Abstract
Background
Time spent in sedentary activities (such as watching television) has previously been associated with several risk factors for cardiovascular disease (CVD) such as increased low-density lipoprotein cholesterol (LDL-C). Little is known about associations with lipoprotein subfractions. Using television and computer screen time in hours per day as a measure of sedentary time, we examined the association of screen time with lipoprotein subfractions.
Methods
Data were used from men and women forming the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) study population. Mixed linear models specified lipoprotein measures as the outcome, and screen time as the predictor for fourteen lipoprotein subfraction measures, and included age, smoking status, pedigree, and fat, carbohydrate daily alcohol and energy intake as covariates. Analyses were run separately for men (n = 623) and women (n = 671). A step-down Bonferroni correction was applied to results. The analysis was repeated for significant results (p < .05), additionally controlling for body mass index (BMI) and moderate and vigorous physical activity.
Results
Linear models indicated that screen time was associated with five lipoprotein parameters in women: the concentration of large VLDL particles (p = .01), LDL particle number (p = .01), concentration of small LDL particles (p = .04), the concentration of large HDL particles (p = .04), and HDL diameter (p = .02). All associations remained after controlling for moderate or vigorous physical activity and BMI.
Conclusions
We show that sedentary time is associated with lipoprotein measures, markers of cardiometabolic disease, independently of physical activity and BMI, in women but not men.
Keywords: sedentary time, diet, fat intake, lipoprotein size, BMI, exercise, television
The relative distribution of the small, medium and large lipoprotein particles within the fractions of very low-, low-, and high-density lipoproteins (VLDL, LDL, and HDL respectively) can indicate metabolic dysfunction. In particular, increases in the concentrations of small LDL and HDL particles, and increases in the concentrations of large VLDL particles occur in in the earliest stages of an insulin resistant (IR) state (Festa et al., 2005; Frazier-Wood et al., 2011; Garvey et al., 2003; Hulthe et al., 2000; Mykkanen et al., 1997). IR is the hallmark of cardiometabolic disease, a combination of metabolic factors including high blood pressure, elevated insulin levels, excess body fat around the waist and abnormal cholesterol levels. The number and size distribution of lipoprotein particles are modifiable through pharmacological intervention, diet and exercise (Beard et al., 1996; Lemieux et al., 2002; Melenovsky et al., 2002; Wood et al., 2006), suggesting they may be targets for interventions aimed at reducing cardiometabolic disease risk.
Parameters pertaining to the size and distribution of lipoprotein subfractions can be accurately quantified using nuclear magnetic resonance (NMR) spectroscopy. Unlike older methods for determining lipoprotein parameters such as ultra-centrifugation, NMR does not require the physical separation of the lipoproteins. Instead, NMR uses subclass distinction in NMR spectral properties. This allows a more accurate quantification of VLDL, LDL, and HDL than spectrum representation through shape-fitting algorithms and gives information about subfraction distributions that closely agrees with that given by gradient-gel electrophoresis (Otvos et al., 1992). Although the expense of gaining lipoprotein data via NMR currently hinders its use in a clinical setting, lipoprotein phenotypes are powerful for examining the correlates of IR in their earliest stages, when interventions may be most effective.
Sedentary time may be operationally defined as time spent with a low metabolic output of 1.0–1.5 metabolic equivalents (METS; Pate et al., 2008). Sedentary time is on the increase in the developed world and has been consistently associated with increased CVD risk in longitudinal studies (reviewed in Thorp et al., 2011). These associations can be seen even when accounting for a reciprocal decrease in time spent in moderate-to-vigorous physical activity (4.5–7.5 METS; Helmerhorst et al., 2009). Thus, sedentary time may be a modifiable risk behavior useful for decreasing CVD prevalence.
The mechanisms by which sedentary time conveys independent disease risk are not understood, despite evidence from a number of animal models that it has a unique physiology (termed “inactivity physiology”) compared with activity time (Bey & Hamilton, 2003; Bey et al., 2003; Hamilton et al., 1998, 2001; Zderic & Hamilton, 2006). Examining biomarkers of cardiometabolic disease, which may have a more homogenous etiology than CVD itself, may shed light on the mechanisms by which sedentary behavior conveys risk for CVD. Increased television viewing time has been associated a cluster of cardiometabolic disease risk factors, including waist circumference, triglycerides, HDL-cholesterol, systolic blood pressure, diastolic blood pressure, and fasting plasma glucose in men but not women (Wijndaele et al., 2010). The effect of sedentary time on lipoprotein subfractions, another cardiometabolic disease biomarker, is not studied. We aimed to examine the associations between screen time, a measure of overall sedentary time, and fourteen lipoprotein measures, separately in the men and women of the GOLDN study. We hypothesized that any associations between screen time and lipoprotein measures would remain when controlling for time spent in moderate and heavy physical activity.
Methods
Participants
The study population is drawn from the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) study. GOLDN consisted of 1,328 men and women from 148 families consisting of a mix of familial relationships including parent-offspring (N = 614), siblings (N = 667), grandparent-grandchild (N = 89), avuncular (N = 617), half-sibling (N = 22), grand avuncular (N = 69), half avuncular (N = 23), first cousins (N = 268), half grand avuncular (N = 12), 1st cousin once removed (N = 81), half 1st cousin (N = 11), half 1st cousin once removed (N = 4) and 2nd cousin (N = 1) relationships. All participants were of European descent and recruited in Minneapolis, Minnesota or Salt Lake City, Utah. The primary aim of the GOLDN study was to characterize the role of genetic and dietary factors on an individual’s response to both a high-fat meal challenge and fenofibrate intervention.
GOLDN consisted of an initial screening visit (visit 0) during which participants were asked to discontinue the use of lipid lowering drugs. Approximately 4–8 weeks later, baseline blood chemistries were measured (visit 1). A day later (visit 2) participants’ fasting blood samples were collected before participating in a high fat meal challenge, from which lipoprotein data were extracted. Thus lipoprotein data are only available from subjects who were willing to participate in the high fat meal intervention. The final sample consisted of 1036 individuals across 187 families; 497 men and 539 women (mean ± SD: 48.8 ± 16.2 y of age). The protocol was approved by the Institutional Review Boards at the University of Minnesota, University of Utah, Tufts University/New England Medical Center and the University of Alabama at Birmingham. Written informed consent was obtained from all participants.
Data Collection
Clinical characteristics including anthropometric measurements were taken at the study clinics where a fasting blood sample was also drawn, as described previously (Kabagambe et al., 2009). Questionnaires were administered to collect demographic data and information on lifestyle attributes and medical history.
Screen Time
Participants were asked, by questionnaire, how much time they spent per day in front of a computer or television screen. The use of screen-time as a proxy for sedentary behavior is employed to distinguish time spent with a low metabolic output of 1.0–1.5 METS, from time spent in light activity at 1.6–2.89 METS. Light activity can involve activities such as cooking or sitting and writing. Sedentary time and light activity are often grouped together, but they are distinct constructs, and their differentiation is important from both research and clinical perspectives (Pate et al., 2008). This combined definition of screen time (computer and televisions time) provided the highest reliability in a meta-analysis of possible screen-time measures (Clark et al., 2009).
Physical Activity
As part of the same questionnaire, participants were asked how much time, per day, they spent in moderate physical activity and in heavy physical activity.
Smoking Status and Dietary Intake
Participants were asked to complete the National Cancer Institute’s (NCI) Food Frequency Questionnaire (FFQ; Millen et al., 2006). Smoking status was measured as never/past/current, and alcohol intake (g/day). Intake of saturated fat, polyunsaturated fat (PUFA), monounsaturated fat (MUFA), and carbohydrate (g/day) were assessed. Energy intake (kcalories/day) were calculated from the responses, and used to calculate the percentage of calories as saturated fat, MUFA, PUFA and carbohydrate respectively.
Biochemical Measurements
All plasma samples used for this analysis were collected after an 8-hr fast and analyzed together at the end of the study.
Lipoprotein Measures
Measurements of VLDL, LDL and HDL diameter and concentrations of each subfraction (small, medium and large) were determined by NMR spectroscopy (Tsai et al., 2004). NMR detects the signal emitted by lipoprotein methyl-group protons when in the field of a magnet charged at 400 MHz. The NMR signal is deconvoluted to obtain estimates of particle numbers for each of several lipoprotein fractions. The weighted average particle diameter for each lipoprotein fraction (VLDL, LDL and HDL) is calculated as the sum of the average lipoprotein particle diameters multiplied by the relative mass percentage, based on the amplitude of the methyl NMR signal and given in nm. The ranges of diameters for small, medium and large particle classification within each fraction of VLDL, LDL and HDL are given in Table 1.
Table 1. Diameter Ranges of Lipoprotein Subclasses When Measured by NMR.
| NMR Lipoprotein Parameter | Diameter Range (nm) |
|---|---|
| VLDL | |
| Large VLDL/chylomicrons | >60 |
| Medium VLDL | 35–60 |
| Small VLDL | 27–35 |
| LDL | |
| Large LDL | 21.2–23 |
| Small LDL | 18–21.2 |
| Medium small LDL | 19.8–21.2 |
| Very small LDL | 18–19.8 |
| HDL | |
| Large HDL | 8.8–13 |
| Medium HDL | 8.2–8.8 |
| Small HDL | 7.3–8.2 |
Note. Adapted from (Jeyarajah, Cromwell, & Otvos, 2006)
Analysis
All analyses were conducted in SAS version 9.3. The distributions of lipoprotein measures were assessed using measures of central tendency (mean and standard deviation) and a qualitative assessment of histograms. Where measures deviated from normality, logarithmic (concentration of large and medium VLDL particles, and medium HDL particles) or square root (overall number of VLDL particles and concentrations of small VLDL and large LDL particles), transformations were applied. All analyses were conducted separately for males and females.
Initial exploratory analyses on the association between screen time and lipoprotein measures were conducted using Pearson correlations. These baseline measures of association between lipoprotein measures and screen time are presented in Table 2. Subsequently we ran mixed linear models to analyze the association between lipoprotein measures as screen time when additionally controlling for key covariates. Lipoprotein measures provided the outcome variables in separate models, and screen time hours per day was the continuous predictor. Covariates included smoking status, alcohol and energy intake, gender and data collection center. Pedigree was included as a fixed effect. Given the independence of some phenotypes, and the relatively large sample size, a step-down Bonferroni correction was applied to all results (Holm, 1979). Only results surviving the step-down Bonferroni correction at p < .05 were considered significant.
Table 2. Initial Correlations (r) Between Lipoprotein Measures and Screen Time in the GOLDN Study Population.
| Correlation Coefficient with Screen Time (r) |
||
|---|---|---|
| Men | Women | |
| VLDL parameters | ||
| particle number, nmol/L | .06 | .15* |
| concentration of large particles, nmol/L | .08 | .19** |
| concentration of medium particles, nmol/L | .07 | .13* |
| concentration of small particles, nmol/L | 0.01 | .13** |
| average diameter, nm | .07 | .08 |
| LDL parameters | ||
| particle number, nmol/L | .12* | .20** |
| concentration of large particles, nmol/L | .01 | −.02 |
| concentration of small particles, nmol/L | .10* | .16** |
| average diameter, nm | −.05 | −.12* |
| HDL parameters | ||
| particle number, nmol/L | −.07 | .02 |
| concentration of large particles, nmol/L | −.06 | −.10 |
| concentration of medium particles, nmol/L | −.04 | .02 |
| concentration of small particles, nmol/L | −.02 | .08 |
| average diameter, nm | −.06 | −.13* |
p < .05;
p < .001
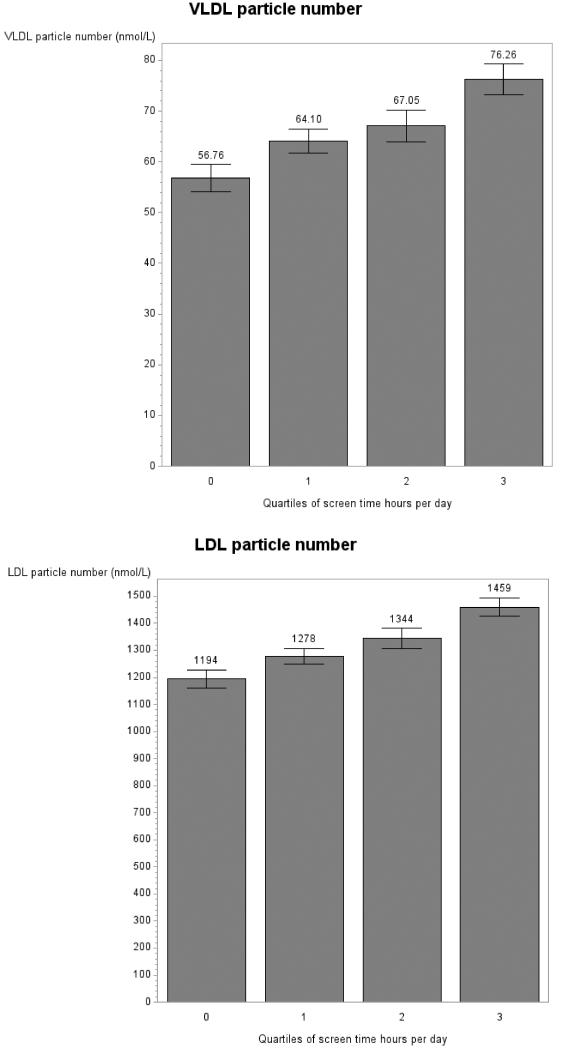
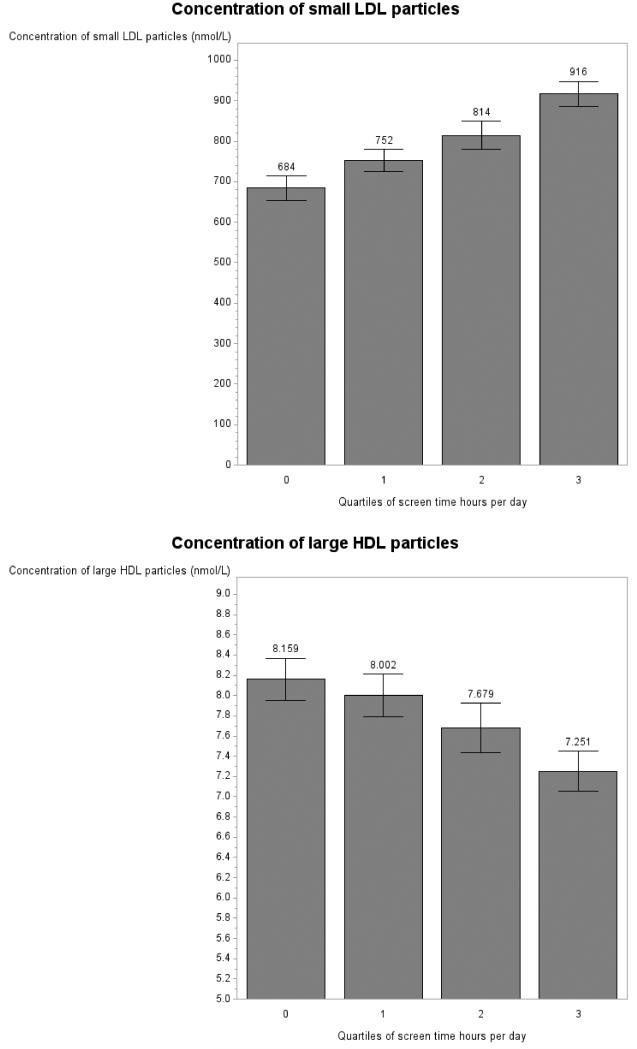
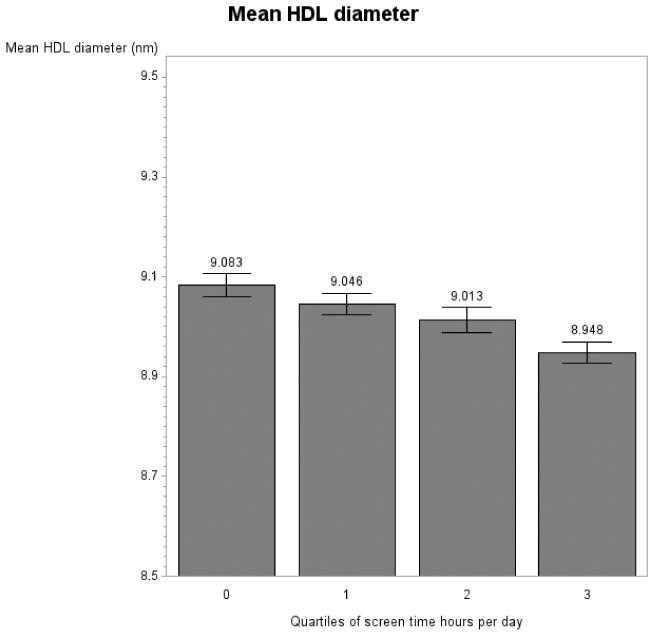
Significant associations between screen time and lipoprotein parameters were visualized using predicted lipoprotein values from the fully adjusted mixed models (Figure 1). Due to the fact that some categories of screen time were reported with low frequency (e.g., 2 women watched 11 hr per day), for the visualization only, data were collapsed into quartiles of screen time. Note that additionally, because of the different scales of the lipoprotein measures, the y axis scales differ accordingly and do not always start at 0.
Figure 1.
Lipoprotein measures by quartiles of screen time per day in women in the GOLDN study for concentration of large VLDL particles (panel A), LDL particle number (panel B), concentration of small LDL particles (panel C), concentration of large HDL particles (panel D) and mean HDL diameter (panel E). Note: Graphs show predicted values after adjustment for age, data collection center and pedigree.
For those lipoprotein measures showing significant associations with screen time, the above models were repeated including hours spent per day in moderate and heavy physical activity as a predictor, and subsequently with hours spent per day in moderate and heavy physical activity and BMI as predictors.
Results
Descriptive Statistics
Men and women were not significantly different in age, alcohol intake or percentage of current smoker (all p > .05; Table 3), however they did report significantly different daily caloric intakes (p < .001; Table 3). Men and women reported different percentages of their diet as saturated fat, MUFA, PUFA and carbohydrate (P<=.001; Table 3). Men and women showed significantly different particle numbers and subfraction concentrations for all lipoprotein measures (all p £ .0001; Table 3). Men and women displayed different mean fraction diameters, which reflect different subfraction distributions for the LDL and HDL fractions (p < .0001; Table 3). Men and women reported significantly differing amounts of heavy physical activity (p < .0001; Table 3), but not amounts of time spend in moderate activity (p = .57) or in front of a computer or television screen (p = .98).
Table 3. Mean (+ SD) or Percentages for Age, Smoking, Dietary Intake and Lipoprotein Measures, and Physical Activity Time for the GOLDN Study Population.
| Men | Women | P-value | |
|---|---|---|---|
| age, y | 48.70 (16.45) | 48.18 (16.26) | .57 |
| smokers, current, % | 3.72 | 3.92 | .90 |
| alcohol (g/day) | .49 (.50) | .51 (.50) | .57 |
| Dietary Intake | |||
| Saturated fat intake, % | 12.15 (2.78) | 11.550 (2.72) | .0003 |
| MUFA intake, % | 13.67 (2.82) | 12.93 (2.82) | <.0001 |
| PUFA intake, % | 7.24 (2.02) | 7.66 (2.26) | .001 |
| carbohydrate intake, % | 47.38 (8.79) | 50.27 (8.38) | <.0001 |
| energy intake (kcal/day) | 2489.9 (1401.5) | 1767.3 (784.4) | <.0001 |
| VLDL Parameters | |||
| particle number, nmol/L | 83.80 (52.41) | 65.69 (47.83) | <.0001 |
| concentration of large particles, nmol/L | 4.88 (9.98) | 3.08 (4.49) | <.0001 |
| concentration of medium particles, nmol/L | 43.65 (40.62) | 31.72 (31.69) | <.0001 |
| concentration of small particles, nmol/L | 35.22 (21.74) | 30.86 (21.92) | .0001 |
| average diameter, nm | 51.38 (7.44) | 51.40 (8.19) | 0.87 |
| LDL Parameters | |||
| particle number, nmol/L | 1445.3 (462.0) | 1313.6 (476.4) | <.0001 |
| concentration of large particles, nmol/L | 319.2 (228.8) | 488.7 (285.2) | <.0001 |
| concentration of small particles, nmol/L | 1077.8 (521.4) | 787.2 (553.3) | <.0001 |
| average diameter, nm | 20.49 (0.79) | 21.1 (0.86) | <.0001 |
| HDL Parameters | |||
| particle number, nmol/L | 29.13 (4.98) | 32.68 (5.68) | <.0001 |
| concentration of large particles, nmol/L | 4.71 (2.71) | 7.8 (3.58) | <.0001 |
| concentration of medium particles, nmol/L | 2 (2.68) | 3.93 (4.10) | <.0001 |
| concentration of small particles, nmol/L | 22.42 (5.02) | 20.95 (5.92) | <.0001 |
| Average diameter, nm | 8.66 (0.39) | 9.03 (0.44) | <.0001 |
| Fasting Lipid Metabolism Parameters | |||
| insulin (mU/L) | 14.19 (8.43) | 13.32 (7.89) | .07 |
| glucose (mg/dL) | 105.5 (20.81) | 97.80 (15.71) | <.0001 |
| triglycerides (mg/dL) | 153.3 (142.0) | 125.2 (82.17) | <.0001 |
| Physical Activity | |||
| moderate physical activity, hr/day | 3.04 (2.68) | 2.95 (2.30) | 0.57 |
| vigorous physical activity, hr/day | 1.08 (1.54) | 0.53 (0.96) | <.0001 |
| Sedentary Behavior | |||
| screen time, hr/day | 2.63 (1.88) | 2.63 (1.81) | 0.98 |
Note. P-value derived from t tests means comparison tests between men and women using transformed data, or C2 tests with 1 degree of freedom.
Association of Screen Time with Lipoprotein Measures
When corrected for the twenty-eight initial tests (fourteen lipoprotein parameters, each examined in each gender) screen time did not associate with lipoprotein measures for men (all p > .05; Table 4). In women, screen time was associated with concentration of large VLDL particles (p = .01; Table 4, Figure 1 A), LDL particle number (p = .01; Table 4, Figure 1 B), concentration of the small LDL subfraction (p = .048; Table 4, Figure 1 C), concentration of large HDL particles (p = .048; Table 4, Figure 1 E) and mean of HDL diameter (p = .02; Table 4, Figure 1 F), after a step-down Bonferroni correction. Dietary intake of saturated fat, MUFA, PUFA and carbohydrates was not associated with lipoprotein measures in our models (p > .05) with the exception of number of VLDL particles in men (p = .04).
Table 4. Results From Mixed Linear Models Examining the Association of Screen Time on Lipoprotein Measures, Controlling for Age, Pedigree, Data Collection Center, Smoking Status and Fat (Saturated, MUFA and PUFA), Carbohydrate, and Alcohol Intake.
| Association with Screen time |
||||||
|---|---|---|---|---|---|---|
| Men |
Women |
|||||
| Lipoprotein Measure | F | P | Step-Down Bonferroni Correction |
F | P | Step-down Bonferroni Correction |
| VLDL parameters | ||||||
| Particle number, nmol/L | .01 | .94 | >.99 | 6.04 | .01 | .30 |
| Concentration of large particles, nmol/L | .52 | .47 | .13 | 12.97 | .0004 | .01* |
| Concentration of medium particles, nmol/L | .30 | .58 | >.99 | .37 | .06 | >.99 |
| Concentration of small particles, nmol/L | 1.49 | .22 | >.99 | 5.59 | .02 | .32 |
| Average diameter, nm | 5.06 | .02 | .49 | 2.71 | .10 | >.99 |
| LDL parameters | ||||||
| Particle number, nmol/L | 1.76 | .19 | >.99 | 13.04 | .0003 | 0.01* |
| Concentration of large particles, nmol/L | 0.04 | .84 | >.99 | 1.64 | .20 | >.99 |
| Concentration of small particles, nmol/L | 1.49 | .22 | >.99 | 10.06 | .002 | 0.04* |
| Average diameter, nm | 0.07 | .79 | >.99 | 2.88 | .09 | >.99 |
| HDL parameters | ||||||
| Particle number, nmol/L | 5.40 | .02 | .32 | 0.72 | .39 | >.99 |
| Concentration of large particles, nmol/L | 1.15 | .28 | >.99 | 9.43 | .002 | 0.04* |
| Concentration of medium particles, nmol/L | 0.35 | .56 | >.99 | 0.43 | .51 | >.99 |
| Concentration of small particles, nmol/L | 2.32 | .13 | >.99 | 0.35 | .56 | >.99 |
| Average diameter, nm | 1.19 | .28 | >.99 | 11.12 | .001 | 0.02* |
Significant associations.
Within the female GOLDN sample, when adjusting for age, pedigree and data collection center, an increase in 1 hr of screen time per day was associated with a an increase of .34 nmol/L (± .10) in large VLDL particles, of 34.97 nmol/L (± 10.37) in LDL particle number, and of 37.23 nmol/L (± 12.65) in the concentration of small LDL particles. An increase in 1 hr of screen time per day was associated with a decrease in the concentration of large HDL particles of .23 nmol/L (± .08) and in HDL diameter of .03 nm (+/.01).
Association of Screen Time with Lipoprotein Measures When Controlling for Physical Activity Time, and BMI in Women
Physical activity was moderately correlated with screen time (r=−.10; p < .001). All significant associations remained when additionally controlling for time spent in moderate and vigorous physical activity (all p < .001; shown). BMI was strongly associated with screen time (p > .0001). However, significant associations between lipoprotein measures and screen time remained significant, although were less strong, when BMI was additionally controlled for (all p > .05; Table 5).
Table 5.
Results From Mixed Linear Models Examining the Association of Screen Time on Lipoprotein Measures, Controlling for Age, Pedigree, Data Collection Center, Smoking Status and Fat (Saturated, MUFA and PUFA), Carbohydrate and Alcohol Intake As Well As Time Spent in Heavy and Moderate Physical Activity (PA) and BMI, in Women
| Screen Time, hr/day | PA, hr/day | BMI (kg/m2) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| F | P | F | P | F | P | |
| Concentration of large VLDL particles, nmol/L | 7.06 | .01 | .22 | .64 | 109.37 | <.0001 |
| LDL particle number, nmol/L | 8.08 | .001 | .11 | .74 | 56.95 | <.0001 |
| Concentration of small LDL particles, nmol/L | 5.52 | .02 | <.01 | .96 | 72.41 | <.0001 |
| Concentration of large HDL particles, nmol/L | 5.09 | .02 | .27 | .60 | 80.93 | <.0001 |
| HDL diameter, nm | 6.61 | .01 | 2.62 | .11 | 116.01 | <.0001 |
Discussion
We examined the association of screen time, a measure of sedentary behavior, with lipoprotein subfraction measures in men and women. No associations were seen in men, but in women higher screen time was associated with five lipoprotein measures: increased large VLDL particles, increased overall and small LDL particles, and a decrease in the concentration of large HDL particles and HDL diameter—the aggregate of which may be the most atherogenic pattern of lipoprotein particles (Frazier-Wood et al., 2012). These associations held when controlling for time spent in heavy and moderate physical activity and BMI.
The association of screen time with this atherogenic pattern of lipoprotein measures confirms previous research that sedentary behavior is a risk factor for CVD (Biddle et al., 2012; Healy et al., 2011; Helmerhorst et al., 2009). The parameters most associated with IR are debated; a recent review suggested that an increase in VLDL particle number and concentration of small LDL particles (reflected additionally as a decrease in LDL diameter) and a decrease in the concentration of large HDL particles (reflected as an decrease in HDL diameter) showed the most consistent associations with IR (Frazier-Wood et al., 2012). However, longitudinal studies indicate that baseline parameters including large VLDL (reflected in an increase in VLDL diameter) may be an additional predictor of incident Type 2 diabetes (Festa et al., 2005). Overall, of the fourteen lipoprotein parameters, screen time was associated with those considered most indicative of IR in our analysis, with the exception of VLDL particle number.
When adjusted for age, gender and ethnicity, a prior study reported that conversion to type 2 diabetes within five years has been associated with a significant increase of 6 nmol/L in VLDL particles, 160 nmol/L in LDL particles, 190 nmol/L in small LDL particles, and a decrease of 1 nmol/L in large HDL particles and of .23 nm in HDL diameter from baseline (Festa et al., 2005). In our study, such differences were accounted for by between 3 and 6 hr of screen time, with the exception of HDL diameter where .23 nm was accounted for by 1 hr of screen time. Thus, if intervention strategies were to be implemented to reduce screen time, realistic daily screen time goals could be set which may reduce the risk of type 2 diabetes.
The associations held when controlling for time spent in moderate and physical activity. How sedentary time coveys risk for CVD over and above activity time is unknown, and it is hoped that studies using more refined phenotypes like lipoprotein subfractions might give such mechanistic clues. Screen time was associated with five out of fourteen lipoprotein parameters in women, suggesting heterogeneity in the etiology of the different lipoprotein measures and some specificity to physiological effects of such sedentary time on lipoproteins. Work by Hamilton and colleagues suggests that the loss of muscle activity experienced when sedentary leads to the suppression of lipoprotein lipase (LPL) activity (Bey & Hamilton, 2003; Hamilton et al., 2004). LPL is involved in triglyceride uptake from VLDL particles and HDL formation; thus with reduced LPL activity one would expect sedentary time to be associated with increased VLDL size, and reduced numbers of HDL particles. Our results partially support this with increased numbers of large VLDL particles (but not overall particle number), and decreased HDL in the large subfraction in women. That the associations remained, although lessened, when BMI was controlled for further suggests a specific deleterious physiology to sedentary behavior. In addition, all models controlled for the percentage of energy intake as carbohydrate, saturated fat, PUFA and MUFA indicating that sedentary behavior is associated with lipoprotein subclasses independently of dietary intake. Although dietary intake of saturated fat is a risk factor for CVD, our finding may not be seen as surprising given results indicating that physical activity level (including inactivity) and caloric intake are associated with lipoproteins in a manner which negates the association of individual macronutrient percentages (Hartung et al., 1980; Williams et al., 1992). The implication of a dyslipidemic profile specific to sedentary time which may not be mediated through BMI or diet, is that an increase in activity level, a reduction in caloric intake, or a change in the macronutrient profile of the diet is not enough to minimize the physiological effects; a decrease in sedentary time must also be effected.
The associations between screen time and lipoprotein parameters were not present in male members of GOLDN. This is in line with previous results using other cardiometabolic disease risk markers, but the reasons for the gender difference are unclear (Wijndaele et al., 2010). Biological markers of CVD risk are more prevalent in men than women, which may arise from a combination of age and estrogen deficiency as women age (Jousilahti et al., 1999; Mercuro et al., 2003). However, the association of biological markers of CVD risk with lifestyle behaviors also associated with CVD risk is not yet considered to differ by gender. If our results are replicated, this could have important public health implications for devising strategies to prevent cardiovascular risk that are sensitive to gender, but more work needs to confirm and explain the physiology underlying our findings. It has been suggested that other correlates of gender, such as poorer diet quality and higher smoking rates might account for the difference, suggesting a ceiling effect to CVD risk. Although, we report no difference in smoking and alcohol intake, we do report a difference in macronutrient intake, indicating that more work needs to be done in this area and better matching between men and women on background characteristics may be needed.
Our analyses were limited by their cross-sectional nature, and longitudinal associations would shed further light on any causal direction between screen time and cardiometabolic disease risk. Our definition of sedentary behavior was ‘screen time’ which distinguishes between sedentary time spent sitting and sedentary time spent with some muscle contraction (e.g., cooking) and the effect of alternative definitions should be investigated. The use of food frequency questionnaire data precluded a sensitive analysis of the effects of diet on lipoprotein subclasses, but the consistent lack of an association between diet and lipoprotein parameters suggests that more objectively measured dietary intake may not change this finding. Finally, although we did not expect physical activity to modify the association between screen time and lipoproteins, that physical activity was not associated with lipoprotein measures at all was surprising; future research should consider including a more objective measure of physical activity.
Screen time, a measure of sedentary behavior, was associated with increased large VLDL and small LDL particles, increased overall LDL particles, and a decreased concentration of large HDL particles and HDL diameter in women in the GOLDN study. Our analyses further implicate the role of sedentary behavior in the development of cardiometabolic risk. Longitudinal associations, the effect of a reduction in screen time, and the reasons underlying the gender differences are important avenues for future research.
Acknowledgments
We are grateful to the staff of the GOLDN study for the assistance in data collection and management.
Sources of funding: This study was funded by NHLBI grant number U01HL072524.
Footnotes
Financial disclosures: None to declare.
Conflict of interest: All authors declare that they have no conflicts of interests.
Contributor Information
Alexis C. Frazier-Wood, Division of Epidemiology, Genetics and Environmental Sciences, University of Texas School of Public Health, Houston, TX
Ingrid B. Borecki, Dept. of Genetics, Washington University School of Medicine, St. Louis, MO
Mary F. Feitosa, Dept. of Genetics, Washington University School of Medicine, St. Louis, MO
Paul N. Hopkins, Dept. of Internal Medicine, University of Utah, Salt Lake City, UT
Caren E. Smith, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA
Donna K. Arnett, Dept. of Epidemiology, University of Alabama at Birmingham, Birmingham, AL
References
- Beard CM, Barnard RJ, Robbins DC, Ordovas JM, Schaefer EJ. Effects of Diet and Exercise on Qualitative and Quantitative Measures of LDL and Its Susceptibility to Oxidation. Arteriosclerosis, Thrombosis, and Vascular Biology. 1996;16(2):201–207. doi: 10.1161/01.atv.16.2.201. PubMed doi:10.1161/01.ATV.16.2.201. [DOI] [PubMed] [Google Scholar]
- Bey L, Akunuri N, Zhao P, Hoffman EP, Hamilton DG, Hamilton MT. Patterns of global gene expression in rat skeletal muscle during unloading and low-intensity ambulatory activity. Physiological Genomics. 2003;13(2):157–167. doi: 10.1152/physiolgenomics.00001.2002. 10.1152/physiolgenomics.00001.2002. PubMed. [DOI] [PubMed] [Google Scholar]
- Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. The Journal of Physiology. 2003;551(Pt 2):673–682. doi: 10.1113/jphysiol.2003.045591. 10.1113/jphysiol.2003.045591. PubMed doi:10.1113/jphysiol.2003.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddle SJH, King J, Yates T. TV viewing, but not total sedentary behaviour, is associated with adverse cardiometabolic biomarkers in adolescents. Evidence-Based Nursing. 2012 doi: 10.1136/ebnurs-2012-100613. 10.1136/ebnurs-2012-100613. PubMed. [DOI] [PubMed] [Google Scholar]
- Clark BK, Sugiyama T, Healy GN, Salmon J, Dunstan DW, Owen N. Validity and reliability of measures of television viewing time and other non-occupational sedentary behaviour of adults: a review. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2009;10(1):7–16. doi: 10.1111/j.1467-789X.2008.00508.x. doi:10.1111/j.1467-789X.2008.00508.x. [DOI] [PubMed] [Google Scholar]
- Festa A, Williams K, Hanley AJ, Otvos JD, Goff DC, Wagenknecht LE, Haffner SM. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation. 2005;111:3465–3472. doi: 10.1161/CIRCULATIONAHA.104.512079. PubMed doi:10.1161/CIRCULATIONAHA.104.512079. [DOI] [PubMed] [Google Scholar]
- Frazier-Wood AC, Garvey WT, Dall T, Honigberg R, Pourfarzib R. Opportunities for using lipoprotein subclass profile by nuclear magnetic resonance spectroscopy in assessing insulin resistance and diabetes prediction. Metabolic Syndrome and Related Disorders. 2012;10(4):244–251. doi: 10.1089/met.2011.0148. PubMed doi:10.1089/met.2011.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier-Wood AC, Glasser S, Garvey WT, Kabagambe EK, Borecki IB, Tiwari HK, Arnett DK. A clustering analysis of lipoprotein diameters in the metabolic syndrome. Lipids in Health and Disease. 2011;10:237. doi: 10.1186/1476-511X-10-237. 10.1186/1476-511X-10-237. PubMed doi:10.1186/1476-511X-10-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Liao Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–462. doi: 10.2337/diabetes.52.2.453. PubMed doi:10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- Hamilton MT, Areiqat E, Hamilton DG, Bey L. Plasma triglyceride metabolism in humans and rats during aging and physical inactivity. International Journal of Sport Nutrition and Exercise Metabolism. 2001;11(Suppl):S97–S104. doi: 10.1123/ijsnem.11.s1.s97. PubMed. [DOI] [PubMed] [Google Scholar]
- Hamilton MT, Etienne J, McClure WC, Pavey BS, Holloway AK. Role of local contractile activity and muscle fiber type on LPL regulation during exercise. The American Journal of Physiology. 1998;275(6 Pt 1):E1016–E1022. doi: 10.1152/ajpendo.1998.275.6.E1016. PubMed. [DOI] [PubMed] [Google Scholar]
- Hamilton MT, Hamilton DG, Zderic TW. Exercise physiology versus inactivity physiology: An essential concept for understanding lipoprotein lipase regulation. Exercise and Sport Sciences Reviews. 2004;32(4):161–166. doi: 10.1097/00003677-200410000-00007. PubMed doi:10.1097/00003677-200410000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung GH, Foreyt JP, Mitchell RE, Vlasek I, Gotto AM. Relation of diet to high-density-lipoprotein cholesterol in middle-aged marathon runners, joggers, and inactive men. The New England Journal of Medicine. 1980;302(7):357–361. doi: 10.1056/NEJM198002143020701. 10.1056/NEJM198002143020701. PubMed doi:10.1056/NEJM198002143020701. [DOI] [PubMed] [Google Scholar]
- Healy GN, Matthews CE, Dunstan DW, Winkler EAH, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. European Heart Journal. 2011;32(5):590–597. doi: 10.1093/eurheartj/ehq451. 10.1093/eurheartj/ehq451. PubMed doi:10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst HJF, Wijndaele K, Brage S, Wareham NJ, Ekelund U. Objectively measured sedentary time may predict insulin resistance independent of moderate- and vigorous-intensity physical activity. Diabetes. 2009;58(8):1776–1779. doi: 10.2337/db08-1773. 10.2337/db08-1773. PubMed doi:10.2337/db08-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6(2):65–70. [Google Scholar]
- Hulthe J, Bokemark L, Wikstrand J, Fagerberg B. The metabolic syndrome, LDL particle size, and atherosclerosis: the Atherosclerosis and Insulin Resistance (AIR) study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:2140–2147. doi: 10.1161/01.atv.20.9.2140. 10.1161/01.ATV.20.9.2140. PubMed doi:10.1161/01.ATV.20.9.2140. [DOI] [PubMed] [Google Scholar]
- Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clinics in Laboratory Medicine. 2006;26(4):847–870. doi: 10.1016/j.cll.2006.07.006. 10.1016/j.cll.2006.07.006. PubMed doi:10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, Age, Cardiovascular Risk Factors, and Coronary Heart Disease: A Prospective Follow-Up Study of 14 786 Middle-Aged Men and Women in Finland. Circulation. 1999;99(9):1165–1172. doi: 10.1161/01.cir.99.9.1165. 10.1161/01.CIR.99.9.1165. PubMeddoi:10.1161/01.CIR.99.9.1165. [DOI] [PubMed] [Google Scholar]
- Kabagambe EK, Ordovas JM, Tsai MY, Borecki IB, Hopkins PN, Glasser SP, Arnett DK. Smoking, inflammatory patterns and postprandial hypertriglyceridemia. Atherosclerosis. 2009;203:633–639. doi: 10.1016/j.atherosclerosis.2008.08.005. PubMeddoi:10.1016/j.atherosclerosis.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux I, Laperrière L, Dzavik V, Tremblay G, Bourgeois J, Després J-P. A 16-week fenofibrate treatment increases LDL particle size in type IIA dyslipidemic patients. Atherosclerosis. 2002;162(2):363–371. doi: 10.1016/s0021-9150(01)00711-0. PubMeddoi:10.1016/S0021-9150(01)00711-0. [DOI] [PubMed] [Google Scholar]
- Melenovsky V, Malik J, Wichterle D, Simek J, Pisarikova A, Skrha J, Ceska R. Comparison of the effects of atorvastatin or fenofibrate on nonlipid biochemical risk factors and the LDL particle size in subjects with combined hyperlipidemia. American Heart Journal. 2002;144(4):E6. doi: 10.1016/s0002-8703(02)00142-4. PubMed. [DOI] [PubMed] [Google Scholar]
- Mercuro G, Zoncu S, Dragoni F. Gender differences in cardiovascular risk factors. Italian heart journal: official journal of the Italian Federation of Cardiology. 2003;4(6):363–6. [PubMed] [Google Scholar]
- Millen AE, Midthune D, Thompson FE, Kipnis V, Subar AF. The National Cancer Institute diet history questionnaire: validation of pyramid food servings. Am J Epidemiol. 2006;163(0002-9262 (Print)):279–288. doi: 10.1093/aje/kwj031. [DOI] [PubMed] [Google Scholar]
- Mykkanen L, Haffner SM, Rainwater DL, Karhapaa P, Miettinen H, Laakso M. Relationship of LDL size to insulin sensitivity in normoglycemic men. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17(1079-5642 (Print)):1447–1453. doi: 10.1161/01.atv.17.7.1447. [DOI] [PubMed] [Google Scholar]
- Otvos JD, Jeyarajah EJ, Bennett DW, Krauss RM. Development of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurement. Clinical Chemistry. 1992;38:1632–1638. PubMed. [PubMed] [Google Scholar]
- Pate RR, O’Neill JR, Lobelo F. The evolving definition of “sedentary”. Exercise and Sport Sciences Reviews. 2008;36(4):173–178. doi: 10.1097/JES.0b013e3181877d1a. 10.1097/JES.0b013e3181877d1a. PubMeddoi:10.1097/JES.0b013e3181877d1a. [DOI] [PubMed] [Google Scholar]
- Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary behaviors and subsequent health outcomes in adults a systematic review of longitudinal studies, 1996-2011. American Journal of Preventive Medicine. 2011;41(2):207–215. doi: 10.1016/j.amepre.2011.05.004. 10.1016/j.amepre.2011.05.004. PubMeddoi:10.1016/j.amepre.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Tsai MY, Georgopoulos A, Otvos JD, Ordovas JM, Hanson NQ, Peacock JM, Arnett DK. Comparison of ultracentrifugation and nuclear magnetic resonance spectroscopy in the quantification of triglyceride-rich lipoproteins after an oral fat load. Clin Chem. 2004;50(0009-9147 (Print)):1201–1204. doi: 10.1373/clinchem.2004.032938. [DOI] [PubMed] [Google Scholar]
- Wijndaele K, Healy GN, Dunstan DW, Barnett AG, Salmon J, Shaw JE, Owen N. Increased car-diometabolic risk is associated with increased TV viewing time. Medicine and Science in Sports and Exercise. 2010;42(8):1511–1518. doi: 10.1249/MSS.0b013e3181d322ac. 10.1249/MSS.0b013e3181d322ac. PubMeddoi:10.1249/MSS.0b013e3181d322ac. [DOI] [PubMed] [Google Scholar]
- Williams PT, Krauss RM, Vranizan KM, Albers JJ, Wood PD. Effects of weight-loss by exercise and by diet on apolipoproteins A-I and A-II and the particlesize distribution of high-density lipoproteins in men. Metabolism: Clinical and Experimental. 1992;41(4):441–449. doi: 10.1016/0026-0495(92)90082-l. PubMed doi:10.1016/0026-0495(92)90082-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RJ, Volek JS, Liu Y, Shachter NS, Contois JH, Fernandez ML. Carbohydrate Restriction Alters Lipoprotein Metabolism by Modifying VLDL, LDL, and HDL Subfraction Distribution and Size in Overweight Men. The Journal of Nutrition. 2006;136(2):384–389. doi: 10.1093/jn/136.2.384. PubMed. [DOI] [PubMed] [Google Scholar]
- Zderic TW, Hamilton MT. Physical inactivity amplifies the sensitivity of skeletal muscle to the lipidinduced downregulation of lipoprotein lipase activity. Journal of applied physiology (Bethesda, Md.: 1985) 2006;100(1):249–57. doi: 10.1152/japplphysiol.00925.2005. [DOI] [PubMed] [Google Scholar]