the greatest PFS benefit with an HR of 0.57 (17).
In addition to the treatment arm, baseline PSA and presence of pain were found to be the strongest prognostic factors for OS and PFS in multivariable analysis, as were hemoglobin and LDH to a lower extent. Each of these factors has been previously validated (20, 21), but, importantly, the tasquinimod treatment group was significantly associated with both improved PFS and OS when these factors were considered and adjusted for (Table 1). In the double-blind phase of this trial, gastrointestinal side effects, fatigue, and muscle and joint pain were the most frequently reported side effects in both arms and more frequent in the tasquinimod arm. Notably, these side effects were transient in nature occurring more often during the first 2 months of treatment; thereafter, gastrointestinal side effects were at a level similar to what was observed using placebo. Notably, the number of grade 3 and 4 side effects was low, and decreased further after 6 months of treatment. This improvement in perceived tolerability could be due to the dropout of men with more severe side effects or due to improved tolerability due to supportive measures, dose reductions, or adaptations to toxicity over time. Overall, the safety profile is highly acceptable for use as monotherapy and minimizes the risk to patients in receiving future systemic therapies. In addition, the safety data in older men suggests that dose reductions may permit the safe continuation of therapy at lower doses that achieve similar drug levels as in younger men, due to reduced clearance.
In conclusion, the current data suggest an overall favorable efficacy and safety profile for tasquinimod and not only justify its evaluation as a single agent in the pre-docetaxel phase III trial, powered to show a clinically meaningful OS benefit, but also justify further combination studies with other active systemic therapies in men with CRPC. Tasquinimod’s mechanism of action is not necessarily prostate cancer specific and further evaluation in other tumor types is also warranted.
Supplementary Material
Translational Relevance.
In this study, we examined overall survival (OS) outcomes and the associations of survival outcomes with a range of standard prognostic and exploratory biomarkers of tasquinimod efficacy in men with metastatic castration-resistant prostate cancer (mCRPC). Tasquinimod is an oral immunomodulatory and antiangiogenic agent currently in phase III randomized controlled testing in the pre-docetaxel mCRPC treatment space; therefore, identifying potential predictive biomarkers of efficacy is clinically relevant. We report that in a multivariate analysis controlling for known prognostic factors, tasquinimod was associated with improvements in both progression-free survival (PFS) and OS, accompanied by favorable effects on known prognostic biomarkers, such as alkaline phosphatase and LDH, and had a favorable long-term safety profile. In addition, we found that low pretreatment levels of thrombospondin-1 and measures of lower disease burden such as prostate-specific antigen (PSA) and alkaline phosphatase were potential predictive biomarkers associated with improvements in survival with tasquinimod over placebo.
Acknowledgments
This study was conducted within the Department of Defense Prostate Cancer Clinical Trials Consortium (DOD PCCTC) and the authors are grateful to the additional investigators who participated in this study, including R. Agajanian, American Institute Of Research, Whittier; G. Ahlgren, Universitetssjukhuset MAS, Malmö; C. Andreou, Andreou Research, Surrey; J. Araujo, MD Anderson Cancer Center, Houston; G. Bernstein, Center for Urological Care, Bryn Mawr; S. Brosman Pacific Institute of Urology, Santa Monica; S. Chang, Vanderbilt University Medical Center, Nashville; F. Chu, San Bernardino Urological Associates, San Bernardino; W. Clark, Alaska Clinical Research Center, Anchorage; R. Clark, North Idaho Urology; C. D’Alene, Giovanni Colombo, Midwest Urology, Peoria; B. Cowan, Urology Associates, Englewood; S. Denmeade, Johns Hopkins Hospital, Baltimore; J. Elist, Pacific Clinical Center, Beverly Hills; H. Fisher, Community Care Physicians, Albany; M. Fleming, Virginia Oncology Associates, Norfolk; J. Frankel, Seattle Urology Research Center, Burien; R. Freid, Lawrenceville Urology, Lawrenceville; R. Harris, Midwest Urology/RMD Research, Melrose Park; E. Hirchberg, Guelph Urology Associates, Guelph; R. Israeli, Staten Island Urological Research P.C., Staten Island; R. Kane, Wake Urological Associates, Raleigh; D. Keiller, Urology Physicians of San Diego, San Diego; J. Marks, Plantation; C. Redfern, Medical Oncology Associates of San Diego; I. Shapira, Beth Isreal Medical Center, New York; D. Shevrin, North Shore University Health System, Evanston; P. Sieber, Urological Associates of Lancaster, Lancaster; F. Snoy, Urology Group of New Mexico, Albuquerque; T. Webster, Office of Dr. Todd Webster, Ontario; J. Williams, Idaho Urologic Institute, Meridian; and E. Woods, Edward Woods, Scarborough. The authors thank the many patients and their families for their participation in this trial.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
Ö. Nordle and G. Forsberg are employees and shareholders of Active Biotech. A.J. Armstrong has received commercial research funding from Active Biotech and is consultant/advisory board member for Ipsen. A.J. Armstrong has received commercial research funding from Active Biotech, Dendreon, Medivation, Janssen, and Sanofi Aventis; A.J. Armstrong holds a consultant/advisory board position with Active Biotech, Dendreon, Medivation, Janssen, and Bayer. M. Häggman is a consultant for Active Biotech. W.M. Stadler has received commercial research funding from Active Biotech. V. Assikis has received honoraria from the Speakers Bureau of Astellas, Janssen, and Dendreon and is a consultant/advisory board member for Astellas, Dendreon, and Janssen. JE Damber receives research funding from Active Biotech and is a consultant to Ipsen. M.A. Carducci is a consultant/advisory board member for Active Biotech. R. Pili receives research funding from Active Biotech. No potential conflicts of interest were disclosed by the other authors.
Authors’ Contributions
Conception and design: A.J. Armstrong, G. Forsberg, M.A. Carducci, R. Pili
Development of methodology: A.J. Armstrong, G. Forsberg, R. Pili
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): A.J. Armstrong, M. Häggman, W.M. Stadler, J.R. Gingrich, V. Assikis, J. Polikoff, J-E. Damber, M.A. Carducci, R. Pili
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): A.J. Armstrong, J-E. Damber, Ö. Nordle, G. Forsberg, M.A. Carducci, R. Pili
Writing, review, and/or revision of the manuscript: A.J. Armstrong, M. Häggman, W.M. Stadler, J.R. Gingrich, J. Polikoff, J-E. Damber, L. Belkoff, Ö. Nordle, G. Forsberg, M.A. Carducci, R. Pili
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): G. Forsberg, Ö. Nordle
Study supervision: A.J. Armstrong, G. Forsberg, M.A. Carducci, R. Pili
References
- 1.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for meta-static castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 2.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de WR, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 5.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de SP, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 7.Dalrymple SL, Becker RE, Isaacs JT. The quinoline-3-carboxamide anti-angiogenic agent, tasquinimod, enhances the anti-prostate cancer efficacy of androgen ablation and taxotere without effecting serum PSA directly in human xenografts. Prostate. 2007;67:790–7. doi: 10.1002/pros.20573. [DOI] [PubMed] [Google Scholar]
- 8.Isaacs JT, Pili R, Qian DZ, Dalrymple SL, Garrison JB, Kyprianou N, et al. Identification of ABR-215050 as lead second generation quinoline-3-carboxamide anti-angiogenic agent for the treatment of prostate cancer. Prostate. 2006;66:1768–78. doi: 10.1002/pros.20509. [DOI] [PubMed] [Google Scholar]
- 9.Isaacs JT. The long and winding road for the development of tasquinimod as an oral second-generation quinoline-3-carboxamide anti-angiogenic drug for the treatment of prostate cancer. Expert Opin Investig Drugs. 2010;19:1235–43. doi: 10.1517/13543784.2010.514262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallberg E, Vogl T, Liberg D, Olsson A, Bjork P, Wikstrom P, et al. S100A9 interaction with TLR4 promotes tumor growth. PLoS ONE. 2012;7:e34207. doi: 10.1371/journal.pone.0034207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjork P, Bjork A, Vogl T, Stenstrom M, Liberg D, Olsson A, et al. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 2009;7:e97. doi: 10.1371/journal.pbio.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 13.Olsson A, Bjork A, Vallon-Christersson J, Isaacs JT, Leanderson T. Tasquinimod (ABR-215050), a quinoline-3-carboxamide anti-angiogenic agent, modulates the expression of thrombospondin-1 in human prostate tumors. Mol Cancer. 2010;9:107. doi: 10.1186/1476-4598-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cursiefen C, Maruyama K, Bock F, Saban D, Sadrai Z, Lawler J, et al. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J Exp Med. 2011;208:1083–92. doi: 10.1084/jem.20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oka M, Iwata C, Suzuki HI, Kiyono K, Morishita Y, Watabe T, et al. Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood. 2008;111:4571–9. doi: 10.1182/blood-2007-10-120337. [DOI] [PubMed] [Google Scholar]
- 16.Isaacs JT, Antony L, Dalrymple SL, Brennen WN, Gerber S, Hammers H, et al. Tasquinimod Is an Allosteric Modulator of HDAC4 survival signaling within the compromised cancer microenvironment. Cancer Res. 2013;73:1386–99. doi: 10.1158/0008-5472.CAN-12-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pili R, Häggman M, Stadler WM, Gingrich JR, Assikis VJ, Bjork A, et al. Phase II randomized, double-blind, placebo-controlled study of tasquinimod in men with minimally symptomatic metastatic castrate-resistant prostate cancer. J Clin Oncol. 2011;29:4022–8. doi: 10.1200/JCO.2011.35.6295. [DOI] [PubMed] [Google Scholar]
- 18.Bratt O, Häggman M, Ahlgren G, Nordle Ö, Bjork A, Damber JE. Open-label, clinical phase I studies of tasquinimod in patients with castration-resistant prostate cancer. Br J Cancer. 2009;101:1233–40. doi: 10.1038/sj.bjc.6605322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong AJ, Garrett-Mayer ES, Yang YC, de WR, Tannock IF, Eisenberger M. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: A TAX327 study analysis. Clin Cancer Res. 2007;13:6396–403. doi: 10.1158/1078-0432.CCR-07-1036. [DOI] [PubMed] [Google Scholar]
- 21.Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 22.Santilli F, Vazzana N, Bucciarelli LG, Davi G. Soluble forms of RAGE in human diseases: clinical and therapeutical implications. Curr Med Chem. 2009;16:940–52. doi: 10.2174/092986709787581888. [DOI] [PubMed] [Google Scholar]
- 23.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–91. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 24.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly WK, Halabi S, Carducci M, George D, Mahoney JF, Stadler WM, et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol. 2012;30:1534–40. doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jennbacken K, Welen K, Olsson A, Axelsson B, Torngren M, Damber JE, et al. Inhibition of metastasis in a castration resistant prostate cancer model by the quinoline-3-carboxamide tasquinimod (ABR-215050) Prostate. 2012;72:913–24. doi: 10.1002/pros.21495. [DOI] [PubMed] [Google Scholar]
- 27.Carducci MA, Saad F, Abrahamsson PA, Dearnaley DP, Schulman CC, North SA, et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110:1959–66. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 28.Nelson JB, Fizazi K, Miller K, Higano C, Moul JW, Akaza H, et al. Phase 3, randomized, placebo-controlled study of zibotentan (ZD4054) in patients with castration-resistant prostate cancer metastatic to bone. Cancer. 2012;118:5709–18. doi: 10.1002/cncr.27674. [DOI] [PubMed] [Google Scholar]
- 29.Rathkopf DE, Smith MR, De Bono JS, Logothetis C, Shore ND, De Souza PL, et al. Updated interim analysis (IA) of COU-AA-302, a randomized phase III study of abiraterone acetate (AA) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) without prior chemotherapy. J Clin Oncol (Meeting Abstracts) 2013;31:5. [Google Scholar]
- 30.Grevers LC, de Vries TJ, Vogl T, Abdollahi-Roodsaz S, Sloetjes AW, Leenen PJ, et al. S100A8 enhances osteoclastic bone resorption in vitro through activation of Toll-like receptor 4: implications for bone destruction in murine antigen-induced arthritis. Arthritis Rheum. 2011;63:1365–75. doi: 10.1002/art.30290. [DOI] [PubMed] [Google Scholar]
- 31.Zreiqat H, Howlett CR, Gronthos S, Hume D, Geczy CL. S100A8/S100A9 and their association with cartilage and bone. J Mol Histol. 2007;38:381–91. doi: 10.1007/s10735-007-9117-2. [DOI] [PubMed] [Google Scholar]
- 32.Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–22. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pili R, Häggman M, Stadler WM, Gingrich JR, Assikis VJ, Bjork A, et al. A randomized, multicenter, international phase II study of tasquinimod in chemotherapy naive patients with metastatic castrate-resistant prostate cancer (CRPC) J Clin Oncol (Meeting Abstracts) 2010;28:4510. [Google Scholar]
- 34.Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch-and hedgehog-dependent tumor-initiating cells. Cancer Cell. 2012;22:373–88. doi: 10.1016/j.ccr.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, Kantoff PW. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the immunotherapy for prostate adenocarcinoma treatment (IMPACT) trial. Urology. 2013;81:1297–302. doi: 10.1016/j.urology.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 36.Firlej V, Mathieu JR, Gilbert C, Lemonnier L, Nakhle J, Gallou-Kabani C, et al. Thrombospondin-1 triggers cell migration and development of advanced prostate tumors. Cancer Res. 2011;71:7649–58. doi: 10.1158/0008-5472.CAN-11-0833. [DOI] [PubMed] [Google Scholar]
- 37.Catena R, Bhattacharya N, El RT, Wang S, Choi H, Gao D, et al. Bone marrow-derived gr1+ cells can generate a metastasis-resistant microenvironment via induced secretion of thrombospondin-1. Cancer Discov. 2013;3:578–89. doi: 10.1158/2159-8290.CD-12-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.