Abstract
Primary antiphospholipid antibody syndrome (APS) is a rare clinical event in the People’s Republic of China. As APS is easily neglected or misdiagnosed, a delayed treatment can result. The patient reported here was a 32-year-old female who died by systemic venous thrombosis on day 11 after a cesarean section delivery. Luckily, the baby survived. A blood test demonstrated that the patient’s platelets were decreased at 19 weeks of gestation. Anti-cardolipin antibody and antiβ2GP1 (anti-β2-glycoprotein-I antibody) were positive at 36 weeks and 2 days of gestation. This patient was diagnosed with APS. Unfortunately, as physicians, we could not provide proper treatment as the patient’s relatives were concerned that the proposed treatment would have negative effects on the infant’s health. This clinical case strongly suggests that physicians need to appreciate that APS is a very serious condition, especially for pregnant women, and that proper treatment should be provided as early as possible to avoid a bad outcome, despite the fact that a cure for this disease is not currently available.
Keywords: APS, thrombosis, Hughes syndrome
Introduction
Antiphospholipid syndrome (APS) or Hughes syndrome is a special autoimmune and hypercoagulable clinical situation resulting from antiphospholipid antibodies.1–5 Primary APS studies indicate that patients with APS do not have clinical evidence of any other related autoimmune disease. Antiphospholipid antibodies are a set of antibodies which can react with a variety of antigens, including lupus anticoagulation antibodies, anticardiolipin antibodies, and anti-acid phospholipid antibodies.1–5 Antiphospholipid antibodies were discovered by bacteriologist August Paul von Wassermann in 1906.6 Phospholipids are a kind of fat existing in all living cells and cell membranes, like blood cells and endothelial cells. The antiphospholipid antibodies mainly cause arteriovenous thrombosis and thrombocytopenia, resulting in a series of clinical symptoms, like stroke, heart attack, kidney damage, deep vein thrombosis, pulmonary embolism, and thrombocytopenia.7 In pregnant women, APS can cause miscarriage, premature birth, and stillbirth.8 Researchers still do not know why some people can produce APS antibodies and why APS antibodies cause blood clots. Currently, there are no effective medicines that can block this autoimmune response clinically, although many novel targeted treatments are developing.3 Physicians can only prevent clots. Herein, we report a special case of an APS-related pregnancy and mistreatment resulting in death.
Case report
A 32-year-old woman at 36 weeks and 2 days of pregnancy was admitted to the hospital on November 20, 2012. The patient had suffered from thrombocytopenia for 4 months and thrombosis of the lower extremities for 1 month. The patient did not have any history of cardiac disease, nephronia, urophthisis, hepatitis, or tuberculosis.
This patient was in her third pregnancy. The first pregnancy was a miscarriage at 18 weeks of gestation, 3 years previous. The second pregnancy resulted in fetal death at 24 weeks gestation, 2 years previous. Thrombocytopenia was found during both first and second pregnancies, platelet counts were ∼90×109/L (standard range [SR], 100×109–300×109/L). Anticardiolipin antibody was positive (>10 RU/mL [SR, <10 RU/mL]) in her second pregnancy.
The patient was diagnosed with intrauterine gestation by color Doppler ultrasound at First Hospital, Bethune Faculty of Medical Sciences of Jilin University after 40 days menelipsis, in her third pregnancy. At 16 weeks of gestation, the titer of the ABO blood group antibody was 1:256. At 19 weeks of gestation, the platelet count was 88×109/L (SR, 100×109–300×109/L). At 29 weeks and 6 days of gestation, the patient complained that she felt a numb pain that became severe after movement, on her left leg. Examination of color Doppler ultrasound discovered intraluminal visible heterogeneous hypoechoic and hyperechoic dots, no clear boundary between diseased area and endothelium of blood vessel, no flow signal in the lumen of blood vessel, and an increase of vein diameter in superficial femoral vein, proximal part of deep femoral vein, popliteal vein, anterior tibial vein, posterior tibial vein, peroneal vein, and muscle vein. These indicated that thrombosis occurred in her deep vein of the left leg. Her physicians suggested that she needed thrombolytic and anticoagulant treatments. The patient and her relatives, however, refused treatments because they were concerned about possible side effects on the baby.
At 34 weeks and 2 days of gestation, the patient’s platelet count was 52×109/L (SR, 100×109–300×109/L). Three days after, the blood pressure was 140/90 mmHg. At 36 weeks and 2 days of gestation, she was admitted to hospital. The fetal heart rate was 150 beats/minute. Fetal non-stress test was normal. The titer of ABO blood group antibody was 1:512. The platelet count was 53×109/L (SR, 100×109–300×109/L). The urinary protein was negative. The anticardiolipin antibody (IgG) was positive (52 RU/mL [SR, <10 RU/mL]). The anti-β2-glycoprotein-I antibody (IgG) was also positive (150 RU/mL [SR, 0–20 RU/mL]). The lupus anticoagulant was negative.
After reviewing the patient’s previous pregnancy history and records, we noted that the patient had two abortions, one missed abortion at 18 weeks, and a fetal death at 24 weeks. The fetal death at 24 weeks meets the clinical criteria of a single fetal death at greater than 10 weeks.2,3,8 At 16 weeks of her third pregnancy, she had venous thrombosis with lower platelet count, which meets another clinical criteria for APS.2,3,8 Clinical examinations demonstrated that her anticardiolipin antibody (52 RU/mL) and anti-β2-glycoprotein-I antibody (150 RU/mL) were positive, which meets laboratory criteria for APS.2,3,8 Therefore, at 34 weeks of the third pregnancy, the patient was diagnosed as APS.
Unfortunately, the patient and her relatives still refused thrombolytic and anticoagulant treatments. Her physicians considered her baby and her situation and decided to stop her gestation.
At 36 weeks and 3 days of gestation, the patient delivered a healthy infant (an Apgar score of 10 at 1 minute, an Apgar score of 10 at 10 minutes, bodyweight 2,440 g, height 47 cm) by cesarean section. After delivery, the patient received 4,000 IU (equal to 40 mg) of low molecular weight heparin, subcutaneously, and was administered fluids. Three days after, the patient felt abdominal pain. Examinations revealed a platelet count at 80×109/L (SR, 100×109–300×1/L) and activated partial thromboplastin time (APTT) at 83.8 seconds (SR, 20–40 seconds). An abdominal computed tomography (CT) scan showed less clear boundary, multiple lamellar irregular low density areas at sizes of 0.7–2.4 cm, edge enhancement during arterial phase (A) and venous phase (V), and low density images during portal venous phase (P) with normal bile duct and gall bladder in the liver (Figure 1). These indicated that the patient had deep vein thrombosis in the liver and this caused infarcts in the liver. To improve the liver situation, the patient took 1.8 g of reduced glutathione once only and 5,000 IU of low molecular weight heparin, once a day.
Figure 1.
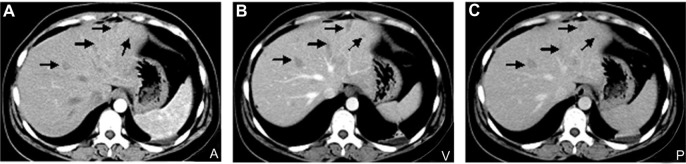
(A,B,C) Images of computed tomography scan at 36 weeks and 3 days of gestation.
Notes: Arrows show that there are multiple plaque-shaped hypodense lesions in the liver at three different phases (A, V, and P).
Abbreviations: A, arterial phase; P, portal venous phase; V, venous phase.
On the next day, examinations uncovered a platelet count at 81×109/L (SR, 100×109–300×1/L), APTT at 79.1 seconds (SR, 23–40 seconds), fibrinogen degradation product at 11.2 μg/mL (SR, 0–5 μg/mL), fibrinogen at 5.29 g/L (SR, 2–4 g/L), aspartate aminotransferase at 151 U/L (SR, 5–40 U/L), and alanine aminotransferase of 173 U/L (SR, 5–40 U/L). On day 5 post-delivery, the patient started to have blurred awareness, right limb shaking, an inability to speak, and to twitch and bite her own tongue. Then, the patient gradually recovered her awareness, which appeared to be normal after 30 minutes. Examinations showed her blood pressure at 160/105 mmHg, heart rate at 110 beats/minute, saturation of blood oxygen at 96%, aspartate aminotransferase at 123 U/L (SR, 5–40 U/L), alanine aminotransferase at 215 U/L (SR, 5–40 U/L), white blood cell count at 7.30×109/L (SR, 4×109–10×109/L) and absolute neutrophil count 6.06×109/L (SR, 0.5×109–0.7×109/L). A CT scan of the head did not demonstrate any bleeding area or thrombotic lesion. A magnetic resonance image of the head discovered an intracerebral multiple abnormal signal, a short T1 signal in the longitudinal sinus, a diameter of the right pupil at 4.0 mm and the left at 3.5 mm. These results suggested that the patient may have had venous sinus thrombosis. The patient had reflection of light, positive bilateral pathologic reflex, and autonomic activities of limps. The patient continued to have anticoagulant treatment (5,000 IU of low molecular weight heparin, twice a day, hypodermic injection, and 1.8 g of reduced glutathione once). At the same time, the patient received dehydration treatment (50 g of mannitol, every 12 hours) to lower intracranial pressure and cefepime hydrochloride to prevent infection.
On day 6 post-delivery, the patient was suddenly unconscious and exhibited paroxysmal-decerebrate-rigidity at 4 am. The patient’s breath became short. The heart rate increased to 130 beats/minute. A CT scan of the head showed low density areas at bilateral lobus occipitalis and lobus temporalis. These results indicated that right cerebral had infarction and hemorrhage, and hemorrhage had broken into ventricles. At 9 am, her heart rate was 152 beats/minute with blood pressure at 114/55 mmHg. Laboratory tests showed APTT at 81.8 seconds (SR, 23–40 seconds), prothrombin activity at 63% (SR, 80%–120%), fibrinogen at 4.67 g/L (SR, 2–4 g/L), and platelet count at 56×109/L (SR, 100×109–300×109/L). Diameter of her pupil was 4.5 mm in the right eye and 2.5 mm in the left eye. Direct and indirect light reflex disappeared on days 6 and 11. On day 11 post-delivery, the patient died, even with all possible rescue treatments.
Discussion
Herein, we report a rare primary APS case, the patient miscarried in her first pregnancy and suffered a fetal death in her second pregnancy with thrombocytopenia and positive of anticardiolipin. Therefore, this patient should have been diagnosed with possible APS in her second pregnancy although she did not have thrombosis or other specific clinical symptoms. Her physician should have been alert and considered follow-up and given her preventive treatment. At 29 weeks in her third pregnancy, we, her physicians, recognized that the thrombosis occurred and prescribed thrombolytic and anticoagulant treatments, but we did not screen all antiphospholipid antibodies and tried to give her an accurate APS diagnosis. At 36 weeks in her third pregnancy, she was finally diagnosed as APS with positive results for both anticardiolipin and anti-β2-glycoprotein-I antibodies plus lower platelet count and thrombosis. With the late diagnosis and the patient’s and her family’s refusal of treatment, the patient died after delivering a healthy baby.
In this case, we, her physicians, missed early opportunities to give her correct diagnosis and preventive treatment. The first opportunity was her second pregnancy. At the very least, we should have been alerted about a possible APS diagnosis for this patient and considered whether we needed to give her preventive treatment or follow-up care, although we did not have all the required criteria for a diagnosis of APS. A previous report suggests that a woman with recurrent pregnancy failure plus positive antiphospholipid antibody needs preventive anticoagulant treatment because the incidence of pregnancy loss can reach 90% in this special population.9 The second opportunity was at 29 weeks in her third pregnancy. We needed to do further detailed clinical tests including the testing for antiphospholipid antibodies in order to find real reasons for the patient’s problems. In her third pregnancy, we had a relatively early chance to treat her problem. We, however, did not insist on anticoagulant treatment because the patient and her relatives refused.
For this patient, we might have given insufficient treatment for the patient’s thrombosis situation after the delivery of the baby because she had already received delayed treatment. We only gave her 4,000 IU of low molecular weight heparin once, after the delivery of the baby. We should have given her a heparin, warfarin, and low dose of aspirin (81 mg/day) combination to save her life. One other factor, which could have affected whether the patient survived, is the time for the termination of pregnancy, although there is no unified standard. In general, the gestation does not need to be more than 37 weeks.10 Depending on our patient’s situation, we should stop her gestation at 34 weeks and 2 days instead of at 36 weeks and 3 days gestation in order to save both baby and mother. We waited too long.
Unfortunately, our patient died on day 11 after the delivery of the baby. Cerebral hemorrhage and infarcts in the liver were the major reasons to cause her death at the final stage. However, APS thrombosis should be the major inducer. APS can cause a hyper-coagulable state, like deep vein thrombosis in her left leg at 19 weeks in her last pregnancy, and deep vein thrombosis was in the liver 3 days after the patient’s delivery, and the hypo-coagulable state afterwards, like cerebral hemorrhage. APS can also cause vascular endothelial cell damage and vasculitides to increase vasopermeability and reduce platelet function to promote hemorrhage.11 In this case, the patient’s infarcts in the liver could have further damaged her coagulation–anticoagulation function, resulting in further promotion of hemorrhage. To prevent infection, we used cefepime (2 g every 8 hours). Cefepime is a beta lactam antibiotic (a fourth-generation cephalosporin). It was reported that beta lactam antibiotic can cause liver cell necrosis.12 Cephalosporin can also inhibit the gamma carboxylation process of hepatic blood coagulation factor II, VII, IX, X to block the activity of blood coagulation factor.13,14 According to the “Criteria for use of Cefepime in Adults” published by the American Society of Hospital Pharmacists in 1994,15 this patient did not meet the criteria. Therefore, we think that cefepime used here could have further damaged the liver cells and could have also affected coagulation, leading to hemorrhage. In the future, we will not use antibiotics in general. In order to prevent infection in similar cases, we would consider using penicillin G to limit liver injury and low dose of prednisone to suppress immune activity and inflammation.
Conclusion
As clinical physicians, we need to elevate our alert threshold in patients who have pregnancy-related problems involving thrombosis or any positive antiphospholipid antibody indication. We need to try to diagnose APS as early as possible to allow for early prevention and treatment. Physicians also need to insist on proper treatments and to give good advice for the time of termination of a pregnancy to save the mother and the baby. APS can cause complex clinical problems. Physicians need to be very careful when prescribing medicine to avoid adverse outcomes.
Acknowledgments
We would like to thank Dr Kavitha Rao and Robert Wellner (NIDDK, NIH, USA) for manuscript editing assistance.
Footnotes
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Mialdea M, Sangle SR, D’Cruz DP. Antiphospholipid (Hughes) syndrome: beyond pregnancy morbidity and thrombosis. J Autoimmune Dis. 2009;6:3–7. doi: 10.1186/1740-2557-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi S, McCrae KR. Recent advances in the antiphospholipid antibody syndrome. Curr Opin Hematol. 2014;21(5):371–379. doi: 10.1097/MOH.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 4.Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346(10):752–763. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- 5.Hughes GR, Harris NN, Gharavi AE. The antiphospholipid syndrome. J Rheumatol. 1986;13(3):486–489. [PubMed] [Google Scholar]
- 6.Wassermann A, Neisser A, Bruck C. Eine serodiagnostische Reaktion bei Syphilis. In: Deutsche medicinische Wochenschrift, Berlin. 1906;32:745–746. [Google Scholar]
- 7.Dusse LM, Silva FD, Freitas LG, et al. Antiphospholipid syndrome: a clinical and laboratorial challenge. Rev Assoc Med Bras. 2014;60(2):181–186. doi: 10.1590/1806-9282.60.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Kutteh WH. Antiphospholipid antibody syndrome and reproduction. Curr Opin Obstet Gynecol. 2014;26(4):260–265. doi: 10.1097/GCO.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 9.Younis JS, Brenner B, Ohel G, et al. Activated protein C resistance and factor V Leiden mutation can be associated with first as well as second-trimester recurrent pregnancy loss. Am J Reprod Immunol. 2000;43(1):31–35. doi: 10.1111/j.8755-8920.2000.430106.x. [DOI] [PubMed] [Google Scholar]
- 10.Coppage KH, Polzin WJ. Severe preeclampsia and delivery outcomes: is immediate cesarean delivery beneficial? Am J Obstet and Gynecol. 2002;186(5):921–923. doi: 10.1067/mob.2002.124041. [DOI] [PubMed] [Google Scholar]
- 11.Du VX, Kelchtermans H, de Groot PG, et al. From antibody to clinical phenotype, the black box of the antiphospholipid syndrome: pathogenic mechanisms of the antiphospholipid syndrome. Thromb Res. 2013;132(3):319–326. doi: 10.1016/j.thromres.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Westphal JF, Vetter D, Brogard JM. Hepatic side-effects of antibiotics. J Antimicrob Chemother. 1994;33(3):387–401. doi: 10.1093/jac/33.3.387. [DOI] [PubMed] [Google Scholar]
- 13.Shearer MJ, Bechtold H, Andrassy K, et al. Mechanism of cephalosporin-induced hypoprothrombinemia: relation to cephalosporin side chain, vitamin k metabolism, and vitamin K status. J Clin Pharmacol. 1988;28(1):88–95. doi: 10.1002/j.1552-4604.1988.tb03106.x. [DOI] [PubMed] [Google Scholar]
- 14.Shevchuk YM, Conly JM. Antibiotic-associated hypoprothrombinemia: a review of prospective, 1966–1988. Rev Infect Dis. 1990;12(6):1109–1126. [PubMed] [Google Scholar]
- 15.Woods M. Criteria for use of cefepime in adults. American Society of Hospital Pharmacists. Am J Health Syst Pharm. 1994;51(4):531–532. [PubMed] [Google Scholar]


