Abstract
Introduction
Minimally invasive surgery for discectomy may accelerate recovery and reduce pain, but it also requires technical expertise and is associated with increased risks. We performed a meta-analysis to determine the effects of minimally invasive versus open surgery on functional outcomes, pain, complications and reoperations among patients undergoing cervical or lumbar discectomy.
Methods
We searched MEDLINE, Embase and the Cochrane Library for reports of relevant randomized controlled trials published to Jan. 12, 2014. Two reviewers assessed the eligibility of potential reports and the risk of bias of included trials. We analyzed functional outcomes and pain using standardized mean differences (SMDs) that were weighted and pooled using a random-effects model.
Results
We included 4 trials in the cervical discectomy group (n = 431) and 10 in the lumbar discectomy group (n = 1159). Evidence overall was of low to moderate quality. We found that minimally invasive surgery did not improve long-term function (cervical: SMD 0.11, 95% confidence interval [CI] −0.09 to 0.31; lumbar: SMD 0.04, 95% CI −0.11 to 0.20) or reduce long-term extremity pain (cervical: SMD −0.21, 95% CI −0.52 to 0.10; lumbar: SMD 0.08, 95% CI −0.16 to 0.32) compared with open surgery. The evidence suggested overall higher rates of nerve-root injury (risk ratio [RR] 1.62, 95% CI 0.45 to 5.84), incidental durotomy (RR 1.56, 95% CI 0.80 to 3.05) and reoperation (RR 1.48, 95% CI 0.97 to 2.26) with minimally invasive surgery than with open surgery. Infections were more common with open surgery than with minimally invasive surgery (RR 0.24, 95% CI 0.04 to 1.38), although the difference was not statistically significant.
Interpretation
Current evidence does not support the routine use of minimally invasive surgery for cervical or lumbar discectomy. Well-designed trials are needed given the lack of high-quality evidence.
Symptomatic cervical and lumbar spinal disc diseases affect at least 5% of the population,1,2 and their high incidence is associated with substantial morbidity, social burden and economic impact.3,4 Conventional open surgical techniques provide good or excellent results in carefully selected patients whose symptoms fail to improve with nonsurgical management.5–8 Minimally invasive surgical techniques differ from conventional open surgery, predominantly with respect to access pathways. The intended procedures for minimally invasive surgery should otherwise be nearly or exactly the same as conventional open techniques in order to reach similar effectiveness. The access pathway should protect the soft tissues and the muscles as much as possible, and minimally invasive techniques aim to be less destructive and less traumatic.
Minimally invasive surgery for discectomy may accelerate functional recovery and reduce pain,9,10 but it may be associated with increased risks of neurologic injury, incidental durotomy and reoperation.11–14 In addition, minimally invasive surgery requires advanced technical expertise and may require specialized equipment and navigation systems and involve increased intraoperative exposure to radiation; therefore, its use should be guided by high-quality evidence.15
Although several recent systematic reviews have examined minimally invasive discectomy, they were each limited in scope or methodologic quality, or both.4,9,11,16–18 Many included observational studies at high risk of bias, others examined procedures other than discectomy, and few focused on patient-centred outcomes such as function and pain. We performed a systematic review and meta-analysis to determine the effect of minimally invasive surgery compared with conventional open surgery for cervical and lumbar discectomy with regard to function, pain, complications and reoperation.
Methods
We followed the protocol outlined in the Cochrane Handbook for Systematic Reviews of Interventions.19 We report our findings in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.20
Literature search
We systematically searched MEDLINE, Embase and the Cochrane Library for articles published up to and including Jan. 12, 2014. Subject headings and subheadings (MeSH terms in MEDLINE and EMTREE terms in Embase) were used in various combinations and supplemented with free text (an example of the search strategy is available in Appendix 1, www.cmajopen.ca/content/2/4/E295/suppl/DC1). Manual searches of the reference lists of included trials and of “related articles” featured in PubMed were conducted to identify additional articles. We searched conference proceedings (North American Spine Society, Canadian Spine Society, American Academy of Orthopaedic Surgeons, Canadian Orthopaedic Association) from the last 3 years and ClinicalTrials.gov to identify relevant unpublished trials.
Study selection
We included RCTs comparing minimally invasive surgery and conventional open surgery for primary discectomy. No restrictions were made regarding patient age or sex, spinal level, surgical technique, instruments for outcome measures, timing of assessment, language21 and publication status. Two reviewers (N.E. and M.K.) independently screened all titles and abstracts of potentially eligible reports using an electronic screening form that was piloted for accuracy and usability in a previous systematic review.22 All discrepancies were resolved by consensus. Both reviewers independently reviewed the full text of all reports identified through screening of titles and abstracts to determine final eligibility.
Data extraction and quality assessment
The same 2 reviewers independently extracted study data using a piloted electronic data collection form. Authors of the included studies were contacted if important data were unclear or not reported.
Outcomes were classified by consensus as critical, important but not critical, or of limited importance.23 Function, extremity pain, axial pain, infection, nerve-root injury, incidental durotomy, reoperation and death were considered critical or important outcomes; data on other outcomes of limited importance were not collected. The selected complications were chosen because they are considered to be patient-important outcomes or were commonly reported in the identified primary studies. Outcomes were dichotomized into short-term (< 6 mo) or long-term (≥ 1 yr) categories according to when the latest outcome data were available.
For the assessment of methodologic quality, both reviewers independently evaluated risk of bias in included trials using the Cochrane Collaboration’s risk-of-bias tool.19 They assessed the quality of evidence in included trials using the GRADE approach.19,24 Data from RCTs were considered high-quality evidence, but the quality could be rated down because of risk of bias, imprecision, inconsistency, indirectness or publication bias.
Data synthesis
We used standardized mean differences (SMDs) to summarize results for function and pain. The SMDs were weighted according to the inverse variance method and pooled using a conservative random-effects model.19,25,26 When standard deviations (SDs) were not available, they were calculated from confidence intervals (CIs), standard errors, p values or ranges where possible, or they were estimated from similar studies or comparable validity and reliability studies.27–32 We pooled data on complications and reoperations from only trials that reported these outcomes, and we calculated risk ratios (RRs) using the Mantel–Haenszel method and a random-effects model.19
Minimal important differences (MIDs) were incorporated to aid the interpretation of treatment effects. The MID describes the smallest effect that an informed patient would perceive as beneficial enough to justify a change in management in the absence of troublesome adverse effects and excessive cost.33–37 The anchor-based MID was estimated to be 10 points for the Oswestry Disability Index for measuring functional outcomes,38 2.5 points for the visual analogue scale for extremity pain (arm or leg)39 and 3.5 points for the visual analogue scale for axial pain (neck or back).39 Each MID was converted to SD units using the median SD for each comparison. A zone of clinical equivalence based on the converted MIDs was projected onto the forest plots to aid interpretability of the pooled SMDs.40
We quantified heterogeneity using the χ2 test for heterogeneity and the I2 statistic.19 I2 values were interpreted according to the Cochrane handbook: 0%–40% might not be important, 30%–60% may represent moderate heterogeneity, 50%–90% substantial heterogeneity and 75%–100% considerable heterogeneity.19 We developed a priori hypotheses to explain potentially high heterogeneity in treatment effect across trials between cervical and lumbar discectomy, between tubular and microendoscopic surgical techniques, and between the presence and absence of blinding.41 We did not perform sensitivity analyses for losses to follow-up because only 1 trial reported such losses, which were negligible in each study arm.13 We did perform sensitivity analyses to test the effect of assumptions made for estimating missing SDs by pooling the SMDs from only trials with completely reported data for each of the long-term outcomes.
We calculated interobserver agreement for reviewer’s assessments of study eligibility with the Cohen κ coefficient42,43 and interobserver agreement for risk-of-bias assessments with the intraclass correlation coefficient; all of the coefficients were calculated with the use of SPSS software (version 21.0; SPSS Inc.). All tests of significance were 2-tailed, and p values of less than 0.05 were considered significant. To assess for publication bias, we visually inspected a funnel plot for the outcome of long-term function.19 The forest plots and the funnel plot were created with the use of Review Manager software (RevMan version 5.2; Nordic Cochrane Centre, Cochrane Collaboration, 2012).
Results
Search results and study characteristics
Of 883 potentially eligible studies identified, 21 were reviewed in full. We included 14 RCTs (n = 1590) in the meta-analysis (Figure 1 and Table 1).13,44–57 The excluded trials are listed in Appendix 2 (www.cmajopen.ca/content/2/4/E295/suppl/DC1). Interobserver agreement for eligibility was satisfactory (κ value = 0.84, 95% CI 0.71 to 0.98). Four RCTs (n = 431) compared cervical discectomy techniques, and 10 (n = 1159) compared lumbar discectomy techniques; none reported on thoracic discectomies. Only 1 trial reported losses to follow-up in each arm.13 Five RCTs did not report complete data on SDs, p values, CIs or alternative measures of spread.44–46,49,54
Figure 1:

Selection of articles for the meta-analysis. MIS = minimally invasive surgery.
Table 1: Characteristics of randomized controlled trials of minimally invasive surgery (MIS) versus conventional open surgery for cervical and lumbar discectomy included in the meta-analysis.
| Trial | Patient characteristics |
Treatment arm; no. of patients and description of treatment |
Duration of follow-up, wk |
|||
|---|---|---|---|---|---|---|
| Mean age, yr | Male sex, % | MIS | Open surgery | Short term | Long term | |
|
Cervical discectomy |
||||||
| Soliman et al., 201344 |
44 |
48.0 |
37; microendoscopic discectomy and fusion |
33; conventional anterior cervical discectomy and fusion |
– |
121 |
| Ruetten et al., 200845 |
34 |
43.0 |
100; full-endoscopic cervical posterior foraminotomy |
100; conventional anterior cervical discectomy and fusion |
26 |
104 |
| Ruetten et al., 200946 |
36 |
NR |
60; full-endoscopic anterior cervical decompression |
60; conventional anterior cervical discectomy and fusion |
26 |
104 |
| Kim et al., 200947 |
63 |
54.3 |
22; tubular posterior discectomy or foraminotomy |
19; open posterior discectomy or foraminotomy |
26 |
104 |
|
Lumbar discectomy |
||||||
| Arts et al., 200913 and 201148 |
53 |
41.5 |
167; tubular discectomy |
161; conventional microdiscectomy |
26 |
104 |
| Brock et al., 200849 |
61 |
51.0 |
66; tubular discectomy |
59; conventional microdiscectomy |
1 |
– |
| Franke et al., 200950 |
60 |
44.0 |
52; microscope-assisted percutaneous nucleotomy |
48; conventional microdiscectomy |
26 |
52 |
| Garg et al., 201151 |
71 |
37.5 |
55; microendoscopic discectomy |
57; conventional open discectomy |
26 |
52 |
| Huang et al., 200552 |
68 |
39.5 |
10; microendoscopic discectomy |
12; conventional open discectomy |
– |
82 |
| Righesso et al., 200753 |
47 |
43.9 |
21; microendoscopic discectomy |
19; conventional open discectomy |
26 |
104 |
| Ruetten et al., 200854 |
42 |
43.0 |
100; endoscopic interlaminar and transforaminal discectomy |
100; conventional microdiscectomy |
26 |
104 |
| Ryang et al., 200855 |
53 |
38.0 |
30; minimal access trocar microdiscectomy |
30; conventional microdiscectomy |
– |
69 |
| Shin et al., 200856 |
40 |
42.0 |
15; microendoscopic discectomy |
15; conventional microdiscectomy |
1 |
– |
| Teli et al., 201057 | 66 | 39.5 | 70; microendoscopic discectomy | 72; conventional microdiscectomy | 26 | 104 |
Note: NR = not reported.
Only 1 trial was found to have a low risk of bias.13,48 The remaining trials were found to have a high [n = 1045–47,49,51–55,57] or uncertain [n = 3 trials44,50,56] risk of bias (Figure 2). Interobserver agreement for the risk-of-bias assessments was satisfactory (intraclass correlation coefficient = 0.70, 95% CI 0.57 to 0.80). Random sequence generation was inadequate or unclear in 9 trials, allocation concealment was inadequate or unclear in 13, and blinding of participants and outcome assessors was not done or was unclear in 10 trials (Figure 2). Ten of the studies did not report performing an intention-to-treat analysis.44–46,49–52,54–56
Figure 2:
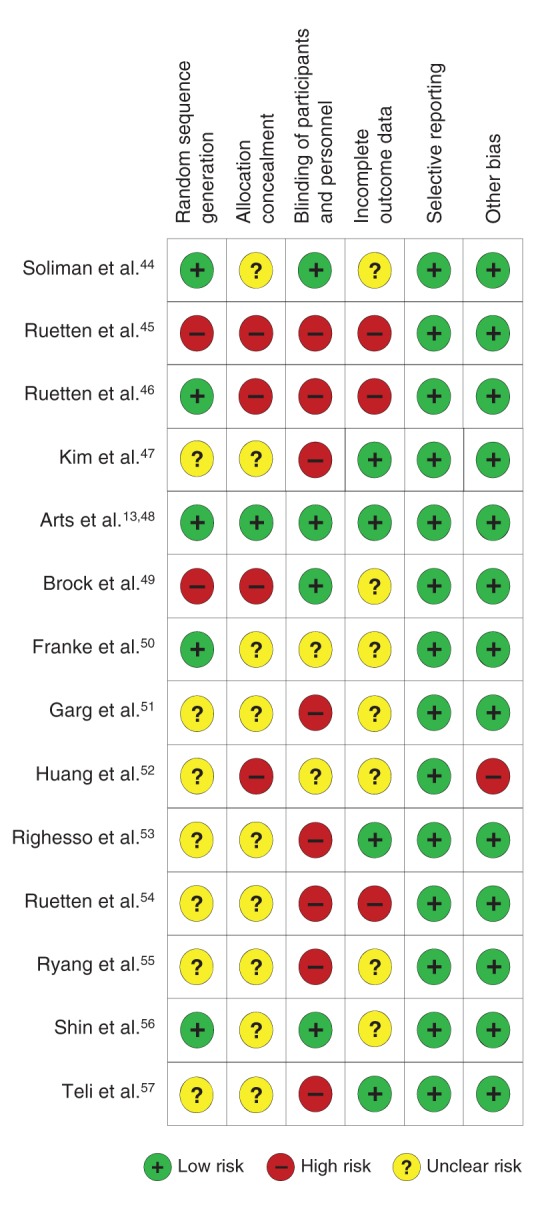
Risk-of-bias assessment of randomized controlled trials included in the meta-analysis. One trial had a low risk of bias,13,48,10 10 had a high risk of bias,45–47,49,51–55,57 and 3 had an uncertain risk of bias.44,50,56
Both reviewers downgraded the quality of evidence for all comparisons of function from high to moderate because of risk of bias (Table 2). Moderate quality indicates moderate confidence in the effect estimate and that there is a possibility that the true effect is substantially different.58 The quality of evidence for all comparisons of pain was downgraded to low because of risk of bias and inconsistency.59 Low quality indicates that confidence in the effect estimate is limited and that the true effect may be substantially different from the estimate. The quality of evidence for adverse events was ranked down to low based on risk of bias and imprecision.60,61
Table 2: Summary of findings for minimally invasive surgery (MIS) compared with conventional open surgery for cervical and lumbar discectomy.
|
Patient or population: patients with degenerative disc disease Intervention: minimally invasive discectomy Comparison: conventional open surgery Outcomes: functional outcomes, pain, complications, reoperation | |||
|---|---|---|---|
| Outcome (instrument) | Anticipated absolute effect, risk with MIS (95% CI) | No. of participants (studies) |
GRADE quality of evidence |
|
Short-term function (ODI, JOA, NASS or RDQ) Follow-up: < 6 mo |
Cervical: Mean short-term function was 0.18 SDs higher (0.10 lower to 0.46 higher)† Lumbar: Mean short-term function was 0.04 SDs lower (0.18 lower to 0.09 higher)† |
Cervical: 200 (1) Lumbar: 839 (7) |
Moderate* |
|
Long-term function (ODI, JOA, NASS or RDQ) Follow-up: < 2 yr |
Cervical: Mean long-term function was 0.11 SDs higher (0.09 lower to 0.31 higher)† Lumbar: Mean long-term function was 0.04 SDs higher (0.11 lower to 0.20 higher)† |
Cervical: 390 (3) Lumbar: 982 (7) |
Moderate* |
|
Short-term extremity pain (VAS) Follow-up: < 6 mo |
Cervical: Mean short-term arm pain was 0.25 SDs lower (1.04 lower to 0.53 higher)† Lumbar: Mean short-term leg pain was 0.15 SDs higher (0.02 lower to 0.31 higher)† |
Cervical: 361 (3) Lumbar: 865 (6) |
Low*‡ |
|
Long-term extremity pain
(VAS) Follow-up: < 2 yr |
Cervical: Mean short-term arm pain was 0.21 SDs lower (0.52 lower to 0.10 higher)† Lumbar: Mean short-term leg pain was 0.08 SDs higher (0.16 lower to 0.32 higher)† |
Cervical: 431 (4) Lumbar: 792 (6) |
Low*‡ |
|
Short-term axial pain (VAS) Follow-up: < 6 mo |
Cervical: Mean short-term neck pain was 0.48 SDs lower (0.94 to 0.01 lower)† Lumbar: Mean short-term back pain was 0.62 SDs lower (1.28 lower to 0.04 higher)† |
Cervical: 361 (3) Lumbar: 825 (5) |
Low*‡ |
|
Long-term axial pain (VAS) Follow-up: < 2 yr |
Cervical: Mean short-term neck pain was 0.01 SDs lower (0.28 to 0.26 lower)† Lumbar: Mean short-term back pain was 0.51 SDs lower (1.59 lower to 0.57 higher)† |
Cervical: 431 (4) Lumbar: 670 (3) |
Low*‡ |
| Adverse events | |||
| Complications, reoperation Follow-up: < 2 yr |
Overall higher rates of nerve-root injury (RR 1.62, 95% CI 0.45 to 5.84), incidental durotomy (RR 1.56, 95% CI 0.80 to 3.05) and reoperation (RR 1.48, 95% CI 0.97 to 2.26), but differences were not statistically significant Infections were more common in open-surgery group (RR 0.24, 95% CI 0.04 to1.38), but difference was not statistically significant |
Cervical and lumbar: Up to 1262 (8) |
Low*§ |
Note: CI = confidence interval, GRADE = Grading of Recommendations Assessment, Development, and Evaluation, JOA = Japanese Orthopaedic Association instrument, MIS = minimally invasive surgery, NASS = North American Spine Society instrument, ODI = Oswestry Disability index, RDQ = Roland Disability Quotient, RR = risk ratio, SD = standard deviation, VAS = visual analogue scale. *Downgraded because of risk of bias. †Effect failed to exceed minimal important difference (smallest effect that an informed patient would perceive as beneficial enough to justify a change in management in the absence of troublesome adverse effects and excessive cost). ‡Downgraded because of inconsistency. §Downgraded because of imprecision. GRADE Working Group grades of evidence: High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate.
In the assessment of publication bias, the funnel plot for long-term function was symmetrical overall across the lumbar studies and suggestive of possible publication bias across the cervical studies (Figure 3). However, the small sample for each comparison limits robust interpretation.19
Figure 3:

Funnel plot of long-term function in trials of minimally invasive surgery versus conventional open surgery for cervical (green circles) and lumbar (blue diamonds) discectomy. SMD = standardized mean difference.
Function
Minimally invasive surgery did not improve short-term function in 1 trial of cervical discectomy45 that reported short-term visual analogue scores from 200 patients (SMD 0.18, 95% CI –0.10 to 0.46). The same was true across 6 trials of lumbar discectomy49–51,53,54,57 involving a total of 719 patients (SMD –0.04, 95% CI –0.18 to 0.11) with low heterogeneity (p = 0.5, I2 = 0%). The pooled estimates did not exceed the threshold of ± 1.00 SD for the MID.
Minimally invasive surgery did not improve long-term function across 3 trials of cervical discectomy44–46 involving 390 patients (SMD 0.11, 95% CI –0.09 to 0.31) with low heterogeneity (p = 0.8, I2 = 0%) and across 7 trials of lumbar discectomy48,50,51,53–55,57 involving a total of 982 patients (SMD 0.04, 95% CI –0.11 to 0.20) with low heterogeneity (p = 0.2, I2 = 26%) (Figure 4). The findings were robust to sensitivity testing (cervical trials: SMD 0.08, 95% CI –0.14 to 0.30; heterogeneity p < 1.0, I2 = 0%; lumbar trials: SMD 0.10, 95% CI –0.07 to 0.26; heterogeneity p = 0.4, I2 = 10%). The pooled estimates did not exceed the threshold of ± 1.00 SD for the MID.
Figure 4:
Pooled long-term (≥ 1 yr) function following minimally invasive surgery (MIS) and conventional open surgery for cervical and lumbar discectomy. Red lines show a zone of clinical equivalence based on a minimal important difference of 10 points on the Oswestry Disability Index.38 Standardized mean differences greater than zero favour MIS. CI = confidence interval.
Extremity pain
Minimally invasive surgery did not improve short-term arm pain across 3 cervical trials45–47 that reported short-term visual analogue scores from 361 patients (SMD –0.25, 95% CI –1.04 to 0.53) with high heterogeneity (p < 0.01, I2 = 91%) or short-term leg pain across 6 trials of lumbar discectomy13,49,53,54,56,57 involving a total of 865 patients (SMD 0.15, 95% CI –0.02 to 0.31) with low heterogeneity (p = 0.2, I2 = 28%). The pooled estimates did not exceed the threshold of ± 1.04 SD for the MID.
No significant improvement in long-term arm pain was found with minimally invasive surgery across 4 trials of cervical discectomy44–47 involving 431 patients (SMD –0.21, 95% CI –0.52 to 0.10) with moderate heterogeneity (p = 0.07, I2 = 57%) or long-term leg pain across 6 trials of lumbar discectomy13,52–55,57 involving 792 patients (SMD 0.08, 95% CI –0.16 to 0.32) with moderate heterogeneity (p = 0.05, I2 = 56%) (Figure 5). These findings were robust to sensitivity testing (cervical: SMD –0.29, 95% CI –0.64 to 0.06; heterogeneity p = 0.1, I2 = 57%; lumbar: SMD 0.13, 95% CI –0.21 to 0.48; heterogeneity p = 0.02, I2 = 64%). The pooled estimates did not exceed the threshold of ± 1.04 SD for the MID.
Figure 5:
Pooled long-term (≥ 1 yr) pain in extremities following minimally invasive surgery (MIS) and conventional open surgery for cervical and lumbar discectomy. The cervical studies reported on pain in the upper extremities, and the lumbar studies reported on pain in the lower extremities. Red lines show a zone of clinical equivalence based on a minimal important difference of 2.5 points on the visual analogue scale.39 Standardized mean differences less than zero favour MIS. CI = confidence interval.
There was substantial heterogeneity in the analyses of short-term extremity pain across the cervical and lumbar trials and in the analysis of long-term extremity pain across the lumbar trials. There was residual heterogeneity when we compared tubular (p > 0.9, I2 = 0%) and endoscopic (p = 0.002, I2 = 71%) techniques. Blinded trials were more consistent than nonblinded trials in showing no difference in effect between minimally invasive and open surgery (blinded: SMD 0.05, 95% CI –0.15 to 0.24; heterogeneity p < 0.9, I2 = 0%; nonblinded: SMD –0.04, 95% CI –0.30 to 0.21; heterogeneity p = 0.004, I2 = 66%).
Axial pain
We found a significant improvement in short-term neck pain with minimally invasive surgery across 3 trials of cervical discectomy45–47 involving 361 patients (SMD –0.48, 95% CI –0.94 to –0.01) with high heterogeneity (p = 0.02, I2 = 75%); however, the pooled estimate did not exceed the threshold of ± 1.06 SD for the MID. Statistical significance was not robust to sensitivity testing (SMD –0.11, 95% CI –0.50 to 0.73; heterogeneity not applicable [1 trial]). We found no significant improvement in short-term back pain following minimally invasive surgery across 5 trials of lumbar discectomy13,49,54,56,57 involving 825 patients (SMD –0.62, 95% CI –1.28 to 0.04) with high heterogeneity (p < 0.01, I2 = 95%). The pooled estimate for the lumbar trials did not exceed the threshold of ± 1.06 SD for the MID.
No significant improvement in long-term neck pain was found across 4 trials of cervical discectomy44–47 involving 431 patients (SMD –0.01, 95% CI –0.28 to 0.26) with moderate heterogeneity (p = 0.3, I2 = 44%) or in long-term back pain across 3 trials of lumbar discectomy48,54,57 involving 670 patients (SMD –0.51, 95% CI –1.59 to 0.57) with high heterogeneity (p < 0.001, I2 = 98%) (Figure 6). The pooled estimates did not exceed the threshold of ± 1.06 SD for the MID.
Figure 6:
Pooled long-term (≥ 1 yr) axial pain following minimally invasive surgery (MIS) and open surgery for cervical and lumbar discectomy. The cervical studies reported on neck pain and the lumbar studies reported on back pain. Red lines show a zone of clinical equivalence based on a minimal important difference of 3.5 points on the visual analogue scale.39 Standardized mean differences less than zero favour MIS. CI = confidence interval.
There was substantial heterogeneity in the analysis of short-term axial pain across the cervical trials and in the analyses of long-term axial pain across the cervical and lumbar trials. There was significant residual heterogeneity when we compared tubular (p = 0.7, I2 = 0%) and endoscopic (p < 0.001, I2 = 95%) techniques. Blinded trials were more consistent than nonblinded trials in showing no difference in effect between minimally invasive and open surgery (blinded: SMD 0.09, 95% –0.11 to 0.29; heterogeneity p = 0.54, I2 = 0%; nonblinded: SMD –0.33, 95% CI –1.04 to 0.39; heterogeneity p < 0.001, I2 = 95%).
Adverse events
Reports of complications varied across the 14 trials (Table 3). Overall, the evidence suggested higher rates of nerve-root injury (RR 1.62, 95% CI 0.45 to 5.84), incidental durotomy (RR 1.56, 95% CI 0.80 to 3.05) and reoperation (RR 1.48, 95% CI 0.97 to 2.26) with minimally invasive than with open surgery, but the differences were not statistically significant. Infections were more common with open surgery (RR 0.24, 95% CI 0.04 to 1.38), but again the difference was not statistically significant. Arts and colleagues13,48 reported 1 death in the group undergoing minimally invasive surgery, but they did not report whether it was related to the patient’s management.
Table 3: Rates of complications and reoperation across the randomized controlled trials of minimally invasive surgery (MIS) versus conventional open surgery for cervical and lumbar discectomy included in the meta-analysis.
| Complication; no. of patients |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Infection |
Nerve-root injury |
Incidental durotomy |
Reoperation |
Death |
||||||
| Trial | MIS | Open | MIS | Open | MIS | Open | MIS | Open | MIS | Open |
|
Cervical |
||||||||||
| Soliman et al.44 |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
| Ruetten et al.45 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
3 |
NR |
NR |
| Ruetten et al.46 |
NR |
NR |
NR |
NR |
NR |
NR |
4 |
3 |
NR |
NR |
| Kim et al.47 |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
| Subtotal |
0 |
0 |
0 |
0 |
0 |
0 |
7 |
6 |
0 |
0 |
|
Lumbar |
||||||||||
| Arts et al.13,48 |
0 |
0 |
3 |
3 |
14 |
7 |
23 |
14 |
1 |
0 |
| Brock et al.49 |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
| Franke et al.50 |
0 |
0 |
0 |
0 |
NR |
NR |
5 |
2 |
NR |
NR |
| Garg et al.51 |
0 |
0 |
0 |
0 |
5 |
5 |
1 |
0 |
NR |
NR |
| Huang et al.52 |
0 |
1 |
1 |
0 |
NR |
NR |
NR |
NR |
NR |
NR |
| Righesso et al.53 |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
| Ruetten et al.54 |
0 |
1 |
0 |
0 |
0 |
0 |
6 |
5 |
NR |
NR |
| Ryang et al.55 |
NR |
NR |
NR |
NR |
0 |
2 |
2 |
4 |
NR |
NR |
| Shin et al.56 |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
| Teli et al.57 |
0 |
4 |
2 |
0 |
6 |
2 |
8 |
3 |
0 |
0 |
| Subtotal |
0 |
6 |
6 |
3 |
25 |
16 |
45 |
28 |
1 |
0 |
| All | 0 | 6 | 6 | 3 | 25 | 16 | 52 | 34 | 1 | 0 |
Note: NR = not reported.
Interpretation
We found moderate-quality evidence that failed to show an advantage attributable to minimally invasive surgery for discectomy in terms of short- and long-term function and low-quality evidence that failed to show an advantage in terms of short- and long-term pain. Low-quality evidence suggested higher rates of nerve-root injury, incidental durotomy and reoperation with minimally invasive than with conventional open surgery and higher rates of infection with open surgery, although the differences were not statistically significant.
These findings are similar to those of previous systematic reviews that examined minimally invasive techniques for spinal surgery.4,9,11,16–18 However, many of those systematic reviews included observational studies at high risk of bias, others examined procedures other than discectomy, and few focused on patient-important outcomes such as function and pain. A recent meta-analysis reported low-quality evidence to suggest that, on average, minimally invasive techniques for lumbar discectomy took 11 minutes longer, conserved 52 mL of blood and reduced the length of stay by 1.5 days; however, these findings were limited by a high risk of bias, low numbers of studies and small samples.9
Limitations
We did not report on operative variables such as blood loss, radiation exposure, operating time and muscle injury. Operative variables are not regarded as patient-important outcomes, they may often be confounded by learning curves or surgeon expertise, and they can be challenging to measure accurately.26,62,63 We also did not address length of stay, return to work and cost-effectiveness, but others have shown that high-quality economic data are lacking.9,64 Of the 14 included trials, follow-up of 1 year or longer was reported in 12 trials, whereas follow-up of 2 years or more was reported in only 8 trials. We considered long-term follow-up to be 1 year or longer to increase the volume of data available for pooling and thus increase the power and precision of our meta-analysis; however, longer follow-up in all trials would have been preferable.
The SMD is vulnerable to between-study heterogeneity and can be susceptible to widely varying SDs.19 Heterogeneity was low for short- and long-term function, which confirmed that combining instruments and tubular or endoscopic techniques was reasonable. Although tubular and endoscopic techniques may seem technically distinct from each other, analyzing them separately did not completely explain the residual unexplained heterogeneity for pain. Further post hoc subgroup hypotheses were not explored given their high susceptibility to spurious findings.65,66 The MIDs may have been limited by context and not completely generalizable across populations or techniques,36 and their specific applicability to patients undergoing cervical procedures or minimally invasive surgical procedures remains unclear.
Implications for practice
Careful patient selection and adequate nerve-root decompression may be the most important principles to optimize patient outcomes. Many spinal surgery procedures are known to have difficult learning curves, and surgeons embarking on minimally invasive surgical techniques should obtain specialized training to minimize complications.62,67–69 Rates of inadequate decompression requiring reoperation and of complications such as nerve-root injury and incidental durotomy should be lower or no worse with minimally invasive surgery than with open surgery to justify changes in individual surgeon’s practices. Conventional open techniques for spinal surgery are themselves technically demanding, and minimally invasive techniques are likely even more challenging.67
A possible explanation for the lack of an observed benefit attributable to minimally invasive discectomy may be that conventional open microdiscectomy is already relatively minimally invasive and that tubular or endoscopic approaches simply lead to less visualization and a higher risk of complications. In experienced hands and in the absence of excessive subcutaneous adipose tissue, conventional open discectomy can be accomplished through small incisions comparable in size to those required for tubular retractors. The purported advantages of minimally invasive surgical techniques, including reduced soft-tissue and muscle damage, reduced perioperative blood loss, reduced infection rate, shorter hospital stay and accelerated recovery, could be more substantial for larger operations such as multilevel and instrumented procedures.10,70,71
Implications for research
Well-designed RCTs are needed to provide high-quality evidence. Recurrent limitations related to biased randomization and allocation concealment, lack of blinding, and reporting of losses must be overcome. Uncommon adverse events and long-term clinical outcomes are often challenging to study in randomized trials and may be more appropriately studied in large observational studies.72 Economic evaluations are required to evaluate all costs and benefits important to patients and payers at clinically important follow-up periods.73,74
Trials of spinal surgery have sparsely reported information about skill or experience and are frequently at risk of expertise bias.67 When clinical trials include surgeons whose experience with a conventional technique exceeds their experience with an experimental technique, outcomes may be biased in favour of the conventional technique.75 Future trials could consider the establishment of minimum thresholds of competency before surgeons are allowed to participate, or they could consider innovative expertise-based designs.76 Reporting of expertise is also critical to establish the generalizability outside of clinical trials.
Conclusion
The current evidence suggests a risk–benefit ratio that does not support the routine use of minimally invasive surgery for cervical and lumbar discectomy. Appropriate patient selection and technically adequate nerve-root decompression may be the most important determinants of long-term outcomes, and surgeons embarking on minimally invasive surgical techniques should consider obtaining specialized training. Given the lack of high-quality evidence, well-designed randomized trials are needed, as are large observational studies and economic evaluations. Future studies should also further examine and clearly report the influence of surgeon expertise on patient-important outcomes.
Supplemental information
For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/2/4/E295/suppl/DC1
Supplementary Material
References
- 1.Konstantinou K, Dunn KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine 2008;33:2464-72. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto M, Fujimura Y, Suzuki N, et al. MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg Br 1998;80:19-24. [DOI] [PubMed] [Google Scholar]
- 3.Cenic A, Kachur E. Lumbar discectomy: a national survey of neurosurgeons and literature review. Can J Neurol Sci 2009;36:196-200. [DOI] [PubMed] [Google Scholar]
- 4.Dasenbrock HH, Juraschek SP, Schultz LR, et al. The efficacy of minimally invasive discectomy compared with open discectomy: a meta-analysis of prospective randomized controlled trials. J Neurosurg Spine 2012;16:452-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atlas SJ, Keller RB, Wu YA, et al. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the Maine Lumbar Spine study. Spine 2005;30:927-35. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the spine patient outcomes research trial (SPORT): a randomized trial. JAMA 2006;296:2441-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson JN, Waddell G. Surgical interventions for lumbar disc prolapse: updated Cochrane review. Spine 2007;32:1735-47. [DOI] [PubMed] [Google Scholar]
- 8.Boselie TF, Willems PC, van Mameren H, et al. Arthroplasty versus fusion in single-level cervical degenerative disc disease. Cochrane Database Syst Rev 2012;9:CD009173. [DOI] [PubMed] [Google Scholar]
- 9.Kamper SJ, Ostelo RW, Rubinstein SM, et al. Minimally invasive surgery for lumbar disc herniation: a systematic review and meta-analysis. Eur Spine J 2014;23:1021-43. [DOI] [PubMed] [Google Scholar]
- 10.Payer M. “Minimally invasive” lumbar spine surgery: a critical review. Acta Neurochir (Wien) 2011;153:1455-9. [DOI] [PubMed] [Google Scholar]
- 11.Kane J, Kay A, Maltenfort M, et al. Complication rates of minimally invasive spine surgery compared to open surgery: a systematic literature review. Semin Spine Surg 2013;25:191-9. [Google Scholar]
- 12.Arts M, Brand R, van der Kallen B, et al. Does minimally invasive lumbar disc surgery result in less muscle injury than conventional surgery? A randomized controlled trial. Eur Spine J 2011;20:51-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arts MP, Brand R, van den Akker ME, et al. Leiden–The Hague Spine Intervention Prognostic Study Group (SIPS) . Tubular diskectomy vs conventional microdiskectomy for sciatica: a randomized controlled trial. JAMA 2009;302:149-58. [DOI] [PubMed] [Google Scholar]
- 14.Hermantin FU, Peters T, Quartararo L, et al. A prospective, randomized study comparing the results of open discectomy with those of video-assisted arthroscopic microdiscectomy. J Bone Joint Surg Am 1999;81:958-65. [DOI] [PubMed] [Google Scholar]
- 15.Allen RT, Garfin SR. The economics of minimally invasive spine surgery: the value perspective. Spine 2010;35(Suppl):S375-82. [DOI] [PubMed] [Google Scholar]
- 16.Fourney DR, Dettori JR, Norvell DC, et al. Does minimal access tubular assisted spine surgery increase or decrease complications in spinal decompression or fusion? Spine 2010;35(Suppl):S57-65. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein CL, Macwan K, Sundararajan K, et al. Comparative outcomes of minimally invasive surgery for posterior lumbar fusion: a systematic review. Clin Orthop Relat Res 2014;472:1727-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith N, Masters J, Jensen C, et al. Systematic review of microendoscopic discectomy for lumbar disc herniation. Eur Spine J 2013;22:2458-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT, Greene S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Oxford (UK): Cochrane Collaboration; 2011. [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Pham B, Lawson ML, et al. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health Technol Assess 2003;7:1-90. [DOI] [PubMed] [Google Scholar]
- 22.Evaniew N, Khan M, Drew B, et al. Intrawound vancomycin to prevent infections after spine surgery: a systematic review and meta-analysis. Eur Spine J 2014; May 18 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 23.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011;64:395-400. [DOI] [PubMed] [Google Scholar]
- 24.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction —GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-94. [DOI] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
- 26.Puhan MA, Soesilo I, Guyatt GH, et al. Combining scores from different patient reported outcome measures in meta-analyses: When is it justified? Health Qual Life Outcomes 2006;4:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furukawa TA, Barbui C, Cipriani A, et al. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 2006;59:7-10. [DOI] [PubMed] [Google Scholar]
- 29.Zanoli G, Stromqvist B, Jonsson B. Visual analog scales for interpretation of back and leg pain intensity in patients operated for degenerative lumbar spine disorders. Spine 2001;26:2375-80. [DOI] [PubMed] [Google Scholar]
- 30.Azimi P, Mohammadi HR, Montazeri A. An outcome measure of functionality and pain in patients with lumbar disc herniation: a validation study of the Japanese Orthopedic Association (JOA) score. J Orthop Sci 2012;17:341-5. [DOI] [PubMed] [Google Scholar]
- 31.Daltroy LH, Cats-Baril WL, Katz JN, et al. The North American Spine Society Lumbar Spine Outcome Assessment Instrument: reliability and validity tests. Spine 1996;21:741-9. [DOI] [PubMed] [Google Scholar]
- 32.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000;25:2940,52; discussion 2952. [DOI] [PubMed]
- 33.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407-15. [DOI] [PubMed] [Google Scholar]
- 34.Copay AG, Subach BR, Glassman SD, et al. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J 2007;7:541-6. [DOI] [PubMed] [Google Scholar]
- 35.Gatchel RJ, Mayer TG. Testing minimal clinically important difference: Consensus or conundrum? Spine J 2010;10:321-7. [DOI] [PubMed] [Google Scholar]
- 36.Bannuru RR, Vaysbrot EE, McIntyre LF. Did the American Academy of Orthopaedic Surgeons osteoarthritis guidelines miss the mark? Arthroscopy 2014;30:86-9. [DOI] [PubMed] [Google Scholar]
- 37.Carragee EJ. The rise and fall of the “minimum clinically important difference.”. Spine J 2010;10:283-4. [DOI] [PubMed] [Google Scholar]
- 38.Copay AG, Glassman SD, Subach BR, et al. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J 2008;8:968-74. [DOI] [PubMed] [Google Scholar]
- 39.Solberg T, Johnsen LG, Nygaard OP, et al. Can we define success criteria for lumbar disc surgery? Estimates for a substantial amount of improvement in core outcome measures. Acta Orthop 2013;84:196-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnston BC, Thorlund K, Schunemann HJ, et al. Improving the interpretation of quality of life evidence in meta-analyses: the application of minimal important difference units. Health Qual Life Outcomes 2010;8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glasziou PP, Sanders SL. Investigating causes of heterogeneity in systematic reviews. Stat Med 2002;21:1503-11. [DOI] [PubMed] [Google Scholar]
- 42.Sackett D, Haynes R, Guyatt G, et al. Clinical epidemiology: a basic science for clinicians. 2nd ed. Boston: Little Brown; 1991. [Google Scholar]
- 43.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. [PubMed] [Google Scholar]
- 44.Soliman HM. Cervical microendoscopic discectomy and fusion: Does it affect the postoperative course and the complication rate? A blinded randomized controlled trial. Spine 2013;38:2064-70. [DOI] [PubMed] [Google Scholar]
- 45.Ruetten S, Komp M, Merk H, et al. Full-endoscopic cervical posterior foraminotomy for the operation of lateral disc herniations using 5.9-mm endoscopes: a prospective, randomized, controlled study. Spine 2008;33:940-8. [DOI] [PubMed] [Google Scholar]
- 46.Ruetten S, Komp M, Merk H, et al. Full-endoscopic anterior decompression versus conventional anterior decompression and fusion in cervical disc herniations. Int Orthop 2009;33:1677-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim KT, Kim YB. Comparison between open procedure and tubular retractor assisted procedure for cervical radiculopathy: results of a randomized controlled study. J Korean Med Sci 2009;24:649-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arts MP, Brand R, van den Akker ME, et al. Tubular diskectomy vs conventional microdiskectomy for the treatment of lumbar disk herniation: 2-year results of a double-blind randomized controlled trial. Neurosurgery 2011;69:135,44; discussion 144. [DOI] [PubMed]
- 49.Brock M, Kunkel P, Papavero L. Lumbar microdiscectomy: subperiosteal versus transmuscular approach and influence on the early postoperative analgesic consumption. Eur Spine J 2008;17:518-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franke J, Greiner-Perth R, Boehm H, et al. Comparison of a minimally invasive procedure versus standard microscopic discotomy: a prospective randomised controlled clinical trial. Eur Spine J 2009;18:992-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garg B, Nagraja UB, Jayaswal A. Microendoscopic versus open discectomy for lumbar disc herniation: a prospective randomised study. J Orthop Surg (Hong Kong) 2011;19:30-4. [DOI] [PubMed] [Google Scholar]
- 52.Huang TJ, Hsu RW, Li YY, et al. Less systemic cytokine response in patients following microendoscopic versus open lumbar discectomy. J Orthop Res 2005;23:406-11. [DOI] [PubMed] [Google Scholar]
- 53.Righesso O, Falavigna A, Avanzi O. Comparison of open discectomy with microendoscopic discectomy in lumbar disc herniations: results of a randomized controlled trial. Neurosurgery 2007;61:545-9, discussion 549. [DOI] [PubMed] [Google Scholar]
- 54.Ruetten S, Komp M, Merk H, et al. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine 2008;33:931-9. [DOI] [PubMed] [Google Scholar]
- 55.Ryang YM, Oertel MF, Mayfrank L, et al. Standard open microdiscectomy versus minimal access trocar microdiscectomy: results of a prospective randomized study. Neurosurgery 2008;62:174-81, discussion 181-2. [DOI] [PubMed] [Google Scholar]
- 56.Shin DA, Kim KN, Shin HC. Yoon do H. The efficacy of microendoscopic discectomy in reducing iatrogenic muscle injury. J Neurosurg Spine 2008;8:39-43. [DOI] [PubMed] [Google Scholar]
- 57.Teli M, Lovi A, Brayda-Bruno M, et al. Higher risk of dural tears and recurrent herniation with lumbar micro-endoscopic discectomy. Eur Spine J 2010;19:443-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401-6. [DOI] [PubMed] [Google Scholar]
- 59.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence — inconsistency. J Clin Epidemiol 2011;64:1294-302. [DOI] [PubMed] [Google Scholar]
- 60.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 6. Rating the quality of evidence — imprecision. J Clin Epidemiol 2011;64:1283-93. [DOI] [PubMed] [Google Scholar]
- 61.Walsh M, Srinathan SK, McAuley DF, et al. The statistical significance of randomized controlled trial results is frequently fragile: a case for a fragility index. J Clin Epidemiol 2014;67:622-8. [DOI] [PubMed] [Google Scholar]
- 62.Wang B, Lu G, Patel AA, et al. An evaluation of the learning curve for a complex surgical technique: the full endoscopic interlaminar approach for lumbar disc herniations. Spine J 2011;11:122-30. [DOI] [PubMed] [Google Scholar]
- 63.Zhao-Yu C, Yan G, Wei C, et al. Reduced blood loss after intra-articular tranexamic acid injection during total knee arthroplasty: a meta-analysis of the literature. Knee Surg Sports Traumatol Arthrosc 2013;Dec. 19 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 64.van den Akker ME, Arts MP, van den Hout WB, et al. Tubular diskectomy vs. conventional microdiskectomy for the treatment of lumbar disk-related sciatica: cost utility analysis alongside a double-blind randomized controlled trial. Neurosurgery 2011;69:829,35; discussion 835-6. [DOI] [PubMed]
- 65.Ghert M, Petrisor B. Subgroup analyses: When should we believe them? J Bone Joint Surg Am 2012;94(Suppl 1):61-4. [DOI] [PubMed] [Google Scholar]
- 66.Sun X, Briel M, Walter SD, et al. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ 2010;340:c117. [DOI] [PubMed] [Google Scholar]
- 67.van Oldenrijk J, van Berkel Y, Kerkhoffs GM, et al. Do authors report surgical expertise in open spine surgery related randomized controlled trials? A systematic review on quality of reporting. Spine 2013;38:857-64. [DOI] [PubMed] [Google Scholar]
- 68.Wang H, Huang B, Li C, et al. Learning curve for percutaneous endoscopic lumbar discectomy depending on the surgeon’s training level of minimally invasive spine surgery. Clin Neurol Neurosurg 2013;115:1987-91. [DOI] [PubMed] [Google Scholar]
- 69.Benzel EC, Orr RD. A steep learning curve is a good thing! Spine J 2011;11:131-2. [DOI] [PubMed] [Google Scholar]
- 70.Assaker R. Minimal access spinal technologies: state-of-the-art, indications, and techniques. Joint Bone Spine 2004;71:459-69. [DOI] [PubMed] [Google Scholar]
- 71.Lehman RA, Jr, Vaccaro AR, Bertagnoli R, et al. Standard and minimally invasive approaches to the spine. Orthop Clin North Am 2005;36:281-92. [DOI] [PubMed] [Google Scholar]
- 72.Hoppe DJ, Schemitsch EH, Morshed S, et al. Hierarchy of evidence: where observational studies fit in and why we need them. J Bone Joint Surg Am 2009;91(Suppl 3):2-9. [DOI] [PubMed] [Google Scholar]
- 73.Koenig L, Dall TM, Gu Q, et al. How does accounting for worker productivity affect the measured cost-effectiveness of lumbar discectomy? Clin Orthop Relat Res 2014;472:1069-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dijksman LM, Poolman RW, Bhandari M, et al. International Evidence-Based Orthopaedic Surgery Working Group . Money matters: what to look for in an economic analysis. Acta Orthop 2008;79:1-11. [DOI] [PubMed] [Google Scholar]
- 75.Devereaux PJ, Bhandari M, Clarke M, et al. Need for expertise based randomised controlled trials. BMJ 2005;330:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scholtes VA, Nijman TH, van Beers L, et al. Emerging designs in orthopaedics: expertise-based randomized controlled trials. J Bone Joint Surg Am 2012;94(Suppl 1):24-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





