Abstract
Using retrospective reports obtained during treatment visits in 138 heavy drinkers, we found that topiramate’s reduction of heavy drinking was moderated by a polymorphism (rs2832407) in GRIK1, which encodes the GluK1 kainate subunit (Kranzler et al., 2014a). A subsequent analysis of that 12-week topiramate treatment trial showed similar effects of medication and genotype on daily drinking reports obtained via interactive voice response technology (IVR; Kranzler et al., 2014b). Specifically, rs2832407*C-allele homozygotes treated with topiramate reported lower levels of drinking than those receiving placebo. This group also had the largest decreases in the expected positive effects of drinking (i.e., expectancies) and desire to drink. To extend that analysis, which focused on how mean levels of desire and expectancies changed over time with treatment, we used a within-person approach to examine whether daily variation in expectancies and desire to drink interact with topiramate treatment and genotype to predict nighttime drinking levels. In contrast to the previous analysis (Kranzler et al., 2014b), here we focus on whether alcohol expectancies and desire to drink moderate the effects of topiramate on drinking. Results showed a three-way interaction of daily expectancies with genotype and medication, such that the protective effect of topiramate on nighttime drinking among rs2832407*C-allele homozygotes was decreased on days characterized by relatively high levels of anticipated positive effects of alcohol. There was no moderating effect of desire to drink or negative alcohol expectancies. Thus, there is specific moderation of the effects of topiramate by both genotype and cognitive process.
Keywords: Topiramate, Alcohol Expectancies, Drinking Behavior, Pharmacogenetics
INTRODUCTION
In a 12-week, parallel-groups, placebo-controlled trial in 138 heavy drinkers, we found that, compared to placebo, treatment with topiramate 200 mg/day reduced heavy drinking days and increased abstinent days (Kranzler et al., 2014a). These findings, obtained using retrospective reports at baseline and treatment visits, were consistent with those from two studies of alcohol-dependent subjects in which topiramate 300 mg/day was superior to placebo in reducing the frequency of drinking and heavy drinking (Johnson et al. 2003; Johnson et al., 2007).
Topiramate has multiple pharmacological effects, including antagonism of glutamate activity at kainate receptors (Skradski & White 2000; Gibbs, Sombati, DeLorenzo, & Coulter, 2000), most potently and selectively those containing the GluK1 and GluK2 subunits (encoded by GRIK1 and GRIK2, respectively: Gryder & Rogawski, 2003; Kaminski, Banerjee, and Rogawski, 2004). In a case-control study, we found that a single nucleotide polymorphism (SNP), rs2832407, in GRIK1 was associated to alcohol dependence (Kranzler et al. 2009). Specifically, the rs2832407*C allele, the major allele in European Americans (EAs), was overrepresented among individuals with alcohol dependence. In the EA subsample (N=122) from the topiramate trial in heavy drinkers, topiramate reduced heavy drinking significantly more than placebo only in rs2832407*C-allele homozygotes (Kranzler et al., 2014a).
In a secondary analysis of data from that trial (Kranzler et al., 2014b), we used patients’ daily reports obtained using interactive voice response technology (IVR) to examine the interaction of topiramate and rs2832407 on changes in alcohol-related cognitions across the 12 weeks of the study. Prior research indicated that topiramate reduces obsessional thoughts and compulsions about using alcohol (Johnson et al., 2008), as measured by scores on the Obsessive-Compulsive Drinking Scale (OCDS; Anton, Moak, & Latham, 1995). Consistent with these findings, in our study of heavy drinkers, some patients who substantially reduced their drinking reported that they were able to do so because they were no longer thinking frequently about drinking. Thus, based on these qualitative statements, we focused on two variables that captured the appeal and saliency of drinking and its effects: desire to drink (which is conceptually similar to obsessional thoughts and craving) and expected positive effects of drinking [i.e., positive alcohol expectancies, which a substantial body of evidence links to subsequent drinking behavior (Jones, Corbin, & Fromme, 2001)]. We found a study day × medication group × genotype group interaction that predicted both outcomes, with rs2832407*C-allele homozygotes who were treated with topiramate showing the largest decreases across the 12-week study period. We did not, however, find that changes in positive alcohol expectancies or desire to drink across the treatment period mediated the medication group × genotype group interaction effects on drinking (Kranzler et al., 2014b). Based on those findings, it appears that there may be a more complicated role for these variables in the effects of topiramate.
The present study was conducted to understand more fully the cognitive mechanisms involved in the effects of topiramate and its moderation by rs2832407. Here, we moved from analysis of how mean levels of these variables changed across study weeks, which was the approach that we used previously (Kranzler et al., 2014b), to examine how day-to-day changes in positive expectancies and desire to drink (i.e., daily deviations from mean levels) interact with medication and genotype to predict same-day drinking behavior. Although desire to drink (or craving) is often conceptualized as having meaningful variation over time (Haass-Koffler, Leggio, & Kenna, 2014), focusing on daily variation in expectancies is somewhat novel. However, theory commonly specifies that expectancy activation is a dynamic process (Goldman, Del Boca, & Darkes, 1999) and studies have shown that expectancies change within person (Cooney, Gillespie, Baker, & Kaplan, 1987; Goldstein, Wall, McKee, & Hinson, 2004; Wall, Hinson, McKee, & Goldstein, 2001). Thus, we believe that the examination of expectancies at the daily level of analysis, as with desire to drink, could be informative. These analyses differ from and augment our prior analyses of the data across the 12 weeks of the study (Kranzler et al., 2014a; Kranzler et al., 2014b), which in addition to showing that the greatest reduction in drinking was among topiramate-treated individuals with the rs2832407*CC genotype, showed that rs2832407*C-allele homozygotes treated with topiramate had larger decreases in mean levels of positive expectancies and desire to drink than those assigned to receive placebo.
In the present analysis, we posited that topiramate would weaken the within-person link between positive expectancies or desire to drink and drinking. Specifically, we predicted that rs2832407*C-allele homozygotes treated with topiramate would demonstrate a weaker positive within-person association between daily expectancies (and desire) and nighttime drinking. As a comparison, we also examined the moderating effects of the anticipation of negative effects of drinking (i.e., negative expectancies).
EXPERIMENTAL PROCEDURES
Overview
In this 12-week, parallel-groups, placebo-controlled trial of topiramate in heavy drinkers, patients were randomly assigned to medication group, and double-blind conditions were maintained throughout the study. At each of nine treatment visits, patients received counseling with medical management (Pettinati et al., 2004), a brief psychosocial intervention. Additional details on the study design are provided in Kranzler et al. (2014).
The study was initiated at the University of Connecticut Health Center (UCHC) and completed at the University of Pennsylvania Treatment Research Center (Penn). The institutional review boards at both institutions approved the consent form and study protocol. Throughout the trial, patients responded to daily IVR surveys that were computer administered. Study participants were paid to complete daily reports and an assessment battery at the end of treatment.
Genotyping Procedure
We extracted DNA from whole blood using the PureGene kit (GentraSystems, Minneapolis, MN) and genotyped rs2832407 using the TaqMan SNP genotyping assay (Life Technologies, Grand Island, NY). All genotypes were repeated, with consistent results. The genotype frequencies in the 122 European Americans were consistent with Hardy-Weinberg equilibrium expectations (χ2=0.61, df=2, p=0.74).
Patients
We screened 200 prospective participants in person, with 138 patients (62.3% male) randomly assigned to treatment with topiramate (N=67, 48.6%) or placebo (N=71, 51.4%). The study sample was middle-aged (mean=51.1 yr, SD=8.2) and predominantly EA (88.4%), married (60.9%), and employed (80.4%), with an average of more than three years of college. During pretreatment, patients had approximately 6 drinking days/week and of which 5 were heavy drinking days (defined as ≥ 4 drinks in a day for women; ≥ 5 drinks in a day for men). The groups were comparable on all pretreatment demographic and clinical measures except age: placebo patients were approximately 3.5 yr older than topiramate patients. To address this difference, we included age as a factor in the analyses, as described below.
Of the 138 patients randomly assigned to treatment with topiramate or placebo, 122 (88.4%) identified themselves as EA. We limited the current analysis to the EA subgroup to avoid confounding due to substantial population differences in the frequency of rs2832407 alleles. Population stratification, which could also confound the interpretation of genetic moderator effects, is comparatively unlikely in European-ancestry groups, which generally do not show high degrees of admixture with other populations and where allele frequency differences are comparatively small (Halder et al., 2009).
Procedures
Subjects who responded to advertisements underwent an initial telephone screening, after which eligible individuals were seen in person and gave written, informed consent to participate. They then underwent a medical and psychiatric evaluation to substantiate that they met inclusion criteria and no exclusion criteria.
Prior to randomization, patients completed questionnaires and were administered research interviews by trained research evaluators; then they received their first counseling sessions and supplies of study medication. During the first six weeks of treatment, patients were seen weekly for medication adjustments, with three biweekly visits after the medication dosage was stabilized. At each study visit, we measured the patient’s breath alcohol concentration, weight, and vital signs; patients completed questionnaires; and a nurse obtained information on concurrent medications, adverse events, and protocol adherence, and delivered the Medical Management (Pettinati et al., 2004) counseling. After 12 weeks of treatment (or earlier for patients that did not complete the treatment), patients again completed questionnaires and were interviewed by the nurse and the evaluator.
Study Treatments
Counseling
The Medical Management manual promotes medication adherence and treatment participation through education and support; it was modified to be consistent with a goal of non-hazardous drinking (Sanchez-Craig, Wilkinson, & Davila, 1995). Men were advised to consume ≤ 3 standard drinks per day and 12 standard drinks per week and women were advised to consume ≤ 2 drinks per day and 8 drinks per week.
Medication
Topiramate treatment was initiated at a dosage of 25 mg at bedtime and the daily dosage was increased at weekly intervals to 50 mg, 75 mg, 100 mg, 150 mg, and 200 mg. After the first week, the medication was administered twice daily in divided doses, with the maximal dosage achieved in week 6. Placebo and topiramate were encapsulated and indistinguishable from one another. Daily Assessments: We used IVR to administer survey questions by telephone (Gurvich, Kenna, & Leggio, 2013). Patients were trained to use the IVR and each was given a wallet- sized, follow-along guide detailing each question, including answer options to assist them with the first few IVR calls. They called daily between 5 and 8 PM to report their desire to drink and their expectancies concerning the effects of alcohol and their alcohol consumption by pressing the keys on the keypad, with responses entered automatically in a database. We chose the time of the calls to reduce the potential for patients to have begun drinking heavily prior to making the calls. Patients who failed to call in during the allotted time received computerized reminder calls. A research assistant monitored calls to ensure that they were made daily and to address problems and questions immediately (to enhance accuracy and adherence). Patients who missed a series of calls were contacted and reminded of the importance to the research of their adhering to the call-in schedule.
Patients were queried each evening about their current desire to drink using three items adapted from the Alcohol Urge Questionnaire (Bohn, Krahn, & Staehler, 1995) (e.g., “Today I felt like I could really use a drink”). Responses were on a 5-point scale ranging from 0 (“not at all”) to 4 (“extremely likely”). We also queried patients daily using three items adapted from expectancy scales (Rohsenow, 1983; Fromme, Stroot, & Kaplan, 1993; Leigh & Stacy, 1993). The first two items assessed anticipated positive outcomes for drinking later that night and correspond to the commonly identified expectancy domains of general pleasure and tension reduction. Patients were asked, “If you were to drink tonight how likely is it that you” (a) “would have a good time?” and (b) “would feel less tense/more relaxed?” The third item assessed anticipated negative outcomes: “If you were to drink tonight, how likely is it that you would become clumsy or uncoordinated?” The response options for these items were the same as those measuring desire to drink. For analysis, we averaged the appropriate items to create a daily desire to drink composite (alpha = 0.79) and a positive expectancy composite (alpha = 0.72).
Patients also reported the number of standard drinks of beer, wine, liquor and “other beverages” that they consumed. To capture all drinking during the preceding 24-hour period, patients were asked to report separately the amounts that they drank the previous day (in total), and any drinking during the current day, up until the time of the IVR report. We calculated nighttime drinking by subtracting daytime drinking from total day drinking. On 4.9% of the days, negative values resulted because patients reported more daytime drinks than total drinks (which were reported the next day). In these situations, the value for nighttime drinking was set to zero. We also winsorized the 0.2% of nighttime drinking values that exceeded 20 drinks to a maximum of 20. We used these data to examine lagged associations between daily expectancies (reported one evening) and nighttime drinking that followed (reported the next day).
Data Analysis
We examined whether daily expectancies and desire to drink interacted with medication condition and genotype to predict nighttime drinking (i.e., drinking after the daily survey). Study day was coded as −83 to 0, medication condition as 0 = placebo and 1 = topiramate, and genotype as 0 = A-allele carrier and 1 = C-allele homozygote. Because there was evidence from our prior clinical trial (Kranzler et al., 2014a) that the two-level rs2832407 genotype was a significant moderator of topiramate’s effects, we combined the AA and AC groups, comparing them with the CC group as a dichotomous genotype.
To evaluate the effect of relative levels of daily expectancies and desire, they were person-mean centered (i.e., each person’s overall mean levels was subtracted from each daily value). Given the count nature of the dependent variable (number of nighttime standard drinks), we used generalized estimating equations to specify a negative binomial distribution and log link. We specified all two-way interactions and the three-way interaction of changes in daily expectancies × medication group × genotype group. All models controlled for sex; the model predicting nighttime drinking also controlled for study day, mean levels of expectancies (or desire to drink), and earlier day drinking. We used “independent” error structure; alternative specifications (exchangeable, autoregressive [AR1]) produced similar results. We used SPSS software.
Although the treatment groups differed significantly on age, there was no impact of age on any of the models, hence age was removed from the models. Inclusion of the study site (UCHC vs. Penn) and a squared diary day predictor (to account for quadratic trends in the outcome) also failed to influence any of the models, so they were removed from the analysis. Our statistical approaches allowed for the unbalanced nature of the data, i.e., no subjects were excluded due to missing daily reports.
RESULTS
IVR Adherence
The 122 patients provided complete data on 7,810 person-days [mean=64.0 daily reports per person (SD=22.0) or 76.2% (SD=26.2) of study days]. They reported drinking on 78.5% of days, consuming a mean of 4.5 drinks (SD = 2.6) per drinking day. Daytime drinking (i.e., prior to the daily survey) was reported on 47.2% of days and subjects reported drinking a mean of 3.7 drinks (SD = 2.1) per daytime drinking period. Nighttime drinking was reported on 56.7% of the days and subjects reported drinking 3.4 drinks (SD = 2.3) per nighttime drinking period. Table 1 shows means and standard deviations (SDs) for the daily variables by medication and genotype groups. It also shows the mean and SD for the number of IVR reporting days, which did not differ by medication group [F(1, 118)=0.38, p=0.54], genotype group [F(1, 118)=1.75, p=0.19], or the medication group × genotype interaction [F(1, 118)=1.07, p=0.30].
Table 1.
Descriptive statistics by genotype and medication groups
|
|
|
|||
|---|---|---|---|---|
| Genotype Group | C-allele Homozygotes | A-allele Carriers | ||
| Medication Group | Placebo | Topiramate | Placebo | Topiramate |
| Person N | 30 | 21 | 36 | 35 |
| Mean person-days | 64.03 (21.86) | 70.76 (13.80) | 62.86 (24.27) | 61.14 (23.56) |
| Mean nighttime drinks per day | 2.50 (1.50) | 1.31 (0.85) | 1.78 (1.44) | 2.02 (1.45) |
| Mean daytime drinks per day | 1.78 (1.66) | 1.48 (1.78) | 1.83 (1.50) | 1.99 (2.25) |
| Mean daily expectancies | 1.74 (0.71) | 1.31 (0.95) | 1.40 (0.78) | 1.47 (0.90) |
| Mean daily desire to drink | 2.04 (0.76) | 1.83 (0.62) | 2.03 (0.49) | 1.91 (0.58) |
Note. Values for drinking, expectancies and desire to drink variables are based on aggregate person-level values and are not weighted for the number of reporting days; standard deviations are in parentheses.
Predicting Nighttime Drinking
We entered the predictors in 3 steps. In the first step, we entered sex, daytime drinking level, study day, mean levels of desire to drink (or expectancies), daily desire to drink (or expectancies), medication group, and genotype group. In block 2, we entered the product terms for the two-way interactions of daily desire to drink (or positive expectancies), medication group and genotype group, and in block 3 we entered the three-way product terms. The results for positive expectancies and desire to drink are shown in Tables 2 and 3 (the coefficients correspond to the step when entered).
Table 2.
Results from negative binomal regressions predicting nighttime drinking from positive expectancies, medication and genotype
| Block | Predictor | B | SE | p | 95% CI | |
|---|---|---|---|---|---|---|
| 1 | Diary Day | −.006 | .0010 | <.001 | −.007 | −.004 |
| Sex | −.033 | .1281 | .799 | −.284 | .218 | |
| Daytime drinks | −.157 | .0286 | <.001 | −.213 | −.101 | |
| Mean Expectancies | .154 | .0835 | .066 | −.010 | .318 | |
| Daily Expectancies | .247 | .0457 | <.001 | .157 | .337 | |
| Medication group | −.310 | .1331 | .020 | −.571 | −.049 | |
| Genotype group | −.030 | .1237 | .808 | −.273 | .212 | |
|
| ||||||
| 2 | Medication group × Genotype group | −.799 | .2475 | .001 | −1.284 | −.314 |
| Daily Expectancies × Medication group | .056 | .0875 | .521 | −.115 | .228 | |
| Daily Expectancies × Genotype group | .096 | .0851 | .258 | −.071 | .263 | |
|
| ||||||
| 3 | Daily Expectancies × Medication group × Genotype group | .437 | .1570 | .005 | .129 | .745 |
Note: Diary day (coded −83 to 0), Sex (coded 0=female, 1=male), Medication group (coded 0=placebo, 1=topiramate) and Genotype group (coded 0=A-allele carrier, 1=C-allele homozygote)
Table 3.
Results from negative binomal regressions predicting nighttime drinking from desire to drink, medication and genotype
| Block | Predictor | B | SE | p | 95% CI | |
|---|---|---|---|---|---|---|
| 1 | Diary Day | −.006 | .0010 | <.001 | −.008 | −.004 |
| Sex | .044 | .1254 | .727 | −.202 | .290 | |
| Daytime drinks | −.177 | .0312 | <.001 | −.238 | −.116 | |
| Mean Desire | .200 | .0969 | .039 | .010 | .390 | |
| Daily Desire | .227 | .0364 | <.001 | .156 | .299 | |
| Medication group | −.306 | .1318 | .020 | −.564 | −.048 | |
| Genotype group | −.020 | .1244 | .871 | −.264 | .224 | |
|
| ||||||
| 2 | Medication group × Genotype group | −.764 | .2465 | .002 | −1.247 | −.281 |
| Daily Desire × Medication group | .081 | .0684 | .235 | −.053 | .215 | |
| Daily Desire × Genotype group | .027 | .0701 | .700 | −.110 | .164 | |
|
| ||||||
| 3 | Daily Desire × Medication group × Genotype group | .141 | .1363 | .302 | −.126 | .408 |
Note: Diary day (coded −83 to 0), Sex (coded 0=female, 1=male), Medication group (coded 0=placebo, 1=topiramate) and Genotype group (coded 0=A-allele carrier, 1=C-allele homozygote).
We found a significant medication group × genotype group interaction (block 2 for both models). Probing of this effect in the expectancy model indicated that in C-allele homozygotes, topiramate-treated patients reported less nighttime drinking than those receiving placebo (b= 0.80 0.80, SE=0.18, p<0.001, 95%CI: −1.16, −0.45). There was no such effect among A- allele carriers (b= −0.004, SE=0.18, p=0.98, 95%CI: −0.35, 0.34). Exponentiation of the medication group slope for the C-allele homozygotes indicated that the nighttime drinking rate for the topiramate group was 45% that of the placebo group (i.e., drinking was approximately 2.23 times higher for the placebo group). Nighttime drinking is a subset of the drinking data that we reported previously (Kranzler et al., 2014b).
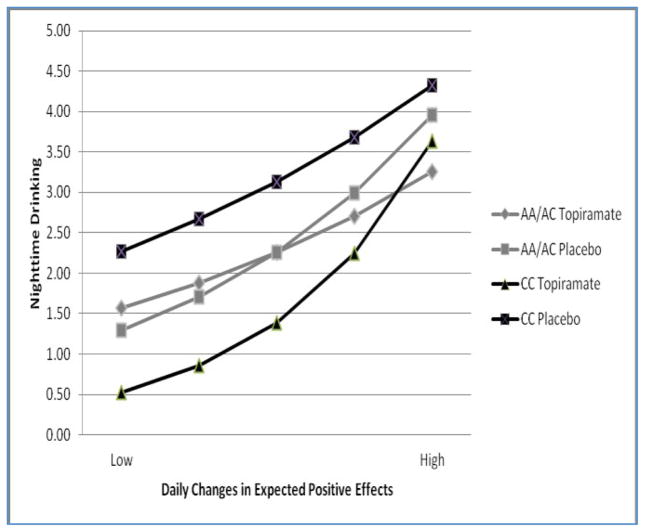
The medication group × genotype group interaction was qualified by a significant three- way interaction with daily positive expectancies (block 3), but not daily desire to drink. The form of the significant three-way interaction can be seen in Figure 1. Opposite to prediction, rs2832407*C-allele homozygotes treated with topiramate showed the strongest positive association between daily positive expectancies and nighttime drinking. The effect was driven by the relatively low levels of drinking for this group (relative to the other groups) on all days except those characterized by very high levels of expectations of positive outcomes.
Figure 1. Prediction of nighttime number of drinks by interactive effects of changes in daily expectancies, genotype, and medication condition.
Values for the X-axis range from 2 points below to 2 points above mean levels on the expectancy scale. In C-allele homozygotes, topiramate-treated patients reported less nighttime drinking than those receiving placebo (p<0.001). There was no such effect among A-allele carriers (p=0.98). The protective effect of topiramate on nighttime drinking among rs2832407*C-allele homozygotes was decreased on days characterized by relatively high levels of anticipated positive effects of alcohol. Note that the p-values were derived from a model that included all 2-way and 3-way interactions; thus, they are the conditional effects of topiramate for each genotype group averaged across all levels of expectancies.
We probed this interaction by focusing on daily expectancies as the moderator of the medication group × genotype group interaction. Specifically, we focused on how the effect of topiramate for CC individuals decreased as the relative levels of expectancies increased. Results indicated that the medication effect was significant for CC individuals on days when expectancies were 1 point above individuals’ mean levels (b= −0.49, SE=0.18, p=0.005, 95%CI: − 0.83, −0.15), but not on days when they were 2 points above mean levels (b= −0.17, SE=0.23, p=0.53, 95%CI: −0.62, 0.28). Approximately 6.0% of responses were at least 1 point above the mean (and on those days topiramate effects in CC individuals were significant), while days characterized by 2 points above participants’ mean levels (where topiramate effects in CC individuals were not significant) were rare (~0.6% of the person days were at least at this level). Thus, the effects of topiramate on nighttime drinking were evident on the vast majority (i.e., more than 94%) of days.
Estimation of these models with negative alcohol expectancies did not reveal a moderating effect. The three-way interaction of daily impairment expectancies × medication group × genotype group did not approach significance (b= 0.088, SE=0.21, p=0.67, 95%CI: − 0.31, 0.49). To exclude the possibility that the significant effect of positive expectancies was due to the greater reliability of the positive expectancy composite (compared to the single item for negative outcomes), we re-estimated (separately) the model using the single items that comprised the positive expectancy composite. The three-way interaction was significant for both the item assessing expectations of good times (b= 0.32, SE=0.15, p=0.038, 95%CI: 0.02, 0.62) and the one assessing tension reduction (b= 0.26, SE=0.11, p=0.014, 95%CI: 0.05, 0.47).
DISCUSSION
In this study, we used daily data obtained via IVR to examine whether daily variation in desire to drink and positive alcohol expectancies interacted with topiramate treatment and genotype to predict later day drinking. These analyses augment our prior analysis of similar measures across the 12 weeks of the study, which showed that topiramate-treated individuals with the rs2832407*CC genotype showed the greatest reductions in mean levels of drinking, positive outcome expectancies, and desire to drink (Kranzler et al., 2014b).
Here, using a within-person approach, we found that the relative level of daily positive expectancies reported in the evening moderated the interaction effect of genotype group × medication group on nighttime drinking. The form of this interaction was not in the direction that we predicted. Rather than finding weaker contingencies between deviations from mean levels of positive expectancies and later-day drinking among topiramate-treated rs2832407*C- allele homozygotes, we found the opposite pattern. However, we believe that the form of the interaction–when conceptualizing positive expectancies as the moderator–provides information that contributes to an understanding of the mechanisms of interest. We found that for rs2832407*C-allele homozygotes, on more than 94% of days, topiramate treatment significantly reduced nighttime drinking more than placebo treatment. However, on days when expected positive effects of drinking were very high (as might occur, for example, in anticipation of going to a drinking-related event such as a party or happy hour), topiramate did not exert a protective effect. In contrast, in A-allele carriers, there was no topiramate effect regardless of expectancy levels.
Taken together with the results from Kranzler et al. (2014b), our findings indicate that topiramate (at least for rs2832407*C-allele homozygotes) reduces overall levels of expectancies, but it does not weaken highly salient positive alcohol expectancies, which seemed to overpower the effect of topiramate (though only on very few days). This effect was limited to rs2832407*C-allele homozygotes (Figure 1), which appears to be attributable to the fact that only that genotype group showed otherwise reduced drinking. That is, in the face of high positive alcohol expectancies, nighttime drinking by rs2832407*C-allele homozygotes treated with topiramate resembled that of the other three groups. An important caveat is that although our daily indicator of desire to drink, and less directly, expected positive effects of drinking, capture “incentive salience” (Robinson & Berridge, 2008), they do not tap a more generalized salience that is not necessarily related to “desire” or “wanting,” (e.g., an alcohol advertisement that is salient for a problem drinker in abstinence-oriented treatment does not necessarily prompt a desire to drink). Daily measures that capture this generalized salience might provide a better opportunity to demonstrate mediating effects that we did not observe in our last study.
Our results answer only a few of the relevant questions about the cognitive mechanisms underlying topiramate’s effect. One possibility is that, although topiramate does not weaken the effect of explicit expectancies (i.e., processes amenable to introspection and self-report), it might interfere with implicit expectancies (i.e., processes that are out of awareness) (Reich, Below, & Goldman, 2010). Topiramate could weaken the link between stimuli and environmental cues that activate implicit expectancies or the link between implicit expectancies and drinking behavior. Either of these processes, over time, could result in decreased levels of explicit expectancies and desire (Kranzler et al., 2014b) and drinking level (Johnson et al., 2003; Johnson et al., 2007; Miranda et al., 2008, Kranzler et al., 2014a).
Despite an overall effect of topiramate on desire to drink in C-allele homozygotes (Kranzler et al., 2014b), evening reports of desire to drink did not moderate the effect of the medication group × genotype interaction on nighttime drinking. The modest sample size (n=122) may have provided inadequate statistical power to detect this effect. The finding does not support the postulated neuropharmacologic mechanism of topiramate’s effects on drinking: namely, that the medication, by blocking AMPA- and kainate-mediated glutamatergic neurotransmission may suppress ethanol-induced release of dopamine in the nucleus accumbens, reducing the reinforcing effect of alcohol, resulting in reduced craving for alcohol (Johnson et al., 2007). A cognitive mechanism that has been suggested as a partial explanation for the effects of topiramate to reduce drinking is that it increases inhibitory control (Rubio, Martínez- Gras, & Manzanares, 2009). In the context of the incentive sensitization model of Robinson and Berridge (2008), an enhancement of inhibitory control could offset the incentive salience associated with “wanting” alcohol.
Our findings implicate the GluK1-containing kainate receptor at the neuropharmacologic level and positive alcohol expectancies at the cognitive level as mechanisms for topiramate’s reduction of drinking. Thus, it appears that the effects of topiramate on drinking, which are more robust than those of naltrexone or acamprosate (Blodgett, Del Re, Maisel, & Finney, 2014), are moderated by both genetic and cognitive factors, so their elucidation is of both theoretical and clinical relevance.
Efforts to replicate and extend these findings would benefit from larger sample sizes, as it is possible that a small percentage of cases drove the observed effects. This could only be accurately evaluated via replication. Further, subsequent studies should utilize already validated measures of expectancies, though it should be noted that our items demonstrated validity in their associations with drinking behavior. Future studies could also measure expectancies and drinking multiple times each day to provide greater precision in predicting daytime drinking, which in our study occurred frequently and was as intense as evening drinking. Subsequent studies of topiramate aimed at understanding its cognitive effects should include patients’ expectancies regarding drinking and their perceived self-efficacy in dealing with those expectancies. Future studies could also attempt to measure aspects of “generalized salience,” which has less to do with “wanting” alcohol and which could serve as a mediator of the effects of topiramate in rs2832407*C-allele homozygotes on nighttime drinking level.
Research examining implicit (i.e., automatic) expectancies using human laboratory paradigms in which stimuli delivered outside of consciousness elicit automatic processes that can be measured using functional magnetic resonance imaging may also help to elucidate the cognitive mechanism of topiramate’s effects. This approach has been used to study the effects of drug and sexual cues presented outside of consciousness (Childress et al., 2008; Wetherill et al., 2014) and showed that these cues elicited increased activity in brain regions supporting reward detection. A similar approach could help to define more clearly the relations between implicit alcohol expectancies and drinking behavior and the effects of topiramate and rs2832407 genotype.
Acknowledgments
Supported by NIH grants P60 AA03510, K24 AA13736, P50 AA12870, and M01 RR06192.
Footnotes
www.clinicaltrials.gov registration: NCT00626925
DISCLOSURES
HK, SA, HT, and JC were responsible for the study concept and design. JG performed the genetic analysis. HK, SA, and HT conducted the data analysis and interpretation of findings. HK and SA drafted the manuscript. HK, SA, and HT provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved the final version for publication.
SA, HT, JG, and JC have no disclosures to make. HRK has been a consultant or advisory board member with Alkermes, Lilly, Lundbeck, Otsuka, Pfizer, and Roche. He has received honoraria from the Alcohol Clinical Trials Initiative (ACTIVE) of the American Society of Clinical Psychopharmacology, which is supported by AbbVie, Ethypharm, Lilly, Lundbeck, and Pfizer.
Staff members of the Clinical Research and Evaluation Unit of the University of Connecticut Alcohol Research Center and the Center for Studies of Addiction of the University of Pennsylvania Perelman School of Medicine (particularly Timothy Pond, M.P.H.) were instrumental in the conduct of the study.
References
- Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: a self- rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcoholism: Clinical and Experimental Research. 1995;19:92–99. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Armeli S, Mohr C, Todd M, Maltby N, Tennen H, Carney MA, Affleck G. Daily evaluation of anticipated outcomes from alcohol use among college students. Journal of Social and Clinical Psychology. 2005;4:767–792. [Google Scholar]
- Blodgett JC, Del Re AC, Maisel NC, Finney JW. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2014;38:1481–1988. doi: 10.1111/acer.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O’Brien CP. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS One. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Gillespie RA, Baker LH, Kaplan RF. Cognitive changes after alcohol cue exposure. Journal of Consulting and Clinical Psychology. 1987;55:150–155. doi: 10.1037/0022-006X.55.2.150. [DOI] [PubMed] [Google Scholar]
- Fromme K, Stroot EA, Kaplan D. Comprehensive effects of alcohol: Development and psychometric assessment of a new expectancy questionnaire. Psychological Assessment. 1993;5:19–26. [Google Scholar]
- Gibbs JW, 3rd, Sombati S, DeLorenzo RJ, Coulter DA. Cellular actions of topiramate: blockade of kainate-evoked inward currents in cultured hippocampal neurons. Epilepsia. 2000;(suppl 1):S10–16. doi: 10.1111/j.1528-1157.2000.tb02164.x. [DOI] [PubMed] [Google Scholar]
- Goldman MS, Del Boca FK, Darkes J. Alcohol expectancy theory: The application of cognitive neuroscience. In: Leonard KE, Blane HT, editors. Psychological theories of drinking and alcoholism. 2. New York: Guilford Press; 1999. pp. 203–246. [Google Scholar]
- Goldstein AL, Wall A, McKee SA, Hinson RE. Accessibility of alcohol expectancies from memory: Impact of mood and motives in college student drinkers. Journal of Studies on Alcohol. 2004;65:95–104. doi: 10.15288/jsa.2004.65.95. [DOI] [PubMed] [Google Scholar]
- Gryder DS, Rogawski MA. Selective antagonism of GluR5 kainate-receptor- mediated synaptic currents by topiramate in rat basolateral amygdala neurons. Journal of Neuroscience. 2003;23:7069–7074. doi: 10.1523/JNEUROSCI.23-18-07069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvich EM, Kenna GA, Leggio L. Use of novel technology-based techniques to improve alcohol-related outcomes in clinical trials. Alcohol & Alcoholism. 2013;48:712–719. doi: 10.1093/alcalc/agt134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Leggio L, Kenna GA. Pharmacological approaches to reducing craving in patients with alcohol use disorders. CNS Drugs. 2014;28:343–360. doi: 10.1007/s40263-014-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder I, Yang BZ, Kranzler HR, Stein MB, Shriver MD, Gelernter J. Measurement of admixture proportions and description of admixture structure in different U.S. populations. Human Mutation. 2009;30:1299–1309. doi: 10.1002/humu.21045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641–1651. doi: 10.1001/jama.298. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Addolorato G, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM for the Topiramate for Alcoholism Advisory Board the Topiramate for Alcoholism Study Group. Improvement of physical health and quality of life of alcohol- dependent individuals with topiramate treatment: US multisite randomized controlled trial. Archives of Internal Medicine. 2008;168:1188–1199. doi: 10.1001/archinte.168.11.1188. [DOI] [PubMed] [Google Scholar]
- Jones BT, Corbin W, Fromme K. A review of expectancy theory and alcohol consumption. Addiction. 2001;96:57–72. doi: 10.1046/j.1360-0443.2001.961575.x. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Banerjee M, Rogawski MA. Topiramate selectively protects against seizures induced by ATPA, a GluR5 kainate receptor agonist. Neuropharmacology. 2004;46:1097–1104. doi: 10.1016/j.neuropharm.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Gelernter J, Anton RF, Arias AJ, Herman A, Zhao H, Burian L, Covault J. Association of markers in the 3’ region of the GluR5 kainate receptor subunit gene to alcohol dependence. Alcoholism: Clinical and Experimental Research. 2009;33:925–930. doi: 10.1111/j.1530-0277.2009.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, Armeli S, Tennen H, Arias AJ, Gelernter J, Pond T, Oncken C, Kampman KM. Topiramate treatment for heavy drinkers: Moderation by a GRIK1 polymorphism. American Journal of Psychiatry. 2014a;171:445–452. doi: 10.1176/appi.ajp.2013.13081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Feinn R, Tennen H, Gelernter J, Covault J. GRIK1 genotype moderates topiramate’s effects on daily drinking level, expectations of alcohol’s positive effects, and desire to drink. International Journal of Neuropsychopharmacology. 2014b;17:1549–1556. doi: 10.1017/S1461145714000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh BC, Stacy AW. Alcohol outcome expectancies: Scale construction and predictive utility in higher order confirmatory models. Psychological Assessment. 1993;5:216–229. [Google Scholar]
- Miranda R, Jr, MacKillop J, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, Swift R, Ray L, McGeary J. Effects of topiramate on urge to drink and the subjective effects of alcohol: a preliminary laboratory study. Alcoholism: Clinical and Experimental Research. 2008;32:489–497. doi: 10.1111/j.1530-0277.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- Pettinati H, Weiss R, Miller W, Donovan D, Ernst D, Rounsaville B. Medical Management Treatment Manual: A Clinical Research Guide for Medically Trained Clinicians Providing Pharmacotherapy as Part of the Treatment for Alcohol Dependence. Bethesda: NIAAA; 2004. COMBINE Monograph Series, Volume 2. DHHS Publication No. NIH 04-5289. [Google Scholar]
- Reich R, Below M, Goldman M. Explicit and implicit measures of expectancy and related alcohol cognitions: A meta-analytic comparison. Psychology of Addictive Behaviors. 2010;24:13–25. doi: 10.1037/a0016556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society B. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ. Drinking habits and expectancies about alcohol’s effects for self versus others. Journal of Consulting and Clinical Psychology. 1983;51:752–756. doi: 10.1037//0022-006x.51.5.752. [DOI] [PubMed] [Google Scholar]
- Rubio G, Martínez-Gras I, Manzanares J. Modulation of impulsivity by topiramate: implications for the treatment of alcohol dependence. Journal of Clinical Psychopharmacology. 2009;29:584–589. doi: 10.1097/JCP.0b013e3181bfdb79. [DOI] [PubMed] [Google Scholar]
- Sanchez-Craig M, Wilkinson DA, Davila R. Empirically based guidelines for moderate drinking: 1-year results from three studies with problem drinkers. American Journal of Public Health. 1995;85:823–828. doi: 10.2105/ajph.85.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skradski S, White HS. Topiramate blocks kainate-evoked cobalt influx into cultured neurons. Epilepsia. 2000;41(suppl 1):S45–47. doi: 10.1111/j.1528-1157.2000.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: a technique for assessing self- reported alcohol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Wall A, Hinson RE, McKee SA, Goldstein A. Examining alcohol outcome expectancies in laboratory and naturalistic bar settings: A within-subject experimental analysis. Psychology of Addictive Behaviors. 2001;15:219–226. doi: 10.1037/0893-164X.15.3.219. [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Childress AR, Jagannathan K, Bender J, Young KA, Suh JJ, O’Brien CP, Franklin TR. Neural responses to subliminally presented cannabis and other emotionally evocative cues in cannabis-dependent individuals. Psychopharmacology (Berl) 2014;231:1397–1407. doi: 10.1007/s00213-013-3342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]