Abstract
Our goal was to improve the efficiency of chemotherapy administration for pediatric oncology patients. We identified prechemotherapy hydration as the process that most often delayed chemotherapy administration. An aggressive hydration protocol, supported by fluid order sets, was developed for patients receiving planned chemotherapy. The mean interval from admission to achieving adequate hydration status was reduced significantly from 4.9 to 1.4 hours with a minor reduction in the time to initiate chemotherapy from 9.6 to 8.6 hours. Chemotherapy availability became the new rate-limiting process.
Keywords: chemotherapy administration, chemotherapy safety, lean-sigma, prechemotherapy hydration, protocols, rapid hydration protocol, six-sigma
Cemotherapy administration for pediatric oncology patients is among the highest-risk processes in the hospital. The regimens are complex, the agents have a narrow therapeutic index, and the tremendous variation in patient sizes leads to a 100-fold range of appropriate doses among patients. An error in chemotherapy administration can prove fatal. According to Dinning and colleagues, more than 48 000 diagnosed cancer patients can expect to have some adverse events related to their care each year. Of these adverse events, historically, 20% have been medication related, and two thirds have been thought to be preventable.1 Because of this inherent risk, many programs have adopted procedures of independent, multidisciplinary checks that attempt to ensure that a human error by a single individual cannot reach the patient. These procedures are time consuming and labor intensive, so efforts to evaluate and improve the efficiency of these systems and processes without compromising patient safety are desirable. An example of such an analysis was the initiative reported by Tracy and colleagues.2 They embarked on a comprehensive project to reduce chemotherapy error. With a unique systems improvement approach and through many concurrent change projects, the group demonstrated significant improvement in chemotherapy safety at their institution.
The goal of the quality improvement (QI) team for the Division of Pediatric Oncology, at the Johns Hopkins Hospital, was to engage in a comprehensive review of current systems and processes related to chemotherapy administration and based on review results, identify target areas for improvement, ensuring that safety and efficiency remain inherent in the process at all times. The focus of the work reported in this article was to improve the process supporting chemotherapy administration by modifying the approach to prechemotherapy hydration and to assess the effectiveness and safety of that intervention.
BACKGROUND
An executive safety committee used a Lean-Sigma Approach to review chemotherapy administration processes in the Division of Pediatric Oncology. The focus of the review was the quality and efficiency of the processes for prescribing, dispensing, and administering planned inpatient chemotherapy. This review revealed a need to improve the efficiency of chemotherapy delivery: the mean interval from arrival of the patient in clinic until initiation of chemotherapy on the inpatient unit was 9.6 hours. Most chemotherapy was dispensed and administered during the evening shift when pharmacy and nursing staffing levels decline. The review of current processes identified the time to meet urine parameters as the major rate-limiting step in achieving adequate prechemotherapy hydration, as patients met protocol mandated hydration goals at a mean of 4.9 hours after admission to the inpatient unit. Rate limiting was defined as that step in the process that most strongly influenced the time to complete the entire process in the safest and most efficient way. Tracy and colleagues identified prolonged prehydration of patients as a major reason for delays in chemotherapy administration. A modification in approach to prechemotherapy hydration through the implementation of a rapid hydration protocol would serve as an innovative and a successful hydration strategy and part of a larger safety initiative for this institution.2
Postreview, the QI team hypothesized that improving prechemotherapy hydration would reduce the time to initiate chemotherapy by up to 4 hours, which in some cases would facilitate earlier discharges and improve patient satisfaction. It was known from previous patient care surveys that delays in treatment were a major source of dissatisfaction for patients and families. The QI team expected that dispensing and administering chemotherapy during the day shift, when there was sufficient pharmacy and nursing staff, would reduce provider stress and increase safety. They also believed that earlier chemotherapy initiation would allow the critical tasks to be performed at a time of day when staffing was optimal. Scavuzzo and Gamba3 described efforts of one institution to streamline its chemotherapy process and address the issue of delay in care through the creation of a “virtual chemotherapy unit.” Through this virtual unit, the group was able to address inconsistencies in order writing across the continuum of care and initiate chemotherapy earlier in the day and with fewer caregiver handoffs, an initiative supporting patient safety and promoting patient satisfaction.3
Heslin et al4 suggested that the success of any QI initiative begins with a relatively small but multidisciplinary group of stakeholders coming together to participate in leadership for change. The Division of Pediatric Oncology convened a multidisciplinary group composed of physician, nursing, and pharmacy representatives to improve the timeliness of chemotherapy administration through prechemotherapy hydration efforts.
Prechemotherapy hydration is an important component in the process of safe chemotherapy administration. Adequate prechemotherapy hydration helps protect the kidneys and bladder against known adverse effects of chemotherapy as well as aids in the excretion of toxic metabolites. Previously, there had been no awareness that a change in approach to prechemotherapy hydration was needed or that it had ability to dramatically accelerate a patient's readiness to receive chemotherapy, potentially affecting the timeliness of care delivery. The result of the review challenged this thinking. The question posed by the group was: “Could the approach to prechemotherapy hydration be more aggressive and still safely improve efficiency in chemotherapy administration?”
The QI team standardized its approach through the development of a hydration protocol. Two goals were set by the team: an intermediate goal to have an impact on the prechemotherapy hydration status of the patient and an overarching goal to improve the timeliness of care delivery. The team measured the effect on the times to meet urine parameters and to initiate chemotherapy. A goal was established to initiate chemotherapy within 4 hours of confirmation that the patient was to be admitted for chemotherapy. With implementation of the hydration protocol and a method for data collection, the team was able to immediately measure outcomes and appreciate the impact of the QI initiative.
DEVELOPMENT OF PROTOCOL
Elements of the protocol
An aggressive prechemotherapy hydration protocol with fluid order sets was developed. There were 2 order sets that were part of the protocol: one was incorporated in the outpatient clinic as a stand-alone order set and the other was incorporated as part of the admission orders on the inpatient units. The fluid order set outlined the details of the protocol. Aggressive hydration was defined as fluid boluses initiated immediately and repeated sequentially and without delay until urine parameters were met. A fluid bolus of normal saline was to be administered at 20 mL/kg IV over 1 hour with a maximum 1000-mL fluid bolus to be repeated every hour until identified urine parameters were met or until the patient had received a total of 60 mL/kg of fluid. Hydration goals were measured on the basis of established urine parameters: specific gravity 1.010 or less, pH 7.0 or more (for methotrexate administration), and urine output 3 mL/kg per hour or more. Once urine parameters were met, prescribed hydration fluids were started. The protocol was to be initiated in the outpatient clinic; patients began hydration in the clinic as soon as admission was confirmed, and they continued hydration once they made the transition to the inpatient unit. A standard mechanism to report patient status and manage safe and efficient handoff of care between settings was implemented. Previously there had been no formal report mechanism in place to clearly articulate hydration efforts made in the clinic or a way to describe effectively the current hydration status of the patient on admission.
Exclusion criteria were identified for those patients with unstable renal, pulmonary, and cardiac function and for whom an aggressive approach to hydration would be unsafe (eg, hypoplastic left heart, patients with 1 kidney, and patients with diabetes insipidus or syndrome of inappropriate antidiuretic hormone).
IMPLEMENTATION OF PROTOCOL
Education
An education plan was implemented to include all prescribers (physician assistants, nurse practitioners, and physicians), nurse clinicians, and pharmacists. The team's clinical nurse specialist provided an educational in-service for all 3 nursing care units: the out-patient clinic, inpatient oncology unit, and pediatric care research unit (PCRU). Concurrent with staff education related to this initiative, patient and family education was provided, emphasizing the importance of adequate prechemotherapy hydration and in maintaining hydration status until the time of chemotherapy initiation. Objectives of the prechemotherapy hydration protocol and use of the order sets were outlined for the staff. The data collection tool was presented and reviewed with the group. Reportable signs and symptoms indicating symptomatic fluid overload were reviewed with staff (eg, acute onset shortness of breath, abnormal breath sounds, tachypnea, tachycardia, and hypertension).
EVALUATION OF EFFECTIVENESS AND SAFETY OF PROTOCOL
Data collection
A comprehensive audit tool was developed for data collection (Fig 1). The data points identified were selected by the group specifically to address 4 critical areas in which success of the system could be easily demonstrated or areas for improvement could be identified: documentation/computation, staffing, clinical, and drug and pharmacy. Landmark times on the tool were the following: when the patient arrived at the clinic, the inpatient admission was confirmed, the urine parameters were met, and the chemotherapy was initiated. The data collection tool was initiated in the outpatient clinic and transitioned with the patient to the inpatient units for further use.
Figure 1.
Data collection tool. A copy was clipped to the satellite chart of each patient scheduled to be admitted for chemotherapy. Nurses recorded the times each step was achieved and reasons for any delays beyond 4 hours from confirmation of the admission to initiation of chemotherapy.
Data collection was carried out over an 8-week period. The study sample consisted of 45 consecutive pediatric oncology patients admitted for planned chemotherapy. There were 2 inpatient units included in this data collection: the inpatient pediatric oncology unit and PCRU. Since the results did not differ between the units, the data from both units were pooled for all analyses. There were no reportable signs or symptoms of fluid overload among patients as a result of the aggressive hydration protocol.
Data analysis
Data were maintained in an Access 2003 database (Microsoft, Seattle, Washington) and analyzed using Excel 2003 and Stata (version 9.2, College Station, Texas). For parameters with approximately normal distributions, means and standard deviations are presented; medians and interquartile ranges are presented to describe nonnormal distributions. The Mann-Whitney U test with a 2-sided α of .05 was used to assess the significance of differences between time distribution data sets collected before and after the hydration intervention. Because the distributions were not normal, the use of tests such as a t test would not be appropriate for this analysis.
RESULTS
Impact on hydration status and meeting urine parameters
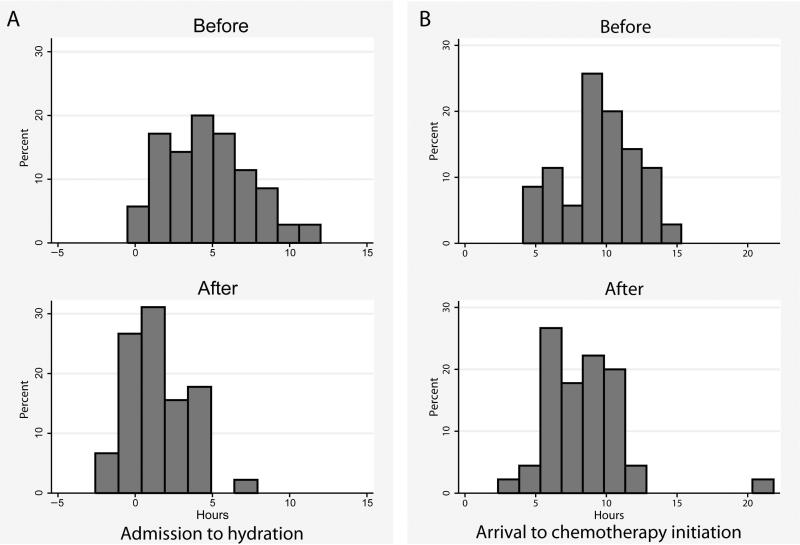
Through the implementation of the aggressive prechemotherapy hydration protocol via fluid order sets, 40 of the 45 patients achieved adequate hydration status and were able to meet identified urine parameters. The time from arrival on the inpatient units to the time urine parameters were met decreased significantly after the implementation of the protocol (Fig 2A). The preaudit data showed that patients met urine parameters at a mean of 4.9 hours after admission, and after protocol implementation, the mean time decreased to 1.4 hours (P < .0001).
Figure 2.
(A) Time to meet urine parameters from time of admission to inpatient unit. Top panel, baseline data. Bottom panel, after implementation of the hydration initiative. P < .0001, Mann-Whitney rank-sum test. (B) Time chemotherapy was initiated from arrival time in clinic. Top panel, baseline data. Bottom panel, after implementation of the hydration initiative. P < .05, Mann-Whitney rank-sum test.
Reduction in time to initiate chemotherapy
There was a smaller, but still statistically significant, reduction in the interval between arrival time to clinic and the time chemotherapy was initiated on the inpatient units. After implementation of the protocol, this interval was reduced from a median of 9.3 hours (interquartile range, 7.9–11.6 hours) to a median of 8.2 hours (interquartile range, 6.5– 10 hours, P < .05) (Fig 2B). The preaudit data showed that the interval between meeting urine parameters and initiation of chemotherapy was a mean of 2.1 hours. After implementation of the protocol, this interval increased to a mean of 4.3 hours.
Factors contributing to persistent delays
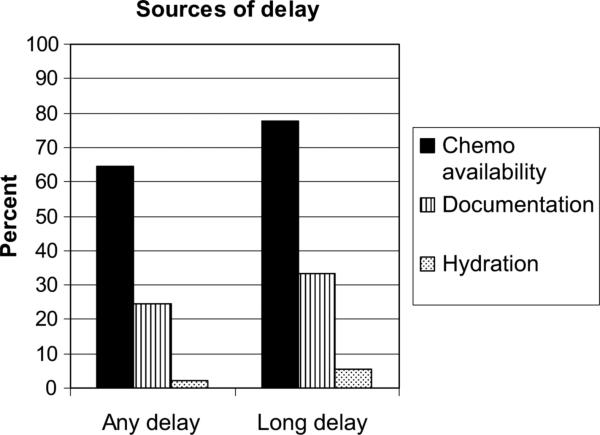
The QI team had established a goal to initiate chemotherapy within 4 hours of confirmation that the patient was to be admitted for chemotherapy. This was to be done by improving the prechemotherapy hydration. However, delays were common: for 18 of the 45 patients, the interval from the time that their inpatient admission was confirmed in clinic to the time they received their first chemotherapy exceeded 8 hours. The QI team analyzed the factors contributing to any delay (over 4 hours) and long delay (over 8 hours) in chemotherapy initiation after implementation of our hydration initiative (Fig 3). Availability of chemotherapy from pharmacy was an issue for 14 of these 18 patients experiencing greater than an 8-hour delay. Only 1 patient did not achieve adequate urine parameters with the hydration initiative.
Figure 3.
Factors contributing to delay identified by unit nurses caring for the patient. Any delay = delay greater than 4 hours to initiate chemotherapy, long delay = delay greater than 8 hours to initiate chemotherapy. Multiple factors could be identified; thus, sum of percentages may exceed 100%.
DISCUSSION
The QI team's hydration initiative proved successful. We were able to adequately hydrate our patients safely and more efficiently than previously. The interval to meet urine parameters on arrival to the inpatient units was reduced significantly, resulting in patients being ready to receive chemotherapy much earlier. No longer was prechemotherapy hydration a rate-limiting factor to delivering timely care to our patients. Tracy and colleagues2 also showed that with the implementation of their rapid hydration protocol, they were able to reduce hydration time from 6.75 to 1.57 hours and without adverse side effects.
The dispensing of chemotherapy from the pharmacy was identified next as the new rate-limiting step in the process of timely chemotherapy initiation. While the QI team confirmed the safety and feasibility of reducing time to meet urine parameters, they demonstrated further the limited impact on the time of chemotherapy administration and identified specific challenges to efficient chemotherapy administration. Chemotherapy availability would be the team's next area for improvement, reinforcing the point that for a successful QI program, there is a need to reevaluate the results of each initiative and uncover other areas for improvement.
It is important to discuss the limitations of our study so that it may serve as a learning experience as we embark on other QI initiatives related to this work. The time chemotherapy was made available was identified as the new rate-limiting step but was not included among the landmark times identified on the tool. The audit tool collected true/false data about whether chemotherapy availability was a factor contributing to the delay in initiating chemotherapy administration. In the present study, there was no mechanism to determine the actual time of delivery of chemotherapy to the unit. In addition, the participation by the entire nursing staff in the auditing process may have led to heightened performance and biased the results toward reduced times. According to Wickstrom and Bendix,5 the Hawthorne effect is a possible explanation for positive results in intervention studies. In this study, behavioral change may have occurred because of an awareness of being observed or active compliance with the supposed wishes of the researchers as a result of the special attention received. Measurement bias also would have been applied during the baseline data collection, so the significant change in time to meet adequate hydration status cannot be attributed solely to the Hawthorne effect.5
The key to the success of any QI initiative is that there is active participation by all primary stakeholders in the process. Heslin et al4 suggested that the support of key leaders gives credibility to the effort and helps with generalized acceptance. The success of this initiative was that through this specific intervention, the QI team was able to measure its impact and, in doing so, identified additional areas of focus to improve performance. According to Britton,6 the key to the success of any QI initiative is to manage your work “up and out”: sharing it with top leadership as well as networking and collaborating with others to create a culture of improvement. In a culture of improvement, failures are considered opportunities for improvement, there is encouragement and support of small projects, risk taking is accepted, and innovations are commonplace.
The results of this study have prompted further evaluation by departmental leadership of current oncology resources in the pediatric pharmacy and the need for allocation of additional FTEs including a chemotherapy technician and expanded coverage by our point-of-care pharmacist. These are ongoing efforts to build support to address chemotherapy availability and continue to meet the goal of providing safe and efficient chemotherapy administration.
Acknowledgments
The authors acknowledge George J. Dover, MD, Chairman, Department of Pediatrics, Johns Hopkins Children's Center, for his support with this quality improvement initiative.
REFERENCES
- 1.Dinning C, Branowicki P, O'Neill JB, Marino BL, Billett A. Chemotherapy error reduction: a multidisciplinary approach to create templated order sets. J Pediatr Oncol Nurs. 2005;22(1):20–30. doi: 10.1177/1043454204272530. [DOI] [PubMed] [Google Scholar]
- 2.Tracy E, DiTaranto S, Womer RB. Evolution of a rapid hydration protocol. J Pediatr Oncol Nurs. 2004;21(1):22–26. doi: 10.1177/1043454203259956. [DOI] [PubMed] [Google Scholar]
- 3.Scavuzzo J, Gamba N. Bridging the gap: the virtual chemotherapy unit. J Pediatr Oncol Nurs. 2004;21(1):27–32. doi: 10.1177/1043454203259951. [DOI] [PubMed] [Google Scholar]
- 4.Heslin MJ, Doster BE, Daily SL, et al. Durable improve ments in efficiency, safety, and satisfaction in the operating room. J Am Coll Surg. 2008;206(5):1083–1089. doi: 10.1016/j.jamcollsurg.2008.02.006. discussion 1089–1090. [DOI] [PubMed] [Google Scholar]
- 5.Wickstrom G, Bendix T. The “Hawthorne effect”— what did the original Hawthorne studies actually show? Scand J Work Environ Health. 2000;26(4):363–367. [PubMed] [Google Scholar]
- 6.Britton LJ, Thrasher S, Gutierrez H. Creating a culture of improvement: experience of a pediatric cystic fibrosis center. J Nurs Care Qual. 2008;23(2):115–120. doi: 10.1097/01.NCQ.0000313759.33654.00. [DOI] [PubMed] [Google Scholar]