Abstract
The spontaneous positioning of colloids on the surfaces of micrometer-sized liquid crystalline droplets and their subsequent polymerization offers the basis of a general and facile method for the synthesis of patchy microparticles. The existence of multiple local energetic minima, however, can generate kinetic traps for colloids on the surfaces of the liquid crystal (LC) droplets and result in heterogeneous populations of patchy microparticles. To address this issue, here we demonstrate that adsorbate-driven switching of the internal configurations of LC droplets can be used to sweep colloids to a single location on the LC droplet surfaces, thus resulting in the synthesis of homogeneous populations of patchy microparticles. The surface-driven switching of the LC can be triggered by addition of surfactant or salts, and permits the synthesis of dipolar microparticles as well as “Janus-like” microparticles. By using magnetic colloids, we illustrate the utility of the approach by synthesizing magnetically-responsive patchy microdroplets of LC with either dipolar or quadrupolar symmetry that exhibit distinct optical responses upon application of an external magnetic field.
Keywords: patchy microparticles, liquid crystal microdroplets, surfactants, ordering transitions, polymerization
1. Introduction
Nano-/microparticles with patterned surface properties (e.g., patchy microparticles or Janus microparticles), when compared to their homogeneous counterparts, exhibit a range of physical properties that are enabling the design of new classes of functional materials.[1-3] For example, charged[4] or magnetic[5] dipolar patchy microparticles can be aligned or directed into self-assembled structures under the influence of an applied external electric or magnetic field, respectively. The responsiveness of these particles to external fields can be used for design of tunable electronic or photonic materials.[1] Additional applications of patchy microparticles include “electronic paper”,[6, 7] self-propelled nano-/microparticles,[8] and biological detectors.[9] Despite their potential utility, few methods exist for synthesis of patchy microparticles. Current methodologies include self-assembly of block copolymers, seeded emulsion polymerization, and microfluidics.[2] In this paper, we advance a methodology for synthesis of homogeneous populations of dipolar patchy microparticles in which the long-range order of thermotropic liquid crystals (LCs) confined to micrometer-sized droplets dispersed in water is reversibly switched so as to direct colloidal microparticles to populate a unique location (patch) on the droplet surface. Subsequent photo-polymerization of the droplets is used to preserve the organization of the LC microparticles.
LCs combine properties commonly associated with crystalline solids (long-range orientational order) and isotropic liquids (high levels of molecular mobility).[10] When nematic LCs are confined to water-dispersed microdroplets, the interfacial interactions of the LC set the orientation of the LCs at the droplet surface (i.e., surface anchoring).[11, 12-14] To accommodate the surface anchoring conditions, the LC within the interior of the droplet assumes a variety of configurations that involve elastic strain of the LC (e.g., splay, bend, etc.)[14-18] and topological defects (localized regions where the LC effectively melts).[15, 16, 19, 20, 21] At equilibrium, the ordering of the LC within the microdroplets reflects minimization of the combined contributions of surface anchoring, bulk elastic deformations and topological defects to the free energy.[14-18] In the study reported in this paper, we demonstrate that reversible manipulation of the surface anchoring of LC droplets decorated with colloids can be used to switch the configurations of LC droplets so as to sweep the colloids into unique locations on the droplet surface for the synthesis of homogeneous populations of patchy microparticles.[12, 14, 22, 23]
The use of reversible switching of LC microdroplet configurations, as described in this paper for the synthesis of patchy microparticles, builds from our recent report of the use of LC droplets as templates for the synthesis of complex particles based on the LC-directed positioning of colloids (1 μm-in-diameter) adsorbed to the surfaces of the LC microdroplets.[24] In particular, for LC droplets with tangential surface anchoring that adopted a bipolar configuration (see below for additional discussion and Figure 1A), long-range elastic forces were observed to direct the colloids to preferentially locate at one of the two defects present at opposite poles of the bipolar droplets (i.e., boojums).[24, 25] Because colloids with different compositions and properties can be positioned on the LC droplet surface (e.g., polystyrene (PS) or silica colloids), the colloids can be used to introduce a desired functional property into the patchy microparticle (e.g., surface chemistry or responsive to external field).[24] While this new generalizable technique for the synthesis of patchy microparticles offers the advantage of scalability over other methods (described above) because synthesis occurs in a bulk aqueous phase, our previous publication reported heterogeneous populations of patchy microparticles because it was not possible to direct the colloids to a single pole of a LC droplet (e.g., some LC droplets had colloids at both poles while others had colloids at only one pole).[24]
Figure 1.
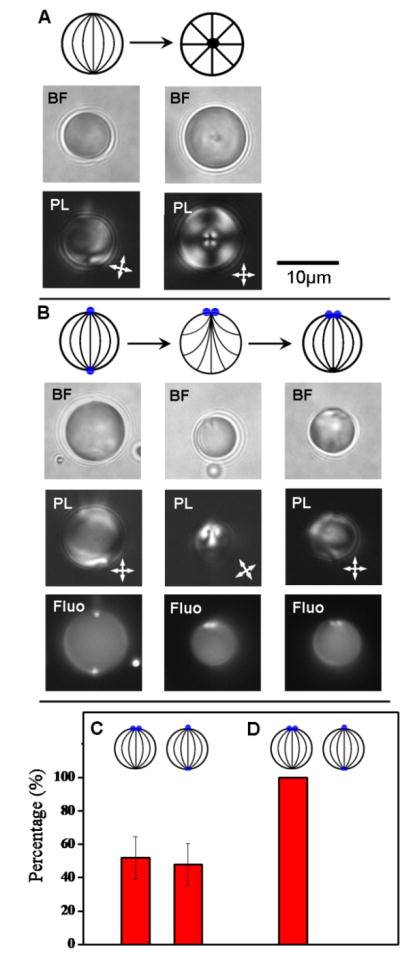
(A) Bipolar-to-radial ordering transition in a water-dispersed droplet of nematic 5CB without colloids adsorbed at the surface (triggered by adsorption of SDS). (B) Method utilized to direct 1 μm-in-diameter PS colloids initially at opposite boojums of a bipolar 5CB droplet to the same boojum via reversible adsorption of SDS. Schematic illustrations and the corresponding bright field (BF) and polarized light (PL) micrographs, and fluorescence (Fluo) micrographs (for B only) are shown from top to bottom for each equilibrium state of the LC droplets. In the schematics, the defects and colloids are represented by solid black or blue circles, respectively. The orientation of the crossed polarizers in PL micrographs is indicated by the white double-headed arrows. (C and D) Percentage of 5CB droplets in each two-colloid configuration (C) before or (D) after moving PS colloids to one defect of the bipolar LC droplets by using SDS to reversibly switch the LC order. The histograms were assembled from analysis of (C) 91 or (D) 48 droplets in 3 independent experiments.
In the present study, we demonstrate that it is possible to take advantage of the above described internal switching of LC droplets to prepare populations of droplets with colloids adsorbed at only one location of the LC droplets. The configurations of the LC droplets are changed by either reversible adsorption of an amphiphilic species to the surfaces of the droplets or addition of salts. Characterization of the dynamics of both the internal switching and subsequent repositioning of the colloids to a single pole, in combination with a simple scaling argument, reveals the driving force for the repositioning is the long-range elasticity of the LC confined to the droplets. We illustrate the utility of this approach by synthesizing “Janus-like” microparticles and magnetically-responsive patchy droplets with either dipolar or quadrupolar symmetry for which the internal configuration of the LC leads to distinct optical responses upon application of an external magnetic field.
2. Results and Discussion
Emulsions of the nematic LC, 4-cyano-4′-pentylbiphenyl (5CB) in the bipolar configuration were prepared by dispersing 5CB in water, and homogenizing the mixture for 30 s. The left column of Figure 1A shows representative bright field (BF) and polarized light (PL) micrographs of a bipolar 5CB droplet obtained from our emulsification procedure. The bipolar droplet contains LC aligned tangential to the droplet surface and possesses two boojum defects at opposite poles.[14, 16, 21-23, 26] After formation of the bipolar droplets, 1 μm-in-diameter fluorescent PS colloids, at the surfaces of which the LC assumes a tangential anchoring, were adsorbed to the surfaces of the droplets through addition of a 1 % (wt/v) dispersion of the PS colloids, followed by another 30 s of homogenization. Fluorescent PS colloids were used to permit the position of the colloids to be observed by fluorescence (Fluo) microscopy (Figure 1B). Consistent with our previous studies,[24, 25] this procedure led to a heterogeneous population of droplets with either two PS colloids located at a single boojum defect or one PS colloid at each boojum (Figure 1C). We comment here that our emulsification procedure resulted in LC droplets with diameters ranging from 1 to 40 μm and that we focus on LC droplets with diameters between 7 and 20 μm in the observations reported below. We also note that monodisperse LC emulsions can be prepared by various techniques (e.g., microfluidics,[27] inkjet printing,[28] filling polymer microcapsules with LC,[29] etc.) and that advances reported below can be combined with these techniques.
Next, a small volume of a stock solution of 100 mM sodium dodecyl sulfate (SDS) was added to the aqueous phase of the emulsions to switch the internal configuration of the LC droplets (a so-called ordering transition) through adsorption of the surfactant at the LC droplet surface.[25] A final concentration of 2 mM SDS was selected to avoid formation of surfactant micelles, which can solubilize LC droplets.[30] After addition of the SDS solution, the droplets were observed to undergo a bipolar-to-preradial ordering transition. The preradial configuration involves LC aligned at an acute angle at the droplet surface (e.g., tilted) and a single defect at a pole of the droplet (Figure 1B, middle column).[14, 16, 18, 21-23, 31] We comment that in the absence of the colloids, adsorption of SDS drives a bipolar-to-radial ordering transition (Figure 1A), and thus our observation of the preradial configuration suggests that the presence of the colloids results in an effective “pinning” of a topological defect to the droplet surfaces.[25] Importantly, independent of the positions of the colloids on the surfaces of bipolar droplets prior to addition of SDS (colloids adsorbed at single boojum or colloids adsorbed at each of the two boojums), after addition of SDS, the colloids were observed to locate at the site of the preradial defect (Figure 1B, middle column).
Finally, the reverse (preradial-to-bipolar) ordering transition was achieved through dilution of the surfactant to a final concentration of < 0.1 mM, and desorption of surfactant from the droplet surfaces (near-monolayer coverage of surfactant is required to change the orientation of the LC from tangential to normal).[13, 22, 32] Upon recovering the bipolar configuration, we did not observe LC droplets with colloids adsorbed at both boojum defects (Figure 1D). Instead, a homogeneous population of LC droplets with colloids adsorbed at a single boojum (Figure 1B, right column) was obtained. This state of the emulsion was found to persist over the duration of our observations (hours). Overall, the results of this experiment demonstrate that surfactant-induced switching of the configurations of LC droplets can be used to synthesize homogeneous populations of bipolar droplets with colloids adsorbed at a single pole.
Next, we characterized the dynamics of the SDS-triggered bipolar-to-preradial ordering transition in 5CB droplets (with one PS colloid adsorbed initially at each boojum defect of the initial bipolar configuration) to provide insight into the mechanism driving the two colloids to the single defect of the preradial configuration. A representative example of the kinetic pathway for this process is shown in Figure 2. The initial time point (t = 0 s) displayed in Figure 2 represents the time at which the 100 mM SDS solution was added to the aqueous phase surrounding the 20 μm-in-diameter 5CB droplet (a drop of SDS solution was added to one end of the sample, and the sample was allowed to equilibrate). Following the addition, SDS was transported (via diffusion and convention) to the surface of the droplet. The first evidence of the LC ordering transition was observed at t = 77 s, at which point a disclination loop formed inside the 5CB droplet, consistent with a previously reported kinetic pathway.[21, 22, 33] Subsequently (t = 77 - 81 s), the disclination loop migrated towards a site previously occupied by one of the two boojums, and shrank to a point to form the preradial configuration. Immediately following our observation of the bipolar-to-preradial ordering transition, we changed the imaging mode of the microscope from polarized light to fluorescence to determine the position of the PS colloids. Surprisingly, only one colloid was observed at the position of the defect of the preradial configuration, while the second colloid remained close to the location of the boojum of the initial bipolar configuration. Over the course of the next 40 s, the latter PS colloid was observed to migrate across the surface of the LC droplet and associate with the first colloid at the defect. From these results, we conclude that one PS colloid pinned the preradial point defect to the surface of the LC droplet, preventing escape of the defect into the core of the droplet (as would be expected in the absence of adsorbed colloids; see Figure 1A),[25] and an additional long-range force drove the second colloid to join the first at the position of the point defect of the preradial configuration.
Figure 2.
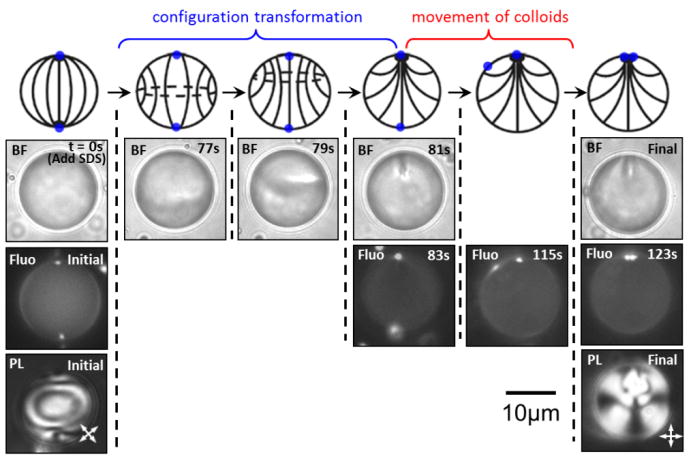
Representative micrographs for the kinetic pathway of a bipolar-to-preradial ordering transition in a nematic 5CB droplet with one 1 μm-in-diameter fluorescent PS colloid adsorbed at each of two boojum defects. BF, Fluo and PL micrographs are shown from top to bottom for the initial and final state. BF or Fluo micrographs are shown for the intermediate states of the process to highlight the evolution of the LC configuration and movement of PS colloids, respectively. Corresponding schematic illustrations are shown at the top.
A key observation of the experiment described above is that the mobile PS colloid (i.e., colloid not pinning the defect to the surface of the droplet) did not randomly diffuse over the surface of the droplet and arrest at the position of the preradial defect. Instead, it followed a nearly direct path from the position of the boojum of the initial bipolar configuration to the preradial defect. This observation led us to hypothesize that the driving force for the migration of the colloid was the elastic distortion of the LC induced by the presence of the colloid at the interface. To test this proposal, a simple scaling argument was formulated to compare the magnitude of the elastic energy associated with the colloid-induced distortion of the LC to the energy dissipated by Stokes' drag during transport of the colloid across the surface of the LC droplet to the preradial defect. Here we comment that a colloid adsorbed at either the defect of a preradial LC droplet or at the opposite pole both distort the LC. Our experimental results reported above and recent coarse grain modelling[25] support, however, the proposition that elastic distortions (and defect core energies) are minimized by the partitioning of a colloid to a defect. Accordingly, we estimate the magnitude of the elastic forces driving the colloid to the defect as:[34, 35]
| (1) |
in which K is the elastic constant of the LC (using the “one constant” approximation), and a is the radius of the PS colloid (0.5 μm). Using a typical value for an elastic constant for a low molecular weight LC, K=10-11 N,[20, 36] we calculate Eelastic ≈ 5×10-18 J. As noted above, the force driving transport of the PS colloid across the surface of the LC droplet is opposed by a Stokes' drag force. Thus, the energy dissipated during the displacement of the colloid can be estimated as the product of this drag force and the distance that the PS colloid travelled to reach the preradial defect (d):[37]
| (2) |
in which η is the apparent dynamic viscosity experienced by the PS colloid at the aqueous—5CB interface, and v is the average velocity of the colloid. We estimate d to be Rπ, where R is the radius of the LC droplet (10 μm), and η to be 30 mPa s (for silica colloids at a planar aqueous—5CB interface, η was measured to fall between 25.8 and 34.9 mPa s[34]). In addition, v can be approximated as:
| (3) |
in which t is the transit time for the PS colloid to reach the preradial defect (40 s, as shown in Figure 2). Combining Equation 2 and 3, we calculate Etransport ≈ 7×10-18 J, which is in close agreement with our estimate for the energy associated with the elastic distortion of the LC around the colloid (5×10-18 J). Overall, this simple scaling argument supports our hypothesis that the elastic forces generated by the LC droplet drive the colloid to the preradial defect.
The technique reported above for synthesis of homogeneous populations of patchy LC droplets used surfactants. Surfactants, however, are often difficult to remove following material synthesis, and their presence can hinder certain applications (e.g., biological sensing[11, 12-14]). Therefore, we sought to switch the internal configurations of the LC droplets through manipulation of the ionic strength and pH of the aqueous solution (without surfactant). Previously, we showed that it is possible to trigger bipolar-to-radial ordering transitions in LC droplets under conditions of high ionic strength (> 100 mM) and alkaline pH (> 12), due to the effects of an electrical double layer (EDL) that forms on the LC-side of the aqueous—LC interface.[38] To explore the use of salts and alkaline conditions for microparticle synthesis, we first formed emulsions of bipolar 5CB droplets with PS colloids adsorbed at either one or both boojums. Next, sodium chloride and sodium hydroxide (the final concentration of sodium chloride was 1 M and the pH was 12.7) were added to the aqueous phase of the emulsions. As was the case for the SDS-triggered ordering transition, addition of salt resulted in a homogeneous population of preradial LC droplets with both colloids adsorbed at the defect (Figure S2 of SI). Finally, the reverse (preradial-to-bipolar) ordering transition, induced by dilution of the bulk aqueous concentration of sodium chloride to below 100 mM, resulted in a homogeneous population of bipolar droplets with two PS colloids trapped at a single boojum defect (Figure S2 of SI). This experiment demonstrates that simple adjustments of ionic strength and pH can also be used to switch the internal configurations of LC droplets to synthesize homogeneous populations patchy LC droplets. We comment here that in both methods described above for synthesis of homogeneous populations of patchy LC droplets (addition of surfactants or simple salts), the colloids remain trapped at a single boojum defect of the bipolar configuration (i.e., do not return to the initial state of the system with one colloid located at each defect) because the colloids are located in a local free energy minimum.[25]
As described in the Introduction, there is a growing range of applications for Janus microparticles (e.g., electronics, photonics, sensing), and thus we next sought to demonstrate how switching of LC droplets can be exploited to synthesize solid “Janus-like” microparticles (Janus microparticles are defined as patchy microparticles exhibiting an approximately 50:50 ratio of anisotropic surface coverage[2, 6]). We began by doping the LC with a small amount of the reactive mesogenic monomer 4-(3-acryloyloxypropyloxy) benzoic acid 2-methyl-1,4-phenylene ester (RM257) and the photo-initiator 2-dimethoxy-2-phenyl acetophenone (DMPAP) prior to formation of the LC-in-water emulsions. Next, the emulsions were formed and PS colloids were subsequently adsorbed to the surfaces of the LC droplets (see also the Experimental Section below for more detail). We found that increasing the homogenization period from 30 s (used in the experiments reported above) to 300 s resulted in more than two PS colloids adsorbed to surfaces of the droplets. As was the case for two adsorbed PS colloids, we observed multiple colloids to adsorb at diametrically opposite boojums of bipolar LC droplets with equal probability (Figure 3A and B). We next added SDS to the emulsions to trigger a bipolar-to-preradial ordering transition, which was observed to sweep the colloids to a single pole of the droplets (the final concentration of SDS was again 2 mM). Finally, the emulsions were exposed to UV light for 40 minutes to photo-polymerize the colloid-coated LC droplets in the preradial configuration.
Figure 3.
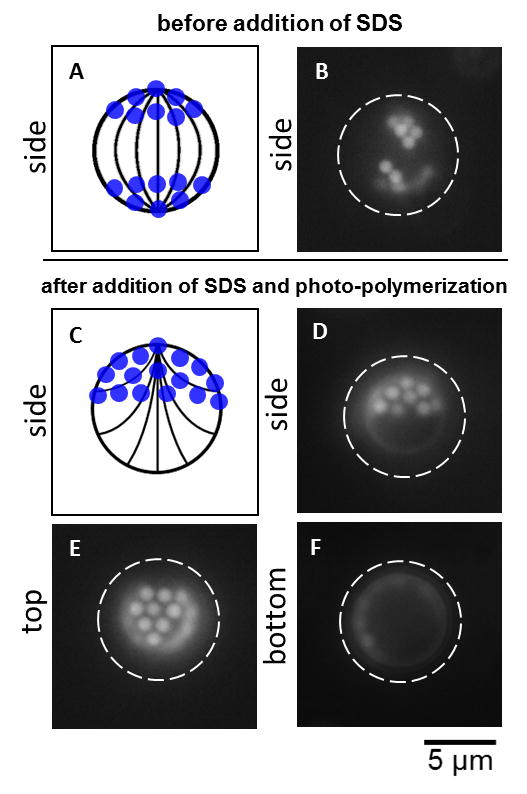
Synthesis of solid “Janus-like” microparticles via switching of LC droplets. (A and B) Side view of the arrangement of PS colloids on the surface of a bipolar LC droplet prior to addition of SDS. A side view is shown to emphasize that colloids were adsorbed at both poles. (C-F) Solid “Janus-like” microparticles with an internal LC structure locked in the preradial configuration (adsorption of SDS) by a polymer network as viewed from the (D) side, (E) top, and (F) bottom. Each image was captured by allowing the microparticles to rotate and subsequently focusing on the apex of the newly oriented microparticle. The white dashed circles indicate the contour of the microparticle. The depth of focus was ∼500 nm. Corresponding schematic illustrations viewed from the side are shown in (A) and (C).
Figure 3C-F shows Fluo micrographs of spherical solid “Janus-like” microparticles with an internal structure of LC locked in the preradial configuration by the polymer network (see Figure S3 of the SI for the corresponding BF and PL micrographs, and Figure S4 for additional examples). The colloids are located on approximately one half of the LC droplet. We note here that the depth of focus of the 100X oil-immersion objective used to collect the images is ∼500 nm. Therefore, PS colloids at different heights along the surface of the spherical “Janus-like” microparticles that fall within this depth of focus appear with similar intensity in Fluo micrographs. Interestingly, the packing arrangements of the colloids on the surfaces of the LC droplets follow a close-packed hexagonal pattern, which is distinct from the star structures recently reported to be formed by 4 μm-in-diameter silica particles, treated to induce a homeotropic anchoring of the LC, trapped at the surfaces of large (150 to 250 μm-in-diameter) bipolar LC droplets dispersed in an aqueous solution of polyvinyl alcohol.[39] Ongoing studies are investigating the origins of this hexagonal pattern, the results of which will be reported elsewhere. From the results displayed in Figure 3 and Figures S3 of the SI, we conclude that our new methodology can be employed to synthesize solid spherical “Janus-like” microparticles with internal structuring characteristic of a LC droplet.
As described above, our methodology for synthesis of patchy microparticles and microparticles is generalizable because the LC droplets can be used to direct the assembly of a range of different organic or inorganic colloids on the droplet surfaces. In the final experiment reported in this paper, we demonstrate synthesis of functional magnetic patchy LC droplets that can be rotated in weak magnetic fields. The patchy LC droplets were synthesized using paramagnetic, 2.5 μm-in-diameter PS colloids (the colloids contained dispersions of iron oxide nanoparticles). Prior to addition of any SDS, we observed bipolar LC droplets with multiple magnetic PS colloids distributed non-uniformly amongst their boojums. We comment here that although the larger size of the magnetic PS colloids (relative to the non-magnetic colloids) allowed the position of the colloids to be resolved in BF micrographs, the exact number of magnetic PS colloids adsorbed at the poles of the droplets could not be determined because the colloids were not fluorescently labeled. When a permanent magnet was held ∼15 cm away from the LC emulsion, the orientational response of the patchy bipolar LC droplets to the weak external magnetic field (∼10-4 Tesla see below) depended on the location of the PS colloids on the surface of the droplets, as shown in Figure 4A. Specifically, bipolar LC droplets with magnetic PS colloids adsorbed at a single pole rotated to an equilibrium state in which the pole with the adsorbed colloids faced the location of the magnet. In contrast, bipolar LC droplets with colloids adsorbed at both poles were observed to align in a bimodal distribution of orientations with droplets oriented such that the two poles were aligned either parallel or perpendicular to magnet (see Figure 4A and B).
Figure 4.
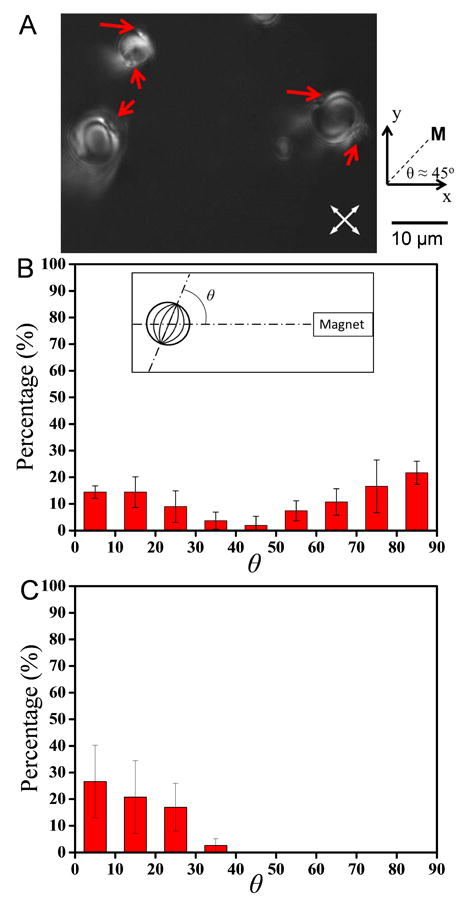
Influence of the positions of magnetic PS colloids adsorbed to the surfaces of bipolar LC droplets on the alignment of the patchy droplets in a weak external magnetic field. (A) Representative PL micrographs of the orientation of droplets with colloids adsorbed at either a single pole (lower droplet on the left) or both poles (top droplet on the left and droplet on the right) in the vicinity of boojum defects. The magnet was held at a ∼45° angle with respect to x-axis and ∼15 cm away from the sample. The red arrows indicate the location of the pole at which the magnetic PS colloids are adsorbed. (B and C) Distribution of rotational orientations observed for bipolar LC droplets in the presence of a magnetic field for droplets with magnetic PS colloids adsorbed at (B) either a single or both pole(s) prior to addition of SDS or (C) trapped at one pole by reversible adsorption of SDS. The rotational orientation is expressed as a function of the angle (θ, see inset in (B)) defined by the location of the magnetic disc relative to the orientation of a line that connects the boojum defects of bipolar LC droplets. The histograms were assembled from analysis of (B) 55 or (D) 39 droplets in 3 independent experiments.
The above observations show that the heterogeneous population of magnetic patchy LC droplets responds non-uniformly to the external magnetic field (Figure 4B). To overcome this drawback, homogeneous populations of LC droplets were prepared by reversible switching of the internal ordering of the LC using SDS (as described above). We note here that we prepared uniform populations of both bipolar and preradial dipolar patchy droplets because these configurations generate distinct optical appearances when viewed between crossed polarizers (Figure 1B). As shown in Figure 4C and Figure S5A and B of the SI, when the entire population of magnetic patchy LC droplets was in either the preradial or bipolar configuration, we observed a uniform orientational response of the LC droplets to the applied field. For example, Figure 5 shows a clock-wise 360° in-plane rotation of a LC droplet in either the preradial or bipolar configuration around an axis normal to the center of the droplets (see SI for corresponding videos). We end with two important comments. First, 0.1 - 1 Tesla magnetic fields have been shown to trigger ordering transitions in LC droplets in which the LC molecules (with a positive dielectric anisotropy) align parallel to the direction of the magnetic field (so-called Fredericks transitions).[16, 40] However, the magnetic field used here was much weaker (∼10-4 Tesla), and the LC droplets were shown to rotate due to interactions between the adsorbed magnetic colloids and the field (rather than interactions between the field and LC). Second, two possible mechanisms exist for the observed rotation of magnetic patchy LC droplets: (i) the magnetic colloids and LC droplet rotate together or (ii) the magnetic colloids adsorbed at the surface rotate first, and the internal configuration of the LC droplet subsequently relaxes to its equilibrium state. We calculate the characteristic time for the LC to adopt the velocity of a magnetic colloid moving across the surface of the droplet (τυ ∼ R2/ν, where ν is the kinematic viscosity of 5CB and is of order 10-5 m2/s)[41] to be much shorter than the time required for relaxation of the molecular orientational order within the LC droplet (τn ∼ η5CBR2/K, where η5CB ∼ 10-2 Pa s is the dynamic viscosity of 5CB and K ∼ 10-11 N is the elastic constant (see above)).[20] For a 10 μm radius LC droplet τυ ∼ 10-5 s ≪ τn ∼ 0.1 s, thus we conclude that viscous forces cause the LC to rotate with the magnetic colloid.
Figure 5.
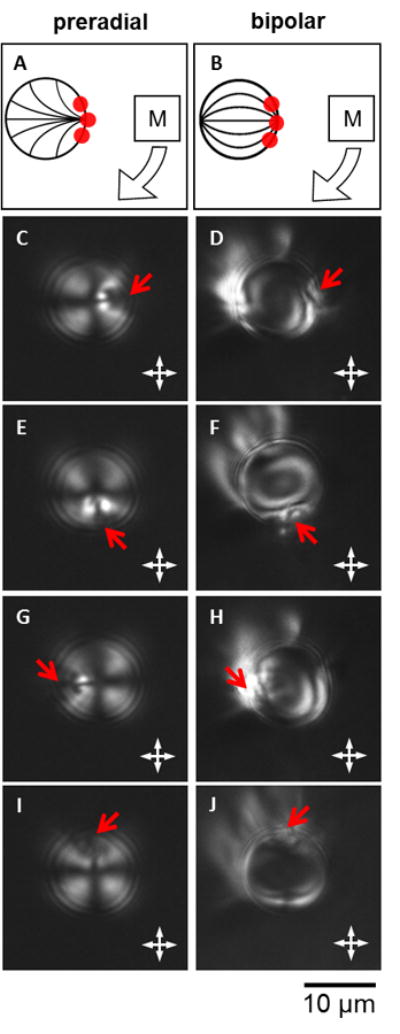
PL micrographs of a clock-wise 360° in-plane rotation of a LC droplet in either a (A, C, E, G, I) preradial or (B, D, F, H, J) bipolar configuration around an axis normal to the center of the droplets. Magnet PS colloids were adsorbed at a single pole (indicated by the red arrow). The rotation was driven by a magnetic disc held ∼15 cm away from the sample. The position of the magnet was to the (C and D) east, (E and F) south, (G and H) west, or (I and J) north of the sample in the plane of the stage. Corresponding schematic illustrations are shown in (A) and (B). Corresponding videos are available in the SI.
3. Conclusion
The experiments reported in this paper demonstrate a generalizable approach to the synthesis of homogeneous dispersions of patchy LC microdroplets and microparticles. The approach uses reversible changes (i.e., switching) of the internal ordering of the LCs to uniformly position colloids on the surfaces of the LC microdroplets. Specifically, a transformation from a bipolar to a preradial configuration, triggered by adsorption of surfactants or addition of simple salts to the aqueous phase of the LC emulsions, induces a long-range elastic force that sweeps adsorbed colloids to a unique location (the location of the single defect of the preradial configuration). The reverse ordering transition results in a homogeneous population of bipolar LC droplets with colloids remaining at the single location (one of the boojum defects) because the colloids are trapped in a local free energy minimum on the surfaces of the droplets. We illustrate the utility of the methodology by demonstrating synthesis of solid “Janus-like” microparticles and also magnetically-responsive patchy bipolar or preradial LC droplets with dipolar symmetry. Because a range of colloidal materials can be positioned on the LC droplets using the methods reported in this paper, and because the LC droplets can be polymerized to form patchy microparticles, the results presented in this paper provide the basis of a general methodology for synthesis of complex multifunctional microparticles. Such microparticles have a range of potential applications, including in biological sensors, chemooptical transduction, directed self-assembly of mesoscopic materials, and drug delivery.
4. Experimental Section
Materials
Sodium dodecyl sulfate (SDS), sodium chloride (NaCl), sodium hydroxide (NaOH), and 2-dimethoxy-2-phenyl acetophenone (DMPAP) were purchased from Sigma-Aldrich (St. Louis, MO). 1 μm-in-diameter fluorescent polystyrene (PS) colloids (λexc=480 nm / λem=520 nm) were purchased from Bangs Laboratories (Fishers, IN). 4-cyano-4′-pentylbiphenyl (5CB) and 4-(3-acryloyoxypropyloxy) benzoic acid 2-methyl-1, 4-phenylene ester (RM257) were obtained from EM Sciences (New York, NY). 2.5 μm-in-diameter magnetic PS colloids were purchased from Spherotech (Lake Forest, IL). Deionization of a distilled water source was performed with a Milli-Q system (Millipore, Bedford, MA).
Preparation of liquid crystal (LC)-in-water emulsions
LC-in-water emulsions were prepared according to previously published methods.[24, 25] Briefly, the emulsions were prepared by emulsifying 4 μL 5CB in 1.98 mL of an aqueous phase using a homogenizer T25 digital ULTRA-TURRAX equipped with a S25 N-10G dispersing element (IKA), for 30 s at 6,500 rpm. This procedure resulted in a homogeneous population of droplets in the bipolar configuration. The emulsions were contained in 19 mm-in-diameter, 51 mm high glass vials.
Adsorption of PS colloids at the surfaces of LC droplets
The PS colloids were adsorbed at the surfaces of the LC droplets through addition of a 20 μL of a 1% wt/v PS colloid dispersion to 1.98 mL of a LC-in-water emulsion, followed by either a 30 or 300 s homogenization process at 6,500 rpm, for experiments with two or many adsorbed colloids, respectively.
Preparation of preradial or bipolar LC droplets with two PS colloids adsorbed at a single defect using sodium dodecyl sulfate (SDS)
For the experiments in which SDS was used to change the internal configuration from bipolar to preradial, 40 μL of a 100 mM aqueous solution of SDS was added to 2 mL of emulsions that contained bipolar LC droplets with two PS colloids adsorbed at the surface. The final concentration of SDS was 2 mM. The reverse (preradial-to-bipolar) ordering transition was achieved through dilution of 100 μL of an emulsion with preradial LC droplets into 2 mL pure water. The final concentration of SDS was below 0.1 mM.
Preparation of preradial or bipolar LC droplets with two PS colloids adsorbed at the surface using electrical double layer (EDL) forces
For the experiments in which EDL forces were used to change the internal configuration from bipolar to preradial, 100 μL of an emulsion with bipolar LC droplets with two PS colloids adsorbed at the surface was added to 2 mL of a 1 M NaCl aqueous solution (with the pH adjusted to 12.7 using NaOH). Subsequently, to induce the reverse (preradial-to-bipolar) ordering transition, we added 100 μL of the emulsion to 2 mL pure water to decrease the concentration of NaCl below 100 mM.
Preparation of polymerized LC patchy microparticles with “Janus-like” surface anisotropy
First, we prepared a photo-reactive RM257/DMPAP/5CB mixture to be used for formation of LC emulsions. The RM257 was mixed with 5CB at a wt/wt percentage of 10%. Then, the photo-initiator DMPAP was added at 5 % wt/wt based on the mass of RM257. After mixing, the experiments were performed in a fashion similar to those performed with two adsorbed PS colloids (see above). However, instead of 5CB, the RM257/DMPAP/5CB mixture was used, and multiple (more than two) colloids were adsorbed by increasing the homogenization time from 30 s to 300 s. Photo-polymerization of the 5CB/RM257/DMPAP mixture was performed using a UV lamp (365 nm) that delivered 2.5 mW/cm2. The emulsion was exposed to UV light for 40 minutes.
Rotation of LC patchy droplets with magnetic PS colloids adsorbed at the surface
A magnetic field was introduced to a LC emulsion by placing a 1.9 cm-in-diameter, 1.3 cm thick magnetic disc (K&J Magnetics Inc., Pipersville, PA) ∼15 cm away from the emulsion. The strength of the magnetic field at the position of the LC emulsion was calculated by the magnet calculator on the website of K&J Magnetics.[42] The rotation of the magnetic patchy LC droplets was driven by an in-plane rotation of the magnetic disc.
Characterization of patchy microparticles by bright field (BF), polarized light (PL), and fluorescence (Fluo) microscopy
To characterize the LC patchy microparticles, a 50 μL volume of emulsion was first dispensed onto a glass coverslip. Next, the LC droplets were imaged using an Olympus IX71 inverted epifluorescence microscope (Center Valley, PA) equipped with a 100x oil-immersion objective, crossed polarizers, a mercury lamp, and a Chroma filter (457 nm ≤ λexc ≤ 502 nm, and 510 nm ≤ λem ≤ 562 nm). BF, PL, and Fluo micrographs of the droplets were collected with a Hamamatsu 1394 ORCAER CCD camera (Bridgewater, NJ) connected to a computer and controlled through SimplePCI imaging software (Compix, Inc., Cranberry Twp., NJ). BF micrographs were collected by removing the polarizer from the optical path of the microscope.
Supplementary Material
Acknowledgments
This work was primarily supported by NSF through DMR-1121288 (Materials Research Science and Engineering Center), the Army Research Office (W911NF-11-1-0251 and W911NF-10-1-0181), and the National Institutes of Health (CA108467, CA105730, AI092004.). Acknowledgment of support is also made to the Department of Energy, Basic Energy Sciences, Biomaterials Program (BESC0004025). The authors thank Professor Daniel J. Klingenberg, Professor Michael D. Graham, Dr. Jonathan K. Whitmer, Tyler F. Roberts, Dr. Rebecca J. Carlton, and Peter C. Mushenheim for helpful discussions.
Footnotes
Supporting Information: Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Xiaoguang Wang, Department of Chemical and Biological Engineering, University of Wisconsin-Madison, 1415 Engineering Drive, Madison, Wisconsin 53706, United States.
Daniel S. Miller, Department of Chemical and Biological Engineering, University of Wisconsin-Madison, 1415 Engineering Drive, Madison, Wisconsin 53706, United States
Prof. Juan J. de Pablo, Institute for Molecular Engineering, University of Chicago, 5801 South Ellis Avenue Chicago, Illinois 60637, United States
Prof. Nicholas L. Abbott, Email: abbott@engr.wisc.edu, Department of Chemical and Biological Engineering, University of Wisconsin-Madison, 1415 Engineering Drive, Madison, Wisconsin 53706, United States.
References
- 1.Pawar AB, Kretzschmar I. Macromol Rapid Commun. 2010;31:150. doi: 10.1002/marc.201090000. [DOI] [PubMed] [Google Scholar]
- 2.Walther A, Müller AH. Chem Rev. 2013;113:5194. doi: 10.1021/cr300089t. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida M, Lahann J. ACS Nano. 2008;2:1101. doi: 10.1021/nn800332g. [DOI] [PubMed] [Google Scholar]
- 4.Crowley JM, Sheridon NK, Romano L. J Electrost. 2002;55:247. [Google Scholar]; Gangwal S, Cayre OJ, Velev OD. 2008;24:13312. doi: 10.1021/la8015222. [DOI] [PubMed] [Google Scholar]; Takei H, Shimizu N. Langmuir. 1997;13:1865. [Google Scholar]
- 5.Dyab AK, Ozmen M, Ersoz M, Paunov VN. J Mater Chem. 2009;19:3475. [Google Scholar]; Erb RM, Jenness NJ, Clark RL, Yellen BB. Adv Mater. 2009;21:4825. doi: 10.1002/adma.200900892. [DOI] [PubMed] [Google Scholar]; Smoukov SK, Gangwal S, Marquez M, Velev OD. Soft Matter. 2009;5:1285. [Google Scholar]
- 6.Nisisako T, Torii T, Takahashi T, Takizawa Y. Adv Mater. 2006;18:1152. [Google Scholar]
- 7.Sheridon N, Richley E, Mikkelsen J, Tsuda D, Crowley J, Oraha K, Howard M, Rodkin M, Swidler R, Sprague R. J Soc Inf Disp. 1999;7:141. [Google Scholar]
- 8.Howse JR, Jones RA, Ryan AJ, Gough T, Vafabakhsh R, Golestanian R. Phys Rev Lett. 2007;99:048102. doi: 10.1103/PhysRevLett.99.048102. [DOI] [PubMed] [Google Scholar]
- 9.Xu C, Xie J, Ho D, Wang C, Kohler N, Walsh EG, Morgan JR, Chin YE, Sun S. Angew Chem, Int Ed. 2008;47:173. doi: 10.1002/anie.200704392. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yoshida M, Roh KH, Mandal S, Bhaskar S, Lim D, Nandivada H, Deng X, Lahann J. Adv Mater. 2009;21:4920. doi: 10.1002/adma.200901971. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekhar S. Liquid Crystals. Cambridge University Press; New York, NY, USA: 1992. [Google Scholar]; Collings PJ. Liquid Crystals : Nature's Delicate Phase of Matter. Princeton University Press; Princeton, NJ, USA: 2002. [Google Scholar]; Collings PJ, Hird M. Introduction to Liquid Crystals Chemistry and Physics. Taylor & Francis; Bristol, PA, USA: 1997. [Google Scholar]; Gennes PGd, Prost J. The Physics of Liquid Crystals. Oxford University Press; New York, NY, USA: 1993. [Google Scholar]
- 11.Bai Y, Abbott NL. Langmuir. 2011;27:5719. doi: 10.1021/la103301d. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lowe AM, Abbott NL. Chem Mater. 2012;24:746. doi: 10.1021/cm202632m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlton RJ, Hunter JT, Miller DS, Abbasi R, Mushenheim PC, Tan L, Abbott NL. Liq Cryst Rev. 2013;1:1. doi: 10.1080/21680396.2013.769310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lockwood NA, Gupta JK, Abbott NL. Surf Sci Rep. 2008;63:255. [Google Scholar]
- 14.Miller DS, Wang X, Abbott NL. Chem Mater. 2013;26:496. doi: 10.1021/cm4025028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crawford GP, Žumer S. Liquid Crystals in Complex Geometries: Formed by Polymer and Porous Networks. Taylor & Francis Inc.; Bristol, PA: 1996. [Google Scholar]
- 16.Drzaic PS. Liquid Crystal Dispersions. World Scientific; River Edge, NJ, USA: 1995. [Google Scholar]
- 17.Lopez-Leon T, Fernandez-Nieves A. Colloid Polym Sci. 2011;289:345. [Google Scholar]
- 18.Gupta JK, Sivakumar S, Caruso F, Abbott NL. Angew Chem, Int Ed. 2009;48:1652. doi: 10.1002/anie.200804500. [DOI] [PubMed] [Google Scholar]
- 19.Alexander GP, BGg Chen, Matsumoto EA, Kamien RD. Rev Mod Phys. 2012;84:497. [Google Scholar]; Lavrentovich OD. Liq Cryst. 1998;24:117. [Google Scholar]
- 20.Kléman M, Lavrentovich OD. Soft Matter Physics : An Introduction. Springer; New York, NY, USA: 2003. [Google Scholar]
- 21.Volovik GE, Lavrentovich OD. Zh Eksp Teor Fiz. 1983;85:1997. [Google Scholar]
- 22.Gupta JK, Zimmerman JS, de Pablo JJ, Caruso F, Abbott NL. Langmuir. 2009;25:9016. doi: 10.1021/la900786b. [DOI] [PubMed] [Google Scholar]
- 23.Prischepa OO, Shabanov AV, Zyryanov VY. JETP Lett. 2004;79:257. [Google Scholar]
- 24.Mondiot F, Wang X, de Pablo JJ, Abbott NL. J Am Chem Soc. 2013;135:9972. doi: 10.1021/ja4022182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitmer JK, Wang X, Mondiot F, Miller DS, Abbott NL, d Pablo JJ. Phys Rev Lett. 2013;111:227801. doi: 10.1103/PhysRevLett.111.227801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berggren E, Zannoni C, Chiccoli C, Pasini P, Semeria F. Phys Rev E. 1994;50:2929. doi: 10.1103/physreve.50.2929. [DOI] [PubMed] [Google Scholar]; Meyer RB. Phys Rev Lett. 1969;22:918. [Google Scholar]; Ondris-Crawford R, Boyko EP, Wagner BG, Erdmann JH, Zumer S, Doane JW. J Appl Phys. 1991;69:6380. [Google Scholar]
- 27.Umbanhowar P, Prasad V, Weitz D. Langmuir. 2000;16:347. [Google Scholar]
- 28.Alino VJ, Tay KX, Khan SA, Yang KL. Langmuir. 2012;28:14540. doi: 10.1021/la3028463. [DOI] [PubMed] [Google Scholar]
- 29.Sivakumar S, Gupta JK, Abbott NL, Caruso F. Chem Mater. 2008;20:2063. [Google Scholar]
- 30.Peddireddy K, Kumar P, Thutupalli S, Herminghaus S, Bahr C. Langmuir. 2012;28:12426. doi: 10.1021/la3015817. [DOI] [PubMed] [Google Scholar]
- 31.Prishchepa OO, Zyryanov VY, Gardymova AP, Shabanov VF. Mol Cryst Liq Cryst. 2008;489:84. [Google Scholar]
- 32.Brake JM, Abbott NL. Langmuir. 2002;18:6101. [Google Scholar]
- 33.Lin IH, Miller DS, Bertics PJ, Murphy CJ, de Pablo JJ, Abbott NL. Science. 2011;332:1297. doi: 10.1126/science.1195639. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tomar V, Hernandez SI, Abbott NL, Hernandez-Ortiz JP, de Pablo JJ. Soft Matter. 2012;8:8679. [Google Scholar]
- 34.Abras D, Pranami G, Abbott NL. Soft Matter. 2012;8:2026. [Google Scholar]
- 35.Bresme F, Oettel M. J Phys: Condens Matter. 2007;19:413101. doi: 10.1088/0953-8984/19/41/413101. [DOI] [PubMed] [Google Scholar]; Stark H. Phys Rep. 2001;351:387. [Google Scholar]
- 36.Joshi AA, Whitmer JK, Guzmán O, Abbott NL, de Pablo JJ. Soft Matter. 2014;10:882. doi: 10.1039/c3sm51919h. [DOI] [PubMed] [Google Scholar]
- 37.Hiemenz PC, Rajagopalan R. Principles of Colloid and Surface Chemistry. Marcel Dekker; New York, NY, USA: 1997. [Google Scholar]; Israelachvili JN. Intermolecular and Surface Forces. Academic Press London; San Diego, CA, USA: 1991. [Google Scholar]
- 38.Carlton RJ, Gupta JK, Swift CL, Abbott NL. Langmuir. 2012;28:31. doi: 10.1021/la203729t. [DOI] [PMC free article] [PubMed] [Google Scholar]; Miller DS, Abbott NL. Soft Matter. 2013;9:374. doi: 10.1039/C2SM26811F. [DOI] [PMC free article] [PubMed] [Google Scholar]; Carlton RJ, Ma CD, Gupta JK, Abbott NL. Langmuir. 2012;28:12796. doi: 10.1021/la3024293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gharbi MA, Nobili M, Blanc C. J Colloid Interface Sci. 2014;417:250. doi: 10.1016/j.jcis.2013.11.051. [DOI] [PubMed] [Google Scholar]
- 40.Candau S, Le Roy P, Debeauvais F. Mol Cryst Liq Cryst. 1973;23:283. [Google Scholar]
- 41.Bird RB, Stewart WE, Lightfoot EN. Transport Phenomena. John Wiley & Sons; New York, NY, USA: 2007. [Google Scholar]; Miller DS, Wang X, Buchen J, Lavrentovich OD, Abbott NL. Anal Chem. 2013;85:10296. doi: 10.1021/ac4021193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.I. K&J Magnetics, Magnetic Field Calculator. [accessed: January, 2014]; http://www.kjmagnetics.com/calculator.asp.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


