Abstract
Polymer-based nanogel formulations offer features attractive for drug delivery, including ease of synthesis, controllable swelling and viscoelasticity as well as drug loading and release characteristics, passive and active targeting, and the ability to formulate nanogel carriers that can respond to biological stimuli. These unique features and low toxicity make the nanogels a favorable option for vascular drug targeting. In this review, we address key chemical and biological aspects of nanogel drug carrier design. In particular, we highlight published studies of nanogel design, descriptions of nanogel functional characteristics and their behavior in biological models. These studies form a compendium of information that supports the scientific and clinical rationale for development of this carrier for targeted therapeutic interventions.
Keywords: nanogel, targeted drug delivery, endothelial targeting, nanocarrier
Introduction
Five decades of intense research efforts yielded an arsenal of diverse carriers aimed to improve delivery of drugs and probes in the body, to minimize side and systemic effects and attain a controllable local action in the desirable therapeutic sites. These carriers include natural agents - e.g., cells such as erythrocytes and molecules such as lipoproteins, and artificial objects - liposomes, carriers based on organic polymers and on inorganic compounds. Few of these carriers entered practical medicine; more are in the clinical trials and many more are in the laboratory design and development. Unlikely one type of the carriers can suite all medical goals. Yet, some characteristics of design, such as multi-functionality, adequate pharmacokinetics, biocompatibility and responsiveness to the microenvironment represent highly attractive features for many, if not every drug carrier (on the condition of convincing positive benefit/cost and benefit/risk balance).
Hydrogel nanoparticles, or nanogels, are typically comprised of highly hydrated, crosslinked hydrophilic polymers 1–4. Nanogels can be formulated to respond to external stimuli, which can lead to changes in various properties, including swelling, permeability, viscoelasticity, and hydrophobicity (or hydrophilicity). The range of external stimuli that can elicit such responses include photosensitivity and light exposure, changes in pH, ionic strength, and temperature as well as exposure to magnetic fields, biological agents and chemicals 5–7. The physicochemical properties of these stimulus-responsive nanogels can then be recovered by either removing or reversing the stimulus. These features can be incorporated into the design of nanogels, helping nanogels to emerge as in a number of fields including medical diagnostics 8, biosensing and imaging 9,10 and tissue engineering 11.
The flexibility and versatility of nanogels offers ample opportunity for their use as targeted drug delivery vehicles 12. Nanogels, like other nanosize drug carriers, exhibit several advantages for drug delivery when compared to other delivery systems. These include the ability to reduce off-target effects, extend drug circulation time due to high stability compared to micelles, control drug release, to target specific tissues via conjugation of the nanogel surface with affinity ligands, to provide protection for the drug cargo from rapid degradation, and to facilitate crossing tissue barriers. Due to their unique physicochemical structure, nanogels can also be created to possess a number of special characteristics, such as i) deformability to enhance binding and retention within the targeting tissue; ii) enhanced stability via their crosslinked structure to prolong their circulation time in the bloodstream; iii) a core-shell structure with a hydrophilic interior network, which permits both small-molecule or biomacromolecule drug loading and protection of hydrophilic compounds; and iv) modular drug loading and release profiles, which can significantly enhance drug loading efficiency as well as bioavailability and thereby reduce drug toxicity and side effects.
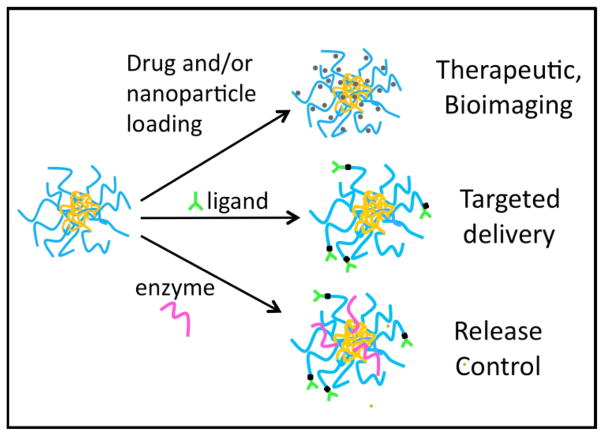
Because drug nanocarriers, including nanogels, are generally too large to be rapidly removed by renal clearance following intravenous administration, they often exhibit extended circulation times that allow for the targeted delivery of their therapeutic cargo to tissues expressing specific disease markers. This is achieved by attaching ligands, antibodies, or other molecules having molecular recognition specificity to the surface of nanocarrier. Drug encapsulation stability within nanocarriers is also highly relevant since thermodynamic parameters such as the percent (%) loading of cargo are not adequate descriptors of delivery vehicle retention within the nanocarrier during circulation in blood and surrounding tissues before binding at the target site. Nanogels meet many, if not all of the key basic requirements of a versatile nanocarrier delivery vehicle, as described in depth below and shown in Figure 1.
Figure 1.
Example of nanogel with a lysozyme core and dextran shell prepared by a Mallard reaction followed by heating (after 14). The hydrophobic core allows for drug (4,14) and nanoparticle (3,28) loading. This nanogel platform allows for conjugation of ligands to the exterior for cell targeting (4) as well as the incorporation of enzymes to control nanogel degradation rate (and therefore, drug or nanoparticle release).
Briefly, the attractive features of nanogels include the following.
Size control: Nanogel size and surface properties can be chemically controlled to limit the rate of clearance by phagocytic cells as well as to enable either passive or active cell targeting. Nanogels must be small enough to traverse capillaries and penetrate tissues through either paracellular or transcellular pathways 13.
Ease of synthesis: The scalability of laboratory-based nanogel development to industrial-scale production for clinical markets and the use of “green” approaches to nanogel manufacturing are important considerations for cost and environmental impact 14.
High encapsulation stability: Drug molecules loaded into the nanogel need to be retained, and not be transported out or leak prematurely while circulating in order to provide maximum therapeutic effects and minimum toxicity or side effects. Cross linking of polymer constituents within the nanogel can be utilized to control drug encapsulation and drug release.
Controlled and sustained drug release: Drug transport should occur at the targeted site, thereby providing both therapeutic efficacy and reduced side effects. Drug loading needs to be sufficiently high to achieve therapeutic goals. Cross linking features prominently in this.
Response to stimuli: Nanogels that are designed to respond to specific stimuli must retain high drug encapsulation stability while circulating, but having reached the targeting site, the drug should release readily in response to the appropriate stimulus.
Targeting. Site-specific delivery of nanogels carriers can be achieved via either coupling to their surface affinity ligands binding to target determinants, or using responsiveness to local factors as above, or via “passive” targeting approaches including extravasation in the pathological sites and retention in the microvasculature.
Low toxicity: The nanogels themselves should be highly biocompatible and free from toxicity, and should be biodegradable with non-toxic degradation products that are readily cleared from the body.
Given these highly desirable aspects for the ideal nanogel drug carrier, we proceed to discuss in greater detail specific nanogel properties, stimulus responsiveness, targeting and toxicity. The features described form the foundation of a useful nanogel delivery vehicle and are fundamental design requirements. Each of these aspects, as well as other characteristics regarding nanogels will be addressed within this review.
Nanogel Properties
Biocompatibility and Degradability
Among the most desired (in fact, sin qua non) features of nanogels, as with any nanotechnology used for therapeutic reasons, is that the materials be biocompatible. That means that they do not provoke any injurious biological responses at the molecular, cellular or organ levels when used. These include typical foreign body responses to small particles that manifest as immunological, thrombogenic or mutagenic activation leading to undesired physiological or anatomical changes such as allergy, blood clot formation or induce disease states such as cancer. Nanogels comprised of known biocompatible polymers or biomacromolecules are thought to have little propensity for driving these adverse biological reactions 15, but their incorporation into nano-sized particles does not automatically mean that their safety as non-toxic agents is assured. Biocompatibility is commonly assessed using cell toxicity and cell viability assays which have shown a lack of cytotoxic effects for many different nanogel formulations 16–19, but this is not uniformly well studied using in vivo preparations. Therefore, it is also important for purposes of clearing nanogels from the body that they also be biodegradable into non-toxic degradation products of sufficiently small size and of chemical composition that do not provoke any of these responses. One approach has been to create nanogels of tetralysine and oligoethylenimine polymers that are degradable when exposed to glutathione at concentrations in the range encountered intracellularly 17, thus anticipating eventual nanogel breakdown into the nontoxic polymers from which the nanogels were originally synthesized.
Swelling behavior
Nanogel swelling in aqueous environments is controlled by multiple factors, including: i) the cross-linking density. At high ionic strengths, the swelling of cationic nanogels was shown to depend largely on cross-linker concentration, whereas at low ionic strength nanogel swelling depended on both the cross-linker as well as the charge concentration 20; and ii) environmental factors such as temperature, pH and ionic strength. Core-shell nanogels consisting of cross-linked poly(ethylene glycol)-b-poly(methacrylic acid) (PEG-b-PMA) were shown to swell with increasing pH due to ionization of carboxylic groups within the PMA 21. Alternatively, PEG-cl-PEI nanogels shrank when pH increased from ~ 8.5 to 10, a result of deprotonation of amino groups within the PEI 22.
Drug loading capacity and drug release
Drug loading into nanogels can be accomplished using a variety of strategies. These include: i) Covalent conjugation of biological agents, which is achievable either during or following nanogel synthesis. Modified enzymes have been copolymerized with acrylamide in both inverse microemulsion 23 and dilute aqueous solutions 24 to obtain nanosized hydrogels. ii) Physical entrapment of compounds within nanogels. This strategy has been used to incorporate proteins into cholesterol-modified pullulan nanogels 25 and siRNA into hyaluronic acid or HA nanogels 26. Doxorubicin has been loaded into and released from amphiphilic cross-linked nanogels whose formulation is based on PEG and pluronic F127 27. iii) Passive/diffusion-based drug loading. Silver nanoparticles 3,28 and dexamethasone 4 have separately been loaded into dextran-lysozyme nanogels by diffusion alone, incubating nanogels in excess drug or nanoparticle solution on a shaker. In general, the drug loading which results from these approaches is relatively modest, typically less than ~ 10% by weight.
Drug release from nanogels occurs by multiple mechanisms, as well. Diffusional release of dexamethasone from dextran-lysozyme nanogels is sufficient to alleviate development of the pulmonary inflammation in a murine model of lung injury 4, while silver nanoparticle exposure from the same nanogel construct inhibits bacterial growth 3. In vitro diffusional doxorubicin release from nanogels was sustained for up to one week 27. Diffusional release is the simplest mechanism to achieve and has previously been used in nanomedicine approaches at a clinical level 29.
Nanogels can also release drugs when the nanogel structure is biologically or chemically degraded. For instance, the release of doxorubicin from pH-sensitive drug-loaded nanogels was significantly accelerated at lower pH values, which led to increased drug uptake by non-small lung carcinoma cells under a slightly acidic pH condition 30. Nanogels can also be developed to release compounds in response to other environmental cues. Disulfide cross-linked POEOMA nanogels that biodegrade into water-soluble polymers and release cargo when exposed to glutathione tripeptide, which is commonly found in cells, have been produced 31.
Size and shape
Nanogel synthesis typically results in spherical particles ranging in size from 20 to 200 nm in diameter, which can be demonstrated by dynamic light scattering and electron microscopy methods 3,4,28. Other shapes are possible to manufacture, using micromolding and photolithographic techniques which also permit control over nanogel size, shape and chemical composition and allow drugs and macromolecules to be loaded as well 32–34. A key advantage to using non-spherical nanogels is that they have the potential to circulate intravascularly for a longer time, given that spherical nanoparticles undergo greater phagocytosis and mechanical retention in the microvasculature than do discoid and ellipsoid nanoparticles 35–37. However, spheroidal hydrogel nanoparticles are more easily produced during chemical synthesis and more amenable to scale-up compared to the micro and nanofabrication methods. Spheroid nanocarriers in the size range 20–200 nm seem amenable to vascular delivery, although surface properties – charge, PEG-coating, proteins conjugated or/and absorbed on the particle – all modulate the rate of hepatic and splenic uptake (main clearing organs of the reticuloendothelial system, RES) 37. Nanogels within this size range circulate for sufficient time to reach their intended vascular targets until they are eventually taken up by the reticuloendothelial system, as is any carrier 38.
Viscoelasticity
Because nanogels are highly solvated, they display both liquid and solid like behavior. These viscoelastic particles can deform in the presence of flow enabling them to navigate more easily past extracellular matrix and within the crowded cellular environment. Whereas bulk gels are readily characterized by traditional rheology methods (e.g., cone and plate rheology), nanorheological methods to characterize the complex modulus are lacking. In the future, nano-indentation methods currently applied to cells and bulk polymeric gels may be extended to nanogels after the influence of substrate and lateral resolution challenges are addressed 39.
Cross linking
Crosslinking of nanogel components can be achieved via physical (i.e., entanglements) or chemical (i.e., covalent) interchain interactions. Physical crosslinks within the nanogels are based on weak interactions between polymer chains, such as van der Waals, electrostatic interactions or hydrogen bonding, and depend on the flexibility of chains as well as the concentration of polymer per unit volume 40. After injection into body fluids, physically cross-linked nanogels can be highly diluted and dissolve, which may result in the premature release of the therapeutic agents, compromising delivery and causing adverse side effects 41. By comparison, physical self-assembly of preformed polymers (or with monomers) followed by chemical cross-linking is a promising method to prepare stable nanogels without using any surfactants or solvents 42,43 This physical self-assembly/chemical cross-linking is especially appropriate for producing biodegradable stimulus responsive nanogels made from biopolymers 34,44,45. In addition to synthetic crosslinkers, functional crosslinkers such as cationic small molecules, can serve the dual purpose of structural stability and drug loading.
Stimulus Responsive Nanogels
Synthesis of stimulus responsive nanogels
Disulfide based cross-linking
Among the various polymer cross-linking methods, self-cross linking reactions as undergone by amphiphilic random copolymers can be utilized to formulate nanogels 46–48. In particular, polymers containing polyethylene glycol (PEG) as the hydrophilic unit and pyridyl disulfide (PDS) as the hydrophobic and cross-linkable unit, spontaneously assemble at the nanoscale under aqueous conditions. Nanogels of various size can be readily produced by varying polymer concentration, crosslinker concentration, and also by taking advantage of the lower critical solution temperature (LCST) polymer behavior. Nanogels have been synthesized via thiol-exchange using lipoic acid-containing dextran in a very similar approach 49. Doxorubicin-loaded nanogels have been prepared using this technique, with the cross-linking achieved catalytically due to addition of dithiolreitol.
Photochemistry-based cross-linking
An alternative cross-linking technique is the use of photochemistry, which has been utilized successfully to stabilize polymer assemblies functionalized with either polymerizable or dimerizable units 50. Hydrophilic block copolymers that contain coumarin, which dimerizes when exposed to UV light, have been used to form micelles, which were then photochemically cross-linked 51. Light-sensitive chemistry has also been used to formulate dendrimer nanocarriers that, with light exposure, release encapsulated drug 52. These techniques are exportable to nanogel formulations, and are especially valuable since the cross-linking activity can be incorporated in such a way that it can be used to exert control over drug release by suppressing enzymatic degradation of substrate with light stimulation at one wavelength or by enhancing enzymatic degradation of substrate as a result of increased decrosslinking with light exposure at another wavelength 53.
Physical cross-linking
Several chemically-distinct nanogels have been synthesized through non-covalent cross-linking methods. Nanogels have been prepared from cholesterol-modified polysaccharides, taking advantage of hydrophobic interactions between cholesterol groups to achieve physical cross-linking 54. Beyond simple hydrophobic interactions, other forces such as host- guest and electrostatic interactions have been utilized to form physically cross-linked nanogels, as are reviewed in 55.
Amine based cross-linking
Amine based cross-linking is an attractive approach for nanogel preparation because amine groups are highly reactive with any number of chemical moieties. There is established methodology for the preparation of shell-crosslinked knedel-like structures using amine crosslinkers. Different amphiphilic block copolymers utilizing poly(acrylic acid) as the hydrophilic, cross-linkable block have been produced 56–58. Several activated esters, including p-nitrophenyl acrylate, pentafluorophenyl acrylate and N-acryloxysuccinimide have also been incorporated as cross-linkable units into copolymers 59,59–61, making these compounds available for nanogel formulation. Reactions involving isocyanate yield another route for cross-linking to create nanogels. For example, pH-responsive cross-linked micelles have been obtained through adding excess 1,8-diaminooctane to micellar aggregates of 3-isopropenyl-α,α-dimethylbenzyl isocyanate bearing copolymers 62.
Click chemistry based cross-linking
Click chemistry has been reported as a means of achieving nanogel synthesis 63. First, amino containing alkynyl groups were immobilized onto the corona of micelles that had been produced via amidation of the acrylic acid groups of poly(acrylic acid)-b-poly(styrene)-based amphiphilic diblock copolymers. Then Click reactions between azido dendrimers and Click-readied micelles were used to covalently cross-link the micelles and produce nanogel networks. Click chemistry has also been used for developing core-cross-linked polyion complex micelles 64.
Classes of Stimulus Responsive Nanogels
pH-responsive nanogels for drug delivery
The pH in biological tissues and body spaces is not uniform, with normal (near neutral) pH of ~7.4 present in blood, acidic pH of ~ 2 or lower in the stomach, and a range of acidic pH values in various tissues and pathological sites - within ischemic tissues, wounds, inflammation sites and in tumors 65,66. Further, pH of some intracellular compartments, such as endosomal-lysosomal vesicular continuum, gradually changes from ~7 to acidic pH of 4–6. These local pH levels can be employed for induction of controllable transformations in the drug carrier, such as disassembly or rearrangements, fusion with or permeation through the membranes, shedding components and drug release. Of course, the spatiotemporal specificity of these pH-mediated transformations is limited due to similarity of the pH in such distinct compartments as tumor interstitium, occluded blood vessel and the lysosomes in any cell in the body.
Nanogels designed to be pH-responsive in order to alter cross-linking or swelling behavior in order to engineer drug delivery when exposure to a critical pH value (pHc) occurs is an attractive means of enhancing encapsulated cargo delivery to specific tissue sites within the body. In this approach, nanogels are formulated to undergo a particular chemical or conformational change at pHc, which reflects the pH microenvironment of the particular tissue site where the deliverable drug is intended to be released. The pHc is selected based on either the pKa (or pKb) of weakly acidic (or basic) groups that are present within the nanogel chemical polyelectrolyte structure. pH-responsive nanogels can be designed to be cationic, leading to swelling when pH < pKb, or they can be designed to be anionic and swell if pH > pKa. Incorporation of additional hydrophobic alkyl residues into the nanogel polyelectrolyte backbone will shift pHc 67. Ionic strength also effects pKa and pKb 68, thereby influencing pHc of pH-responsive nanogels. This impacts drug delivery since biological fluids and diseased tissues may exhibit high ionic strength, which is a significant determinant of the swelling ratios of pH-responsive nanogels 20.
Typically, pH-responsive nanogels are comprised of cross-linked polyelectrolytes having weakly acidic and/or weakly basic groups which serve as proton donors, proton receptors, or in combination as both. Minor changes in the local pH can alter the degree of ionization within the polyelectrolyte chain. Changes in osmotic pressure inside the nanogel resulting from alterations in the degree of ionization can lead to nanogel swelling or deswelling. Core–shell nanogels produced from cross-linked PEG-b-PMA have been shown to swell due to ionization of carboxylic groups of PMA in response to increasing pH 21. Alternatively, nanogels made from cross-linked poly(ethylene oxide) (PEO) and polyethyleneimine (PEI) (PEG-cl-PEI) deswelled with increasing pH as a result of deprotonation of the PEI amino groups 69. Manipulation of the cross-linking via pH change drives these swelling behaviors, with the swelling ratio generally decreasing as the number of cross-links within the nanogel increases 20.
A major motivating factor in designing pH-responsive nanogels to enable drug delivery is that the pH in normal tissue (~ 7.4) is higher than the extracellular pH (pHe) within many tumors (5.8 < pHe < 7.2) 70. Additionally, intracellular cytosolic pH, which is generally slightly acidic, is higher than the pH of ~ 5.0–5.5 that is present within lysosomes or endosomes within cells 71. Nanogels carrying cancer chemotherapy agents have been designed to respond to the variation in pH exposure as they are transported into these environments, with pH changes triggering the release of the toxic cargo 72,73. Based on the nanogel properties, the drug release can be engineered to occur in extracellular tissue 74 or intracellularly into endosomes or lysosomes following cellular uptake 75–77.
Another design point for pH-responsiveness is the presence of both positive and negative charges along the polymer chain of nanogels comprised of amphoteric polyelectrolytes. This feature makes such nanogels interesting for both drug loading release as well as swelling due to the presence of an isoelectric point (IEP). The IEP influences the equilibrium swelling ratio in pH dependent fashion 78 and also exert a significant effect on the ability of macromolecular drugs to be loaded into, and released from nanogels 79. Because polyelectrolyte formulations can accommodate electrostatic interactions with large oppositely charged biomacromolecules, such compounds can be loaded into the nanogel interior 80. The loading can be highly efficient 81 and can include immobilized polynucleotides loaded into pH-responsive nanogels specifically for the purpose of gene delivery 20,82. Another useful behavior of polyampholyte nanogels is their characteristic swelling that occurs as salt concentration increases at the IEP, which makes them practical for drug delivery considering that biological fluids have high ionic strength 83.
Temperature responsive nanogels for drug delivery
Nanogels that respond to temperature undergo rapid changes (increase or decrease) in particle size that occurs when the polymer(s) of which they are comprised undergo a volume phase transition, an event that occurs at the volume phase transition temperature (VPTT). Positively temperature responsive nanogels show a marked and fast increase in size when temperature rises to the VPTT, whereas negatively temperature responsive nanogels rapidly shrink in size with temperature increase above their VPTT. Negatively temperature responsive nanogels have been formulated from poly(N-isopropylacrylamide) (PNIPAM) 84; however, positively temperature responsive nanogels are preferred for drug delivery applications because of the ability of swelling, and not deswelling, behavior to release compounds that are otherwise are entrapped within the collapsed nanogels. Before this is triggered by a localized change in temperature, entrapped drugs are retained within the collapsed nanogel, thus restricting undesired early or premature release. Transport of drug out of the swollen nanogel occurs primarily by diffusion following temperature-induced nanogel size expansion, and this method of drug release is considered to be more efficient than the expulsion of drug from a collapsed negatively temperature responsive nanogel 85,86.
When designing temperature responsive drug delivery nanogels, the VPTT needs to be marginally higher than normal tissue temperature in order for exposure to a slight temperature increase to promote drug release. This is relevant because temperature is usually elevated in inflamed tissues where therapeutics are being delivered, and hyperthermia can also be induced locally by a number of external heating techniques. Nanogels having a VPTT above normal body temperature are the subject of recent interest for these reasons 87,88, but historically many temperature responsive nanogels for drug delivery have been designed to release their cargo when exposed to an increase in temperature 85,89–91. Examples of temperature sensitive nanogel formulations include those comprised of poly(N-isopropylacrylamide), oligo(ethylene glycol) acrylate and 2-(5,5-dimethyl-1,3-dioxan-2-yloxy) ethyl acrylate. Temperature modulation remains an interesting and novel approach for altering tissue characteristics, nanogel swelling as well as drug loading and unloading for disease treatment.
Photoactive/light responsive nanogels
Yet another method to induce changes in biomaterials is to manufacture them from polymers bearing photoactive groups that alter bonding or conformation in response to light exposure. A number of light-responsive functional groups, including triphenylmethane, spirobenzopyran, cinnamonyl and azobenzene undergo size or shape change, or form ionic or zwitterionic moieties when irradiated 92,93. When nanogels formulated from these types of reactive polymers are exposed to the appropriate light wavelength, a phase transition ensues with structural or polarity change occurring with the functional groups. Drug delivery based on this approach are limited to systems that are activated with near infrared (NIR) light, since stimulation with light in the visible and UV regions do not penetrate beyond the skin into deep tissues, and the UV and visible wavelengths can also damage human tissue even at low power.
Use of NIR light, which is transmitted well through skin and many other tissues at the millimeter-to-centimeter length scale, has been used to stimulate hybrid nanogels comprised of temperature responsive polymers and noble metals, including Au and Ag nanoparticles (NP). The presence of the metal constituent leads to localized heating with light absorption, and this promotes phase transition within the polymer constituent, which can then lead to drug release. The utilization of Au-NP in such systems is desired because it has very limited known toxicity 94, and it does not self-quench while providing a large optical cross-section for imaging purposes 95. Being highly stable at the nanoscale, gold has great purpose for funtionalization via surface modification with polymers, organic molecules and biomacromolecules through thiol chemistry 96.
Light responsive, Au-NP containing nanogels have been developed for use as photothermal therapeutics, and these photoactive nanogels can be selectively activated for drug release in a specific disease region by externally applied photo irradiation 97. Additionally, PEGylated nanogels containing Au-NP in a cross-linked network core of poly[2-(N,N-diethylamino)ethyl methacrylate] have been formulated for cancer treatment 98. These nanogels were shown to become cytotoxic only when light activated, secondary to heat being generated by intracellular nanogels. This demonstrates that light responsive nanogels containing metal NPs may be useful for both delivery and release of cargo drug as well as localized heating for thermal therapy 99. This dual mode therapeutic approach based on hybrid nanogels has been shown to enhance therapeutic efficacy.
Gold nanorods have been incorporated into polymeric hydrogels to create stimuli-responsive materials for biomedical applications 100. For example, a cross-linked tert-butyl acrylate network containing gold nanorods were heated by exposure to 770 nm laser at 0.3 W and underwent a shape transition within several minutes 101. Also, peptide hydrogels containing gold nanorods showed release of encapsulated dextran upon exposure to a 808 nm laser 102.
Biomolecule recognition-responsive nanogels
Molecular recognition within biological systems is a native system for inducing specific changes in biological tissues, cells and biomolecules as a result of reaction to, or modification of, a particular molecule or ion that is associated with eliciting some biological function. Mimicking these native biological functions can be achieved by incorporating the stimulus-inducing biomolecule into biomaterials 103, including nanogels, in order to induce the desired responses for purposes such as promoting encapsulated drug release. For instance, biomolecule recognition-responsive hydrogels have been produced to elicit responses to nucleic acids 104, peptides 105, proteins 106 and glucose 107. The latter are of significant interest in diabetes research and the development of nanotechnology-based insulin delivery systems that offer new options for the clinical treatment of this prevalent disease 108.
A few types of nanogels that respond to glucose have been developed. Polysulfide nanogels bearing glucose oxidase (GOx)–pluronic conjugates have been synthesized 109. The GOx enzyme is an oxido-reductase that catalyses the oxidation of glucose to hydrogen peroxide and D-glucono-δ-lactone, which decreases local pH (see above for more detail regarding pH-responsive nanogels). When glucose is present, GOx also elicits oxidation of sulfides, which induces nanogel swelling. Both inducible swelling and pH change can be utilized to promote drug release from GOx-containing nanogels 110.
Nanogels have also been designed for biomolecule-responsive behavior based on specific complex interactions that occur involving glucose and functional groups such as phenylboronic acid (PBA) within the nanogel network 111. PBA groups are present in aqueous solution exist in both charged and uncharged forms. Only the charged form yields a stable complex with glucose, while the uncharged form readily undergoes hydrolysis. Stable glucose-associated complex formation shifts the equilibrium, increasing the number of charged groups and increasing the hydrophilicity of the polymer chain. This promotes nanogel swelling, and associated drug release. A number of studies detailing the synthesis of nanogels based on PBA-glucose interactions have been published 112,113. Shapes such as PBA-based glucose responsive nanocapsules have also been produced 114, as have PBA-based amphoteric nanogels that also electrostatically bind insulin 115. These amphoteric nanogels provide an additional potential therapeutic benefit since they release more insulin in the presence of higher glucose levels.
Nanogels have now been developed for the differential delivery of antimicrobials that are released in the presence of lipase-secreting bacteria 116. This is a broadly applicable approach for treatment of both intracellular and extracellular infections that is based on the presence of a hydrophobic poly(ε-caprolactone) fence structure in the nanogel that prevents antibiotic release until the fence is degraded in the presence of lipase. The resultant encapsulated drug release has been demonstrated to be bactericidal as desired.
Magnetic field responsive nanogels
Another form of hybrid nanogels are those that are magnetic field responsive by virtue of their containing magnetic NPs comprised of either Fe2O3 or Fe3O4. Like Au or Ag NP containing nanogels, magnetic NP containing nanogels may undergo heating upon exposure to an alternating magnetic field. Magnetic nanoparticles may also undergo tissue site localization due to application of a high magnetic field gradient. For drug delivery purposes, superparamagnetic formulations lack any magnetism when not exposed to a magnetic field, making their site direction of function of magnetic field exposure or lack thereof. However, these formulations can have innate toxicity depending on features such as size, shape and chemical composition 117. Entrapment of magnetic NPs into nanogels can be achieved by emulsion polymerization or in situ synthesis methods 118–120, but homogenous distribution of the magnetic NPs within the nanogels is not guaranteed. Resultant nonuniform magnetic NP content may affect the nanogel magnetic field responsiveness for site localization and/or heat production.
Magnetic nanogels having a core–shell structure have been produced as demonstrated by transmission electron microscopy 121. These, as well as other formulations, have significant potential for use in drug delivery in a manner similar to that utilized with photoresponsive/light sensitive nanogels. For instance, nanogel can first be directed to the intended therapeutic site by direct application of a permanent magnetic field 122, and once accumulated in the target site the nanogels release their therapeutic cargo 122. Magnetic poly(vinyl pyrrolidone) nanogels have been loaded with Bleomycin A5 Hydrochloride (BLM) and injected into rabbits having a squamous cell carcinoma 123. A permanent magnet was placed directly over the tumor surface for one day after nanogel injection. Over the next two weeks, the tumor shrank significantly in size due to magnetic field site-directed nanogel accumulation and drug release within the tumor.
Magnetic NPs incorporated into nanogels can also be utilized for thermal therapy, because upon exposure to an alternating magnetic field, they can generate heat 124. This has been expanded for the development of thermoresponsive magnetic composite nanomaterials for multimodal cancer therapy 125. Using a combination of several response-initiated features, an alternating magnetic field was applied to magnetic PNIPAM nanogels to create sufficient heat to drive the local temperature above the nanogel polymer VPTT. As a result of the thermal effects, significant nanogel deswelling occurred, and this promoted release of encapsulated doxorubicin. Such novel use of multiple modalities, including magnetically directed site delivery of the nanogels, thermotherapy and drug release for chemotherapy, makes this a very innovative means of treating disease 126.
Targeting Nanogels
One of the most recognized (albeit not yet fully realized in practical medicine) benefits of utilizing nanoparticles including nanogels as drug carriers is to enable targeted delivery to the desirable sites of intervention – organs, tissues, cells and their compartments, pathological formations such as thrombi, etc. Delivery to these sites is impeded by clearance from the bloodstream via “non-specific” uptake in tissues including the vascular system (i.e., binding to endothelial and blood cells), reticulo-endothelial system (RES, including liver, spleen and lymphatic nodes) and excretory organs including kidneys, lungs, and the bile tract 31,32. These mechanisms highly effective and difficult to circumvent; since they exert important host defense and detox functions their unguided inhibition is generally undesirable. Two cardinal principles to favor delivery to the desirable therapeutic sites over these “sinks” are passive and active targeting.
Passive Targeting
In some cases, carriers “passively” accumulate in desired sites. Nanoparticles typically exhibit circulation times that can be systematically varied and if desirable, extended to allow for the sufficient time of blood perfusion to deliver cargo to a target site. In addition, the increased retention of nanoparticles within the blood pool, due to their inability to diffuse across continuous (i.e. non-fenestrated) endothelium, is often associated with a reduction in off-target toxicity 127. Currently, it is purported that most pre-clinical and all FDA-approved nanoparticles for oncologic purposes passively accumulate at their target site (pathophysiological targeting) as a consequence of enhanced permeability and retention (EPR).
EPR stems from the abnormal increase in vascular permeability that arises during the pathogenesis of a wide range of diseases including inflammation and solid tumors, allowing nanoparticles to deposit in the extracellular space. In some cases, such as in cancer, nanoparticle retention may be heightened due to impaired lymphatic drainage. The EPR effect is highly variable between diseases, organs, and even within a single pathological site. For example, EPR is not commonly observed in gastric and pancreatic cancer 128, and in most tumors at least some of the vasculature remains intact. In contrast, some regions may be extremely permeable, allowing submicron nanoparticles to penetrate into the interstitium 129. There are variable data and opinions on size limitations for the EPR; most sources refer to 20–200 nm diameter range 31,130, yet the rate of permeation from blood and diffusion in the leaky tissues certainly depends on the type of pathology (cancer, inflammation), its stage and other individual characteristics that vary from patient to patient and next to impossible to model adequately in animals.
Regardless, it is widely acknowledged that there is a positive correlation between EPR and circulation time 131. Circulation time is typically extended through the introduction of surface coating materials (e.g. polyethylene glycol) that minimize opsonization and recognition by the reticuloendothelial system (RES) 132. However, it is also recognized that nanoparticle charge, shape, and composition can all effect nanoparticle biodistribution and permeability to the EPR areas 131,133.
To facilitate nanoparticle delivery via EPR, a number of strategies have been used to increase vascular permeability. One such approach involves the use of vasoactive agents (e.g. bradykinin, thrombin) to initiate a cascade of cellular events that lead to a disruption of cellular junctions 134. Alternatively, external stimuli, such as ionizing radiation and photodynamic therapy, have also been used to disrupt vascular integrity in a more targeted manner 135,136. A different strategy involves lowering the high interstitial pressure observed in tumors. For example, vasoconstrictors (angiotensin) have been used to raise systemic pressure and reduce the pressure differential between the tumor and the surrounding vasculature 137. Recently, it has been proposed that normalizing blood vessels within tumors, by making them less leaky, may also lead to lower interstitial fluid pressure, thus allowing small nanoparticles to enter tumors more rapidly 138.
Another example of “passive targeting” is retention of carriers in the vascular area downstream of the injection site due to mechanical or electrostatic retention 34. This approach is used for imaging of blood perfusion using radiolabeled biodegradable particles such as albumin microspheres with diameter 20–50 μm, which are entrapped mechanically in the microvasculature 35. Further, cationic liposomes that bind to negatively charged endothelial glycocalyx have been explored for delivery of genetic materials 36.
Drugs released from carriers that employ “passive targeting” diffuse by the gradient to surrounding tissues and cells. Diffusional barriers (e.g., interstitial components and pressure) and removal from the site of interest by perfusion impede the effectiveness of mass transfer to the cells of interest. Furthermore, passive targeting provides little, if any, guidance in cellular delivery and subsequent subcellular localization. Although passive targeting continues to be the primary mechanism by which most new nanoparticle formulations reach their target site, there has been a general movement towards the use of active targeting to complement EPR.
Active Targeting
“Active targeting” mediated by affinity ligands coupled to a carrier is a more precise and universal approach. It uses ligands that bind to molecules present or enriched in a cell, tissue, or pathological structure of interest (target determinants). Antibodies, their fragments and recombinant polypeptides including single chain antigen binding fragments (scFv), nutrients, hormones, mediators, receptor ligands, peptides, aptamers, and nucleic acids have been explored as targeting ligands 37,38,41,139,140. Recently lipid derivatives such as choline-group containing molecules have also shown significant promise for active targeting 141,142.
Target determinants must meet several criteria in order to be useful for drug delivery. First, it should provide binding or anchoring of carriers. For this purpose, determinant molecules should be present in the site of interest in sufficient surface density on the target cell, tissue or structure accessible to permit anchoring of ligand-directed carriers. Determinants selectively expressed or exposed under the pathological conditions in the site of interest allow selective delivery. Of note, some determinants disappear from the surface of target cells under pathological conditions. Next, binding to the target determinants should not cause side effects that may impede or defeat the purpose of the intended medical intervention. Third, anchoring to the target determinants should favor subsequent addressing to the sub-cellular compartments where the drug cargo is supposed to act.
Traditionally, active targeting has meant the targeting of cell surface receptors that are unique to the target site 143. However, there now exists a much wider range of biomarkers that have been exploited to drive nanoparticle accumulation and trigger the release of cargo, including extracellular enzymes (e.g. matrix metalloproteases) 144,145 and microenvironmental factors (e.g. pH, reactive oxygen species, etc.) 146–149. The foreseen advantages of active targeting include higher accumulation of nanoparticles at the target site, higher specificity, and an increase in the cellular internalization of nanoparticles, all of which are expected to lead to an improved therapeutic index. While it is generally accepted that actively targeting vascular biomarkers can improve nanoparticles accumulation and specificity at a disease site, the benefit of targeting extravascular targets (e.g. cancer or stroma cells) remains highly debated 150. This is largely because nanoparticles may not always have access to these targets, e.g. due to the presence of an intact endothelium, or biomarker expression may be absent, low, or heterogeneous. However, when biomarkers are accessible and abundant, targeting is thought to improve tissue retention and confer specificity towards target cells. Active targeting may also provide a synergistic cytotoxic effect, when the targeting moiety works independent of the nanoparticle’s therapeutic cargo to trigger cell death 151. It has also been suggested that nanoparticles internalized by receptor-mediated endocytosis may avoid removal via glycoprotein efflux pumps, which could further improve the therapeutic index of nanoparticles and possibly help overcome multi-drug resistance 152. Moreover, it has been postulated that the targeting of different epitopes on a particular biomarker could be used to modulate a nanoparticles intracellular trafficking and destination, conferring a second-level of targeting 150.
Bioconjugate Chemistry
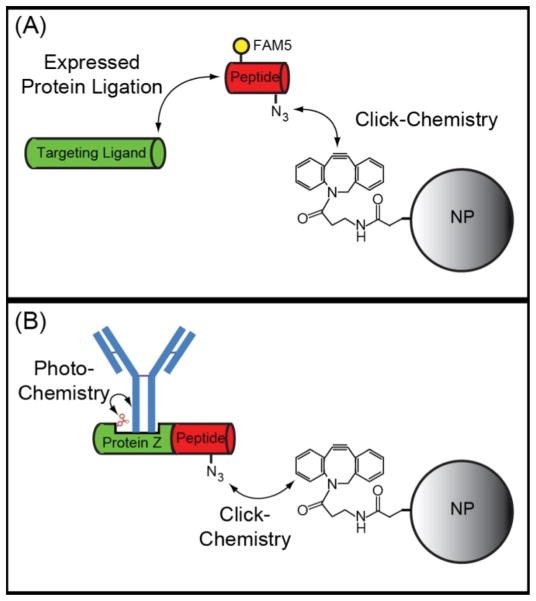
A general requirement for the preparation of targeted nanoparticles is the need to attach targeting moieties to the nanoparticle surface. This can be a costly endeavor when employing conventional bioconjugate chemistries, e.g. those relying on carbodiimide, maleimide, and N-hydroxysuccinimide reactions, since these approaches are highly inefficient with typically <10% of a ligand being conjugated to the nanoparticle surface 153,154. The emergence of highly-efficient click chemistries has helped overcome this limitation 154, but even click chemistries are not site-specific, non-stoichiometric, and not always compatible with certain classes of protein scaffolds. This lack of control can lead to a heterogeneous population of nanoparticles, with variable binding capacity, due to differences in ligand density, orientation, and activity 155. Since ligand presentation has been shown to strongly influence both avidity and specificity, with optimal formulations typically possessing an intermediate number of active ligands on the nanoparticle surface 156,157, it is becoming increasingly recognized that control over nanoparticle bioconjugation is essential to maximize nanoparticle performance and minimize cost. This has led to the development of several techniques that allow for the efficient and site-specific attachment of targeting ligands onto a nanoparticle surface. The first attempts to achieve efficient and site-specific labeling of nanoparticles involved the preparation of targeting ligands with polyhistidine tags, which were bound to nanoparticles modified with Ni-nitrilotriacetic (Ni-NTA) and nanoparticles that naturally contain zinc on their surface (e.g. CdSe/ZnS quantum dots) 158–160. More recently, expressed protein ligation (EPL) has been used to site-specifically modify recombinantly expressed proteins with click-chemistry moieties (e.g. azide, alkyne) that are subsequently clicked to nanoparticles that have been functionalized with a complementary group (Figure 2A) 156,161.
Figure 2.
Schematic of EPL-click chemistry bioconjugation techniques. (A) EPL results in the chemoselective attachment of peptides functionalized with an azide group to recombinantly expressed targeting ligands. Additional functional groups such as fluorescent dyes (e.g. FAM5) can also be included on the peptide. The azido-labeled targeting ligands can then be efficiently and site-specifically attached to alkyne-modified nanoparticles (NP) via click chemistry. The constrained alkyne, Aza-dibenzocyclooctyne, is shown, which allows for copper-free click chemistry. (B) The EPL-click chemistry approach can be combined with non-natural amino acid incorporation to produce an antibody binding protein, Protein Z, with a photocrosslinker (e.g. benzoylphenylalanine) in the Fc binding domain. This allows for the site-specific and covalent attachment of full-length antibodies to nanoparticles.
EPL has been achieved using both protein splicing enzymes (i.e. inteins) and transpeptidases (i.e. sortase); however, it is feasible that other enzymes (e.g. formylglycine generating enzyme, transferases) could also be used in a similar manner. In fact, a variety of enzymes have already been employed for the site-specific modification of proteins for the preparation of protein-drug conjugates 162. To enable the attachment of full-length antibodies to a nanoparticle surface, EPL-click chemistry was recently combined with non-natural amino acid incorporation to produce an antibody-binding protein, Protein Z, with a photocrosslinker in the Fc-biinding domain and an azide at the c-terminus (Figure 2B) 163. This allowed Protein Z to be covalently linked to IgG upon photoactivation and for the subsequent site-specific attachment of IgG to alkyne-modified nanoparticles.
Nanogel toxicity and nanotoxicology
A major health concern for all applications of nanobiotechnology is the nanotoxicology of the materials that are introduced into clinical therapeutics as well as the risks to those workers exposed in manufacturing and delivery processes. Ultimately the inherent toxicity of nanogel drug delivery vehicles will determine their clinical usefulness. In order to design a toxicity-free drug delivery vehicle, the polymer materials and other chemicals, biomacromolecules and NPs from which it is made must be materials that either lack toxicity to begin with or which are metabolized into non-toxic degradation products long before any deleterious effects occur following their introduction into the body. It is already known that methacrylate- and acrylate-based polymeric systems are readily hydrolyzed into mostly non-toxic poly-methacrylic and poly-acrylic acids and small molecule alcohols 164,165. The toxicity of nanogels comprised of these polymers is expected to be low, as a result of the minimal toxicity of the hydrolyzed small molecule degradation products. Nanogels comprised of sugars (e.g., dextran) and stable proteins (e.g., lysozyme) are also expected to have very minimal toxic potential 3,4,28. Other low toxicity nanogel systems have been synthesized via cross-linking of a self-assembling block copolymer consisting of a block of poly(oligoethylene glycol) and a block of randomly co-polymerized vinyl benzyl chloride and pentafluorophenyl acrylate 59. Cells exposed to these nanogels retained very high (> 90%) viability at high concentrations of nanogel exposure (8 mg/ml), providing initial evidence of low toxicity.
As any other nanocarrier drug delivery object, even nanogels based on fully benign materials may, in theory, exert side effects due to: i) a huge surface/mass ratio that may adversely alter both the rate of degradation and reactive capacity of the nanocarrier’s surface; and, ii) interactions with cells - surface activation, uptake, intracellular degradation or deposition, all of which may abnormally activate, damage or kill the cells taking up the carriers. Of course, the latter concern relates mostly to the cleaning and target cells; therefore, in oncologic applications adverse effects towards the target cells may be viewed as an additional bonus
Conclusions
In designing nanogels for targeted drug delivery, there are a number of design issues involving nanogel properties, responsiveness characteristics influencing localization and drug release, targeting and toxicity that must be addressed for any resultant drug delivery vehicle produced to be a safe and effective nanotechnology for clinical use in disease treatment. Herein we have emphasized the need to develop and understand a variety of important physical and biological mechanisms that can be tapped via nanogel design to transport a sufficient amount of drug efficiently and effectively across any number of physiological barriers to accumulate in specific therapeutic sites. The future impact of nanogel-based drug delivery involves minimally toxic or nontoxic methods that reduce side effects and enhance site-specific delivery with appropriate levels of drug release to achieve therapeutic goals. Although nanogels can be very stable, highly biocompatible and stimulus responsive, their practical clinical application is at present still limited. Several important criteria must still be considered in their future development. These include: their size, shape and surface modification, all of which will influence their circulation duration, molecular recognition by diseased tissues and cellular uptake; biodegradability, which will influence both their drug delivery and nanotoxicology; and their responsiveness to different stimuli such as temperature or pH changes, biomolecule levels, irradiation or magnetic field exposure that can be exploited to enhance drug delivery as nanogels are site directed or characteristically altered to promote drug release as they transit from normal to diseased tissues. One particular advantage of nanogels versus many alternative drug nanocarriers is their theoretically ideal suitability for targeting based on their viscoelasticity. First, affinity ligands conjugated to the end groups of mobile polymeric chains have higher probability of effective engagement with the target binding sites both individually and, more importantly, collectively. The latter factor includes the ability to spatially adjust ligand molecules congruently to organization of the binding sites in the target – clusters, groups of clusters, protrusions and invaginations of the plasmalemma. In this aspect, nanogels are expected to exert higher avidity than rigid carriers of similar size carrying similar number of ligands on their surface. Second, nanogel viscoelasticity may enhance accessibility to binding sites hidden in the tissue compartments beyond the reach of rigid particles, while nanogels squeeze into these difficult targets. Third, it is likely that positive impact of these factors will be magnified in conditions associated with flow and other mechanical forces, when rapid multivalent anchoring is the key. As nanogels for drug delivery having these characteristics are developed, their incorporation into clinical medicine will proceed with great rapidity.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants R01 EB006818 and U01 EB016027. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1 TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Wagner V, Dullaart A, Bock AK, Zweck A. Nature Biotechnology. 2006;24:1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 2.Kabanov AV, Vinogradov SV. Angewandte Chemie International Ed. 2009;48:5418–5429. doi: 10.1002/anie.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coll Ferrer MC, Dastgheyb S, Hickok NJ, Eckmann DM, Composto RJ. Acta Biomaterialia. 2014;10:2105–2111. doi: 10.1016/j.actbio.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coll Ferrer MC, Zern B, Composto RJ, Muzykantov’ VR, Eckmann DM. PLOS One. 2014 doi: 10.1371/journal.pone.0102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Las Heras AC, Pennadam S, Alexander C. Chem Soc Rev. 2005;34:276–285. doi: 10.1039/b406727d. [DOI] [PubMed] [Google Scholar]
- 6.Onaca O, Enea R, Hughes DW, Meier W. Macromolecular Bioscience. 2009;9:129–139. doi: 10.1002/mabi.200800248. [DOI] [PubMed] [Google Scholar]
- 7.Bawa P, Pillay V, Choonara YE, du Toit LC. Biomedical Materials. 2009;4:022001. doi: 10.1088/1748-6041/4/2/022001. [DOI] [PubMed] [Google Scholar]
- 8.Oishi M, Sumitani S, Nagasaki Y. Bioconjugate Chem. 2007 Oct;18:1379–1382. doi: 10.1021/bc7002154. [DOI] [PubMed] [Google Scholar]
- 9.Peng HS, Stolwijk JA, Sun LN, Wegener J, Wolfbeis OS. Angewandte Chemie International Ed. 2010;49:4246–4249. doi: 10.1002/anie.200906926. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa U, Nomura SM, Kaul SC, Hirano T, Akiyoshi K. Biochemical & Biophysical Research Communications. 2005;331:917–921. doi: 10.1016/j.bbrc.2005.03.228. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi C, Hasegawa U, Saita Y, Hemmi H, Hayata T, Nakashima K, Ezura Y, Amagasa T, Akiyoshi K, Noda M. Journal of Cellular Physiology. 2009;220:1–7. doi: 10.1002/jcp.21760. [DOI] [PubMed] [Google Scholar]
- 12.Vinogradov SV. Nanomedicine. 2010;5:165–168. doi: 10.2217/nnm.09.103. [DOI] [PubMed] [Google Scholar]
- 13.Goncalves C, Pereira P, Gama M. Materials. 2010;3:1420–1460. [Google Scholar]
- 14.Li J, Yu SY, Yao P, Jiang M. Langmuir. 2008;24:3486–3492. doi: 10.1021/la702785b. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Maheshwari R, Kiick KL. Macromolecules. 2009;42:3–13. doi: 10.1021/ma801782q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen WQ, Chang YL, Liu GY, Wang HF, Cao AN, An ZS. Macromolecules. 2011;44:2524–2530. [Google Scholar]
- 17.Urakami H, Hentschel J, Seetho K, Zeng H, Chawla K, Guan Z. Biomacromolecules. 2013;14:3682–3688. doi: 10.1021/bm401039r. [DOI] [PubMed] [Google Scholar]
- 18.Yuan YY, Du JZ, Song WJ, Wang F, Yang XZ, Xiong MH, Wang J. J Mater Chem. 2012;22:9322–9329. [Google Scholar]
- 19.Coll Ferrer MC, Sobolewski P, Composto RJ, Eckmann DM. Journal of Nanotechnology in Engineering and Medicine. 2013;4:011002–1–8. doi: 10.1115/1.4023246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAllister K, Sazani P, Adam M, Cho MJ, Rubinstein M, Samulski RJ, DeSimone JM. J Am Chem Soc. 2002;124:15198–15207. doi: 10.1021/ja027759q. [DOI] [PubMed] [Google Scholar]
- 21.Bontha S, Kabanov AV, Bronich TK. Journal of Controlled Release. 2006;114:163–174. doi: 10.1016/j.jconrel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Bronich TK, Vinogradov SV, Kabanov AV. Nano Lett. 2001;1:535–540. [Google Scholar]
- 23.Khmelnitsky YL, Neverova IN, Gedorovich AV, Polyakov VA, Levashov AV, Martinek K. European Journal of Biochemistry. 1992;210:751–757. doi: 10.1111/j.1432-1033.1992.tb17477.x. [DOI] [PubMed] [Google Scholar]
- 24.Yan M, Ge J, Liu Z, Ouyang P. J Am Chem Soc. 2006;128:11008–11009. doi: 10.1021/ja064126t. [DOI] [PubMed] [Google Scholar]
- 25.Akiyoshi K, Sasaki Y, Sunamoto J. Bioconjugate Chem. 1999;10:321–324. doi: 10.1021/bc9801272. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Choi SH, Kim SH, Park TG. Journal of Controlled Release. 2008;125:25–32. doi: 10.1016/j.jconrel.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Missirlis D, Kawamura R, Tirelli N, Hubbell JA. European Journal of Pharmaceutical Sciences. 2006;29:120–129. doi: 10.1016/j.ejps.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Coll Ferrer MC, Jr, Ferrier RC, Eckmann DM, Composto RJ. Journal of Nanoparticle Reserarch. 2013;15:1323–1–7. doi: 10.1007/s11051-012-1323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kabanov AV, Batrakova EV, Alakhov VY. Journal of Controlled Release. 2002;82:189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 30.Oh NM, Oh KT, Baik HJ, Lee BR, Lee AH, Youn YS, Lee ES. Colloids and Surfaces B: Biointerfaces. 2010;78:120–126. doi: 10.1016/j.colsurfb.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 31.Oh JK, Drumright R, Siegwart DJ, Matyjaszewski K. Progress in Polymer Science. 2008;33:448–477. doi: 10.1016/j.progpolymsci.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gratton SEA, Pohlhaus PD, Lee J, Guo J, Cho MJ, DeSimone JM. Journal of Controlled Release. 2007;121:10–18. doi: 10.1016/j.jconrel.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talei Franzesi G, Ni B, Ling Y, Khademhosseini A. J Am Chem Soc. 2006;128:15064–15065. doi: 10.1021/ja065867x. [DOI] [PubMed] [Google Scholar]
- 34.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. J Am Chem Soc. 2005;127:10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 35.Euliss LE, DuPont JA, Gratton S, DeSimone J. Chem Soc Rev. 2006;35:1095–1104. doi: 10.1039/b600913c. [DOI] [PubMed] [Google Scholar]
- 36.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Nat Nano. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petros RA, DeSimone JM. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 38.Yallapu MM, Reddy MK, Labhasetwar V. In: Biomedical applications of nanotechnology. Labhasetwar V, Leslie-Pelecky DL, editors. Chapter 6 Wiley; Hoboken, NJ: 2007. [Google Scholar]
- 39.Mahaffy RE, Shih CK, MacKintosh FC, Kas J. Physical Review Letters. 2000;85:880–883. doi: 10.1103/PhysRevLett.85.880. [DOI] [PubMed] [Google Scholar]
- 40.Neradovic D, van Nostrum CF, Hennink WE. Macromolecules. 2001;34:7589–7591. [Google Scholar]
- 41.Kwon GS, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Pharm Res. 1993;10:970–974. doi: 10.1023/a:1018998203127. [DOI] [PubMed] [Google Scholar]
- 42.Ma Q, Remsen EE, Kowalewski T, Wooley KL. J Am Chem Soc. 2001;123:4627–4628. doi: 10.1021/ja0156542. [DOI] [PubMed] [Google Scholar]
- 43.Bronich TK, Keifer PA, Shlyakhtenko LS, Kabanov AV. J Am Chem Soc. 2005;127:8236–8237. doi: 10.1021/ja043042m. [DOI] [PubMed] [Google Scholar]
- 44.Tang M, Dou H, Sun K. Polymer. 2006;47:728–734. [Google Scholar]
- 45.Oh JK, Lee DI, Park JM. Progress in Polymer Science. 2009;34:1261–1282. [Google Scholar]
- 46.Jiwpanich S, Ryu JH, Bickerton S, Thayumanavan S. J Am Chem Soc. 2010;132:10683–10685. doi: 10.1021/ja105059g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryu JH, Jiwpanich S, Chacko R, Bickerton S, Thayumanavan S. J Am Chem Soc. 2010;132:8246–8247. doi: 10.1021/ja102316a. [DOI] [PubMed] [Google Scholar]
- 48.Ryu JH, Chacko RT, Jiwpanich S, Bickerton S, Babu RP, Thayumanavan S. J Am Chem Soc. 2010;132:17227–17235. doi: 10.1021/ja1069932. [DOI] [PubMed] [Google Scholar]
- 49.Li YL, Zhu L, Liu Z, Cheng R, Meng F, Cui JH, Ji SJ, Zhong Z. Angewandte Chemie International Ed. 2009;48:9914–9918. doi: 10.1002/anie.200904260. [DOI] [PubMed] [Google Scholar]
- 50.Pioge S, Nesterenko A, Brotons G, Pascual S, Fontaine L, Gaillard C, Nicol E. Macromolecules. 2011;44:594–603. [Google Scholar]
- 51.He J, Yan B, Tremblay L, Zhao Y. Langmuir. 2011;27:436–444. doi: 10.1021/la1040322. [DOI] [PubMed] [Google Scholar]
- 52.Yesilyurt V, Ramireddy R, Thayumanavan S. Angewandte Chemie - International Edition. 2011;50:3038–3042. doi: 10.1002/anie.201006193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raghupathi KR, Azagarsamy MA, Thayumanavan S. Chemistry - A European Journal. 2011;17:11752–11760. doi: 10.1002/chem.201101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akiyoshi K, Deguchi S, Moriguchi N, Yamaguchi S, Sunamoto J. Macromolecules. 1993;26:3062–3068. [Google Scholar]
- 55.Sasaki Y, Akiyoshi K. Chemical Record. 2010;10:366–376. doi: 10.1002/tcr.201000008. [DOI] [PubMed] [Google Scholar]
- 56.Huang H, Remsen EE, Wooley KL. Chem Commun. 1998:1415–1416. [Google Scholar]
- 57.Joralemon MJ, Smith NL, Holowka D, Baird B, Wooley KL. Bioconjugate Chem. 2005;16:1246–1256. doi: 10.1021/bc0501505. [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Du W, Sun G, Wooley KL. Macromolecules. 2008;41:6605–6607. [Google Scholar]
- 59.Duong HTT, Marquis CP, Whittaker M, Davis TP, Boyer C. Macromolecules. 2011;44:8008–8019. [Google Scholar]
- 60.Hu YC, Pan CY. J Mater Chem. 2005;21:4288–4289. [Google Scholar]
- 61.Li Y, Lokitz BS, Armes SP, McCormick CL. Macromolecules. 2006;39:2726–2728. [Google Scholar]
- 62.Kim Y, Pourgholami MH, Morris DL, Stenzel MH. J Mater Chem. 2011;21:12777–12783. [Google Scholar]
- 63.Joralemon MJ, O’Reilly RK, Hawker CJ, Wooley KL. J Am Chem Soc. 2005;127:16899. doi: 10.1021/ja053919x. [DOI] [PubMed] [Google Scholar]
- 64.Zhang J, Zhou Y, Zhu Z, Ge Z, Liu S. Macromolecules. 2008;41:1444–1454. [Google Scholar]
- 65.Vaupel P, Kallinowski F, Okunieff P. Cancer Research. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 66.Watson P, Jones AT, Stephens DJ. Advanced Drug Delivery Reviews. 2005;57:43–61. doi: 10.1016/j.addr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 67.Schmaljohann D. Advanced Drug Delivery Reviews. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 68.Mandel M. European Polymer Journal. 1970;6:807–822. [Google Scholar]
- 69.Bronich TK, Vinogradov SV, Kabanov AV. Nano Lett. 2001;1:535–540. [Google Scholar]
- 70.Ojugo ASE, McSheehy PMJ, McIntyre DJO, McCoy C, Stubbs M, Leach MO, Judson IR, Griffiths JR. NMR Biomed. 1999;12:495–504. doi: 10.1002/(sici)1099-1492(199912)12:8<495::aid-nbm594>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 71.Van Dyke RW. Sub-Cellular Biochemistry. 1996;27:331–360. doi: 10.1007/978-1-4615-5833-0_10. [DOI] [PubMed] [Google Scholar]
- 72.Ganta S, Devalapally H, Shahiwala A, Amiji M. Journal of Controlled Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 73.Raemdonck K, Demeester J, De Smedt S. Soft Matter. 2009;5:707–715. [Google Scholar]
- 74.Na K, Lee ES, Bae YH. Bioconjugate Chem. 2007;18:1568–1574. doi: 10.1021/bc070052e. [DOI] [PubMed] [Google Scholar]
- 75.Oishi M, Hayashi H, Iijima M, Nagasaki Y. J Mater Chem. 2007;17:3720–3725. [Google Scholar]
- 76.Hayashi H, Iijima M, Kataoka K, Nagasaki Y. Macromolecules. 2004;37:5389–5396. [Google Scholar]
- 77.Hu Y, Litwin T, Nagaraja AR, Kwong B, Katz J, Watson N, Irvine DJ. Nano Lett. 2007;7:3056–3064. doi: 10.1021/nl071542i. [DOI] [PubMed] [Google Scholar]
- 78.Braun O, Selb J, Candau F. Polymer. 2001;42:8499–8510. [Google Scholar]
- 79.Deng L, Zhai Y, Guo S, Jin F, Xie Z, He X, Dong A. J Nanopart Res. 2009;11:365–374. [Google Scholar]
- 80.Kabanov VA, Skobeleva VB, Rogacheva VB, Zezin AB. J Phys Chem B. 2003;108:1485–1490. [Google Scholar]
- 81.Oh KT, Bronich TK, Kabanov VA, Kabanov AV. Biomacromolecules. 2006;8:490–497. doi: 10.1021/bm060599g. [DOI] [PubMed] [Google Scholar]
- 82.Vinogradov SV, Zeman AD, Batrakova EV, Kabanov AV. Journal of Controlled Release. 2005;107:143–157. doi: 10.1016/j.jconrel.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ogawa K, Nakayama A, Kokufuta E. Langmuir. 2003;19:3178–3184. [Google Scholar]
- 84.Pelton R. Advances in Colloid and Interface Science. 2000;85:1–33. doi: 10.1016/s0001-8686(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 85.Li JK, Wang N, Wu XS. Journal of Controlled Release. 1998;56:117–126. doi: 10.1016/s0168-3659(98)00089-3. [DOI] [PubMed] [Google Scholar]
- 86.Wang N, Wu XS. Pharmaceutical Development and Technology. 1997;2:135–142. doi: 10.3109/10837459709022618. [DOI] [PubMed] [Google Scholar]
- 87.Saunders BR, Laajam N, Daly E, Teow S, Hu X, Stepto R. Advances in Colloid and Interface Science. 2009;147–148:251–262. doi: 10.1016/j.cis.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 88.Zhou T, Wu W, Zhou S. Polymer. 2010;51:3926–3933. [Google Scholar]
- 89.Ge H, Ding Y, Ma C, Zhang G. J Phys Chem B. 2006;110:20635–20639. doi: 10.1021/jp060914t. [DOI] [PubMed] [Google Scholar]
- 90.Wang N, Wu XS. Pharmaceutical Development and Technology. 1997;2:135–142. doi: 10.3109/10837459709022618. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y, Zhu W, Wang B, Ding J. Journal of Controlled Release. 2005;105:260–268. doi: 10.1016/j.jconrel.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 92.Alvarez-Lorenzo C, Bromberg L, Concheiro A. Photochemistry and Photobiology. 2009;85:848–860. doi: 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 93.Li MH, Keller P. Soft Matter. 2009;5:927–937. [Google Scholar]
- 94.Sherman AI, Ter Pogossian M. Cancer. 1953;6:1238–1240. doi: 10.1002/1097-0142(195311)6:6<1238::aid-cncr2820060618>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 95.Daniel MC, Astruc D. Chem Rev. 2003;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 96.Shan J, Tenhu H. Chem Commun. 2007:4580–4598. doi: 10.1039/b707740h. [DOI] [PubMed] [Google Scholar]
- 97.Kawano T, Niidome Y, Mori T, Katayama Y, Niidome T. Bioconjugate Chem. 2009;20:209–212. doi: 10.1021/bc800480k. [DOI] [PubMed] [Google Scholar]
- 98.Nakamura T, Tamura A, Murotani H, Oishi M, Jinji Y, Matsuishi K, Nagasaki Y. Nanoscale. 2010;2:739–746. doi: 10.1039/b9nr00329k. [DOI] [PubMed] [Google Scholar]
- 99.Wu W, Shen J, Banerjee P, Zhou S. Biomaterials. 2010;31:7555–7566. doi: 10.1016/j.biomaterials.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 100.Hore MJA, Composto RJ. Macromolecules. 2013;47:875–887. [Google Scholar]
- 101.Hribar KC, Metter RB, Ifkovits JL, Troxler T, Burdick JA. Small. 2009;5:1830–1834. doi: 10.1002/smll.200900395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Charati MB, Lee I, Hribar KC, Burdick JA. Small. 2010;6:1608–1611. doi: 10.1002/smll.201000162. [DOI] [PubMed] [Google Scholar]
- 103.Mohammed JS, Murphy WL. Adv Mater. 2009;21:2361–2374. [Google Scholar]
- 104.Murakami Y, Maeda M. Biomacromolecules. 2005;6:2927–2929. doi: 10.1021/bm0504330. [DOI] [PubMed] [Google Scholar]
- 105.Bysell H, Schmidtchen A, Malmsten M. Biomacromolecules. 2009;10:2162–2168. doi: 10.1021/bm9003354. [DOI] [PubMed] [Google Scholar]
- 106.Ulijn RV. J Mater Chem. 2006;16:2217–2225. [Google Scholar]
- 107.Tanna S, Sahota TS, Sawicka K, Taylor MJ. Biomaterials. 2006;27:4498–4507. doi: 10.1016/j.biomaterials.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 108.Ravaine V, Ancla C, Catargi B. Journal of Controlled Release. 2008;132:2–11. doi: 10.1016/j.jconrel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 109.Rehor A, Botterhuis NE, Hubbell JA, Sommerdijk NAJM, Tirelli N. J Mater Chem. 2005;15:4006–4009. [Google Scholar]
- 110.Motornov M, Zhou J, Pita M, Gopishetty V, Tokarev I, Katz E, Minko S. Nano Lett. 2008;8:2993–2997. doi: 10.1021/nl802059m. [DOI] [PubMed] [Google Scholar]
- 111.Lapeyre V, Gosse I, Chevreux S, Ravaine V. Biomacromolecules. 2006;7:3356–3363. doi: 10.1021/bm060588n. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Y, Guan Y, Zhou S. Biomacromolecules. 2006;7:3196–3201. doi: 10.1021/bm060557s. [DOI] [PubMed] [Google Scholar]
- 113.Hoare T, Pelton R. Macromolecules. 2007;40:670–678. [Google Scholar]
- 114.Zhang Y, Guan Y, Zhou S. Biomacromolecules. 2007;8:3842–3847. doi: 10.1021/bm700802p. [DOI] [PubMed] [Google Scholar]
- 115.Hoare T, Pelton R. Biomacromolecules. 2008;9:733–740. doi: 10.1021/bm701203r. [DOI] [PubMed] [Google Scholar]
- 116.Xiong MH, Bao Y, Yang XZ, Wang YC, Sun B, Wang J. J Am Chem Soc. 2012;134:4355–4362. doi: 10.1021/ja211279u. [DOI] [PubMed] [Google Scholar]
- 117.Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN. Chem Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 118.Murakami Y, Maeda M. Biomacromolecules. 2005;6:2927–2929. doi: 10.1021/bm0504330. [DOI] [PubMed] [Google Scholar]
- 119.Satarkar NS, Biswal D, Hilt JZ. Soft Matter. 2010;6:2364–2371. [Google Scholar]
- 120.Medeiros SF, Santos AM, Fessi H, Elaissari A. International Journal of Pharmaceutics. 2011;403:139–161. doi: 10.1016/j.ijpharm.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 121.Gahjarwar AK, Wong JE, Muller-Schulte D, Bahadur D, Richtering W. J Nanosci Nanotechnol. 2009;9:5355–5361. doi: 10.1166/jnn.2009.1265. [DOI] [PubMed] [Google Scholar]
- 122.Sun H, Zhang L, Zhang X, Zhang C, Wei Z, Yao S. Biomed Microdevices. 2008;10:281–287. doi: 10.1007/s10544-007-9134-7. [DOI] [PubMed] [Google Scholar]
- 123.Adriane K, Huang J, Ding G, Chen J, Liu Y. Journal of Drug Targeting. 2006;14:243–253. doi: 10.1080/10611860600720616. [DOI] [PubMed] [Google Scholar]
- 124.Wang X, Gu H, Yang Z. Journal of Magnetism and Magnetic Materials. 2005;293:334–340. [Google Scholar]
- 125.Purushotham S, Ramanujan RV. Acta Biomaterialia. 2010;6:502–510. doi: 10.1016/j.actbio.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 126.Ciofani G, Riggio C, Raffa V, Menciassi A, Cuschieri A. Medical Hypotheses. 2009;73:80–82. doi: 10.1016/j.mehy.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 127.O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Alland L, Tendler C CAELYX Breast Cancer Study Group . Annals of Oncology. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 128.Kano MR, Bae Y, Iwata C, Morishita Y, Yashiro M, Oka M, Fujii T, Komuro A, Kiyono K, Kaminishi M, Hirakawa K, Ouchi Y, Nishiyama N, Kataoka K, Miyazono K. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3460–3465. doi: 10.1073/pnas.0611660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Torchilin VP. Eur J Pharm Sci. 2000;11:S81–S91. doi: 10.1016/s0928-0987(00)00166-4. [DOI] [PubMed] [Google Scholar]
- 130.Talei Franzesi G, Ni B, Ling Y, Khademhosseini A. J Am Chem Soc. 2006;128:15064–15065. doi: 10.1021/ja065867x. [DOI] [PubMed] [Google Scholar]
- 131.Crayton SH, Elias DR, Al Zaki A, Cheng Z, Tsourkas A. Biomaterials. 2012;33:1509–1519. doi: 10.1016/j.biomaterials.2011.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanomedicine. 2011;6:715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Simone EA, Dziubla TD, Muzykantov VR. Expert Opinion on Drug Delivery. 2008;5:1283–1300. doi: 10.1517/17425240802567846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cloughesy TF, Black KL. J Neuro-Oncol. 2008;26:125–1321300. [Google Scholar]
- 135.Chen B, Pogue BW, Luna JM, Hardman RL, Hoopes PJ, Hasan T. Clinical Cancer Research. 2006;12:917–923. doi: 10.1158/1078-0432.CCR-05-1673. [DOI] [PubMed] [Google Scholar]
- 136.Li C, Ke S, Wu QP, Tansey W, Hunter N, Buchmiller LM, Milas L, Charnsangavej C, Wallace S. Clinical Cancer Research. 2000;6:2829–2934. [PubMed] [Google Scholar]
- 137.Chrastina A, Massey KA, Schnitzer JE. WIREs Nanomed Nanobiotechnol. 2011 Aug;3:421–437. doi: 10.1002/wnan.143. [DOI] [PubMed] [Google Scholar]
- 138.Chauhan VP, Stylianopoulos T, Martin JD, Popovic Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D, Jain RK. Nature Nanotechnology. 2012;7:388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Neradovic D, van Nostrum CF, Hennink WE. Macromolecules. 2001;34:7589–7591. [Google Scholar]
- 140.Ma Q, Remsen EE, Kowalewski T, Wooley KL. J Am Chem Soc. 2001;123:4627–4628. doi: 10.1021/ja0156542. [DOI] [PubMed] [Google Scholar]
- 141.Glunde K, Bhujwalla ZM, Ronen SM. Nat Rev Cancer. 2011;11:835–848. doi: 10.1038/nrc3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou W, Shao J, Jin Q, Wei Q, Tang J, Ji J. Chem Commun. 2010;46:1479–1481. doi: 10.1039/b915125g. [DOI] [PubMed] [Google Scholar]
- 143.Steiche SD, Caldorera-Moore M, Peppas NA. Eur J Pharm Sci. 2013;48:416–427. doi: 10.1016/j.ejps.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Olson ES, Jiang T, Aguilera TA, Nguyen QT, Ellies LG, Scadeng M, Tsien RY. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4311–4316. doi: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Romberg B, Hennink WE, Storm G. Pharmaceutical Research. 2008;25:55–71. doi: 10.1007/s11095-007-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Crayton SH, Tsourkas A. ACS Nano. 2011;5:9592–9601. doi: 10.1021/nn202863x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reshetnyak YK, Andreev OA, Segala M, Markin VS, Engelman DM. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15340–15345. doi: 10.1073/pnas.0804746105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.de Gracia LC, Joshi-Barr S, Nguyen T, Mahmoud E, Schopf E, Fomina N, Almutairi A. J Am Chem Soc. 2012;134:15758–15764. doi: 10.1021/ja303372u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee D, Bae S, Hong D, Lim H, Yoon JH, Hwang O, Park S, Ke Q, Khang G, Kang PM. Scientific Reports. 2013;3:2233. doi: 10.1038/srep02233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang J, Lee CH, Ko HJ, Suh JS, Yoon HG, Lee K, Huh YM, Haam S. Angewandte Chemie International Ed in English. 2007;46:8836–8839. doi: 10.1002/anie.200703554. [DOI] [PubMed] [Google Scholar]
- 152.Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, Lu Y, Atwal J, Elliott JM, Prabhu S, Watts RJ, Dennis MS. Science Translational Medicine. 2011;3:84ra44. doi: 10.1126/scitranslmed.3002230. [DOI] [PubMed] [Google Scholar]
- 153.Kocbek P, Obermajer N, Cegnar M, Kos J, Kristl J. Journal of Controlled Release. 2007;120:18–26. doi: 10.1016/j.jconrel.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 154.Thorek DL, Elias DR, Tsourkas A. Molecular Imaging. 2009;8:221–229. [PubMed] [Google Scholar]
- 155.Hakem IF, Leech AM, Johnson JD, Donahue SJ, Walker JP, Bockstaller MR. J Am Chem Soc. 2010;132:16593–16596. doi: 10.1021/ja107139c. [DOI] [PubMed] [Google Scholar]
- 156.Elias DR, Poloukhtine A, Popik V, Tsourkas A. Nanomedicine. 2013;9:194–201. doi: 10.1016/j.nano.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Haun JB, Hammer DA. Langmuir. 2008;24:8821–8832. doi: 10.1021/la8005844. [DOI] [PubMed] [Google Scholar]
- 158.Dennis AM, Bao G. Nano Lett. 2008;8:1439–1445. doi: 10.1021/nl080358+. [DOI] [PubMed] [Google Scholar]
- 159.Hainfeld JF, Liu W, Halsey CM, Freimuth P, Powell RD. Journal of Structural Biology. 1999;127:185–198. doi: 10.1006/jsbi.1999.4149. [DOI] [PubMed] [Google Scholar]
- 160.Mattoussi H, Mauro JM, Goldman ER, Anderson GP, Sundar VC, Mikulec FV, Bawendi MG. J Am Chem Soc. 2000;122:12142–12150. [Google Scholar]
- 161.Elias DR, Cheng Z, Tsourkas A. Small. 2010;6:2460–2468. doi: 10.1002/smll.201001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.van Vught R, Pieters RJ, Breukink E. Computational and Structural Biotechnology Journal. 2014;9:e201402001. doi: 10.5936/csbj.201402001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hui JZ, Zaki AA, Cheng Z, Popik V, Zhang H, Luning P, Eline T, Tsourkas A. Small. 2014;10:3354–3363. doi: 10.1002/smll.201303629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Langer R, Tirrell DA. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 165.Peppas NA, Langer R. Science. 1994;263:1715–1720. doi: 10.1126/science.8134835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.