Abstract
The aims were to compare the efficacy and tolerability of a new benzene-poly-carboxylic acids complex with cis-diammineplatinum (II) dichloride (BP-C1) versus placebo and to investigate the long-term tolerability of BP-C1 in the treatment of patients with metastatic breast cancer.
Material and methods
A randomized, double-blind, placebo-controlled multicenter study was performed with a semi-crossover design. Patients allocated to placebo switched to BP-C1 after 32 days of treatment. Patients who completed 32 days of BP-C1 treatment were offered the opportunity to continue on BP-C1 for an additional 32 days in an open-label extension. Patients were then followed up for another 28 days. Thirty patients were given daily intramuscular injections of 0.035 mg/kg of body weight BP-C1 or placebo for 32 days. Biochemistry, hematology, National Cancer Institute Common Terminology Criteria for Adverse Events (CTC-NCI), European Organisation for Research and Treatment of Cancer quality of life questionnaire (QOL-C30 and the breast-cancer–specific BR23) data were recorded at screening and after every 16 days of treatment. Computed tomography was performed at screening and every 32 days.
Results
The sum of target lesions increased 2.4% in the BP-C1 group and 14.3% in the placebo group. Only the increase in the placebo group was significant (P=0.013). The difference between the groups was significant in favor of BP-C1 (P=0.04). There was a significant difference (P=0.026) in favor of BP-C1 regarding Response Evaluation Criteria In Solid Tumors (RECIST) classification. The sum of lesions increased slightly in the patients receiving 64 days of continuous BP-C1 treatment, of whom 68.4% were classified as responders. The sum CTC-NCI toxicity score increased nonsignificantly in the BP-C1 group but significantly in the placebo group (P=0.05). The difference in increase between groups did not meet the level of significance (P=0.12). The sum toxicity score was reduced in the patients receiving 64 days of BP-C1 from 9.2 at screening to 8.9 at Day 48, but it increased again to 10.1 by Day 64 and 10.6 during the 28-day follow-up. “Breast cancer-related pain and discomfort” and “Breast cancer treatment problem last week” were significantly reduced (P=0.02) in the BP-C1 group but increased slightly in the placebo group; between-group differences were significant in favor of BP-C1 (P=0.05). “Breast cancer related pain and discomfort”, “Breast cancer treatment problem last week,” and “Physical activity problem” were significantly reduced during the 64 days of BP-C1 treatment (P≤0.05).
Conclusion
For patients suffering from stage IV metastatic breast cancer, treatment with BP-C1 reduces cancer growth, is well tolerated, improves quality of life, and produces few adverse events, which were mainly mild and manageable.
Keywords: tumor growth reduction, improved Quality of Life, safe, few transient adverse effects
Introduction
Breast cancer is a nonhomogeneous disease with variations in its biological profile and prognosis. Prognostic factors for the individual patient should be based on various biological markers, such as described by Goldhirsch et al.1 Other important prognostic factors are tumor size, age of the patient, histological grade, and lymph node involvement or blood-borne metastases. Stage IV (approximately 30% of all cases),2–5 or advanced, metastatic breast cancer (MBC) is a condition in which the cancer has metastasized to one or more other organs of the body. Stage IV MBC is considered incurable, but many treatments are available. Thus, chemotherapy is used in patients with triple-negative breast cancer, in patients no longer responding to hormone therapy, and to patients suffering from life-threatening metastases, either alone6–10 or in various combinations.11,12
A new agent containing benzene-poly-carboxylic acids complex with cis-diammineplatinum (II) dichloride, BP-C1, has recently been introduced as a cost-beneficial treatment with increased efficacy and low toxicity for patients with stage IV breast cancer. As such, BP-C1 is also suitable for treatment of MBC in the third world.13 Preliminary data from a previous study showed that BP-C1 has a huge ability to stop tumor growth in these patients without causing any serious toxicity or increase in toxicity14 other than mainly mild transient side effects that can easily be managed. The aims of this study were to compare the efficacy and tolerability of BP-C1 versus placebo during 32 days of continuous treatment in patients suffering from MBC and to estimate the long-term tolerability and efficacy of BP-C1 in the treatment of MBC.
Material and methods
The study population consisted of female patients suffering from histologically verified breast cancer classified as stage IV with measurable metastasis (ie, MBC), who were between 18–80 years of age, who had previously undergone at least third-line chemotherapy, and had an expected survival time of at least 3 months. Patients were excluded if they had bilirubin >34 μmol/L or alanine aminotransferase >3 times the upper limit of normal, serum creatinine >120 μmol/L, hemoglobin <6.0 mmol/L, platelet count <100,000/mm3 or leukocytes <3×109/L, or had an abnormal coagulation capacity. Additionally, patients with verified brain metastasis, synchronous cancer, clinically significant abnormal electrocardiograph (ECG) results, or a Karnofsky Performance Status Scale (KPSS) score <60% were not included; patients under systemic treatment with corticosteroids or other immunosuppressive drugs the last 21 days and patients with uncontrolled bacterial, viral, fungal, or parasite infection were also excluded.
Design and randomization
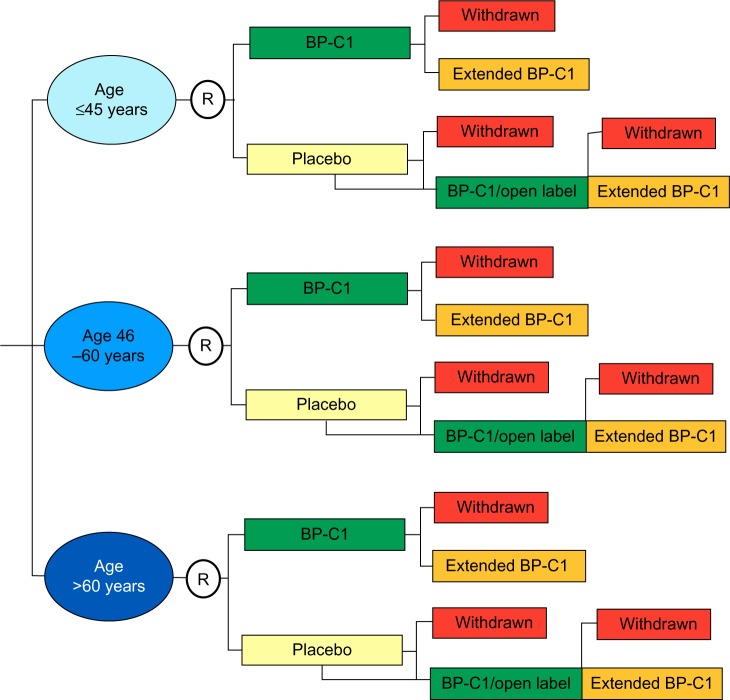
The first part of the study was performed as a randomized, double-blind, placebo-controlled multicenter trial with a stratified semi-crossover design (Figure 1).14 Age group and center were used as stratification factors. The patients within each stratum were allocated 1:1 to BP-C1 or placebo for 32 days by block randomization, with a random block size between four and eight.15 The randomization code was broken after 32 days and the patients allocated to placebo were crossed over to BP-C1 treatment for an additional 32 days. In case of clinically detected disease progression during the first 32 days, the patient could be directly crossed to BP-C1.
Figure 1.
Study design.
Notes: The blue ellipsoids illustrate the strata and open circles indicate randomization. The colored rectangles illustrate the treatment procedure.
Abbreviations: BP-C1, benzene-poly-carboxylic acids complex with cis-diammineplatinum (II) dichloride; R, randomization.
The second part of the study was an open-label multicenter trial with one treatment group. The patients who completed 32 days of BP-C1 treatment with maximum moderate increase in toxicity and clinical benefit from the treatment were offered participation in the second part of the study. All the patients were given the same BP-C1 dose as in the previous treatment sequence, for an additional 32 days.
The main variables were percent change in the total size of target lesions measured by computed tomography (CT) using the Response Evaluation Criteria In Solid Tumors (RECIST) v1.1 criteria, and tolerability measured and classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events Toxicity Criteria (CTC-NCI) v2.0. Quality of life (QOL) was measured using the European Organisation for Research and Treatment of Cancer (EORTC) quality of life questionnaire (QLQ-C30) and the EORTC quality of life breast-cancer–specific questionnaire (QLQ-BR23). The QOL variables were developed from the QOL questionnaires as recommended. The sum of scores within each of the three parts in the QLQ-C30 and QLQ-BR23 questionnaires resulted in the six variables “Physical activity problems,” “Discomfort last week,” “Health and life quality,” “Problems related to the breast cancer treatment last week,” “Sexual interest and activity in the last 4 weeks,” and “Breast cancer-related pain and discomfort last week.” Additionally, KPSS was recorded.
Study procedures
Patients who met the inclusion criteria and who gave their written consent to participate entered into a screening phase of up to 21 days. During this period, the patients were clinically investigated to ensure they met the inclusion criteria, and laboratory screening was performed in order to ensure that patients were not disqualified by the exclusion criteria. During the screening phase, CTs of the chest and abdomen were performed and, if bone or brain metastases were suspected, magnetic resonance images were taken. Additionally CTC-NCI, KPSS, QLQ-C30, and QLQ-BR23 ratings were recorded.
The trial treatment started at the end of the screening period, denoted as Day 1. Each patient was given an identification number, which hid the treatment randomization code. The trial injections started at Day 1 and the patients received one daily intramuscular injection for 32 days. The cumulative BP-C1 dose was 1.12 mg/kg of body weight (BW). This represents a daily dose of 0.035 mg/kg BW or 0.07 mL/kg BW. Clinical and laboratory examination took place after 16 and 32 days of treatment, denoted as Day 16 (16±2 days) and Day 32 (33±1 days), respectively. Blood sampling for laboratory examination was conducted and CTC-NCI, KPSS, adverse events (AEs), and QOL questionnaires were collected and recorded. CTs of the chest and abdomen were performed at Day 32. If clinical signs of disease progression were seen during the treatment period, an additional CT was performed. Patients were classified as complete responders, partial responders, stable disease (SD) or progressive disease (PD) in accordance with the RECIST procedure.
At the end of the 32-day treatment period, the randomization code was broken and patients originally allocated to the placebo group were switched to an additional 32 days of BP-C1 treatment and followed up in the same way as prescribed for the first treatment period. The patients in the original BP-C1 group who showed clinical benefit from the treatment and whose CTC-NCI score increased no more than moderately were offered an opportunity to continue for an additional 32 days of BP-C1 treatment. All of the included patients were followed up 28 days after the final injection. New CTs of the chest and abdomen were taken and blood samples for laboratory examination were drawn; CTC-NCI, KPS, AEs, and QOL questionnaires were collected and recorded.
Statistical analysis
All assumed continuously distributed variables are expressed as mean (standard deviation) and 95% confidence intervals (CIs) constructed in accordance with the Student procedure.16 Discrete and categorical variables are expressed in contingency tables.17 Changes in discrete variables are given in cross-tables. In case of missing observations, the Last Observation Carried Forward procedure was used.18–21
Based on the assumption that BP-C1 is at least as efficient as placebo, all comparisons between groups are one-tailed. Differences were considered significant at P<0.05. Comparison of the treatment groups with regard to the assumed continuously distributed variables was performed by analysis of covariance with the stratification factors and the initial observation as covariates.19 Contingency table analysis was used for comparison of the groups with regard to discrete and categorical variables.
Results
The study sample for the first part of the study consisted of 30 female MBC patients with a mean age of 56.5 years (range: 34.2–73.3 years), a duration of disease of 6.3 years (range: 1.1–19.0 years), and a body mass index (BMI) of 28.4 kg/m2 (range, 20.1–38.3 kg/m2) (Table 1). All patients had previously undergone at least third-line chemotherapy and several other available types of cancer treatment. Upon randomization, 15 patients were allocated to BP-C1 and 15 to placebo for 32 days. In the BP-C1 group, ten patients had a general condition classified as “good” and five as “fair”. Ten patients had an abnormal ECG and four had an enlarged liver. In the placebo group, the general condition was classified as “good” in eleven patients and “fair” in five. An abnormal ECG was discovered in ten patients and five had an enlarged liver. The two groups were clinically comparable with regard to all the initially recorded baseline characteristics, previous cancer treatments, and clinical findings (Table 1).
Table 1.
Baseline characteristics and previous cancer treatments
| Factor specification | Controlled clinical study
|
Expanded BP-C1 treatment duration
|
|
|---|---|---|---|
| BP-C1 (n=15) |
Placebo (n=15) |
BP-C1 (n=19) |
|
| Demographic factors and vital signs | |||
| Age (years) | 55.3 (11.0) | 57.7 (9.4) | 57.1 (10.0) |
| 34.2–68.2 | 39.5–73.3 | 35.9–71.8 | |
| Duration of disease (years) | 6.6 (5.0) | 6.0 (2.9) | 7.0 (4.2) |
| 1.1–19.0 | 1.2–10.4 | 1.2–19.0 | |
| BMI (kg/m2) | 27.8 (4.6) | 29.0 (6.2) | 29.3 (5.8) |
| 20.6–38.1 | 20.1–38.3 | 20.1–38.3 | |
| Systolic blood pressure (mmHg) | 130 (12.4) | 128 (10.0) | 129 (9.9) |
| 104–150 | 110–147 | 110–147 | |
| Diastolic blood pressure (mmHg) | 78 (9.4) | 80 (6.8) | 79 (6.4) |
| 51–90 | 70–90 | 70–90 | |
| Heart rate (beats/minute) | 83 (11.3) | 80 (7.8) | 79 (6.8) |
| 68–115 | 68–98 | 68–96 | |
| Respiratory rate (breaths/minute) | 17 (1.6) | 17 (2.0) | 17 (1.0) |
| 14–20 | 14–22 | 15–19 | |
| Previous cancer treatment | |||
| Surgery | 13 | 14 | 17 |
| Hormone therapy | 12 | 14 | 18 |
| Antibody therapy | 3 | 1 | 3 |
| Radiotherapy | 11 | 10 | 13 |
| Others | 1 | 3 | 4 |
Notes: Demographic factors and vital signs are expressed in mean (standard deviation) and total range. Previous cancer treatment values are expressed in number of patients.
Abbreviations: BMI, body mass index; BP-C1, benzene-poly-carboxylic acids complex with cis-diammineplatium (II) dichloride.
The study sample for the second part of the study included 19 female MBC patients with a mean age of 57.1 years (range: 35.9–71.8 years), a duration of disease of 7.0 years (range: 1.2–19.0 years), and a BMI of 29.3 kg/m2 (range 20.1–38.3 kg/m2). All patients had previously undergone breast cancer surgery and at least three sequences with chemotherapy and hormone therapy (Table 1). The general condition was “good” in 13 patients and “fair” in six. Abnormal ECGs were discovered in 12 patients, and six patients had an enlarged liver.
Tumor growth and RECIST
BP-C1 versus placebo
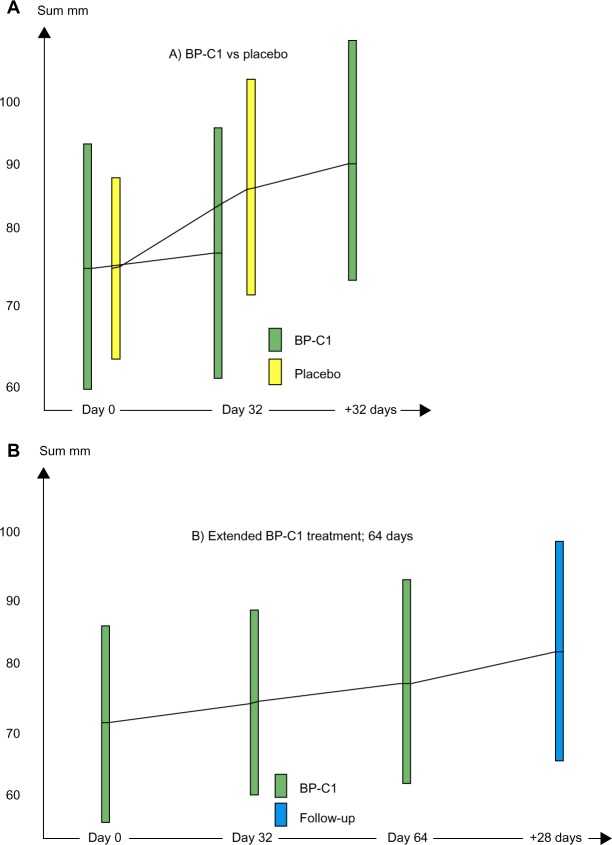
The sum of lesions increased from 76.0 (95% CI =55.3–96.7) to 77.1 (95% CI =56.5–97.7) in the BP-C1 group and from 69.7 (95% CI =54.6–84.8) to 78.3 (95% CI =91.7–94.8) in the placebo group during the first 32 days of treatment (Figure 2A). This represents an increase of 2.4% (95% CI =−3.9 to 8.8) in the BP-C1 group and 14.3% (95% CI =2.3–26.4) in the placebo group. Only the increase in the placebo group was significant (P=0.013). The difference between the groups in sum lesion increase was significant in favor of BP-C1 (P=0.04). Additionally, a significant difference (P=0.026) was detected in favor of BP-C1 regarding classification in accordance with the RECIST criteria 1.1 (Table 2).
Figure 2.
The development in sum of the largest diameters of target lesions, in millmeters.
Notes: Results are expressed by mean values with 95% confidence intervals illustrated by columns. The green bars show BP-C1, the yellow bars show placebo, and the blue bar shows results after 28 days of follow-up. (A) BP-C1 versus placebo followed by BP-C1 treatment. (B) Extended 64 days of BP-C1 treatment.
Abbreviation: BP-C1, benzene-poly-carboxylic acids complex with cis-diammineplatium (II) dichloride.
Table 2.
Comparison of the two groups regarding RECIST classification
| Treatment | PD | SD | PR | P-value | |
|---|---|---|---|---|---|
| Comparison of BP-C1 versus placebo | BP-C1 | 0 | 14 | 1 | 0.026 |
| Placebo | 4 | 11 | 0 | ||
| Placebo patients crossing to BP-C1 | 2 | 13 | 0 |
Note: Results are expressed as number of patients.
Abbreviations: BP-C1, benzene-poly-carboxylic acids complex with cis-diammineplatium (II) dichloride; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria In Solid Tumors; SD, stable disease.
The patients in the placebo group were switched to active BP-C1 treatment for an additional 32 days, and the sum of lesions increased thereafter to 85.0 (95% CI =65.5–104.5). This represents a significant increase of 11.4% (95% CI =1.6–21.2). Three PD patients under placebo treatment were reclassified as SD during the additional BP-C1 treatment.
Extended BP-C1 treatment
In the group of patients receiving 64 days of continuous BP-C1 treatment, the sum of lesions increased nonsignificantly from 72.0 (95% CI =56.6–87.4) to 79.6 (95% CI =63.2–95.9) (Figure 2B). From the end of treatment to the final examination 28 days later, the sum of lesions increased further to 82.2 (95% CI =65.4–98.9). Thirteen patients were classified as SD and six as PD.
Tolerability
BP-C1 versus placebo
The maximum CTC-NCI score increased in three patients and decreased in two patients during the 32 days of BP-C1 treatment. In the group treated with placebo, the maximum CTC-NCI score increased in one patient and decreased in one patient during the same treatment period. In the placebo-group patients who later crossed over to BP-C1 treatment for an additional 32 days, the maximum CTC-NCI score increased in two patients and decreased in two patients. One of the patients from the placebo group did not complete the additional 32 days of BP-C1 treatment.
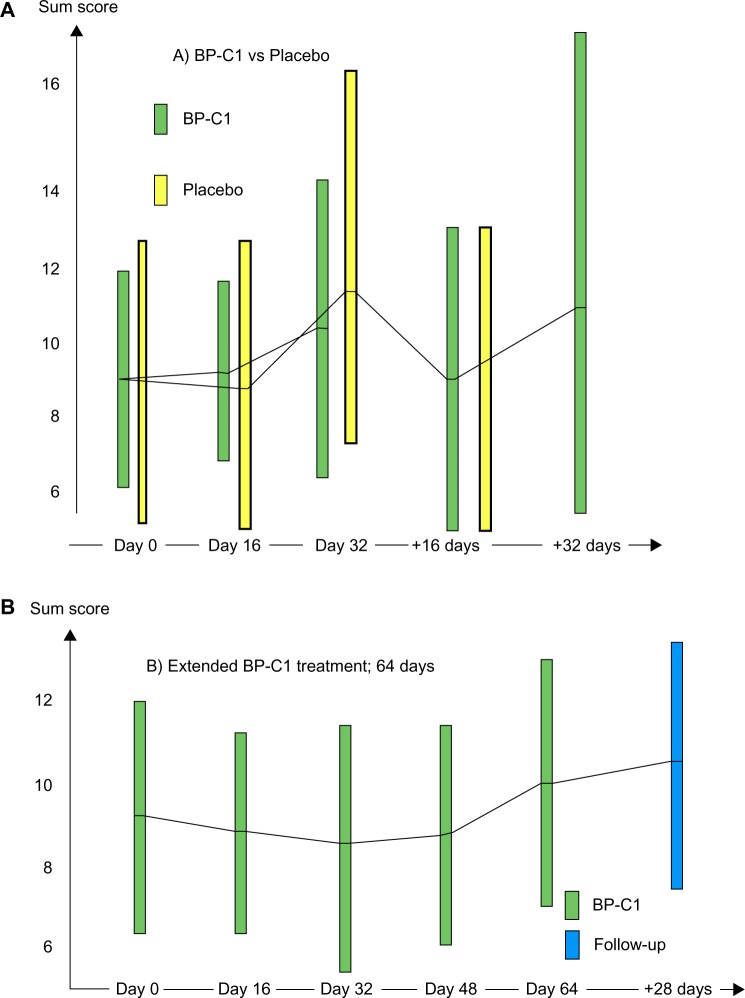
The mean sum CTC-NCI score in the BP-C1 group increased nonsignificantly from 9.0 (95% CI =6.0–12.0) at screening to 10.4 (95% CI =6.2–13.6) after 32 days of treatment (Figure 3A). In the placebo group, the sum CTC-NCI score increased significantly (P=0.05) from 10.1 (95% CI =6.4–13.7) to 12.5 (95% CI =7.3–17.6) during the same 32-day treatment period. These changes represent increases of 13.6% (95% CI =−8.8 to 36.0) in the BP-C1 group and 27.7% (95% CI = −3.3 to 58.7) in the placebo group during the first 32 days of treatment. The results were in favor of BP-C1, but the difference in increase did not meet the level of significance (P=0.12).
Figure 3.
The development in sum of National Cancer Institute Common Terminology Criteria for Adverse Events score.
Notes: Results are expressed by mean values with 95% confidence intervals illustrated by bars. The green bars show BP-C1, the yellow bars show placebo, and the blue bar shows the results after 28 days of follow-up. (A) BP-C1 versus placebo followed by BP-C1 treatment. (B) Extended 64 days of BP-C1 treatment.
Abbreviation: BP-C1, benzene-poly-carboxylic acids complex with cis-diammineplatium (II) dichloride.
Once the patients in the placebo group were switched to BP-C1 treatment, the sum CTC-NCI score reduced slightly from 12.5 to 12.2 (95% CI =5.3–19.0) during the second 32 days of active treatment.
Extended BP-C1 treatment
The maximum CTC-NCI score increased in three patients and decreased in two during 64 days of BP-C1 treatment. During the 28 days follow-up after end of treatment, maximum CTC-NCI score increased in three patients and decreased in one.
The mean sum CTC-NCI score was reduced from 9.2 (95% CI =6.2–12.1) at screening to 8.8, 8.6, and 8.9 (95% CI =6.1–11.8) after 16 days, 32 days, and 48 days of treatment, respectively (Figure 3B). From Day 48 to Day 64, the mean sum CTC-NCI increased again to 10.1 (95% CI =7.5–12.7) and further to 10.6 (95% CI =7.7–13.5) during the 28 days follow-up after end of treatment. None of these changes were significant.
Adverse events
Sixteen mild and six moderate AEs were classified as “possibly” or “probably” related to the BP-C1 treatment (Table 3). In the placebo group, six mild and two moderate AE were classified as “possibly” or “probably” related to the treatment.
Table 3.
Classification, frequency, severity and causality of recorded adverse events during 32 days of BP-C1 or placebo treatment
| Treatment | AE classification | Severity
|
Causality
|
|||
|---|---|---|---|---|---|---|
| Mild | Moderate | Possible | Probable | Definite | ||
| BP-C1 | Abnormal weight gain | 1 | 1 | |||
| Administration site pain | 1 | 1 | ||||
| Blood glucose increased | 1 | 1 | ||||
| Lactate dehydrogenase | 1 | 1 | 2 | |||
| increased | ||||||
| Constipation | 1 | 1 | ||||
| Decreased appetite | 1 | 1 | ||||
| Dizziness | 1 | 1 | ||||
| Dysgeusia | 1 | 1 | ||||
| Fatigue | 1 | 1 | ||||
| Flushing | 1 | 1 | ||||
| Headache | 1 | 1 | 1 | 1 | ||
| Hyperhidrosis | 1 | 1 | ||||
| Hypertension | 1 | 1 | ||||
| Lethargy | 1 | 1 | ||||
| Nausea | 3 | 1 | 2 | |||
| Vomiting | 1 | 1 | ||||
| Sum | 16 | 4 | 6 | 13 | 1 | |
| Placebo | Dyspepsia | 1 | 1 | |||
| Hematuria | 1 | 1 | ||||
| Hypophosphatemia | 1 | 1 | ||||
| Hypoalbuminemia | 1 | 1 | ||||
| Protein urine present | 1 | 1 | ||||
| Proteinuria | 1 | 1 | ||||
| Sum | 6 | 0 | 0 | 5 | 1 | |
Note: Results are expressed as number of adverse events.
Abbreviations: AE, adverse event; BP-C1, benzene-poly-carboxylic acids complex with cis-diammineplatium (II) dichloride.
Quality of life questionnaires
BP-C1 versus placebo
“Breast cancer-related pain and discomfort last week” was significantly reduced (P=0.02) in the BP-C1 group and slightly increased in the placebo group during the initial 32 days of treatment (Table 4). Comparison of the groups detected a significant difference in change of “Breast cancer-related pain” in favor of BP-C1 (P=0.05). A similar pattern was detected for “Breast cancer treatment problems last week.” This variable was significantly reduced in the BP-C1 group (P=0.02) and slightly increased in the placebo group. “Discomfort last week” increased in the placebo group (P=0.07) but stayed nearly constant in the BP-C1 group. “Sexual interest and activity in the last 4 weeks” increased slightly in the BP-C1 group but reduced slightly in the placebo group. No significant changes within groups and between groups were detected. “Physical activity problems” and “Health and life quality” were unchanged in both groups during the 32 days of treatment (Table 4).
Table 4.
Comparison between groups and development within groups with regard to the sum of scores within each of the three parts of the QLQ-C30 and QLQ-BR23
| Variable | Treatment | Screening | Day 16 | Day 32 | Screening – Day 32 |
|---|---|---|---|---|---|
| Physical activity problems | BP-C1 (n=15) |
8.0 (1.8) 7.0 to 9.0 |
7.4 (1.6) 6.5 to 8.3 |
8.2 (3.8) 6.1 to 10.3 |
−0.2 (3.3) −2.2 to 1.6 |
| Placebo (n=15) |
8.0 (2.5) 6.6 to 9.4 |
7.9 (2.3) 6.6 to 9.1 |
8.4 (3.8) 7.2 to 9.6 |
−0.4 (1.5) −1.2 to 0.4 |
|
| Discomfort last week | BP-C1 (n=15) |
35.3 (9.7) 29.9 to 40.7 |
33.6 (6.7) 29.9 to 37.3 |
35.9 (13.3) 28.5 to 43.2 |
−0.6 (6.2) −4.0 to 2.8 |
| Placebo (n=15) |
33.1 (10.3) 27.4 to 38.8 |
34.6 (7.7) 30.3 to 38.9 |
36.7 (8.5) 32.0 to 41.4 |
−3.5 (12.4) −10.4 to 3.3 |
|
| Health and life quality | BP-C1 (n=15) |
9.2 (2.7) 7.7 to 10.7 |
9.6 (1.7) 8.6 to 10.6 |
8.8 (3.0) 7.1 to 10.5 |
0.4 (2.1) −0.7 to 1.5 |
| Placebo (n=15) |
8.1 (2.6) 6.6 to 9.5 |
8.6 (2.4) 7.3 to 9.9 |
7.9 (2.2) 6.7 to 9.1 |
0.2 (2.7) −1.4 to 1.6 |
|
| BP-C1 treatment problems last week | BP-C1 (n=15) |
22.3 (4.9) 19.6 to 24.4 |
19.6 (3.7) 17.6 to 21.6 |
19.9 (5.8) 16.7 to 23.1 |
2.4 (4.1) 0.1 to 4.7 |
| Placebo (n=15) |
20.9 (4.8) 18.2 to 23.5 |
19.6 (4.2) 17.3 to 21.9 |
19.5 (4.1) 17.3 to 21.8 |
1.3 (4.4) −1.1 to 3.8 |
|
| Sexual interest and activity, last 4 weeks | BP-C1 (n=15) |
2.8 (1.0) 2.2 to 3.4 |
3.1 (1.4) 2.4 to 3.9 |
3.1 (2.3) 1.8 to 4.3 |
−0.3 (2.1) −1.4 to 0.9 |
| Placebo (n=15) |
3.3 (1.9) 2.2 to 4.3 |
2.9 (1.9) 1.9 to 4.0 |
3.0 (1.8) 2.0 to 4.0 |
0.3 (2.2) −0.9 to 1.5 |
|
| BC-related pain and discomfort last week | BP-C1 (n=15) |
11.1 (4.8) 8.4 to 13.7 |
10.4 (4.5) 7.7 to 13.0 |
9.8 (3.8) 7.6 to 12.0 |
1.4 (2.3) 0.04 to 2.7 |
| Placebo (n=15) |
10.1 (3.4) 8.2 to 12.0 |
10.2 (2.7) 8.7 to 11.7 |
10.3 (2.5) 8.9 to 11.7 |
−0.2 (3.1) −1.9 to 1.5 |
Note: Results are expressed as mean (standard deviation) and 95% confidence intervals.
Abbreviations: BC, breast cancer; BP-C1, benzene-poly-carboxylic acids complex with cis-diammineplatium (II) dichloride; QLQ-BR23, European Organisation for Research and Treatment of Cancer quality of life breast-cancer–specific questionnaire; QLQ-C30, European Organisation for Research and Treatment of Cancer quality of life questionnaire.
Expanded BP-C1 treatment
“Breast cancer related pain and discomfort last week”, Breast cancer treatment problem last week” and “Physical activity problem” (Table 5) significantly reduces during the 64 days of BP-C1 treatment (P≤0.05). Additionally, “Discomfort last week” was reduced but not significantly (P=0.08).
Table 5.
Development in quality of life during 64 days of BP-C1 treatment and 28 days of follow-up
| Variable | Screening (n=19) |
Day 16 (n=19) |
Day 32 (n=19) |
Day 48 (n=18) |
Day 64 (n=18) |
Final (n=19) |
P-value |
|---|---|---|---|---|---|---|---|
| Physical activity problems | 7.9 | 7.3 | 7.2 | 7.1 | 7.0 | 7.3 | 0.02 |
| 7.1–8.8 | 6.5–8.1 | 6.4–8.4 | 6.2–8.0 | 6.3–7.8 | 6.4–8.2 | 0.05 | |
| Discomfort last week | 33.7 | 32.8 | 32.7 | 32.6 | 32.2 | 33.7 | 0.08 |
| 30.2–37.2 | 29.7–35.9 | 29.4–36.0 | 29.1–36.1 | 28.8–35.6 | 29.6–37.8 | 0.23 | |
| Health and life quality | 9.1 | 8.8 | 8.9 | 9.5 | 9.2 | 8.4 | 0.50 |
| 7.9–10.2 | 7.8–9.8 | 7.9–9.9 | 8.3–10.7 | 8.0–10.3 | 7.0–9.9 | 0.07 | |
| BC treatment problems last week | 20.8 | 20.1 | 20.1 | 19.4 | 19.6 | 20.1 | 0.05 |
| 18.8–22.9 | 18.1–22.0 | 17.8–22.3 | 17.2–21.7 | 17.5–21.6 | 17.8–22.4 | 0.39 | |
| Sexual interest and activity, last 4 weeks | 2.9 | 3.1 | 3.0 | 2.7 | 2.8 | 2.8 | 0.13 |
| 2.2–3.7 | 2.4–3.9 | 2.4–3.6 | 2.1–3.4 | 2.0–3.5 | 2.1–3.6 | 0.35 | |
| BC-related pain and discomfort last week | 11.7 | 11.4 | 11.1 | 10.9 | 10.7 | 11.7 | 0.03 |
| 9.6–13.8 | 9.4–13.4 | 9.3–12.8 | 8.6–13.2 | 8.7–12.7 | 9.6–13.9 | 0.47 |
Notes: Results are expressed as mean and 95% confidence intervals. The upper P-values refer to changes from screening to Day 64 and the lower to changes from screening to final examination 28 days after the last injection.
Abbreviations: BC, breast cancer; BP-C1, benzene-poly-carboxylic acids complex with cis-diammineplatium (II) dichloride.
Karnofsky Performance Status Scale
BP-C1 versus placebo
Except for one patient in the BP-C1 group, all the patients had a KPSS score of 80 or higher. During the first 32 days of BP-C1 treatment, KPSS score was reduced from 90 to 80 in two patients and from 100 to 90 in one patient. In the placebo group, KPSS score was reduced from 80 to 70 in one patient and from 90 to 80 in one patient.
After the switch to BP-C1 for the placebo group, the KPSS score was reduced from 80 to 70 for two patients, from 90 to 80 for one patient, and from 100 to 90 for one patient. No significant differences between groups or changes within groups were detected.
Expanded BP-C1 treatment
During the 64 days of BP-C1 treatment, KPSS was reduced from 90 to 80 in two patients, from 100 to 90 in one patient, and from 100 to 80 in one patient. The reduction was not significant.
Discussion
This trial demonstrated that, compared to placebo, BP-C1 controls tumor growth in patients suffering from stage IV metastatic breast cancer, significantly reduces total toxicity, and significantly improves quality of life. Treatment with BP-C1 is effective and very well tolerated and produces only very few and mild transient adverse effects. Furthermore, BP-C1 can safely be given continuously over 64 days without any serious AE or significant increase in AEs or toxicity. Another major advantage of BP-C1 is that it can be administered in the home of the patient, thereby avoiding the frustrations associated with treatment in an outpatient clinic, such as waiting for blood sampling, waiting for results, waiting for the actual treatment, and meeting and receiving treatment from different staff members.21 Thus, this new drug produces promising results in patients with stage IV MBC, and so far the results are strikingly positive with a large potential impact for the patient and thereby also for their relatives, which has not been seen in previous cancer medicine studies.
Five per cent of newly diagnosed cases of breast cancer are metastatic, and 30% of treated patients experience a systemic recurrence.22 The rates of systemic recurrence vary within different trials, but generally distant metastases are dominant.14 Systemic treatment of breast cancer is available in various modalities, yet the use of palliative systemic therapy for MBC is challenging. The treatment should control the tumor growth and potential side effects should be easy to treat; ideally, the treatment should improve the quality of life of the patients in general. Such an ideal agent has not been available. However, BP-C1 seems to fulfill such demands to be used as a palliative treatment of stage IV MBC. The prognosis for patients with MBC is poor, with a 5-year survival rate of about 20%. Thus, MBC is a substantial problem for women with breast cancer.22
In the present study a group of patients with histologically verified MBC were treated with BP-C1 regardless of the patients’ tumor characteristics (receptor status, differentiation), and previous treatment. All patients had been treated with at least third-line treatment. End points were tumor control according to RECIST 1.1 criteria, toxicity, and quality of life according to EORTC and CTC-NCI.
In a nonrandomized multicenter Phase I trial with 3-level Response Surface Pathway carried out in the Far East, BP-C1 was able to show a 62.5% response rate, including one complete responder, among patients treated with high doses of BP-C1 compared to 28.6% in a low-dose group. BP-C1 was daily administered intramuscularly for 32 days. Minimum Efficacy Cumulative Dose was estimated to 0.96 mg/kg BW and defined the lower limit of the high-dose group.13
The main compound in BP-C1 is BP-Cx-1 which has the ability to be able to penetrate the cell membrane and thereby penetrate into the cytoplasm.23 From the cytoplasm, BP-Cx-1 penetrates into the cell nuclei. BP-C1 seems also to possess the ability to complex with and transport metal ions across membranes – a so-called membranothropic effect.23 Because of its ionophoric characteristics, it has a wide application in chemistry and biology. It can be supposed that the ability of benzene-polycarboxylic acids to penetrate into cell nuclei can be relevant for understanding of the mechanism of action of BP-C1 towards malignant tumors.
Furthermore, it was recently shown that exposure of human breast cancer cells (MCF-7 and T47-D) to BP-C1 significantly reduced cell viability, induced apoptosis, and activated caspase 8 and caspase 9. Moreover, gene expression experiments indicated that BP-C1 increased the expression of proapoptotic genes (CASP8AP, TNFRSF21, NFkB2, FADO, BCL10, and CASP8) and lowered the level of mRNA of inhibitory apoptotic genes (BCL2L11, BCL2L2, and XiAP).24 Furthermore, BP-C1 has been shown to have an immune-modulating effect that may contribute to the anticancer effect of this compound and its parent compound BP-Cx-1. The effect of the two compounds was investigated on human peripheral blood mononuclear cells from healthy donors using different immunological tests reflecting the major functions of the main cells of the immune system – lymphocytes and monocytes. It was demonstrated that monocytes are the major cells responding to BP-C1 and BP-Cx-1. Activation of monocytes leads to two major effects: 1) a significant production of cytokines (tumor necrosis factor-α, interferon-γ, granulocyte macrophage colony-stimulating factor, interleukin (IL)-1β and IL-6) that are able to increase antitumor activity of lymphocytes, and 2) activation of the ability of monocytes to inhibit tumor cell growth. In addition, direct effect on lymphocytes was also demonstrated, exemplified by a significant induction of production of IL-25. No release of IL-10, IL-12, or tumor necrosis factor-β was observed, ie, no cytokine storm was observed. The new anticancer agent and its carrier molecule BP-C1 and BP-Cx-1 are able to activate multiple immunological mechanisms of antitumor response. No cytokine storm could be demonstrated.25
MBC carries a poor prognosis and the treatment has so far been limited to chemotherapy with all of its well-known side effects.26–31 This trial demonstrates that this new anticancer agent BP-C1 can treat patients with MBC successfully by controlling tumor growth, thereby improving quality of life with none or very few mild transient adverse effects. BP-C1 represents a unique opportunity in the treatment of this group of extremely sick patients.
BP-C1 is classified as a class 2 drug in the production chain, and patients with severe breast cancer disease and metastases have therefore been treated in their homes by a visiting nurse without any problems. Most of the patients experienced treatment at home as a very positive event, especially that they were not confronted with sick patients, which these patients experienced as a severe relief.
Conclusion
We have tested a drug that prevents advanced breast cancer from worsening, potentially providing an important new treatment option for women suffering from MBC and their families. This agent deserves an adaptive registration by the regulatory bodies so more clinical trials can be carried out until final registration can be obtained. Thus, larger studies on the effect of BP-C1 depending on receptor status of the tumor cells must be carried out under the timeframe of an adaptive registration. Finally, it can also be concluded that BP-C1 increases QOL significantly in patients with stage IV breast cancer without any increase in toxicity and that the substance can safely be administered continuously for 64 days.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ, Panel members Threshold for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer. Ann Oncol. 2009;20(8):1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig C, Stoelben E, Hasse J. Disease-free survival after resection of lung metastases in patients with breast cancer. Eur J Surg Oncol. 2003;29(6):532–535. doi: 10.1016/s0748-7983(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 4.Hoe AL, Royle GT, Taylor I. Breast liver metastases – incidence, diagnosis and outcome. J R Soc Med. 1991;84(12):714–716. doi: 10.1177/014107689108401207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boogerd W, Hart AA, Tjahja IS. Treatment and outcome of brain metastasis as first site of distant metastasis from breast cancer. J Neurooncol. 1997;35(2):161–167. doi: 10.1023/a:1005818323996. [DOI] [PubMed] [Google Scholar]
- 6.Jørgensen CL, Nielsen TO, Bjerre KD, et al. PAM50 breast cancer intrinsic subtypes and effect of gemcitabine in advanced breast cancer patients. Acta Oncol. 2014;53(6):776–787. doi: 10.3109/0284186X.2013.865076. [DOI] [PubMed] [Google Scholar]
- 7.Sledge GW, Jr, Loehrer PJ, Sr, Roth BJ, Einhorn LH. Cisplatin as first-line therapy for metastatic breast cancer. J Clin Oncol. 1988;6(12):1811–1814. doi: 10.1200/JCO.1988.6.12.1811. [DOI] [PubMed] [Google Scholar]
- 8.Vogel CL, Nabholtz JM. Monotherapy of metastatic breast cancer: a review of newer agents. Oncologist. 1999;4(1):17–33. [PubMed] [Google Scholar]
- 9.Ershler WB. Capecitabine monotherapy: safe and effective treatment for metastatic breast cancer. Oncologist. 2006;11(4):325–335. doi: 10.1634/theoncologist.11-4-325. [DOI] [PubMed] [Google Scholar]
- 10.Lao J, Madani J, Puértolas T, et al. Liposomal doxorubicin in the treatment of breast cancer patients: a review. J Drug Deliv. 2013;2013:456409. doi: 10.1155/2013/456409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuxen MK, Cold S, Tange UB, Balslev E, Nielsen DL. Phase II study of neoadjuvant pegylated liposomal doxorubicin and cyclophosphamide ± trastuzumab followed by docetaxel in locally advanced breast cancer. Acta Oncol. 2014;53(10):1440–1445. doi: 10.3109/0284186X.2014.921727. [DOI] [PubMed] [Google Scholar]
- 12.Shulman LN, Berry DA, Cirrincione CT, et al. Comparison of doxorubicin and cyclophosphamide versus single-agent paclitaxel as adjuvant therapy for breast cancer in women with 0 to 3 positive axillary nodes: CALGB 40101 (Alliance) J Clin Oncol. 2014;32(22):2311–2317. doi: 10.1200/JCO.2013.53.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewi S, Larsen S, Srimuninnimit V, Lu YS, Manuaba T, Lindkaer-Jensen S. Benzene-poly-carboxyli acid complex with cis-diammineplatinum (II) dichloride in the treatment of stage IV breast cancer patients. The Open Breast Cancer Journal. 2013;5:7–15. [Google Scholar]
- 14.Carlsen KH, Kramer J, Fagertun HE, Larsen S. Loratadine and terfenadine in perennial allergic rhinitis. Treatment of nonresponders to the one drug with the other drug. Allergy. 1993;48(6):431–436. doi: 10.1111/j.1398-9995.1993.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 15.Pocock SJ. Clinical Trials: A Practical Approach. New York, NY: John Wiley & Sons Ltd; 1989. [Google Scholar]
- 16.Altman DG. Practical Statistics for Medical Research. London, UK: Chapman and Hall; 1990. [Google Scholar]
- 17.Agresti A. Categorical Data Analysis. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2002. [Google Scholar]
- 18.Panel on Handling Missing Data in Clinical Trials and Committee on National Statistics, editor. The Prevention and Treatment of Missing Data in Clinical Trials. National Academy Press; 2010. 2011. Conclusions and recommendations; pp. 110–112. [Google Scholar]
- 19.Shao J, Zhong B. Last observation carry-forward and last observation analysis. Stat Med. 2003;22(15):2429–2441. doi: 10.1002/sim.1519. [DOI] [PubMed] [Google Scholar]
- 20.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th ed. Boston, MA: Pearson Education, Inc; 2007. [Google Scholar]
- 21.Poulsen L. Patients with breast cancer are treated at home. Sygeplejersken. 2014;5:78–85. [Google Scholar]
- 22.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 23.Perminova IV. Interaction of 3H-Labeled Benzene-Polycarboxylic Acids (BP-Cx-1) with Tumours and Normal Cell Cultures. Report Moscow State University; 2012. [Google Scholar]
- 24.Fares F, Azzam N, Fares B, Larsen S, Lindkaer-Jensen S. Benzene-poly-carboxylic acid complex, a novel anti-cancer agent induces apoptosis in human breast cancer cells. PLoS One. 2014;9(2):e85156. doi: 10.1371/journal.pone.0085156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirkin A, Dzhandzhugazyan KN, Fang JJ, Lindkaer-Jensen S. Benzene-poly-carboxylic acids complex with cis-diammineplatinum [II] dichloride [BP-C1], an innovative anti-cancer agent, activates multiple immunological mechanisms of an antitumor response. International Biologics: Target and Therapy. 2014 In press. [Google Scholar]
- 26.Guarneri V, Conte PF. The curability of breast cancer and the treatment of advanced disease. Eur J Nucl Med Mol Imaging. 2004;31(Suppl 1):S149–S161. doi: 10.1007/s00259-004-1538-5. [DOI] [PubMed] [Google Scholar]
- 27.Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ. 2014;348:g366. doi: 10.1136/bmj.g366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 29.Cheng YC, Ueno NT. Improvement of survival and prospect of cure in patients with metastatic breast cancer. Breast Cancer. 2012;19(3):191–199. doi: 10.1007/s12282-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cameron DA, Gabra H, Leonard RC. Continuous 5-fluorouracil in the treatment of breast cancer. Br J Cancer. 1994;70(1):120–124. doi: 10.1038/bjc.1994.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carey LA, Rugo HS, Marcom PK, et al. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol. 2012;30(21):2615–2623. doi: 10.1200/JCO.2010.34.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]