Abstract
The goal of drug delivery is to improve the safety and therapeutic efficacy of drugs. This review focuses on delivery platforms that are either derived from endogenous pathways, long-circulating biomolecules and cells or that piggyback onto long-circulating biomolecules and cells. The first class of such platforms is protein-based delivery systems—albumin, transferrin, and fusion to the Fc domain of antibodies—that have a long-circulation half-life and are designed to transport different molecules. The second class is lipid-based delivery systems—lipoproteins and exosomes—that are naturally occurring circulating lipid particles. The third class is cell-based delivery systems—erythrocytes, macrophages, and platelets—that have evolved, for reasons central to their function, to exhibit a long life-time in the body. The last class is small molecule-based delivery systems that include folic acid. This paper reviews the biology of these systems, their application in drug delivery, and the promises and limitations of these endogenous systems for drug delivery.
Keywords: Albumin, Drug Delivery, Erythrocyte, Exosome, Fc Fusion, Folic acid, Lipoprotein, Macrophage, Platelet, Transferrin
Introduction
Many conventional drugs have issues that limit their clinical utility; these include poor aqueous solubility, fast clearance, low bioavailability, poor biodistribution, and low intracellular permeability. The goal of drug delivery is to develop engineered systems that address one or more of these problems so as to improve their safety and therapeutic efficacy. Diverse delivery systems have been developed (Jayant and Tamara 2006; Liong et al. 2008; MacEwan et al. 2010; Menjoge et al. 2010; Yang et al. 2011), that range in size from the molecular to the macroscopic with different levels of sophistication. The simplest systems consist of a binary combination of a drug and carrier while the most complex multifunctional systems include one or more drugs, targeting moieties that enhance their intracellular uptake and trafficking, and moieties for in vivo imaging. The goal of this article is not to review this vast field; instead, we focus on one conceptually unique class of drug carriers that capitalize on endogenous pathways, biomolecules and cells to ferry a drug to its in vivo target.
These endogenous drug carriers can be classified into four platforms. The first class is protein-based delivery systems, which include albumin, transferrin and fusions to the Fc domain of antibodies (Fc fusions). They have a long in vivo circulation half-life in the body, and in some instances—such as albumin and transferrin—are also designed to transport different molecules in the body. The second class, lipid-based delivery system, which include lipoproteins and exosomes, are the native transport vehicles for lipids and intercellular signaling molecules, respectively. The third class is cell-based delivery systems, such as erythrocytes, macrophages, and platelets that have a long life-time in the body. The last class is small molecule-based delivery systems; the emblematic example of this class is a vitamin, folic acid that is exploited for targeted drug delivery. Designed and optimized by nature, these systems also embody many of the desirable attributes of engineered drug delivery systems, such as non-toxicity, non-immunogenicity, biocompatibility and biodegradability. This paper reviews the biology of these systems, their application in drug delivery, and the promises and limitations of these endogenous systems as drug delivery vehicles.
Protein-based drug delivery systems
Human plasma is the most complex body fluid, containing approximately 100,000 proteins with concentrations spanning a dynamic range of 12 orders of magnitude (Mitchell 2010). Albumin and immunoglobulin G (IgG) are the most abundant serum proteins with the longest half-lives. Albumin and transferrin are the most important transport proteins in plasma that supply tissues with nutrients and metal ions. These endogenous transport proteins have been co-opted as long circulating drug carriers, as discussed in this section. Understanding the mechanism of the long half-life of IgG’s has led to development of the Fc-fusion protein platform.
Albumin
Human serum albumin (HSA) is a single chain 585 amino acid protein with a molecular weight of 66.7 kDa and is composed of three homologous, largely helical (67%) domains. It is synthesized in the liver and is the most abundant serum protein with a concentration of 35–50 mg/mL in human serum, constituting 55–60% of total serum protein. HSA plays many roles in the circulatory system; it maintains the colloid osmotic pressure, buffers the pH, scavenges free radicals and has anticoagulant properties. In addition to these roles, albumin also has been described as the body’s tramp steamer (Peters 1996), acting as a multifunctional carrier and solubilizer of many endogenous small molecules such as bilirubin, metals, vitamins, hormones, and fatty acids. In human serum, HSA has an average half-life of 19–22 days compared with a few days for other circulating proteins. The exceptionally long half-life of albumin is mediated through two mechanisms. First, its size is above the threshold for renal clearance (Cheng 2013), so that is not excreted through the kidney. Second, its pH-dependent interaction with the neonatal Fc receptor (FcRn) rescues it from intracellular degradation (Anderson et al. 2006; Chaudhury et al. 2003). Albumin has an added benefit as a carrier in that it often masks fused proteins and peptides and subsequently renders them less immunogenic and less susceptible to protease cleavage (Thorpe et al. 2011).
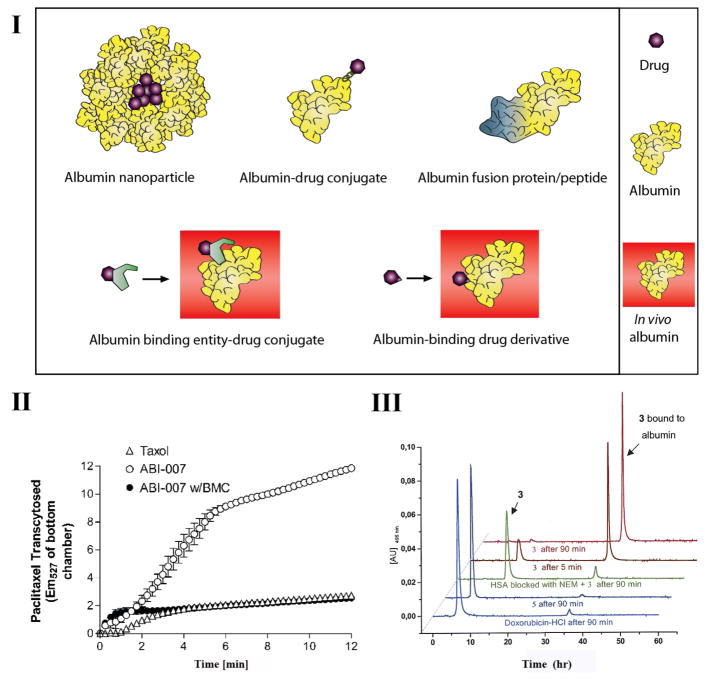
Albumin is emerging as a promising and versatile carrier to improve the pharmacokinetic profile of drugs because of its unique physiological properties. The application of albumin in drug delivery is currently realized by five main approaches: i) encapsulation of drugs into albumin nanoparticles; ii) covalent conjugation of drugs to albumin; iii) recombinant albumin fusions; iv) conjugation of drug molecules to albumin-binding entities; and v) development of albumin binding drug derivatives (Fig. 1. I).
Figure 1.
Albumin-based drug delivery. I: Schematic showing five main approaches that exploit albumin for drug delivery. II: Chromatograms of DOXO-EMCH (3), (6-succinimidocaproyl) hydrazone of doxorubicin (5), and doxorubicin after incubation with human serum albumin and human serum albumin preincubated with N-ethylmaleimide (NEM) for 5 and 90 minutes at 37 °C. Reprinted with permission from (Kratz et al. 2002). Copyright 2002 American Chemical Society. III: Endothelial transcytosis of ABI-007 (Abraxane) (●) and Cremophor-based paclitaxel (○). Cremophor-based paclitaxel transport was all paracellular, whereas ABI-007 transport was both paracellular and transcellular. The transcellular transport of ABI-007 was inhibited by methyl h-cyclodextrin (BMC), a known inhibitor of the gp60/caveolar transcytosis pathway. Reprinted by permission from the American Association for Cancer Research: Desai N et al., Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel, DOI: 10.1158/1078-0432.CCR-05-1634.
The encapsulation of—primarily hydrophobic—drugs in albumin nanoparticles is driven by three imperatives. First, encapsulation into albumin nanoparticles sequesters hydrophobic drugs within the nanoparticle, and thereby improves their solubility and plasma pharmacokinetics. Second, tumor cells take up and metabolize serum albumin as a source of nutrients (Wunder et al. 1997). The third reason is driven by the peculiarities of tumor physiology. The selective accumulation of macromolecular drugs, including albumin-based therapeutics, in solid tumors is mediated by the Enhanced Permeation and Retention (EPR) effect (also termed “passive” targeting) (Matsumura and Maeda 1986). This effect is a consequence of the aberrant vascular architecture of tumors (Yuan 1998), with pores ranging from 100 to 1000 nm in diameter (Wang et al. 2011), combined with impaired lymphatic drainage (Alitalo and Carmeliet 2002), so that long-circulating molecules like albumin and albumin nanoparticles can diffuse out of the tumor blood vessels into the extravascular compartment of tumors and are then retained there. These properties lead to accumulation of albumin-based nanoparticles in tumors (Desai et al. 2006), and provide the rationale for albumin-based targeting of solid tumors. In addition to their passive accumulation by the EPR effect, albumin based therapeutics can also exploit albumin receptor (gp60)-mediated transcytosis (Schnitzer 1992) and binding to the albumin-binding protein SPARC (Framson and Sage 2004) that is overexpressed in a wide variety of tumors (active targeting). These features have led to the development of albumin-based nanoparticle technology (nab-technology) that is a platform for the encapsulation of lipophilic drugs into albumin-based nanoparticles (Fu et al. 2009). In 2005, nab-Paclitaxel (ABI-007; Abraxane®) was approved for the treatment of metastatic breast cancer. An interesting feature of Abraxane is that following its disintegration in plasma, paclitaxel remains absorbed to albumin molecules (Gradishar 2006) and continues to benefit from the favorable pharmacokinetics of albumin. The intratumor paclitaxel concentration was found to be 33% higher for nab-paclitaxel compared with the free drug (Desai et al. 2006; Yardley 2013). In addition, compared with a cremophor formulation of paclitaxel, Abraxane exhibited markedly higher endothelial cell binding and transcytosis. This enhanced endocytosis was abrogated by methyl h-cyclodextrin (BMC), a known inhibitor of the gp60/caveolar transport, suggesting gp60/caveolar-mediated transcytosis as the mechanism of transendothelial cell transport of Abraxane (Fig. 1. II) (Desai et al. 2006).
Drug molecules can also be covalently coupled to HSA. HSA is a highly soluble protein and is also very stable to changes in pH (in the range of 4–9) and temperature (up to 60 °C for up to 10 h) without denaturation. These physico-chemical properties make albumin well suited for conjugation of small molecule therapeutics. For example, a docetaxel-albumin conjugate was synthesized by coupling the drug to the lysine residues of albumin and showed a higher antitumor cytotoxicity and an improved biodistribution profile compared with the free drug (Esmaeili et al. 2009). Targeting ligands such as galactose (Han et al. 1999), folic acid (Dosio et al. 2009) and the RGD peptide (Dubey et al. 2011; Temming et al. 2006) have also been incorporated into albumin-drug conjugates to further target the conjugate to the liver, tumor, and tumor vasculature, respectively.
Fusing peptides and proteins to HSA at the gene level is an alternative and elegant strategy that is applicable to genetically encodable drugs. The challenges in the development of this technology are somewhat paradoxical. Although HSA is the most abundant protein in the body, it is produced exclusively by hepatocytes in the liver (Rothschild et al. 1988). Initial efforts to produce it in microbial expression systems were largely unsuccessful (Lawn et al. 1981; Quirk et al. 1989; Saunders et al. 1987). This bottleneck was solved independently by researchers at a startup company, Delta Biotechnology and by scientists at Rhone-Poulenc Rorer, who developed specialized yeast expression systems that allowed high-level production of HSA by secretion into the growth medium (Fleer et al. 1991; Sleep et al. 1991; Sleep et al. 1990). This technology subsequently led to the development of recombinant albumin fusions, whereby a gene encoding HSA is fused to that of a peptide or protein drug to prolong its circulatory half-life. This technology too was developed independently by Delta Biotechnology and Rhone-Poulenc Rorer (Ballance 1990; Yeh et al. 1992). Albiglutide, an HSA fusion of glucagon-like peptide (GLP-1) has been submitted for marketing authorization by GSK in the US and Europe (Baggio et al. 2004). Several other HSA fusions of protein drugs are being developed by GSK Inc. and Teva Inc. and are in the clinical pipeline (Sleep et al. 2013). Another example is MM-111, an HSA fusion protein that contains two scFv domains directed against epidermal growth factors ErbB2 and ErbB3 that are overexpressed in a majority of cancers (José and Sandra 2009). Developed by Merrimack Pharmaceuticals, MM-111 is in phase I clinical studies for treatment of breast cancer (2010, NCT01097460) and in a phase II clinical trial for treatment of advanced gastroesophageal cancer (2013, NCT01774851).
As an alternative to chemical conjugation or recombinant fusion to HSA, drugs can be modified with HSA binding moieties that piggyback onto HSA in vivo. This approach simplifies the synthesis of the conjugate. Moreover, because of the smaller size and lower viscosity of these drug conjugates, it offers easier formulation and administration. Some albumin binding moieties include streptococcal protein G-derived albumin-binding domain (ABD), small molecule albumin-binding tags, and fatty acids. Of these, fatty acids are the most popular, as albumin possesses 2–3 high-affinity fatty acid binding sites and 2–3 secondary fatty acid binding sites. Detemir (Chapman and Perry 2004)(Levemir®) and Liraglutide (Victoza®)(Rossi and Nicolucci 2009) are albumin-binding myristic acid derivatives of insulin and GLP-1, respectively, and are clinically approved for treatment of type 1 and type 2 diabetes, respectively.
Another variant of an in situ albumin technology is an albumin-binding prodrug platform that exploits the cysteine-34 of endogenous HSA (Fiehn et al. 2008; Kratz 2011). Approximately 70% of plasma HSA contains a solvent-accessible cysteine-34 so that it comprises the largest free thiol pool in plasma. In this approach, a drug is derivatized with a thiol-reactive moiety such as a maleimide and an acid-sensitive or enzymatically cleavable linker that is cleaved and releases the drug within the tumor. INNO-206, an acid-sensitive (6-maleimidocaproyl) hydrazone derivative of doxorubicin (DOXO-EMCH) is in phase III trial for treatment of soft tissue sarcoma (2014, NCT02049905) (Kratz 2011). To investigate the covalent conjugation of DOXO-EMCH to HSA, Kratz et al. incubated DOXO-EMCH with HSA at 37 °C for 5 and 90 minutes and analyzed the samples by chromatography. As shown in Fig. 1. III, most of DOXO-EMCH reacted with HSA within the first 5 min resulting in a peak eluting at 36 minutes. Blocking the thiol group of cysteine-34 in HSA with an excess of N-ethylmaleimide (NEM) prior to incubation with DOXO-EMCH, decreased binding to HSA significantly, demonstrating that the reaction of DOXO-EMCH with HSA is specific for the thiol group of cysteine-34. Furthermore, no binding to HSA was observed with intact doxorubicin or the (6-succinimidocaproyl) hydrazone derivative of doxorubicin that does not contain a thiol-reactive group, which rules out physical interactions of the doxorubicin linker with HSA as the binding mechanism (Kratz et al. 2002). ConjuChem, Inc. has expanded on this platform for the delivery of peptide therapeutics and calls it the drug affinity complex (DAC™) technology.
Transferrin
Transferrin is the iron transport protein in plasma. It delivers iron from sites of adsorption in the intestine and sites of hemoglobin catabolism in spleen, bone marrow, and liver to red cell precursors and sites of iron storage in the bone marrow, liver and spleen. Serum transferrin is synthesized exclusively by liver hepatocytes and consists of a single 679 amino acid polypeptide chain with two iron-binding domains and two oligosaccharide chains with a molecular weight of 79.5 kDa. It has a concentration of 2.5–3.5 mg/mL blood and a half-life of 7 days in circulation. Iron in circulation is loaded onto transferrin (holo-transferrin) and is taken up by cells via transferrin receptor-mediated endocytosis of holo-transferrin. At the extracellular pH of 7.4, holo-transferrin binds to the transferrin receptor with high affinity and undergoes endocytosis. Within endosomes, an acidic pH of ~5.5 induces the release of iron from holo-transferrin and leaves iron-free transferrin (apo-transferrin) bound to its receptor. At this acidic pH, apo-transferrin binds to the transferrin receptor with higher affinity than holo-transferrin and the apo-transferrin-receptor complex is recycled to the cell surface. Upon reaching the neutral pH of the extracellular environment, apo-transferrin is released from the receptor, allowing it once again to sequester iron. The cell cycling time of transferrin is 1–2 minutes and in the context of this pH-dependent cycle, each transferrin molecule undergoes 100–200 circuits in its lifetime. There are two types of transferrin receptors: transferrin receptor 1 (TfR1) and transferrin receptor 2 (TfR2). TfR1 is a transmembrane homodimer that is expressed in all cells with the exception of erythrocytes and can bind up to two transferrin molecules. The other type, TfR2 is specifically expressed in the liver and acts as the hemostatic iron sensor in hepatocytes.
For drug delivery applications, transferrin can be exploited as a targeting carrier by itself or attached to another carrier. In addition, to avoid competition between recombinantly synthesized transferrin-drug conjugates with endogenous transferrin in vivo, anti-transferrin receptor antibodies such as R17217 and OX26 have been used as the targeting ligand. Transferrin has several unique features as a carrier. First, rapidly proliferating cells such as cancer cells often express a high level of TfR1 and TfR2 (Richardson et al. 2009) to meet their increased need for iron as a nutrient and as a co-factor of DNA synthesis enzymes, and this provides an attractive opportunity for tumor-specific delivery of anticancer agents. Second, TfR1 is present on the blood–brain barrier (BBB) and controls the influx of transferrin across the BBB (Rouault and Cooperman 2006), so that transferrin or anti-TfR antibodies have been proposed as a “molecular Trojan horse” to transport therapeutics into the brain (Soni et al. 2008; Ulbrich et al. 2009). Doxorubicin (Lubgan et al. 2009), cisplatin (Luo et al. 2012a), chlorambucil (Beyer et al. 1998), and mitomycin C (Tanaka et al. 2001) have been conjugated to transferrin for delivery to tumor cells. A transferrin conjugate of doxorubicin has been shown to reverse multidrug resistance by altering the cytotoxicity mechanism of the free drug by targeting the plasma membrane rather than the nucleus (Lubgan et al. 2009). Transferrin conjugates of toxins such as ricin A chain (Candiani et al. 2001), saporin (Ippoliti et al. 1995), cholera toxin, and diphtheria toxin (Weaver and Laske 2003) have been synthesized for tumor targeting. Tf-CRM107 (TransMID™) is a fusion of a mutant of diphtheria toxin with transferrin (CRM107) and was studied in a phase III clinical trial for treatment of glioblastoma (2007, NCT00083447) (Laske et al. 1997). The trial was however terminated in 2007 after an analysis of early data showed that it was extremely unlikely that TransMID™ would meet the trial criteria for efficacy (Noonan 2007).
Sasaki et al. developed a transferrin conjugate of artemisinin, an anti-malarial drug, as an anti-cancer agent. Artemisinin reacts with iron and forms cytotoxic free radicals. Tumor cells avidly take up more free iron than normal cells to proliferate and are hence more sensitive to the cytotoxic effects of artemisinin. The artemisinin conjugate of transferrin shuttles iron and artemisinin into tumor cells selectively (Lai et al. 2009; Lai et al. 2005). Artemisinin was also conjugated to a transferrin-receptor targeting peptide, HAIYPRH (Oh et al. 2009). Transferrin can also be conjugated to other drug-loaded carriers including liposomes (Soni et al. 2008; Wu et al. 2007), dendrimers (Li et al. 2012), cyclodextrin (Bellocq et al. 2003) and polymeric nanoparticles (Gupta et al. 2007; Tsuji et al. 2012; Zheng et al. 2010). Mebiopharm Inc. has developed transferrin conjugated liposomal formulations of the anticancer drugs oxaliplatin (MBP-426), methotrexate (MBP-Y003), docetaxel (MBP-Y004), and gemcitabine (MBP-Y005). A phase I/II clinical trial is ongoing to evaluate MBP-426 in patients with second line gastric, gastroesophageal, or esophageal adenocarcinoma (2009, NCT00964080).
Fc fusions
Fc-fusion proteins are molecules consisting of the immunoglobulin Fc domain that is genetically fused to a bioactive protein. Fusion to the immunoglobulin Fc domain extends the half-life of the therapeutic protein through a naturally occurring receptor-mediated process called FcRn recycling. This process is the key contributor to the long half-life of the two most abundant plasma proteins, albumin and immunoglobulin G (IgG). Albumin and IgG account together for about 70% of plasma proteins and have exceptionally long half-lives of approximately 20 days in humans. Though functionally unrelated, it is well established that albumin and IgG are independently salvaged from intracellular degradation and clearance through FcRn recycling (Brambell 1970; Chaudhury et al. 2003).
The FcRn (neonatal Fc receptor), also known as the Brambell receptor, is an MHC class I related receptor that is highly expressed in the early acidic endosomes of vascular endothelial cells and hematopoietic cells and to a lesser extent on the surface of other cell types, including intestinal cells, epithelial cells, muscle, lung, skin, and hepatocytes. However, due to the neutral pH on the cell surface, albumin and IgG do not bind to FcRn on the cell surface and are instead taken up through fluid phase pinocytosis. Within acidified endosomes of vascular endothelial cells, FcRn binds albumin and IgG, and FcRn bound albumin and IgG are protected against lysosomal degradation and are recycled back to the cell surface by exocytosis, where exposure to the neutral pH of blood triggers their release from FcRn back to circulation (Fig. 2. I) (Roopenian and Akilesh 2007).
Figure 2.
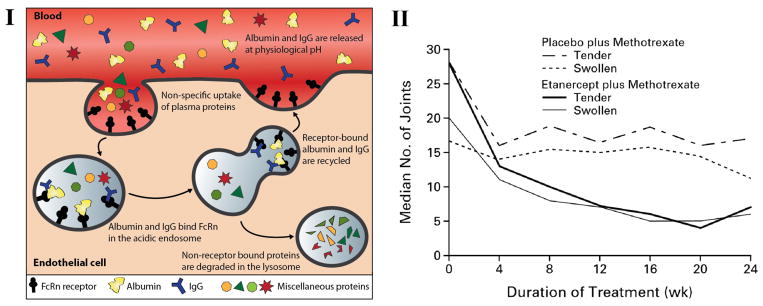
Fc-fusion drug delivery. I: Schematic showing FcRn-mediated recycling of albumin and IgG. II: Therapeutic effect of Etanercept. Median number of patients with tender and swollen joints in a trial with 89 patients with persistently active rheumatoid arthritis. From The New England Journal of Medicine, Weinblatt et al., A Trial of Etanercept, a Recombinant Tumor Necrosis Factor Receptor:Fc Fusion Protein, in Patients with Rheumatoid Arthritis Receiving Methotrexate, 340, 340, 253–259. Copyright © (1999) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Many therapeutic proteins and peptides have very short half-lives limiting their exposure to target tissues and consequently their utility in clinical settings. To overcome this limitation, the Fc fusion platform was introduced in 1989 (Capon et al. 1989) and it has shown success in clinical applications. In addition to an extended half-life, fusion to an Fc domain provides a convenient affinity handle for purification by protein-A affinity chromatography—a well-established method for antibody purification in the manufacturing setting (Roland Kontermann and Dübel 2010). The protein partner is fused to the flexible hinge region of Fc domain at the gene level. The hinge serves as a flexible spacer and allows the Fc domain and the target protein to function independently. The function of the biologic drugs that are fused to the Fc receptor fall into various categories including receptors (e.g. TNFα receptor (Peppel et al. 1991), activin receptor II (Ruckle et al. 2009), TACI receptor (Bracewell et al. 2009), interleukin-1(IL-1) receptor (Hoffman et al. 2008), ligands (e.g. P-selectin glycoprotein ligand-1 (PSGL-1) (Amersi et al. 2002; Farmer et al. 2005)), hormones (e.g. human growth hormone (hGH) (Lee et al. 2007), follicle stimulating hormone (FSH) (Low 2005)), enzymes (e.g. DNAase I (Dwyer et al. 1999)), single chain antibodies (e.g. anti-Tn antigen antibody (Kubota et al. 2010) and anti–carcinoembryonic antigen antibody (Kenanova et al. 2005)), cytokines (e.g. interleukin-2 (IL-2) (Millington et al. 2012), interferon beta (IFNβ) (Vallee et al. 2012)), peptides (e.g. atrial natriuretic peptide (ANP) (Mezo et al. 2012), glucagon-like peptide (GLP-1)(Kumar et al. 2006), and coagulation factors (e.g. coagulation factor VIII (Peters et al. 2013)). Most of the Fc-fusion proteins that are currently marketed or in clinical development target receptor-ligand interactions. One class, which include etanercept for treatment of rheumatoid arthritis (Lethaby et al. 2013; Weinblatt et al. 1999), aflibercept for treatment of wet macular degeneration (Thomas et al. 2013), rilonacept for treatment of cryopyrin-associated periodic syndrome (Kapur and Bonk 2009), and belatacept for the prophylaxis of organ rejection in kidney transplants (Vincenti et al. 2011)) work as antagonists to block receptor–ligand interactions. In a 24-week, double-blind trial with 89 patients with persistently active rheumatoid arthritis, the addition of etanercept to methotrexate therapy improved the portion of patients that met the ACR 20 criteria—American College of Rheumatology criteria for a 20 percent improvement in measures of disease activity—from 27 percent in patients receiving a placebo plus methotrexate to 71 percent in those treated with etanercept plus methotrexate (Fig. 2. II) (Weinblatt et al. 1999). The second class of Fc fusions function as agonists to stimulate receptor function, such as romiplostim, developed for treatment of chronic immune thrombocytopenic purpura (Kuter et al. 2008)).
Fc fusion proteins were initially designed as homodimers containing two copies of the therapeutic protein fused to a dimer of Fc. In the case of large therapeutic proteins, steric hindrance between the two therapeutic molecules with one another or with the Fc domain was a problem. In addition, large Fc fusion proteins potentially have a slow diffusion rate. Thus, a second class of Fc fusion proteins were developed, which consisted of one therapeutic protein molecule fused to a dimer of Fc. This fusion reduced steric hindrance and exhibited increased diffusion. A third class of Fc fusion proteins are cytokine traps consisting of two distinct cytokine receptor domains fused to an Fc dimer (Economides et al. 2003). Cytokine traps were developed based on the notion that many cytokine receptors require more than one receptor chain to bind their ligands to achieve high affinity. One example of a cytokine trap is rilonacept that consists of ligand-binding domains of interleukin-1 (IL-1) receptor and IL-1 accessory protein (IL-1AcP); rilonacept was approved as an orphan drug for treatment for treatment of cryopyrin-associated periodic syndrome (Kapur and Bonk 2009). Peptibodies are the fourth class of Fc fusion proteins (Shimamoto et al. 2012). Developed by Amgen Inc., peptibodies consist of one or two copies of a bioactive peptide fused to N- or C-terminus of an Fc domain. Unlike other Fc fusion proteins that are typically expressed in mammalian cells, peptibodies can be produced in E. coli. The most advanced peptibody is romiplostim, a thrombopoietin mimetic peptibody approved for the treatment of chronic immune thrombocytopenic purpura (Kuter et al. 2008). The fifth class of Fc fusion proteins—MIMETOBODY™ polypeptides—that is a fusion of a bioactive peptide with an Fc domain It often requires protein engineering steps to improve the therapeutic potency, solubility and stability of the fusion (Picha et al. 2014). The MIMETOBODY™ technology has been applied to develop the erythropoietin mimetic, CNTO 530 (Sathyanarayana et al. 2009).
Lipid-based drug delivery systems
Lipid-based carriers are the second class of native human carriers and consist of lipoproteins and exosomes that function as transport vehicles for lipids and intercellular signal molecules (proteins and RNAs), respectively. The surface of these carriers consists of a phospholipid monolayer for lipoproteins and a bilayer for exosomes. While lipoproteins have a hydrophobic core suitable to host poorly soluble drugs, exosomes have a hydrophilic core suitable to host soluble drugs.
Lipoproteins
Lipoproteins are submicron- to micron-sized and mostly spherical complexes consisting of a hydrophobic core surrounded by a hydrophilic outer shell that facilitate lipid transport and exchange throughout the body to tissues where they are required. The core is composed of phospholipids, fat-soluble antioxidants, vitamins, and cholesteryl ester, and the shell contains free cholesterol, phospholipid and apolipoprotein molecules. Most apolipoproteins are amphiphilic and contain hydrophobic domains that interact with lipids as well as hydrophilic domains that allow interaction with the aqueous plasma environment. Apolipoproteins are classified into four main groups (ApoA, B, C, and E) and are integral to lipoprotein transport and metabolism; they stabilize the structure of lipoproteins and determine their metabolic fate by serving as ligands for cell surface receptors or as co-factors and modulators of enzymes involved in lipid metabolism (Dasgupta and Wahed 2013).
Lipoproteins are classified according to their density into five groups: chylomicrons, very low-density lipoproteins (VLDLs), intermediate-density lipoproteins, (IDLs), low-density lipoproteins (LDLs), and high-density lipoproteins, (HDLs) (Table 1). Lipoprotein density is directly related to protein content (chylomicrons < VLDL < IDL < LDL < HDL) (Dasgupta and Wahed 2013). These classes of lipoproteins are assembled in different ways. Chylomicron is the largest (75–12000 nm) of the lipoproteins (Torchilin 2006). This particle assembles in the small intestine and carries dietary lipids from enterocytes to other tissues. VLDL is the next largest lipoprotein nanoparticle (30–80 nm) (Torchilin 2006); it assembles in the small intestine from dietary lipids, or in the liver from lipids synthesized de novo or recycled to the liver during lipoprotein catabolism, and transport lipids to other tissues. IDL is a transient product formed during the lipolytic conversion of VLDL to LDL and is not detectable in normal plasma. LDL has a diameter of 18–25 nm (Torchilin 2006) and is the most prevalent lipoprotein in humans. It is formed from VLDL in plasma and transports endogenous cholesterol to the liver and extrahepatic tissues via LDL receptor-mediated endocytosis. Finally, HDL is the smallest of the lipoproteins with a size of 8–12 nm (Torchilin 2006), and is secreted from the liver and small intestine. It scavenges excess cholesterol from tissues and transports it to the liver. LDL receptors, also referred to as apoB-100/apoE receptors, are expressed in all cells, but are most abundant in hepatocytes. LDL and IDL bind to LDL receptors and form a complex that is internalized into endosomes by receptor-mediated endocytosis. The acidic pH of the endosome then triggers the dissociation and release of LDL and VLDL. The free receptor is then recycled to the cell surface prior to the fusion of endosomes with lysosomes. Once fused with lysosomes, the protein and lipid components of the lipoprotein dissociate and are hydrolyzed. The liberated lipids are used by the cell for the synthesis of new lipoproteins, plasma membranes, and bile acids (Ridgway et al. 2008).
Table 1.
Types of lipoproteins and their physico-chemical properties (Brody 1999; Dasgupta and Wahed 2013; Lichtenstein and Jones 2012; Torchilin 2006).
| Lipoprotein | Size (nm) | Molecular weight (Da) | Itinerary | Lipid (%) | Protein (%) | Apoprotein |
|---|---|---|---|---|---|---|
| Chylomicron | 75–1200 | 400×106 | Intestine to tissues | 97–99 | 1.5–2.5 | apoA, apoB-48, apo C, apoE |
| VLDL | 30–80 | 10–80×106 | Liver to tissues | 90–95 | 5–10 | apoB-100, apoC, apoE |
| IDL | 25–35 | 5–10×106 | Liver to tissues | 80–85 | 15–20 | apoB-100, apoC, apoE |
| LDL | 18–25 | 2.2–3.5×106 | Liver to tissues | 75–80 | 20–25 | apoB-100 |
| HDL | 5–12 | 1.5–4×105 | Tissues to liver | 45–60 | 40–55 | apoA, apoC, apoE |
Lipoproteins are a versatile and potent platform for the delivery of therapeutics to sites of disease for the following reasons. First, they are native to the body and hence do not trigger an immune response (Vyas and Sihorkar 2000), Second, they contain an inner lipid core that provides a compartment for the encapsulation of hydrophobic drugs. Third, their sub-micron size allows selective accumulation in tumors via the EPR effect. Finally, overexpression of lipoprotein receptors in some tumors further enhances the tumor specificity of lipoproteins by active targeting (Firestone 1994). Among lipoproteins, chylomicrons have the largest size and thus enable high drug loading. Chylomicrons have been proposed as delivery vehicles to target drugs to the liver for treatment of infectious diseases such as hepatitis B (Rensen et al. 1995) and malaria (Dierling and Cui 2005). VLDLs have the highest lipid content among the 100-nm sized lipoproteins, which makes them excellent candidates to solubilize and shuttle hydrophobic drugs, such as doxorubicin, to solid tumors. Shawer et al. developed VLDL-mimetic phospholipid-submicron emulsion (PSME) that contains a cholesteryl ester of carborane (BCH) for boron neutron capture therapy of cancer. The VLDL-resembling PSME interacts with native LDL and facilitated the delivery of BCH into cancer cells in vitro (Shawer et al. 2002).
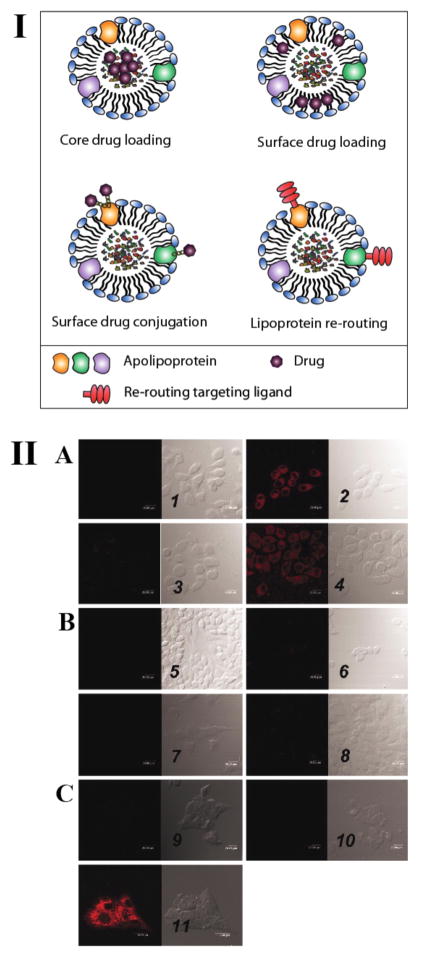
LDLs have a longer half-life (2–4 days) compared with chylomicrons and VLDLs. They are the most widely studied lipoproteins for active targeting of chemotherapeutic agents as various tumor cells express a high level of LDL receptor (LDLR) to meet their high cholesterol demand for proliferation (Favre 1992). Drug molecules can be either incorporated into the hydrophobic core of LDLs (core loading), or intercalated into the outer shell (surface loading), or conjugated covalently to apolipoproteins (covalent modification) (Fig. 3. I). When loaded into LDLs, the anticancer drugs 5-fluorouracil (5-FU), 5-iododeoxyuridine (IUdR), doxorubicin (Dox) and vindesine exhibited enhanced cytotoxicity compared with the free drug (Kader and Pater 2002).
Figure 3.
Lipoprotein-based drug delivery: I: Schematic showing various approaches that exploit lipoproteins for drug delivery. II: Lipoprotein rerouting to folate receptor (FR) expressing cells. Confocal microscopy studies: The numbers refer to each pair of images, with the fluorescence image (Left) and the bright field image (Right) shown. (A) DiI-LDL-FA uptake by the FR+ cell line: KB cells alone (1), KB cells+ DiI-LDL-FA (2), KB cells + DiI-LDL-FA + 250-fold excess of free FA (3), and cells + DiI-LDL-FA + 50-fold excess of native LDL (4). (B) DiI-LDL-FA uptake by the FR− cell line: CHO cells alone (5), CHO cells + DiI-LDL-FA (6), HT-1080 cells alone (7), and HT-1080 cells + DiI-LDL-FA (8). (C) DiI-LDL-FA uptake by HepG2 cells (LDLR+): HepG2 cells alone (9), HepG2 cells + DiI-LDL-FA (10), and HepG2 cells + DiI-LDL (11).
Native LDLs are heterogeneous in size and composition, are difficult to isolate from human plasma in large quantities, and have a short storage life. To overcome these problems, synthetic LDLs have been constructed from a lipid emulsion combined with a synthetic bifunctional peptide containing a lipid binding domain and an LDLR-binding domain. A lipophilic pro-drug, paclitaxel oleate was incorporated into these synthetic LDLs and demonstrated successful intracellular delivery via LDLR mediated endocytosis (Nikanjam et al. 2007). Although promising, the use of LDL as a targeting vehicle is limited to tumors that overexpress the LDL receptor (LDLR). Moreover, expression of LDLR by some normal tissues can undermine the specificity of LDLR targeting. To address this limitation, a LDL rerouting approach has been developed to target receptors other than LDLR that are more specific to tumors. In this approach, a ligand specific to a receptor of interest is conjugated to the apolipoproteins in the LDL structure, which then allows the synthetic LDL to target tumors with higher specificity (Chen et al. 2007; Corbin 2013; Zheng et al. 2005). In one implementation of this approach, Zheng et al. attached folate to a synthetic LDL to enable targeting of cancer cells in tumors that overexpress the folate receptor (FR+). They loaded the optical probe, DiI on the surface of FA-LDLs and native LDLs to visualize their cellular uptake by KB cells (FR+), CHO cells (FR−), HT-1080 cells (FR−) and HepG2 cells (LDLR+). DiI-FA-LDLs showed a strong accumulation in FR+ cells, but no accumulation in FR− cells and LDLR+ cells, demonstrating blockage of binding to the LDL receptors and rerouting to folate receptors. (Fig. 3. II) (Zheng et al. 2005).
Loading drugs into naturally occurring LDLs is a tedious process, as it requires blood to be drawn from the patient, followed by isolation of LDLs and ex vivo drug loading. In addition, the loading procedure—either in a liquid medium or as a dry film—can change the conformation of apolipoproteins and consequently cause loss of LDL targeting (Hammel et al. 2003). A proposed solution to these issues is to load drugs into LDLs in vivo. To this end, one approach is to take advantage of lipid transfer proteins (LTPs). LTPs in circulation mediate the transfer of lipids among various plasma lipoproteins and can similarly remove lipophilic drugs from lipid nanoparticulate carriers and shuttle them to plasma LDLs (Seki et al. 2004). This approach requires that the drug have a high affinity for LTPs, so that some drugs may need to be covalently modified with lipid moieties such as cholesterol to impart a high enough lipophilicity to them.
Exosomes
The term “exosome” was first coined in 1987 (Trams et al. 1981). Exosomes are naturally occurring membrane vesicles around 40–100 nm in diameter. Exosomes are derived from multivesicular bodies (MVBs) that are formed by the inward budding of the endosomal membrane in the cytosol. The intraluminal vesicles in the MVBs are either degraded after fusion of MVBs with lysosomes or secreted as exosomes after fusion of MVBs with the plasma membrane. Exosomes are secreted by normal and diseased cells and are present in blood and other bodily fluids. Exosomes contain a variety of molecules including signal peptides and/or proteins, mRNA, microRNA, and lipids and mediate intercellular communication by providing autocrine, paracrine, or endocrine signals in different ways. Exosomes can fuse with and be internalized by target cells and release their cargo proteins or miRNA resulting in intracellular signaling. In addition, exosomes can fuse with the plasma membrane and transfer surface receptors of the donor cell to the target cell. Exosomes can also bind to cell surface receptors via their surface ligands and activate their target cells. Exosomes also act to protect cells by exporting potentially harmful molecules (Record et al. 2011).
The identification of exosomes as transporters of proteins and nucleic acids has created excitement in the field of drug delivery. Exosomes offer several advantages over conventional drug delivery systems as they evade rapid clearance by mononuclear phagocytes and show great stability in blood. In addition, exosomes are 70–120 nm in diameter (Redman et al. 2012) and hence can take advantage of the EPR effect and extravasate selectively into the tumor or inflamed tissues. Furthermore, the ability of exosomes to deliver their cargo to specific cells can provide specificity for drug delivery. Alvarez et al. successfully developed brain-targeted exosomes containing anti-BACE-1 siRNA to treat Alzheimer’s disease (Alvarez-Erviti et al. 2011). They fused the exosomal surface protein LAMP2B to Rabies virus glycoprotein (RVG) that binds specifically to the nicotinic acetylcholine receptor (AchR) on neurons and on the vascular endothelium of the BBB. Immature dendritic cells (DCs) were harvested from mouse bone marrow, and were transfected with the LAMP2B-RVG construct. Exosomes were purified from the engineered DCs and were loaded with exogenous siRNA. Despite the current excitement about exosomes, there are several obstacles to developing effective exosome-based drug delivery systems. Further study is required to identify the endogenous exosomal cargoes that may mediate potential adverse side effects. The applicability of exosomes for drug delivery in different diseases critically depends on developing appropriate loading strategies for different therapeutics such as proteins, shRNA, miRNA antagonists, and small molecule drugs. Finally, scalable technologies for purification and loading of exosomes remain to be developed and optimized.
Cell-based drug delivery systems
Cell-based carriers have received a great deal of interest as drug delivery vehicles because of a combination of attractive properties. Autologous cells are biocompatible and non-immunogenic. Furthermore, compared to synthetic carriers, cell based carriers have a considerably longer life span in circulation (Batrakova et al. 2011). They are completely biodegradable as they undergo apoptosis upon aging or damage. Additionally, cellular carriers have a high loading capacity for cargo molecules due to their large volume. However, issues with all cell-based carriers are their limited shelf-life, the need for ex vivo manipulation and the need for stringent storage conditions. Herein, we describe the most common cellular delivery systems derived from erythrocytes, macrophages and platelets that have been manipulated to encapsulate different bioactive molecules.
Erythrocytes
Erythrocytes are the most numerous blood cells (5 million cells/mm3 blood) and are responsible for oxygen transport. Erythrocytes are derived in the bone marrow and lose their nucleus and organelles during maturation. Mature erythrocytes have a life span of 100–120 days in the circulatory system and once worn-out, they are broken down selectively by macrophages of the reticuloendothelial system (RES) in the spleen, liver and bone marrow. Erythrocytes have several useful attributes that make them attractive as drug carriers (Porth 2011; Sembulingam and Sembulingam 2012). They are monodisperse in size, with a typical biconcave shape and a diameter of 7–8 μm and an average volume of 90 μm3. The biconcave shape provides a high surface to volume ratio of about 1.7 μm−1 (about 60% in excess of a sphere with an equal volume), which facilitates diffusion of oxygen and metabolic products across its membrane (Jones 1979). With a well-organized cytoskeleton and strict membrane-cytoskeleton association they are reversibly deformable so that they can squeeze through capillaries with diameters as small as 3.5 μm. Depending on the cargo and loading conditions, loaded erythrocytes can have a long circulation half-life of more than 100 days. Furthermore, erythrocytes have a simple metabolic system with a number of endogenous enzymes that enable them to act as active carriers that can convert encapsulated pro-drugs into diffusible active drugs.
Erythrocytes also have some limitations as drug carriers that need to be overcome. The main limitations are rapid leakage of encapsulated cargo and decreased erythrocyte plasticity during the encapsulation process and hence decreased resistance to osmotic and mechanical damage (Hamidi et al. 2001; Pitt et al. 1983). Loaded therapeutics can also alter the physiology and pharmacokinetics of erythrocytes. Furthermore, erythrocyte based carriers have a higher variability in composition and mechanical properties compared with synthetic carriers (Hodson et al. 2008; Tomaiuolo et al. 2012) and also harbor the risk of biological contamination due to their blood origin (Adams et al. 2003).
Figure 4. I illustrates different strategies that take advantage of eyrthrocytes for drug delivery. Drugs with sufficient water solubility and metabolic stability against intra-erythrocytic enzymes can be encapsulated into erythrocytes. Various methods have been developed for encapsulation of therapeutics into erythrocytes such as hypo-osmotic lysis methods (dilution, dialysis, preswelling), electroinsertion, endocytosis and membrane perturbation (Krishnaveni. G et al. 2013). These encapsulation methods seek to inflict minimal damage to erythrocytes. Alteration to their cellular integrity may result in the rapid phagocytosis of erythrocytes by macrophages in the reticuloendothelial system (RES) of the liver, spleen and bone marrow. To reduce membrane fragility resulting from osmotic shock during the encapsulation process, loaded erythrocytes can be chemically cross-linked with cross-linking agents such as glutaraldehyde (Luo et al. 2012b) and bis(sulfosuccinimidyl)suberate (BS3) and 3,3′ dithiobis(sulfosuccinimidyl propionate) (DTSSP) (Jordan et al. 1997). Recently, protein transduction domain (PTD) mediated cell entry has been exploited to load proteins into erythrocytes. Linking PTDs to drugs provides a non-invasive way to ferry attached therapeutics into erythrocytes without damage to the cell membrane (Kwon et al. 2009).
Figure 4.
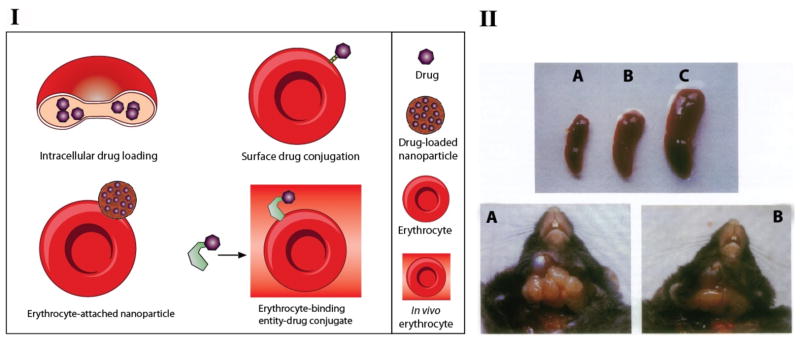
Erythrocyte-based drug delivery. I: Schematic showing various approaches that exploit erythrocytes for drug delivery. II: Therapeutic effect of ddCTP-loaded erythrocytes for anti-HIV treatment. (Upper) Spleen of a control C57BL/6 mouse (A), a mouse infected with LP-BM5 murine retrovirus (C), and a mouse infected with LP-BM5 and treated with ddCTP-loaded erythrocytes every 10 days (B). All spleens were examined 3 months after infection. (Lower) Mediastinal lymphoadenopathy in an infected mouse (A) and in a mouse infected and treated with ddCTP-loaded erythrocytes (B). Reprinted by permission from the author (Magnani et al. 1992b).
Erythrocytes normally, do not extravasate from the circulation into tissues except to the sites of erythrocyte degradation in RES organs. Therefore, drug delivery by erythrocytes is mostly limited to RES and intravascular targets, but many other important pathological sites such as solid tumors and CNS are normally inaccessible to erythrocytes. In addition, drug encapsulation inevitably causes some damage to erythrocytes, which makes it difficult to target loaded erythrocytes away from the RES toward other targets. Because of these limitations, erythrocyte-based systems are primarily focused on delivery of drugs to RES especially macrophages (Lotero et al. 2003; Mishra and Jain 2000; Rossi et al. 2005). In addition to their role in host defense against infections, macrophages themselves are the target site and even act as reservoirs for persistent parasites and viruses in infectious diseases such as leishmaniasis, malaria, AIDS and CMV. Examples of the antiviral and antiparasitic drugs that have been loaded into erythrocytes include primaquine for treatment of malaria (Alanazi et al. 2011), dideoxycytidine and dideoxycytidine 5′-triphosphate (ddCTP) (Magnani et al. 1995; Magnani et al. 1992b), glutathione (Fraternale et al. 2002), and Fludarabine (Magnani et al. 2003) for treatment of AIDS, and the antibiotic amikacin (Briones et al. 2009). Magnani et al. evaluated the efficacy of ddCTP-loaded erythrocytes for inhibition of LP-BMS infectivity in vivo. LP-BMS is a murine leukemia virus that causes an immunodeficiency syndrome in mice that resembles AIDS in humans. As shown in Fig. 4. II, treatment with ddCTP-loaded erythrocytes resulted in significant reduction of lymphoadenopathy (enlargement of lymph nodes) and splenomegaly (enlargement of the spleen), which are reminiscent of the clinical features of HIV infection (Magnani et al. 1992b).
To induce increased phagocytosis, the surface of loaded erythrocytes can be modified by (i) coating with antibodies, a mechanism that promotes phagocytosis of erythrocytes in Epstein-Barr virus (EBV)-associated haemophagocytic syndrome (Eichler et al. 1986; Hsieh et al. 2007), (ii) depleting sialic acid that is a natural mechanism for RES recognition of aged erythrocytes, and (iii) inducing the formation of hemichrome, a mixture of heme and denatured globin that forms in blood disorders such as thalassemia and leads to phagocytosis of erythrocytes by macrophages (Jain. 2000). A few studies have encapsulated anticancer drugs such as 5-fluorouracil (Wang et al. 2009), methotrexate (Jain. 2000), and L-Asparaginase (Agrawal et al. 2013; Kravtzoff et al. 1996) into erythrocytes. L-Asparaginase loaded erythrocytes have shown promising results in preliminary clinical studies for treatment of acute myeloid leukemia (Agrawal et al. 2013). Furthermore, erythrocytes have been used to carry enzyme therapeutics as a replacement therapy for diseases associated with enzyme deficiencies, such as Gaucher’s disease (Beutler et al. 1977; Ninfali et al. 1992). Dexamethasone encapsulated into autologous erythrocytes ex vivo (EryDex) has been developed by EryDel S.p.A., for treatment of ataxia telangiectasia and has successfully completed a Phase II clinical trial (2011, NCT01255358) (Chessa et al. 2014).
As an alternative to encapsulation approaches, drug molecules can also be coupled to the erythrocyte surface using a variety of covalent and non-covalent cross-linkers. Surface coupling approaches can avoid the damage caused by encapsulation procedures and circumvent issues of controlled drug release. However, because the role of erythrocytes is to circulate in the blood stream, they do not have a mechanism to home in to specific sites; therefore, erythrocyte-drug conjugates are mainly directed at pathogen clearance from blood and intravascular pathological conditions such as abnormalities in immune response, coagulation and fibrinolysis (Corinti et al. 2002; Ganguly et al. 2005). Protein antigens coupled to erythrocytes were effective in triggering an immunological response and hence can be explored for development of peptide vaccines (Magnani et al. 1992a). Plasminogen activators (PAs) have also been conjugated to erythrocytes for thromboprophylaxis (Ganguly et al. 2005). In addition, polymeric nanoparticles have been attached ex vivo to erythrocytes as a strategy that combines the advantage of the long circulation half-life of erythrocytes and robustness of polymeric nanoparticles (Chambers and Mitragotri 2007). A recent approach takes advantage of erythrocytes in vivo by the conjugation of drugs to moieties with specific affinity to erythrocytes. ERY1 is a phage display derived peptide that binds the erythrocyte surface with high specificity and has been introduced as a strategy to improve the pharmacokinetic profile of therapeutic proteins (Kontos and Hubbell 2010). In addition, plasminogen activators (PAs) have been fused to a single-chain variable fragment (scFv) that binds glycophorin A (GPA); GPA is a transmembrane sialoglycoprotein that bears the antigens of the MNS blood group system and is present primarily and at a high concentration on the surface of erythrocytes (Zaitsev et al. 2010). Similarly, a monoclonal antibody (mAb) against complement receptor type 1 (CR1) has also been conjugated to erythrocytes (Zaitsev et al. 2006) for thromboprophylaxis (prevention of thrombosis); CR1 is an erythrocyte transmembrane glycoprotein that bears the antigens of the Knop blood group system. As the drugs are anchored to and hitchhike on the erythrocytes in vivo, this approach eliminates the need for erythrocyte isolation and ex vivo manipulation, and hence eliminates the risk of contamination that arises from ex vivo procedures. Furthermore, similar to the in vivo albumin conjugation strategy, this approach has the additional advantage of a smaller size and lower viscosity of drug products leading to easier formulation and administration.
Macrophages
Macrophages are important players of the immune system; they act as the first-line of defense in innate immunity by identifying and phagocytosing pathogens, and play a key role in adaptive immunity by presenting antigens to helper T cells and producing cytokines. They are characterized by their large size (5–50 μm in diameter), irregular shape, remarkable immunological versatility, and more importantly, their ability to ingest foreign matter (Serhan et al. 2010). Macrophages arise in the bone marrow and enter the circulation and migrate and reside virtually in all tissues where they mature into tissue-specific macrophages. Circulating macrophages have a half-life of 1 to 3 days, while tissue-resident macrophages have a much longer half-life, ranging from months to years depending on the tissue they inhabit (James 2006; Luft 2008).
Significant efforts have been invested to exploit macrophages for drug delivery, as they have unique features that are attractive for drug delivery. First, because macrophages are widely present in various tissues and are routinely recruited to diseased sites, they can target a variety of pathological conditions including cancer, atherosclerosis, and other inflammatory, infectious and autoimmune disorders. Second, macrophages phagocytose nanoparticles efficiently, so that they can be efficiently loaded with nanoparticle formulations of drugs. Third, macrophages have inherent hypoxia targeting ability (Lewis and Murdoch 2005) and hence are promising for targeted delivery of anticancer drugs to hypoxic tumor regions that are usually associated with poor drug delivery. Fourth, they exhibit high motility and can migrate across impermeable barriers, including the blood brain barrier (Zhao et al. 2011). Fifth, macrophages are activated by a variety of stimuli at a diseased site, which can trigger release of intracellular contents including drugs.
Macrophages loaded with therapeutics serve as “Trojan Horses”, as they can ferry therapeutics into tumors and other diseased sites. Nanoparticle based formulations are particularly useful for delivering therapeutics to macrophages as they are recognized as foreign particles and are phagocytosed by macrophages. Macrophages can be used for drug delivery as either immediate or intermediate carriers. As immediate carriers, macrophages are isolated and loaded with therapeutics ex vivo and are then re-infused into the host. This approach has been used to load a number of nanoformulated therapeutics such as indinavir nanoparticles, Au nanoshells, and catalase nanozymes for HIV treatment (Dou et al. 2006), solid tumors (Choi et al. 2007) and brain disorders, respectively (Brynskikh et al. 2010; Dou et al. 2009; Haney et al. 2013; Zhao et al. 2011). As intermediate carriers, drug formulations are strategically designed to be recognized and internalized in vivo by macrophages by decoration with surface receptors that target macrophages, such as macrophage mannose receptors (Matsui et al. 2010), integrin receptors (Jain et al. 2003), and scavenger receptors (Etzerodt et al. 2012). However, these intracellular drug loading approaches have some limitations. First, therapeutics loaded into macrophages can cause cytotoxicity and hamper macrophage function. Second, the slow release rate of drugs and their degradation by enzymes inside macrophages are the other two main shortcomings of macrophage delivery. To address these issues, one strategy is to create nanoparticles that “backpack” onto the surface of macrophages, so as to avoid the problems that stem from internalization of therapeutics into macrophages (Doshi et al. 2011; Holden et al. 2010). However, evading phagocytic internalization to achieve long-term immobilization on the macrophages’ surface is challenging and requires precise tuning of the shape, orientation, and mechanical properties of these nanoscale backpacks.
Platelets
Platelets are small, anucleated, discoid fragments with a diameter of 1–4 μm and are derived from megakaryocytes found in the bone marrow. The function of platelets is integral to primary hemostasis, a process in which platelets adhere to damaged endothelium, are activated, release granule constituents and then aggregate to form a thrombus. Platelets also participate in many physiologic and pathogenic processes including antimicrobial host defense, inflammation, angiogenesis, tissue repair/regeneration, and tumor growth and metastasis. Under normal conditions, circulating platelets are present at a concentration of 150,000–450,000/μL blood with a life span of 7–10 days. At any given time, up to one third of the platelets are sequestered as a reserve in the spleen and can be released during periods of physiologic stress. Platelets are the natural carriers of numerous biologically active proteins, which are contained within their cytoplasmic granules and are released upon platelet activation (Manoharan and Manoharan 2003 ; Mohan 2008).
For drug delivery, platelets can be loaded with therapeutic proteins and serve as circulating depots. In an interesting twist to this approach, platelets were loaded with FVIII clotting factor via gene transfer for the treatment of bleeding disorders such as hemophilia and Bernard–Soulier syndrome. Using platelets to deliver coagulation factors takes advantage of their natural function in thrombus formation at sites of vascular injury, so that they allow direct delivery of the drug to the site of injury where they accumulate and release their contents (High 2006; Shi et al. 2007; Shi et al. 2004). In addition to their role in coagulation, platelets interact with and are activated by tumor cells and hence play a multifaceted role in tumor progression and metastasis (Mehta 1984; Nash et al. 2002). Because drug loaded platelets similarly interact with tumor cells, it provides a unique opportunity to use drug-loaded platelets for drug delivery to solid tumors, where platelets can be activated by tumor cells and release their drug payload. Taking this approach, doxorubicin was loaded into platelets; the loaded doxorubicin did not hamper platelet activity and showed a higher antitumor efficiency than the free drug in Ehrlich ascites carcinoma (EAC) bearing mice (Sarkar et al. 2013).
Small molecule-based drug delivery systems
Folic acid
Folic acid (FA), or vitamin B9 is an essential vitamin in eukaryotic cells. Folic acid derivatives act as coenzymes in single carbon transfer reactions that involve the de novo synthesis of purines, synthesis of thymidylate, conversion of homocysteine to methionine, catabolism of histidine to glutamic acid, and interconversion of serine and glycine. Like other vitamins, folic acid cannot be synthesized by the human body and therefore must be supplied through the diet (Caballero 2009).
Cellular uptake of folate is mediated by two mechanisms: (i) direct transport by the reduced folate carrier (RFC); and (ii) receptor-mediated endocytosis by the folate receptor (FR). RFC is a 65 kD transmembrane anionic exchanger that is present in virtually all cells of the body. This carrier has a low affinity for folate (Km = 1–10 μM) and also exhibits substrate preference for reduced folates such as 5-methyltetrahydrofolate (5-MTHF) (Litwack 2008; Zhao et al. 2009). FRs are 38 kD glycoproteins that exist in three isoforms (α, β and γ). FR-α and FR-β are attached to the plasma membrane via a glycosylphosphatidylinositol (GPI) anchor, whereas FR-γ is primarily a secreted protein that is secreted at a low level by the spleen, thymus and bone marrow. The expression of FR-β is limited mostly to the placenta and some hematopoietic cells of the myeloid lineage. FR-α is the most abundant isoform in adults and is expressed primarily in epithelial cells of the choroid plexus, proximal kidney tubules, uterus, lung and breast. Among the three FR isoforms, FR-α possesses the highest affinity for folic acid and 5-MTHF with a KD < 1 nM. Folate bound to the FR is endocytosed to endosomes, where endosomal acidification results in a conformational change of the receptor and enables folate to be released. Membrane-bound FR is recycled back to the cell surface allowing for the uptake additional folates (Antony 1992; Chen et al. 2013).
With the exception of proximal tubules of the kidney and the choroid plexus of the brain, in normal epithelia, FR is expressed on the apical membrane surface of cells where it is inaccessible to blood circulation. However, in malignant and inflammatory disease, epithelial cells lose their polarity and hence FRs can be located at the basal surface. In addition, many types of tumor overexpress FRs (up to 107 FRs/cell) (Parker et al. 2005) to meet their increased need for DNA synthesis and proliferation. In addition to cancer cells, activated macrophages also express a high level of FR. Although activated macrophages primarily serve to protect the body against pathogens, they participate in the development of inflammatory pathologies such as rheumatoid arthritis. These features make folate an attractive targeting ligand to direct drugs to tumors and sites of inflammation. Furthermore, folate-conjugated drugs specifically taken up by FRs rather than RFCs and hence are expected to cause limited toxicity in normal tissues (Fig. 5. I). Although anti-FR monoclonal antibodies can also be used for drug targeting, folate has several advantages as a targeting agent: folate is a small, readily available and inexpensive molecule that has highly stability, lacks immunogenicity and is relatively easy to conjugate to different drugs.
Figure 5.
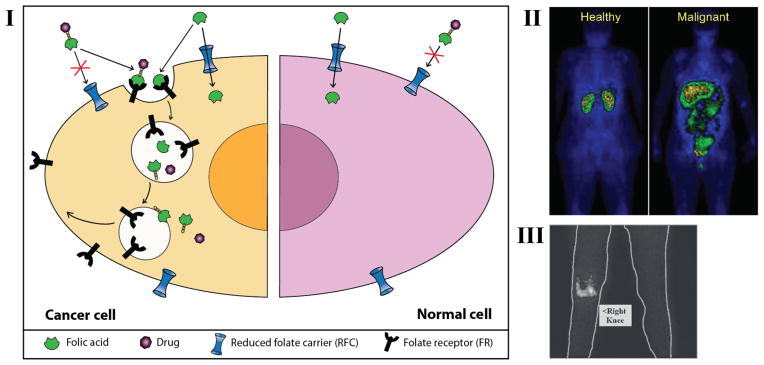
Folic acid-based drug delivery. I: Schematic showing cellular uptake of folic acid and folic acid-drug conjugate in normal cells and cancer cells. II. Scintigraphic image of a healthy individual and an ovarian cancer patient. Images were taken four hours following intravenous administration of 2 mg of 111In-DTPA-folate. While uptake of the 111In-DTPA-folate is primarily confined to the kidneys in the healthy individual, uptake in the ovarian cancer patient is also observed in the malignant tissue. III. Scintigraphic image of a cancer patient diagnosed with arthritis in the right knee obtained 4 hours following intravenous administration of 2 mg 111In-DTPA-folate. This serendipitous observation demonstrated for the first time the potential of folate targeting for imaging and treatment of rheumatoid arthritis (Paulos et al. 2004). Reprinted from Advanced Drug Delivery Reviews, 56, Paulos et al., Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis, 1205–1217, Copyright (2004), with permission from Elsevier and Endocyte.
For drug delivery applications, folate can be conjugated either to drugs or to other drug carriers. The latter approach utilizes synthetic carriers and hence is not summarized here, as the scope of this review is limited to endogenous carriers. Doxorubicin (Yoo and Park 2004), camptothecin (Henne et al. 2006), and mitomycin C (Reddy et al. 2006) are examples of chemotherapeutic drugs that have been directly conjugated to folic acid. Two folate-targeted conjugates, EC145 (Dosio et al. 2010) and EC0225 (Sharma et al. 2010) have been developed by Endocyte Inc. and are currently being evaluated in clinical trials. EC145 is a folate-vinca alkaloid conjugate and is in phase II clinical trials against non small cell lung cancer (2012, NCT01577654), and advanced ovarian and endometrial cancers (2007, NCT00507741). EC0225 is a folate-targeted dual drug conjugate, incorporating two anticancer drugs: a vinca alkaloid unit and mitomycin. It has completed a phase I clinical trial for the treatment of refractory or metastatic tumors (2007, NCT00441870). Several folate-based conjugates, such as 111In-DTPA-folate (Wang et al. 1997) and 99mTc-EC-folate (Xia et al. 2009) have been studied for tumor imaging. In healthy individuals, 111In-DTPA-folate showed accumulation only in kidneys with little or no accumulation in other organs, whereas in ovarian cancer patients, 111In-DTPA-folate also accumulated in the tumor masses in the peritoneal cavity, demonstrating the potential of folate targeting for tumor specific imaging and drug delivery (Fig. 5. II) (Paulos et al. 2004). While studying the ability of 111In-DTPA-folate to image tumors, it was observed that 111In-DTPA-folate also accumulated in the arthritic joints. This serendipitous observation suggested that folic acid can also target drugs to arthritic joints in humans (Fig. 5. III) (Paulos et al. 2004). A folate-targeted imaging agent, FolateScan (99mTc-EC20) has successfully completed a phase II clinical trial to detect inflammation in patients with rheumatoid arthritis (NCT00588393) which further demonstrates that activated macrophages can be targeted with folic acid-drug conjugates for treatment of rheumatoid arthritis and other inflammatory diseases (Xia et al. 2009).
There are some critical considerations in designing folate-targeted drug conjugates. First, drugs need to be attached to folic acid via a spacer to prevent steric hindrance by the drug so as to preserve the affinity of folate for its receptor (Dohmen et al. 2012b; Yoo and Park 2004). Second, for most drugs, it is critical to release the drug from folate following endocytosis in order to be functionally active. This can be accomplished by conjugating the drug to folic acid via a cleavable linker such as disulfide bonds (Henne et al. 2006; Leamon et al. 2005; Satyam 2008; Vlahov et al. 2006), or pH sensitive linkers (Yang et al. 2007). Third, another bottleneck in folate-mediated delivery is the release of therapeutics from the endosome into the cytoplasm. This is particularly an issue for hydrophilic drugs that cannot diffuse out across the endosomal membrane. SiRNA conjugated to folic acid showed enhanced uptake in FR-overexpressing cells, but was trapped in endosomes and hence did not result in a significant silencing effect (Dohmen et al. 2012b; Thomas et al. 2009). To address this issue of efficient endosomal escape, Dohmen et al. attached folic acid to a polycationic backbone composed of oligo(ethanamino)amide, that mediates endosomal escape by the proton sponge effect. They then complexed the folate-targeted polycation with siRNA conjugated to an endosomolytic influenza peptide, that becomes hydrophobic at the acidic pH of the endosome, fuses with and disrupts the endosomal membrane and releases its contents into the cytoplasm (Dohmen et al. 2012a).
Conclusions
The use of blood circulating proteins, lipids, cells, and small molecules for drug delivery is an expanding field of research that is starting to find its way to the clinic. Being biocompatible, biodegradable, and non-immunogenic, these biomolecular and cellular blood components meet many of the requirements of drug carriers. Furthermore, because they circulate throughout the body, they provide easy access to tissues and diseased sites. Most of these blood components act as carriers that transport nutrients, iron, oxygen and signals to the target tissues. For drug delivery applications, these natural carriers can be exploited ex vivo or in vivo. In the ex vivo approach, these natural carriers are harvested from systemic circulation, loaded with the drug and are re-infused into plasma. The in vivo approach, on the other hand, loads the drug molecules onto these carriers in vivo; this is achieved by attaching or loading drug molecules onto entities with an affinity for the carrier, so that following infusion into plasma, they piggyback onto the in vivo carrier and ride to their target site. It is likely that in the near future, we will witness a surge in research to develop new drug formulations based on these native human carriers. In addition, other plasma components such as fibrinogen, tuftsin, haptoglobin, ceruloplasmin, vitamins and hormones are waiting to be explored for their potential in drug delivery applications.
Acknowledgments
We thank Dr. Jim Ballance of PhaseBio Pharmaceuticals for help in tracing the evolution of the albumin fusion technology. We acknowledge the support of NIH grants NIH R01 EB-00188 and NIH R01 EB007205 to A.C.
References
- Wunder A, Stehle G, Sinn H, Schrenk H, HoffBiederbeck D, Bader F, Friedrich E, Peschke P, MaierBorst W, Heene D. Enhanced albumin uptake by rat tumors. International Journal of Oncology. 1997;11(3):497–507. doi: 10.3892/ijo.11.3.497. [DOI] [PubMed] [Google Scholar]
- Adams T, Alanazi F, Lu DR. Safety and Utilization of Blood Components as Therapeutic Delivery Systems. Current Pharmaceutical Biotechnology. 2003;4(5):275–282. doi: 10.2174/1389201033489720. [DOI] [PubMed] [Google Scholar]
- Agrawal V, Hee Woo J, Borthakur G, Kantarjian H, Frankel EA. Red Blood Cell-Encapsulated L-Asparaginase: Potential Therapy of Patients with Asparagine Synthetase Deficient Acute Myeloid Leukemia. Protein and Peptide Letters. 2013;20(4):392–402. [PubMed] [Google Scholar]
- Alanazi F, Harisa G-D, Maqboul A, Abdel-Hamid M, Neau S, Alsarra I. Biochemically altered human erythrocytes as a carrier for targeted delivery of primaquine: an In Vitro study. Archives of Pharmacal Research. 2011;34(4):563–571. doi: 10.1007/s12272-011-0406-7. [DOI] [PubMed] [Google Scholar]
- Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1(3):219–227. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood M. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature biotechnology. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Amersi F, Farmer D, Shaw G, Kato H, Coito A, Kaldas F, Zhao D, Lassman C, Melinek J, Ma J, et al. P-selectin glycoprotein ligand-1 (rPSGL-Ig)-mediated blockade of CD62 selectin molecules protects rat steatotic liver grafts from ischemia/reperfusion injury. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2002;2(7):600–608. doi: 10.1034/j.1600-6143.2002.20704.x. [DOI] [PubMed] [Google Scholar]
- Anderson C, Chaudhury C, Kim J, Bronson C, Wani M, Mohanty S. Perspective-- FcRn transports albumin: relevance to immunology and medicine. Trends in immunology. 2006;27(7):343–348. doi: 10.1016/j.it.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Antony AC. The biological chemistry of folate receptors. Blood. 1992;79(11):2807–20. [PubMed] [Google Scholar]
- Baggio L, Huang Q, Brown T, Drucker D. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53(9):2492–2500. doi: 10.2337/diabetes.53.9.2492. [DOI] [PubMed] [Google Scholar]
- Ballance D, James Fusion proteins containing n-terminal fragments of human serum albumin 1990 [Google Scholar]
- Batrakova EV, Gendelman HE, Kabanov AV. Cell-mediated drug delivery. Expert Opin Drug Deliv. 2011;8(4):415–33. doi: 10.1517/17425247.2011.559457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocq NC, Pun SH, Jensen GS, Davis ME. Transferrin-Containing, Cyclodextrin Polymer-Based Particles for Tumor-Targeted Gene Delivery. Bioconjugate Chemistry. 2003;14(6):1122–1132. doi: 10.1021/bc034125f. [DOI] [PubMed] [Google Scholar]
- Beutler E, Dale G, Guinto D, Kuhl W. Enzyme replacement therapy in Gaucher’s disease: preliminary clinical trial of a new enzyme preparation. Proceedings of the National Academy of Sciences of the United States of America. 1977;74(10):4620–4623. doi: 10.1073/pnas.74.10.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer U, Roth T, Schumacher P, Maier G, Unold A, Frahm A, Fiebig H, Unger C, Kratz F. Synthesis and in vitro efficacy of transferrin conjugates of the anticancer drug chlorambucil. Journal of medicinal chemistry. 1998;41(15):2701–2708. doi: 10.1021/jm9704661. [DOI] [PubMed] [Google Scholar]
- Bracewell C, Isaacs J, Emery P, Ng W. Atacicept, a novel B cell-targeting biological therapy for the treatment of rheumatoid arthritis. Expert opinion on biological therapy. 2009;9(7):909–919. doi: 10.1517/14712590903033919. [DOI] [PubMed] [Google Scholar]
- Brambell FWR. The transmission of passive immunity from mother to young. North-Holland Pub. Co; 1970. [Google Scholar]
- Briones E, Colino CI, Millán CG, Lanao JM. Increasing the selectivity of amikacin in rat peritoneal macrophages using carrier erythrocytes. European Journal of Pharmaceutical Sciences. 2009;38(4):320–324. doi: 10.1016/j.ejps.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Brody T. Nutritional Biochemistry. Academic Press; 1999. [Google Scholar]
- Brynskikh A, Zhao Y, Mosley R, Li S, Boska M, Klyachko N, Kabanov A, Gendelman H, Batrakova E. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson’s disease. Nanomedicine (London, England) 2010;5(3):379–396. doi: 10.2217/nnm.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero B. Guide to Nutritional Supplements. Academic Press; 2009. p. 548. [Google Scholar]
- Candiani C, Tommasi M, Fracasso G, Lorenzetti I, Adami A, Benoni G, Tridente G, Colombatti M. Pharmacokinetics of intrathecal transferrin-ricin a chain immunotoxin. Life sciences. 2001;69(3):335–346. doi: 10.1016/s0024-3205(01)01118-3. [DOI] [PubMed] [Google Scholar]
- Capon D, Chamow S, Mordenti J, Marsters S, Gregory T, Mitsuya H, Byrn R, Lucas C, Wurm F, Groopman J. Designing CD4 immunoadhesins for AIDS therapy. Nature. 1989;337(6207):525–531. doi: 10.1038/337525a0. [DOI] [PubMed] [Google Scholar]
- Chambers E, Mitragotri S. Long Circulating Nanoparticles via Adhesion on Red Blood Cells: Mechanism and Extended Circulation. Experimental Biology and Medicine. 2007;232(7):958–966. [PubMed] [Google Scholar]
- Chapman T, Perry C. Insulin detemir: a review of its use in the management of type 1 and 2 diabetes mellitus. Drugs. 2004;64(22):2577–2595. doi: 10.2165/00003495-200464220-00008. [DOI] [PubMed] [Google Scholar]
- Chaudhury C, Mehnaz S, Robinson J, Hayton W, Pearl D, Roopenian D, Anderson C. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. The Journal of experimental medicine. 2003;197(3):315–322. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Ke J, Zhou XE, Yi W, Brunzelle JS, Li J, Yong E-L, Xu HE, Melcher K. Structural basis for molecular recognition of folic acid by folate receptors. Nature. 2013 doi: 10.1038/nature12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Corbin IR, Li H, Cao W, Glickson JD, Zheng G. Ligand Conjugated Low-Density Lipoprotein Nanoparticles for Enhanced Optical Cancer Imaging in Vivo. Journal of the American Chemical Society. 2007;129(18):5798–5799. doi: 10.1021/ja069336k. [DOI] [PubMed] [Google Scholar]
- Cheng WTaK. Advanced Drug Delivery in Cancer Therapy. In: Ashim Mitra CHL, Cheng Kun, editors. Advanced Drug Delivery. John Wiley & Sons; 2013. [Google Scholar]
- Chessa L, Leuzzi V, Plebani A, Soresina A, Micheli R, DAgnano D, Venturi T, Molinaro A, Fazzi E, Marini M, et al. Intra-Erythrocyte Infusion of Dexamethasone Reduces Neurological Symptoms in Ataxia Teleangiectasia Patients: Results of a Phase 2 Trial. Orphanet Journal of Rare Diseases. 2014;9(1):5. doi: 10.1186/1750-1172-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M-R, Stanton-Maxey K, Stanley J, Levin C, Bardhan R, Akin D, Badve S, Sturgis J, Robinson J, Bashir R, et al. A cellular Trojan Horse for delivery of therapeutic nanoparticles into tumors. Nano letters. 2007;7(12):3759–3765. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- Corbin I. Ligand-Coupled Lipoprotein for Ovarian Cancer-Specific Drug Delivery. In: Malek A, Tchernitsa O, editors. Ovarian Cancer. Humana Press; 2013. pp. 467–480. [DOI] [PubMed] [Google Scholar]
- Corinti S, Chiarantini L, Dominici S, Laguardia ME, Magnani M, Girolomoni G. Erythrocytes deliver Tat to interferon-γ-treated human dendritic cells for efficient initiation of specific type 1 immune responses in vitro. Journal of Leukocyte Biology. 2002;71(4):652–658. [PubMed] [Google Scholar]
- Dasgupta A, Wahed A. Clinical Chemistry, Immunology and Laboratory Quality Control: A Comprehensive Review for Board Preparation, Certification and Clinical Practice. Academic Press; 2013. [Google Scholar]
- Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, Tao C, De T, Beals B, Dykes D, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clinical Cancer Research. 2006;12(4):1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- Dierling A, Cui Z. Targeting primaquine into liver using chylomicron emulsions for potential vivax malaria therapy. International journal of pharmaceutics. 2005;303(1–2):143–152. doi: 10.1016/j.ijpharm.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Dohmen C, Edinger D, Fröhlich T, Schreiner L, Lächelt U, Troiber C, Rädler J, Hadwiger P, Vornlocher H-P, Wagner E. Nanosized Multifunctional Polyplexes for Receptor-Mediated SiRNA Delivery. ACS Nano. 2012a;6(6):5198–5208. doi: 10.1021/nn300960m. [DOI] [PubMed] [Google Scholar]
- Dohmen C, Fröhlich T, Lächelt U, Röhl I, Vornlocher H-P, Hadwiger P, Wagner E. Defined Folate-PEG-siRNA Conjugates for Receptor-specific Gene Silencing. Molecular Therapy — Nucleic Acids. 2012b;1(1) doi: 10.1038/mtna.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi N, Swiston A, Gilbert J, Alcaraz M, Cohen R, Rubner M, Mitragotri S. Cell-based drug delivery devices using phagocytosis-resistant backpacks. Advanced materials (Deerfield Beach, Fla) 2011;23(12):9. doi: 10.1002/adma.201004074. [DOI] [PubMed] [Google Scholar]
- Dosio F, Arpicco S, Stella B, Brusa P, Cattel L. Folate-mediated targeting of albumin conjugates of paclitaxel obtained through a heterogeneous phase system. International journal of pharmaceutics. 2009;382(1–2):117–123. doi: 10.1016/j.ijpharm.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Dosio F, Milla P, Cattel L. EC-145, a folate-targeted Vinca alkaloid conjugate for the potential treatment of folate receptor-expressing cancers. Current opinion in investigational drugs (London, England : 2000) 2010;11(12):1424–1433. [PubMed] [Google Scholar]
- Dou H, Destache C, Morehead J, Mosley R, Boska M, Kingsley J, Gorantla S, Poluektova L, Nelson J, Chaubal M, et al. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood. 2006;108(8):2827–2835. doi: 10.1182/blood-2006-03-012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou H, Grotepas C, McMillan J, Destache C, Chaubal M, Werling J, Kipp J, Rabinow B, Gendelman H. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. Journal of immunology (Baltimore, Md: 1950) 2009;183(1):661–669. doi: 10.4049/jimmunol.0900274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey P, Singodia D, Verma R, Vyas S. RGD modified albumin nanospheres for tumour vasculature targeting. The Journal of pharmacy and pharmacology. 2011;63(1):33–40. doi: 10.1111/j.2042-7158.2010.01180.x. [DOI] [PubMed] [Google Scholar]
- Dwyer M, Huang A, Pan C, Lazarus R. Expression and characterization of a DNase I-Fc fusion enzyme. The Journal of biological chemistry. 1999;274(14):9738–9743. doi: 10.1074/jbc.274.14.9738. [DOI] [PubMed] [Google Scholar]
- Economides A, Carpenter L, Rudge J, Wong V, Koehler-Stec E, Hartnett C, Pyles E, Xu X, Daly T, Young M, et al. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nature medicine. 2003;9(1):47–52. doi: 10.1038/nm811. [DOI] [PubMed] [Google Scholar]
- Eichler HG, Gasic S, Bauer K, Korn A, Bacher S. In vivo clearance of antibody-sensitized human drug carrier erythrocytes. Clin Pharmacol Ther. 1986;40(3):300–3. doi: 10.1038/clpt.1986.180. [DOI] [PubMed] [Google Scholar]
- Esmaeili F, Dinarvand R, Ghahremani M, Amini M, Rouhani H, Sepehri N, Ostad S, Atyabi F. Docetaxel-albumin conjugates: preparation, in vitro evaluation and biodistribution studies. Journal of pharmaceutical sciences. 2009;98(8):2718–2730. doi: 10.1002/jps.21599. [DOI] [PubMed] [Google Scholar]
- Etzerodt A, Maniecki MB, Graversen JH, Møller HJ, Torchilin VP, Moestrup SK. Efficient intracellular drug-targeting of macrophages using stealth liposomes directed to the hemoglobin scavenger receptor CD163. Journal of Controlled Release. 2012;160(1):72–80. doi: 10.1016/j.jconrel.2012.01.034. [DOI] [PubMed] [Google Scholar]
- Farmer D, Shen X-D, Amersi F, Anselmo D, Ma J, Ke B, Gao F, Dry S, Fernandez S, Shaw G, et al. CD62 blockade with P-Selectin glycoprotein ligand-immunoglobulin fusion protein reduces ischemia-reperfusion injury after rat intestinal transplantation. Transplantation. 2005;79(1):44–51. doi: 10.1097/01.tp.0000146965.64706.e8. [DOI] [PubMed] [Google Scholar]
- Favre G. Targeting of tumor cells by low density lipoproteins: principle and use of ellipticin derivatives. Comptes rendus des seances de la Societe de biologie et de ses filiales. 1992;186(1–2):73–87. [PubMed] [Google Scholar]
- Fiehn C, Kratz F, Sass G, Müller-Ladner U, Neumann E. Targeted drug delivery by in vivo coupling to endogenous albumin: an albumin-binding prodrug of methotrexate (MTX) is better than MTX in the treatment of murine collagen-induced arthritis. Annals of the rheumatic diseases. 2008;67(8):1188–1191. doi: 10.1136/ard.2007.086843. [DOI] [PubMed] [Google Scholar]
- Firestone RA. Low-Density Lipoprotein as a Vehicle for Targeting Antitumor Compounds to Cancer Cells. Bioconjugate Chemistry. 1994;5(2):105–113. doi: 10.1021/bc00026a002. [DOI] [PubMed] [Google Scholar]
- Fleer R, Yeh P, Amellal N, Maury I, Fournier A, Bacchetta F, Baduel P, Jung G, L’Hôte H, Becquart J. Stable multicopy vectors for high-level secretion of recombinant human serum albumin by Kluyveromyces yeasts. Bio/technology (Nature Publishing Company) 1991;9(10):968–975. doi: 10.1038/nbt1091-968. [DOI] [PubMed] [Google Scholar]
- Framson P, Sage E. SPARC and tumor growth: where the seed meets the soil? Journal of cellular biochemistry. 2004;92(4):679–690. doi: 10.1002/jcb.20091. [DOI] [PubMed] [Google Scholar]
- Fraternale A, Casabianca A, Orlandi C, Cerasi A, Chiarantini L, Brandi G, Magnani M. Macrophage protection by addition of glutathione (GSH)-loaded erythrocytes to AZT and DDI in a murine AIDS model. Antiviral Research. 2002;56(3):263–272. doi: 10.1016/s0166-3542(02)00128-6. [DOI] [PubMed] [Google Scholar]
- Fu Q, Sun J, Zhang W, Sui X, Yan Z, He Z. Nanoparticle albumin-bound (NAB) technology is a promising method for anti-cancer drug delivery. Recent patents on anti-cancer drug discovery. 2009;4(3):262–272. doi: 10.2174/157489209789206869. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Krasik T, Medinilla S, Bdeir K, Cines DB, Muzykantov VR, Murciano JC. Blood Clearance and Activity of Erythrocyte-Coupled Fibrinolytics. Journal of Pharmacology and Experimental Therapeutics. 2005;312(3):1106–1113. doi: 10.1124/jpet.104.075770. [DOI] [PubMed] [Google Scholar]
- Gradishar W. Albumin-bound paclitaxel: a next-generation taxane. Expert opinion on pharmacotherapy. 2006;7(8):1041–1053. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- Gupta Y, Jain A, Jain SK. Transferrin-conjugated solid lipid nanoparticles for enhanced delivery of quinine dihydrochloride to the brain. Journal of Pharmacy and Pharmacology. 2007;59(7):935–940. doi: 10.1211/jpp.59.7.0004. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Tajerzadeh H, Dehpour A-R, Rouini M-R, Ejtemaee-Mehr S. In vitro characterization of human intact erythrocytes loaded by enalaprilat. Drug Delivery. 2001;8(4):223–230. doi: 10.1080/107175401317245903. [DOI] [PubMed] [Google Scholar]
- Hammel M, Laggner P, Prassl R. Structural characterisation of nucleoside loaded low density lipoprotein as a main criterion for the applicability as drug delivery system. Chemistry and physics of lipids. 2003;123(2):193–207. doi: 10.1016/s0009-3084(03)00002-1. [DOI] [PubMed] [Google Scholar]
- Han J-H, Oh Y-K, Kim D-S, Kim C-K. Enhanced hepatocyte uptake and liver targeting of methotrexate using galactosylated albumin as a carrier. International journal of pharmaceutics. 1999;188(1):39–47. doi: 10.1016/s0378-5173(99)00206-9. [DOI] [PubMed] [Google Scholar]
- Haney M, Zhao Y, Harrison E, Mahajan V, Ahmed S, He Z, Suresh P, Hingtgen S, Klyachko N, Mosley R, et al. Specific transfection of inflamed brain by macrophages: a new therapeutic strategy for neurodegenerative diseases. PloS one. 2013;8(4) doi: 10.1371/journal.pone.0061852. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Henne WA, Doorneweerd DD, Hilgenbrink AR, Kularatne SA, Low PS. Synthesis and activity of a folate peptide camptothecin prodrug. Bioorganic & Medicinal Chemistry Letters. 2006;16(20):5350–5355. doi: 10.1016/j.bmcl.2006.07.076. [DOI] [PubMed] [Google Scholar]
- High K. The leak stops here: platelets as delivery vehicles for coagulation factors. The Journal of clinical investigation. 2006;116(7):1840–1842. doi: 10.1172/JCI29193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson L, Skeaff C, Fielding B. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Progress in lipid research. 2008;47(5):348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Hoffman H, Throne M, Amar N, Sebai M, Kivitz A, Kavanaugh A, Weinstein S, Belomestnov P, Yancopoulos G, Stahl N, et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis and rheumatism. 2008;58(8):2443–2452. doi: 10.1002/art.23687. [DOI] [PubMed] [Google Scholar]
- Holden CA, Yuan Q, Yeudall WA, Lebman DA, Yan H. Surface engineering of macrophages with nanoparticles to generate a cell–nanoparticle hybrid vehicle for hypoxia-targeted drug delivery. International Journal of Nanomedicine. 2010;5:25–36. [PMC free article] [PubMed] [Google Scholar]
- Hsieh W-C, Chang Y, Hsu M-C, Lan B-S, Hsiao G-C, Chuang H-C, Su I-J. Emergence of Anti-Red Blood Cell Antibodies Triggers Red Cell Phagocytosis by Activated Macrophages in a Rabbit Model of Epstein-Barr Virus-Associated Hemophagocytic Syndrome. The American Journal of Pathology. 2007;170(5):1629–1639. doi: 10.2353/ajpath.2007.060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippoliti R, Lendaro E, D’Agostino I, Fiani M, Guidarini D, Vestri S, Benedetti P, Brunori M. A chimeric saporin-transferrin conjugate compared to ricin toxin: role of the carrier in intracellular transport and toxicity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1995;9(12):1220–1225. doi: 10.1096/fasebj.9.12.7672515. [DOI] [PubMed] [Google Scholar]
- Jain S, Mishra V, Singh P, Dubey PK, Saraf DK, Vyas SP. RGD-anchored magnetic liposomes for monocytes/neutrophils-mediated brain targeting. International Journal of Pharmaceutics. 2003;261(1–2):43–55. doi: 10.1016/s0378-5173(03)00269-2. [DOI] [PubMed] [Google Scholar]
- Jain PMN. Surface Modified Methotrexate Loaded Erythrocytes for Enhanced Macrophage Uptake. 2000;8(4):217–224. doi: 10.3109/10611860008997900. [DOI] [PubMed] [Google Scholar]
- James K. And You Thought You Were Safe. Papermill Press Ltd; 2006. [Google Scholar]
- Jayant K, Tamara M. Polymer–drug conjugates: Progress in polymeric prodrugs. Progress in Polymer Science. 2006:31. [Google Scholar]
- Jones D. The important of surface area/volume ratio to the rate of oxygen uptake by red cells. The Journal of General Physiology. 1979;74(5):643–646. doi: 10.1085/jgp.74.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan J, Murciano J, Lotero A, Herraez A, Diez J. In vitro properties and organ uptake of rat band 3 cross-linked erythrocytes. Biochimie. 1997;79(1):53–61. doi: 10.1016/s0300-9084(97)87625-0. [DOI] [PubMed] [Google Scholar]
- José B, Sandra MS. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nature Reviews Cancer. 2009;9(7):463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- Kader A, Pater A. Loading anticancer drugs into HDL as well as LDL has little affect on properties of complexes and enhances cytotoxicity to human carcinoma cells. Journal of controlled release : official journal of the Controlled Release Society. 2002;80(1–3):29–44. doi: 10.1016/s0168-3659(01)00536-3. [DOI] [PubMed] [Google Scholar]
- Kapur S, Bonk M. Rilonacept (arcalyst), an interleukin-1 trap for the treatment of cryopyrin-associated periodic syndromes. P & T : a peer-reviewed journal for formulary management. 2009;34(3):138–141. [PMC free article] [PubMed] [Google Scholar]
- Kenanova V, Olafsen T, Crow DM, Sundaresan G, Subbarayan M, Carter NH, Ikle DN, Yazaki PJ, Chatziioannou AF, Gambhir SS, et al. Tailoring the Pharmacokinetics and Positron Emission Tomography Imaging Properties of Anti–Carcinoembryonic Antigen Single-Chain Fv-Fc Antibody Fragments. Cancer Research. 2005;65(2):622–631. [PMC free article] [PubMed] [Google Scholar]
- Kontos S, Hubbell J. Improving protein pharmacokinetics by engineering erythrocyte affinity. Molecular pharmaceutics. 2010;7(6):2141–2147. doi: 10.1021/mp1001697. [DOI] [PubMed] [Google Scholar]
- Kratz F. INNO-206 (DOXO-EMCH), an Albumin-Binding Prodrug of Doxorubicin Under Development for Phase II Studies. Current Bioactive Compounds. 2011;7(1):33–38. [Google Scholar]
- Kratz F, Warnecke A, Scheuermann K, Stockmar C, Schwab J, Lazar P, Drückes P, Esser N, Drevs J, Rognan D, et al. Probing the Cysteine-34 Position of Endogenous Serum Albumin with Thiol-Binding Doxorubicin Derivatives. Improved Efficacy of an Acid-Sensitive Doxorubicin Derivative with Specific Albumin-Binding Properties Compared to That of the Parent Compound. Journal of Medicinal Chemistry. 2002;45(25):5523–5533. doi: 10.1021/jm020276c. [DOI] [PubMed] [Google Scholar]
- Kravtzoff R, Ropars C, Desbois I, Chassaigne M, Lamagnere JP, Colombat P, Muh JP, Valat C. Improved pharmacodynamics of l-asparaginase-loaded in human red blood cells. European Journal of Clinical Pharmacology. 1996;49(6):465–470. doi: 10.1007/BF00195932. [DOI] [PubMed] [Google Scholar]
- Krishnaveni G, Krishnamoorthy B, Muthukumaran M. Future Prospectus in Targetting Drug Delivery: A Review on Resealed Erytrocytes - A Promising Carrier Scholars Academic Journal of Pharmacy. 2013;2(2):81–88. [Google Scholar]
- Kubota T, Matsushita T, Niwa R, Kumagai I, Nakamura K. Novel Anti-Tn Single-Chain Fv-Fc Fusion Proteins Derived from Immunized Phage Library and Antibody Fc Domain. Anticancer Research. 2010;30(9):3397–3405. [PubMed] [Google Scholar]
- Kumar M, Hunag Y, Glinka Y, Prud’Homme G, Wang Q. Gene therapy of diabetes using a novel GLP-1/IgG1-Fc fusion construct normalizes glucose levels in db/db mice. Gene therapy. 2006 doi: 10.1038/sj.gt.3302836. [DOI] [PubMed] [Google Scholar]
- Kuter D, Bussel J, Lyons R, Pullarkat V, Gernsheimer T, Senecal F, Aledort L, George J, Kessler C, Sanz M, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371(9610):395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]
- Kwon YM, Chung HS, Moon C, Yockman J, Park YJ, Gitlin SD, David AE, Yang VC. L-Asparaginase encapsulated intact erythrocytes for treatment of acute lymphoblastic leukemia (ALL) Journal of Controlled Release. 2009;139(3):182–189. doi: 10.1016/j.jconrel.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H, Nakase I, Lacoste E, Singh N, Sasaki T. Artemisinin-transferrin conjugate retards growth of breast tumors in the rat. Anticancer research. 2009;29(10):3807–3810. [PubMed] [Google Scholar]
- Lai H, Sasaki T, Singh N, Messay A. Effects of artemisinin-tagged holotransferrin on cancer cells. Life sciences. 2005;76(11):1267–1279. doi: 10.1016/j.lfs.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Laske D, Youle R, Oldfield E. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nature medicine. 1997;3(12):1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- Lawn R, Adelman J, Bock S, Franke A, Houck C, Najarian R, Seeburg P, Wion K. The sequence of human serum albumin cDNA and its expression in E. coli. Nucleic acids research. 1981;9(22):6103–6114. doi: 10.1093/nar/9.22.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamon CP, Reddy JA, Vlahov IR, Vetzel M, Parker N, Nicoson JS, Xu L-C, Westrick E. Synthesis and Biological Evaluation of EC72: A New Folate-Targeted Chemotherapeutic. Bioconjugate Chemistry. 2005;16(4):803–811. doi: 10.1021/bc049709b. [DOI] [PubMed] [Google Scholar]
- Lee C, Woo J, Cho K, Kang S, Kang S, Choi Y. Expression and characterization of human growth hormone-Fc fusion proteins for transcytosis induction. Biotechnology and applied biochemistry. 2007;46(Pt 4):211–217. doi: 10.1042/BA20060083. [DOI] [PubMed] [Google Scholar]
- Lethaby A, Lopez-Olivo MA, Maxwell L, Burls A, Tugwell P, Wells GA. Etanercept for the treatment of rheumatoid arthritis. Cochrane Database of Systematic Reviews. 2013;(5) doi: 10.1002/14651858.CD004525.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, Murdoch C. Macrophage Responses to Hypoxia: Implications for Tumor Progression and Anti-Cancer Therapies. The American Journal of Pathology. 2005;167(3):627–635. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, He H, Jia X, Lu W-L, Lou J, Wei Y. A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials. 2012;33(15):3899–3908. doi: 10.1016/j.biomaterials.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Lichtenstein AH, Jones PJH. Lipids: Absorption and Transport. In: Erdman JW Jr, IAM, Zeisel SH, editors. Present Knowledge in Nutrition. John Wiley & Sons; 2012. [Google Scholar]
- Liong M, Lu J, Kovochich M, Xia T, Ruehm S, Nel A, Tamanoi F, Zink J. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS nano. 2008;2(5):889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwack G. Folic Acid and Folates. Academic Press; 2008. [DOI] [PubMed] [Google Scholar]
- Lotero L, Olmos G, Diez J. Delivery to macrophages and toxic action of etoposide carried in mouse red blood cells. Biochimica et biophysica acta. 2003;1620(1–3):160–166. doi: 10.1016/s0304-4165(02)00536-6. [DOI] [PubMed] [Google Scholar]
- Low SC. Heterodimeric follicle stimulating hormone-fc (fsh-fc) fusion proteins for the treatment of infertility 2005 [Google Scholar]
- Lubgan D, Jozwiak Z, Grabenbauer G, Distel L. Doxorubicin-transferrin conjugate selectively overcomes multidrug resistance in leukaemia cells. Cellular & molecular biology letters. 2009;14(1):113–127. doi: 10.2478/s11658-008-0037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft F. Curbing the appetites of the big eaters. Journal of molecular medicine (Berlin, Germany) 2008;86(4):351–352. doi: 10.1007/s00109-008-0321-7. [DOI] [PubMed] [Google Scholar]
- Luo L-Z, Jin H-W, Huang H-Q. Transferrin-cisplatin specifically deliver cisplatin to HepG2 cells in vitro and enhance cisplatin cytotoxicity. Journal of proteomics. 2012a;77:237–250. doi: 10.1016/j.jprot.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Luo R, Mutukumaraswamy S, Venkatraman S, Neu B. Engineering of erythrocyte-based drug carriers: control of protein release and bioactivity. Journal of materials science Materials in medicine. 2012b;23(1):63–71. doi: 10.1007/s10856-011-4485-2. [DOI] [PubMed] [Google Scholar]
- MacEwan S, Callahan D, Chilkoti A. Stimulus-responsive macromolecules and nanoparticles for cancer drug delivery. Nanomedicine (London, England) 2010;5(5):793–806. doi: 10.2217/nnm.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani M, Balestra E, Fraternale A, Aquaro S, Paiardini M, Cervasi B, Casabianca A, Garaci E, Perno C-F. Drug-loaded red blood cell-mediated clearance of HIV-1 macrophage reservoir by selective inhibition of STAT1 expression. Journal of Leukocyte Biology. 2003;74(5):764–771. doi: 10.1189/jlb.0403156. [DOI] [PubMed] [Google Scholar]
- Magnani M, Cassabianca A, Rossi L, Fraternale A, Brandi G, Silvotti L, Piedimonte G. Inhibition of HIV-1 and LP-BM5 replication in macrophages by dideoxycytidine and dideoxycytidine 5′-triphosphate. Antiviral Chemistry and Chemotherapy. 1995;6(5):312–319. [Google Scholar]
- Magnani M, Chiarantini L, Vittoria E, Mancini U, Rossi L, Fazi A. Red blood cells as an antigen-delivery system. Biotechnology and applied biochemistry. 1992a;16(2):188–194. [PubMed] [Google Scholar]
- Magnani M, Rossi L, Brandi G, Schiavano GF, Montroni M, Piedimonte G. Targeting antiretroviral nucleoside analogues in phosphorylated form to macrophages: in vitro and in vivo studies. Proceedings of the National Academy of Sciences. 1992b;89(14):6477–6481. doi: 10.1073/pnas.89.14.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoharan A, Manoharan S. Essential of Clinical Haematology. Jaypee Brothers; 2003. [Google Scholar]
- Matsui M, Shimizu Y, Kodera Y, Kondo E, Ikehara Y, Nakanishi H. Targeted delivery of oligomannose-coated liposome to the omental micrometastasis by peritoneal macrophages from patients with gastric cancer. Cancer Science. 2010;101(7):1670–1677. doi: 10.1111/j.1349-7006.2010.01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–92. [PubMed] [Google Scholar]
- Mehta P. Potential role of platelets in the pathogenesis of tumor metastasis. Blood. 1984;63(1):55–63. [PubMed] [Google Scholar]
- Menjoge A, Kannan R, Tomalia D. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug discovery today. 2010;15(5–6):171–185. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Mezo A, McDonnell K, Low S, Song J, Reidy T, Lu Q, Amari J, Hoehn T, Peters R, Dumont J, et al. Atrial natriuretic peptide-Fc, ANP-Fc, fusion proteins: semisynthesis, in vitro activity and pharmacokinetics in rats. Bioconjugate chemistry. 2012;23(3):518–526. doi: 10.1021/bc200592c. [DOI] [PubMed] [Google Scholar]
- Millington T, Koulmanda M, Ng C, Boskovic S, Nadazdin O, Benichou G, Zheng X, Strom T, Madsen J. Effects of an agonist interleukin-2/Fc fusion protein, a mutant antagonist interleukin-15/Fc fusion protein, and sirolimus on cardiac allograft survival in non-human primates. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2012;31(4):427–435. doi: 10.1016/j.healun.2012.01.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P, Jain N. Surface Modified Methotrexate Loaded Erythrocytes for Enhanced Macrophage Uptake. Journal of Drug Targeting. 2000;8(4):217–224. doi: 10.3109/10611860008997900. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Proteomics retrenches. Nature biotechnology. 2010;28(7):665–670. doi: 10.1038/nbt0710-665. [DOI] [PubMed] [Google Scholar]
- Mohan H. Essential Pathology for Dental Students. Jaypee Brothers; 2008. [Google Scholar]
- Nash G, Turner L, Scully M, Kakkar A. Platelets and cancer. The lancet oncology. 2002;3(7):425–430. doi: 10.1016/s1470-2045(02)00789-1. [DOI] [PubMed] [Google Scholar]
- Nikanjam M, Blakely E, Bjornstad K, Shu X, Budinger T, Forte T. Synthetic nano-low density lipoprotein as targeted drug delivery vehicle for glioblastoma multiforme. International journal of pharmaceutics. 2007;328(1):86–94. doi: 10.1016/j.ijpharm.2006.07.046. [DOI] [PubMed] [Google Scholar]
- Ninfali P, Rossi L, Baronciani L, Tirillini B, Ropars C, Magnani M. Acetaldehyde, ethanol and acetone concentrations in blood of alcohol-treated mice receiving aldehyde dehydrogenase-loaded erythrocytes. Alcohol and Alcoholism. 1992;27(1):19–23. [PubMed] [Google Scholar]
- Noonan M. Celtic Pharma Terminates Transmid Trial KSB311R/CIII/001 2007 [Google Scholar]
- Oh S, Kim B, Singh N, Lai H, Sasaki T. Synthesis and anti-cancer activity of covalent conjugates of artemisinin and a transferrin-receptor targeting peptide. Cancer letters. 2009;274(1):33–39. doi: 10.1016/j.canlet.2008.08.031. [DOI] [PubMed] [Google Scholar]
- Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Analytical Biochemistry. 2005;338(2):284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Paulos CM, Turk MJ, Breur GJ, Low PS. Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis. Advanced Drug Delivery Reviews. 2004;56(8):1205–1217. doi: 10.1016/j.addr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Peppel K, Crawford D, Beutler B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. The Journal of experimental medicine. 1991;174(6):1483–1489. doi: 10.1084/jem.174.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R, Toby G, Lu Q, Liu T, Kulman J, Low S, Bitonti A, Pierce G. Biochemical and functional characterization of a recombinant monomeric factor VIII-Fc fusion protein. Journal of thrombosis and haemostasis : JTH. 2013;11(1):132–141. doi: 10.1111/jth.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T. Biochemistry, genetics, and medical applications. San Diego, CA: Academic Press; 1996. All about albumin. [Google Scholar]
- Picha K, Huang C, Bugelski P, O’Neil K. Engineering Peptide Therapeutics Using MIMETIBODY™ Technology. Methods in molecular biology (Clifton, NJ) 2014;1088:125–145. doi: 10.1007/978-1-62703-673-3_9. [DOI] [PubMed] [Google Scholar]
- Pitt E, Johnson CM, Lewis DA, Jenner DA, Offord RE. Encapsulation of drugs in intact erythrocytes: An intravenous delivery system. Biochemical Pharmacology. 1983;32(22):3359–3368. doi: 10.1016/0006-2952(83)90363-5. [DOI] [PubMed] [Google Scholar]
- Porth C. Essentials of Pathophysiology: Concepts of Altered Health States. Lippincott Williams & Wilkins; 2011. [Google Scholar]
- Quirk A, Geisow M, Woodrow J, Burton S, Wood P, Sutton A, Johnson R, Dodsworth N. Production of recombinant human serum albumin from Saccharomyces cerevisiae. Biotechnology and applied biochemistry. 1989;11(3):273–287. [PubMed] [Google Scholar]
- Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochemical pharmacology. 2011;81(10):1171–1182. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Reddy J, Westrick E, Vlahov I, Howard S, Santhapuram H, Leamon C. Folate receptor specific anti-tumor activity of folate–mitomycin conjugates. Cancer Chemotherapy and Pharmacology. 2006;58(2):229–236. doi: 10.1007/s00280-005-0151-z. [DOI] [PubMed] [Google Scholar]
- Redman CWG, Tannetta DS, Dragovic RA, Gardiner C, Southcombe JH, Collett GP, Sargent IL. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta. 2012;33(Supplement):S48–S54. doi: 10.1016/j.placenta.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Rensen P, van Dijk M, Havenaar E, Bijsterbosch M, Kruijt J, van Berkel T. Selective liver targeting of antivirals by recombinant chylomicrons--a new therapeutic approach to hepatitis B. Nature medicine. 1995;1(3):221–225. doi: 10.1038/nm0395-221. [DOI] [PubMed] [Google Scholar]
- Richardson D, Kalinowski D, Lau S, Jansson P, Lovejoy D. Cancer cell iron metabolism and the development of potent iron chelators as anti-tumour agents. Biochimica et biophysica acta. 2009;1790(7):702–717. doi: 10.1016/j.bbagen.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Ridgway N, McLeod R, Vance JE, Vance D. Biochemistry of Lipids, Lipoproteins and Membranes. Elsevier; 2008. p. 624. [Google Scholar]
- Kontermann Roland, Dübel S. Antibody Engineering. Springer; 2010. [Google Scholar]
- Roopenian D, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nature reviews Immunology. 2007;7(9):715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- Rossi L, Serafini S, Antonelli A, Pierigé F, Carnevali A, Battistelli V, Malatesta M, Balestra E, Caliò R, Perno C-F, et al. Macrophage depletion induced by clodronate-loaded erythrocytes. Journal of drug targeting. 2005;13(2):99–111. doi: 10.1080/10611860500064123. [DOI] [PubMed] [Google Scholar]
- Rossi M, Nicolucci A. Liraglutide in type 2 diabetes: from pharmacological development to clinical practice. Acta bio-medica : Atenei Parmensis. 2009;80(2):93–101. [PubMed] [Google Scholar]
- Rothschild M, Oratz M, Schreiber S. Serum albumin. Hepatology (Baltimore, Md) 1988;8(2):385–401. doi: 10.1002/hep.1840080234. [DOI] [PubMed] [Google Scholar]
- Rouault T, Cooperman S. Brain iron metabolism. Seminars in pediatric neurology. 2006;13(3):142–148. doi: 10.1016/j.spen.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Ruckle J, Jacobs M, Kramer W, Pearsall A, Kumar R, Underwood K, Seehra J, Yang Y, Condon C, Sherman M. Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24(4):744–752. doi: 10.1359/jbmr.081208. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Alam M, Shaw J, Dasgupta A. Drug Delivery Using Platelet Cancer Cell Interaction. Pharmaceutical research. 2013 doi: 10.1007/s11095-013-1097-1. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana P, Houde E, Marshall D, Volk A, Makropoulos D, Emerson C, Pradeep A, Bugelski P, Wojchowski D. CNTO 530 functions as a potent EPO mimetic via unique sustained effects on bone marrow proerythroblast pools. Blood. 2009;113(20):4955–4962. doi: 10.1182/blood-2008-08-172320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyam A. Design and synthesis of releasable folate–drug conjugates using a novel heterobifunctional disulfide-containing linker. Bioorganic & Medicinal Chemistry Letters. 2008;18(11):3196–3199. doi: 10.1016/j.bmcl.2008.04.063. [DOI] [PubMed] [Google Scholar]
- Saunders C, Schmidt B, Mallonee R, Guyer M. Secretion of human serum albumin from Bacillus subtilis. Journal of bacteriology. 1987;169(7):2917–2925. doi: 10.1128/jb.169.7.2917-2925.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J. gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis. The American journal of physiology. 1992;262(1 Pt 2):54. doi: 10.1152/ajpheart.1992.262.1.H246. [DOI] [PubMed] [Google Scholar]
- Seki J, Sonoke S, Saheki A, Koike T, Fukui H, Doi M, Mayumi T. Lipid transfer protein transports compounds from lipid nanoparticles to plasma lipoproteins. International journal of pharmaceutics. 2004;275(1–2):239–248. doi: 10.1016/j.ijpharm.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Sembulingam K, Sembulingam P. Essentials of Medical Physiology. JP Medical Ltd; 2012. [Google Scholar]
- Serhan CN, Ward PA, Gilroy DW. Fundamentals of Inflammation (Google eBook) Cambridge University Press; 2010. [Google Scholar]
- Sharma S, Sausville E, LoRusso P, Vogelzang N, Samlowski W, Carter J, Forman K, Bever S, Messmann R. A phase I study of EC0225 administered weeks 1 and 2 of a 4-week cycle. 2010:3082. [Google Scholar]
- Shawer M, Greenspan P, Øie S, Lu DR. VLDL-resembling phospholipid-submicron emulsion for cholesterol-based drug targeting. Journal of Pharmaceutical Sciences. 2002;91(6):1405–1413. doi: 10.1002/jps.10117. [DOI] [PubMed] [Google Scholar]
- Shi Q, Wilcox D, Fahs S, Fang J, Johnson B, Du L, Desai D, Montgomery R. Lentivirus-mediated platelet-derived factor VIII gene therapy in murine haemophilia A. Journal of thrombosis and haemostasis : JTH. 2007;5(2):352–361. doi: 10.1111/j.1538-7836.2007.02346.x. [DOI] [PubMed] [Google Scholar]
- Shi Q, Wilcox D, Morateck P, Fahs S, Kenny D, Montgomery R. Targeting platelet GPIbalpha transgene expression to human megakaryocytes and forming a complete complex with endogenous GPIbbeta and GPIX. Journal of thrombosis and haemostasis : JTH. 2004;2(11):1989–1997. doi: 10.1111/j.1538-7836.2004.00961.x. [DOI] [PubMed] [Google Scholar]
- Shimamoto G, Gegg C, Boone T, Quéva C. Peptibodies: A flexible alternative format to antibodies. mAbs. 2012;4(5):586–591. doi: 10.4161/mabs.21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleep D, Belfield G, Ballance D, Steven J, Jones S, Evans L, Moir P, Goodey A. Saccharomyces cerevisiae strains that overexpress heterologous proteins. Bio/technology (Nature Publishing Company) 1991;9(2):183–187. doi: 10.1038/nbt0291-183. [DOI] [PubMed] [Google Scholar]
- Sleep D, Belfield G, Goodey A. The secretion of human serum albumin from the yeast Saccharomyces cerevisiae using five different leader sequences. Bio/technology (Nature Publishing Company) 1990;8(1):42–46. doi: 10.1038/nbt0190-42. [DOI] [PubMed] [Google Scholar]
- Sleep D, Cameron J, Evans L. Albumin as a versatile platform for drug half-life extension. Biochimica et biophysica acta. 2013;1830(12):5526–5534. doi: 10.1016/j.bbagen.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Soni V, Kohli DV, Jain SK. Transferrin-conjugated liposomal system for improved delivery of 5-fluorouracil to brain. Journal of Drug Targeting. 2008;16(1):73–78. doi: 10.1080/10611860701725381. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Fujishima Y, Kaneo Y. Receptor mediated endocytosis and cytotoxicity of transferrin-mitomycin C conjugate in the HepG2 cell and primary cultured rat hepatocyte. Biological & pharmaceutical bulletin. 2001;24(3):268–273. doi: 10.1248/bpb.24.268. [DOI] [PubMed] [Google Scholar]
- Temming K, Lacombe M, Schaapveld R, Orfi L, Kéri G, Poelstra K, Molema G, Kok R. Rational design of RGD-albumin conjugates for targeted delivery of the VEGF-R kinase inhibitor PTK787 to angiogenic endothelium. ChemMedChem. 2006;1(11):1200–1203. doi: 10.1002/cmdc.200600201. [DOI] [PubMed] [Google Scholar]
- Thomas M, Kularatne SA, Qi L, Kleindl P, Leamon CP, Hansen MJ, Low PS. Ligand-Targeted Delivery of Small Interfering RNAs to Malignant Cells and Tissues. Annals of the New York Academy of Sciences. 2009;1175(1):32–39. doi: 10.1111/j.1749-6632.2009.04977.x. [DOI] [PubMed] [Google Scholar]
- Thomas M, Mousa S, Mousa S. Comparative effectiveness of aflibercept for the treatment of patients with neovascular age-related macular degeneration. Clinical ophthalmology (Auckland, NZ) 2013;7:495–501. doi: 10.2147/OPTH.S29974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe A, Freeman H, Pradhananga SL, Wilkinson IR, Ross RJM. Long-Acting Growth Hormone Analogues. In: Ho K, editor. Growth Hormone Related Diseases and Therapy: A Molecular and Physiological Perspective for the Clinician. Springer; 2011. pp. 361–373. [Google Scholar]
- Tomaiuolo G, Rossi D, Caserta S, Cesarelli M, Guido S. Comparison of two flow-based imaging methods to measure individual red blood cell area and volume. Cytometry Part A : the journal of the International Society for Analytical Cytology. 2012;81(12):1040–1047. doi: 10.1002/cyto.a.22215. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. Nanoparticulates as Drug Carriers Imperial. College Press; 2006. [Google Scholar]
- Trams E, Lauter C, Salem N, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochimica et biophysica acta. 1981;645(1):63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Yoshitomi H, Usukura J. Endocytic mechanism of transferrin-conjugated nanoparticles and the effects of their size and ligand number on the efficiency of drug delivery. Journal of Electron Microscopy. 2012 doi: 10.1093/jmicro/dfs080. [DOI] [PubMed] [Google Scholar]
- Ulbrich K, Hekmatara T, Herbert E, Kreuter J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB) European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft für Pharmazeutische Verfahrenstechnik e V. 2009;71(2):251–256. doi: 10.1016/j.ejpb.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Vallee S, Rakhe S, Reidy T, Walker S, Lu Q, Sakorafas P, Low S, Bitonti A. Pulmonary administration of interferon Beta-1a-fc fusion protein in non-human primates using an immunoglobulin transport pathway. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2012;32(4):178–184. doi: 10.1089/jir.2011.0048. [DOI] [PubMed] [Google Scholar]
- Vincenti F, Dritselis A, Kirkpatrick P. FRESH FROM THE PIPELINE: Belatacept. Nature Reviews Drug Discovery. 2011;(10):655–656. doi: 10.1038/nrd3536. [DOI] [PubMed] [Google Scholar]
- Vlahov IR, Santhapuram HKR, Kleindl PJ, Howard SJ, Stanford KM, Leamon CP. Design and regioselective synthesis of a new generation of targeted chemotherapeutics. Part 1: EC145, a folic acid conjugate of desacetylvinblastine monohydrazide. Bioorganic & Medicinal Chemistry Letters. 2006;16(19):5093–5096. doi: 10.1016/j.bmcl.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Vyas SP, Sihorkar V. Endogenous carriers and ligands in non-immunogenic site-specific drug delivery. Advanced Drug Delivery Reviews. 2000;43(2–3):101–164. doi: 10.1016/s0169-409x(00)00067-3. [DOI] [PubMed] [Google Scholar]
- Wang G-P, Guan Y-S, Jin X-R, Jiang S-S, Lu Z-J, Wu Y, Li Y, Li M, Luo F. Development of novel 5-fluorouracil carrier erythrocyte with pharmacokinetics and potent antitumor activity in mice bearing malignant ascites. Journal of Gastroenterology and Hepatology. 2009;25(5) doi: 10.1111/j.1440-1746.2009.06155.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Byrne JD, Napier ME, DeSimone JM. More Effective Nanomedicines through Particle Design. Small. 2011;7(14):1919–1931. doi: 10.1002/smll.201100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Luo J, Lantrip DA, Waters DJ, Mathias CJ, Green MA, Fuchs PL, Low PS. Design and Synthesis of [111In]DTPA–Folate for Use as a Tumor-Targeted Radiopharmaceutical. Bioconjugate Chemistry. 1997;8(5):673–679. doi: 10.1021/bc9701297. [DOI] [PubMed] [Google Scholar]
- Weaver M, Laske D. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. Journal of neuro-oncology. 2003;65(1):3–13. doi: 10.1023/a:1026246500788. [DOI] [PubMed] [Google Scholar]
- Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, Jackson CG, Lange M, Burge DJ. A Trial of Etanercept, a Recombinant Tumor Necrosis Factor Receptor:Fc Fusion Protein, in Patients with Rheumatoid Arthritis Receiving Methotrexate. New England Journal of Medicine. 1999;340(4):253–259. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- Wu J, Lu Y, Lee A, Pan X, Yang X, Zhao X, Lee RJ. Reversal of Multidrug Resistance by Transferrin-Conjugated Liposomes Co-encapsulating Doxorubicin and Verapamil. Journal of Pharmacy & Pharmaceutical Sciences. 2007;10(3):350–357. [PubMed] [Google Scholar]
- Xia W, Hilgenbrink AR, Matteson EL, Lockwood MB, Cheng J-X, Low PS. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 2009;113(2):438–446. doi: 10.1182/blood-2008-04-150789. [DOI] [PubMed] [Google Scholar]
- Yang F, Jin C, Jiang Y, Li J, Di Y, Ni Q, Fu D. Liposome based delivery systems in pancreatic cancer treatment: from bench to bedside. Cancer treatment reviews. 2011;37(8):633–642. doi: 10.1016/j.ctrv.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Yang J, Chen H, Vlahov IR, Cheng J-X, Low PS. Characterization of the pH of Folate Receptor-Containing Endosomes and the Rate of Hydrolysis of Internalized Acid-Labile Folate-Drug Conjugates. Journal of Pharmacology and Experimental Therapeutics. 2007;321(2):462–468. doi: 10.1124/jpet.106.117648. [DOI] [PubMed] [Google Scholar]
- Yardley D. nab-Paclitaxel mechanisms of action and delivery. Journal of controlled release : official journal of the Controlled Release Society. 2013;170(3):365–372. doi: 10.1016/j.jconrel.2013.05.041. [DOI] [PubMed] [Google Scholar]
- Yeh P, Landais D, Lemaître M, Maury I, Crenne J, Becquart J, Murry-Brelier A, Boucher F, Montay G, Fleer R. Design of yeast-secreted albumin derivatives for human therapy: biological and antiviral properties of a serum albumin-CD4 genetic conjugate. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(5):1904–1908. doi: 10.1073/pnas.89.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HS, Park TG. Folate-receptor-targeted delivery of doxorubicin nano-aggregates stabilized by doxorubicin–PEG–folate conjugate. Journal of Controlled Release. 2004;100(2):247–256. doi: 10.1016/j.jconrel.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Yuan F. Transvascular drug delivery in solid tumors. Seminars in Radiation Oncology. 1998;8(3):164–175. doi: 10.1016/s1053-4296(98)80042-8. [DOI] [PubMed] [Google Scholar]
- Zaitsev S, Danielyan K, Murciano J-C, Ganguly K, Krasik T, Taylor RP, Pincus S, Jones S, Cines DB, Muzykantov VR. Human complement receptor type 1–directed loading of tissue plasminogen activator on circulating erythrocytes for prophylactic fibrinolysis. Blood. 2006;108(6):1895–1902. doi: 10.1182/blood-2005-11-012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev S, Spitzer D, Murciano J-C, Ding B-S, Tliba S, Kowalska MA, Marcos-Contreras OA, Kuo A, Stepanova V, Atkinson JP, et al. Sustained thromboprophylaxis mediated by an RBC-targeted pro-urokinase zymogen activated at the site of clot formation. Blood. 2010;115(25):5241–5248. doi: 10.1182/blood-2010-01-261610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert reviews in molecular medicine. 2009;11:e4. doi: 10.1017/S1462399409000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Haney M, Mahajan V, Reiner B, Dunaevsky A, Mosley R, Kabanov A, Gendelman H, Batrakova E. Active Targeted Macrophage-mediated Delivery of Catalase to Affected Brain Regions in Models of Parkinson’s Disease. Journal of nanomedicine & nanotechnology. 2011:S4. doi: 10.4172/2157-7439.S4-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Chen J, Li H, Glickson JD. Rerouting lipoprotein nanoparticles to selected alternate receptors for the targeted delivery of cancer diagnostic and therapeutic agents. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(49):17757–17762. doi: 10.1073/pnas.0508677102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Yu B, Weecharangsan W, Piao L, Darby M, Mao Y, Koynova R, Yang X, Li H, Xu S, et al. Transferrin-conjugated lipid-coated PLGA nanoparticles for targeted delivery of aromatase inhibitor 7α-APTADD to breast cancer cells. International Journal of Pharmaceutics. 2010;390(2):234–241. doi: 10.1016/j.ijpharm.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]