Abstract
A concise enantioselective synthesis of (-)-paroxetine (Paxil) and (-)-femoxetine has been achieved. Key to these syntheses is a N-heterocyclic carbene catalyzed homoenolate addition to a nitroalkene followed by in situ reduction of the nitro-group to rapidly access δ-lactams.
Due to their abundance in natural products and medicinal agents, the rapid, stereodefined construction of piperidines has received considerable attention from the synthetic community. 1, 2 Specifically, the pursuit of methodologies to furnish 3- and 4- substituted piperidine rings has been the subject of particularly intense focus due to its application in the synthesis of paroxetine. Paroxetine (Paxil) is a selective serotonin reuptake inhibitor (SSRI) that was discovered in 1970 and introduced to market in 1992 as a treatment for depression, anxiety, and panic disorder. 3 A related SSRI, femoxetine, was discovered concurrently with paroxetine, but was not pursued.3
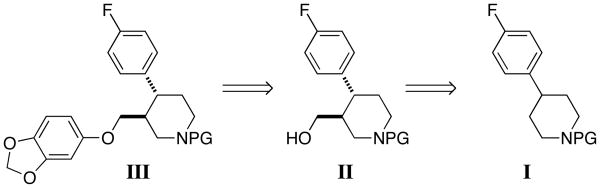
Since the initial report, many syntheses of paroxetine have appeared relying upon the establishment of a single enantiomer of N-protected trans-4-(4-fluorophenyl)-3-piperidinemethanol II, followed by coupling with sesamol and deprotection. Approaches to set the stereochemistry often depend on the chiral pool, 4 resolution, 5 chiral auxiliaries, 6 chiral base, 7 and asymmetric catalysis. 8 These methods have proven effective and are represented in the literature accordingly. However, a highly convergent synthesis in which all the carbons of paroxetine are introduced in a single stereocontrolled step is lacking.
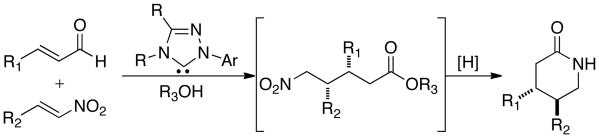
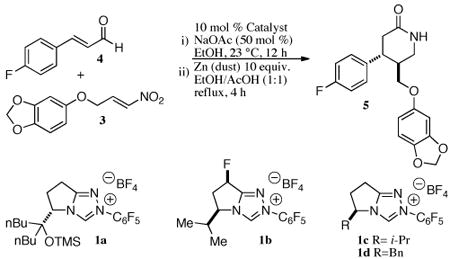
Recently, we reported a N-heterocyclic carbene (NHC) catalyzed homoenolate addition of enals to nitroalkenes allowing access to syn δ-nitroesters in good yield, excellent dr, and excellent ee.9,10 Furthermore, we developed a protocol for the in situ reduction of the syn δ-nitroesters to the corresponding trans δ-lactam. This method provides rapid access to trans 3,4-disubstituted piperidones. We envisioned that utilization of this method would allow for exceptional levels of efficiency in the syntheses of paroxetine and femoxetine. Our method is notable, as it allows for the concomitant formation of the piperidine ring and establishment of the contiguous stereocenters in a single reaction (Scheme 1).
Scheme 1. NHC-Catalyzed Nitroester/Lactam Formation.
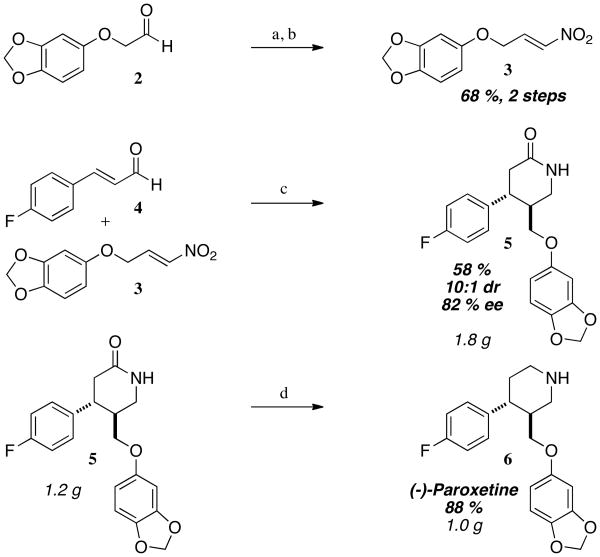
We began our synthesis of paroxetine by forming requisite nitro alkene 3 (Scheme 2). From commercially available aldehyde 2 we performed a Henry reaction with nitromethane followed by elimination with trifluoroacetic anhydride to furnish nitroalkene 3 in 68 % yield over two steps. With this nitroalkene in hand we were ready to attempt the key NHC-catalyzed step. Using conditions we optimized and reported in our previous publication using catalyst 1a we observed no desired lactam product 5. We hypothesized that the bulky bis-butyl/OTMS moiety of the catalyst may be too large to facilitate the reaction and explored other catalyst scaffolds (Table 1). We found that fluorinated catalyst 1b11 provides the desired lactam 5 in 82 % ee, 10:1 dr, and 58 % yield. Satisfied with these results we scaled the reaction to 9 mmol and were pleased to find the reaction proceeds smoothly, delivering 1.8 grams of product in predictable yield and selectivities. We then subjected 1.2 grams (3.55 mmol) of the lactam precursor 5 to a LAH reduction, which delivered paroxetine (Paxil) 6 in 88 % yield (1.0 grams, 3.13 mmol), for an overall yield of 35 % over 4 steps in 82 % ee.
Scheme 2.
Preparation of paroxetine. Reagents and conditions: a) CH3NO2, KOtBu (20 mol%), tBuOH/THF, 0 to 23 °C, quantitative; b) trifluoroacetic anhydride, Et3N, CH2Cl2, 0 °C, 68 %; c) NHC 1b, NaOAc (50 mol%), EtOH, 23 °C, 12 h, then Zn (dust), EtOH/AcOH, reflux, 58 %, 10:1 dr, 82 % ee; d) LiAlH4, THF, 0 to 66 °C, 88 %.
Table 1.
Chiral Catalyst Screen.
| ||||
|---|---|---|---|---|
| Entry | Catalyst | yielda | dr (trans/cis)b | ee (%)c |
| 1 | 1a | - | N/A | N/A |
|
| ||||
| 2 | lb | 58 | 10:1 | 82 |
|
| ||||
| 3 | 1c | 48 | 8:1 | 71 |
| 4 | 1d | 51 | 7:1 | 69 |
Yields are isolated yields after chromatography.
Diastereoselectivity determined by 1H NMR of the unpurified reaction mixture.
Enantiomeric excess was determined by HPLC analysis on a chiral stationary phase.
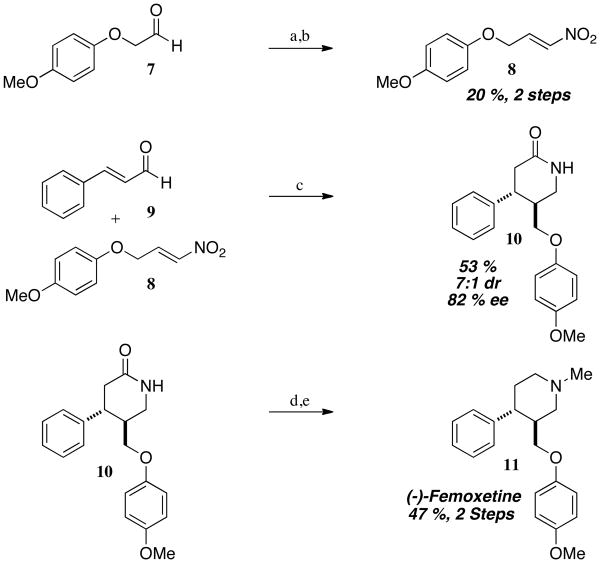
Due to its structural similarity with paroxetine, we also explored a short synthesis of femoxetine. We first synthesized nitroalkene 8 from a two-step Henry/elimination sequence to furnish nitroalkene 8 in 20 % yield over two steps (Scheme 3). We then subjected nitroalkene 8 and cinnamaldehyde to the key NHC-catalyzed step followed by in situ reduction and were pleased to observe lactam 10 in 53 % yield, 7:1 dr, and 82 % ee. Methylation of the lactam with methyl iodide followed by LAH reduction completes our synthesis of femoxetine 11 in 5 steps and 82 % ee.
Scheme 3.
Preparation of femoxetine. Reagents and conditions: a) CH3NO2, KOtBu (20 mol%), tBuOH/THF, 0 to 23 °C, quantitative; b) trifluoroacetic anhydride, Et3N, CH2Cl2, 0 °C, 20 %; c) NHC 1b, NaOAc (50 mol%), EtOH, 23 °C, 12 h, then Zn (dust), EtOH/AcOH, reflux, 53 %, 7:1 dr, 82 % ee; d) NaH, CH3I, THF, 23 °C, 55 %; e) LiAlH4, THF, 0 to 66 °C, 87 %.
In conclusion, we have developed rapid syntheses of (-)-paroxetine and (-)-femoxetine by employing a NHC catalyzed coupling of enals and nitroalkenes. The use of fluorinated NHC catalyst 1b in a one-pot sequence provides access to lactam products in moderate yield and good stereoselectivities, which were quickly elaborated to both paroxetine and femoxetine. Further, we have demonstrated that this approach is amenable to gram scale. To the best of our knowledge, this work represents the shortest synthesis of paroxetine to date.
Experimental Section
Preparation of Lactam 5: To a 100 mL flame dried round bottom flask containing a magnetic stirbar was added nitroalkene 3 (2.01 g, 9 mmol, 1.0 equiv), NHC 1b (377 mg, 0.9 mmol, 10 mol%), sodium acetate (370 mg, 4.5 mmol, 0.5 equiv), 4-fluorocinnamaldehyde (2.03 g, 13.5 mmol, 1.5 equiv), followed by 30 mL ethanol. The flask was then fitted with a rubber septum and stirred under an atmosphere of argon for 12 hours at 23 °C. After 12 hours, the septum was removed and zinc dust (5.85 g, 90 mmol, 10 equiv) was added followed by 30 ml of acetic acid. The flask was then heated under reflux with a reflux condenser and heating mantle. After four hours, the heat source was removed and the reaction was allowed to cool. Upon cooling, the reaction was filtered through celite and rinsed with 30 mL EtOAc. The filtrate was then diluted with an additional 20 mL EtOAc and quenched with 60 mL saturated NaHCO3. The organic layer was then separated, washed with brine (1 × 60 mL), dried (Na2SO4), and concentrated in vacuo. The crude residue was then purified by column chromatography (5 % MeOH in CH2Cl2) to yield 58 % (4R,5S)-5-((benzo[d][1,3]dioxol-5-yloxy)methyl)-4-(4-fluorophenyl)piperidin-2-one as an off-white solid (82% ee, 10:1 dr).
Supplementary Material
Figure 1. Typical Synthetic Approach Towards Paroxetine.
Acknowledgments
We thank NIGMS (GM72586) for generous support. KEO thanks NIH (GM096749) for funding.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/ajoc.201xxxxxx.
References
- 1.(a) Buffat MGP. Tetrahedron. 2004;60:1701–1729. [Google Scholar]; (b) Poupon E, Nay B. Biomimetic Organic Synthesis. Ch. 1 Wiley-VCH; New York USA: 2011. [Google Scholar]; (c) Kathiravan MK, Salake AB, Chothe AS, Dudhe PB, Watode RP, Mukta MS, Gadhwe S. Bioorg Med Chem. 2012;20:5678–5698. doi: 10.1016/j.bmc.2012.04.045. [DOI] [PubMed] [Google Scholar]; (d) Khadem S, Marles RJ. Molecules. 2012;17:191–206. doi: 10.3390/molecules17010191. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Jordan AM, Roughly SD. J Med Chem. 2011;54:3451–3479. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- 2.For select recent approaches to stereocontrolled piperidine synthesis, see: Duttwyler S, Lu C, Rheingold AL, Bergman RG, Ellman JA. J Am Chem Soc. 2012;134:4064–4067. doi: 10.1021/ja2119833.Kumar P, Louie J. Org Lett. 2012;14:2026–2029. doi: 10.1021/ol300534j.Martin TJ, Rovis T. Angew Chem Int Ed. 2013;52:5368–5371. doi: 10.1002/anie.201301741.Huy PH, Koskinen AMP. Org Lett. 2013;15:5178–5181. doi: 10.1021/ol4026588.Wang SG, You SL. Angew Chem Int Ed. 2014;53:2194–2197. doi: 10.1002/anie.201309876.Peng Z, Wong JWW, Hansen EC, Puchlopek-Dermenci ALA, Clarke HJ. Org Lett. 2014;16:860–863. doi: 10.1021/ol403630g.Chen W, Wilde RG, Seidel D. Org Lett. 2014;16:730–732. doi: 10.1021/ol403431u.
- 3.(a) Barnes RD, Wood-Kaczmar MW, Curzons AD, Lynch IR, Richardson JE, Burton PC. U.S. Patent 24721723. 1986; (b) Gunasekara NS, Noble S, Benfield P. Drugs. 1988;55:85. doi: 10.2165/00003495-199855010-00007. [DOI] [PubMed] [Google Scholar]; (c) Bourin M, Chue P, Guillon Y. CNS Drug Reviews. 2001;7:25–47. doi: 10.1111/j.1527-3458.2001.tb00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Katzman MA, Tricco AC, McIntosh D, Filteau MJ, Bleau P, Chokha PR, Kjernisted KD, Mok H, Pham B. J Clin Psychiatry. 2007;68:1845–1859. doi: 10.4088/jcp.v68n1204. [DOI] [PubMed] [Google Scholar]
- 4.(a) Cossy J, Mirguet O, Gomez Pardo D, Desmurs JR. Tetrahedron Lett. 2001;42:5705. [Google Scholar]; (b) Cossy J, Mirguet O, Gomez Pardo D, Desmurs R. Eur J Org Chem. 2002:3543. [Google Scholar]
- 5.(a) Sugi K, Itaya N, Katsura T, Igi M, Yamazaki S, Ishibashi T, Yamaoka T, Kawada Y, Tagami Y, Otsuki M, Ohshima T. Chem Pharm Bull. 2000;48:529. doi: 10.1248/cpb.48.529. [DOI] [PubMed] [Google Scholar]; (b) de Gonzalo G, Brieva R, Sánchez VM, Bayod M, Gotor V. J Org Chem. 2001;66:8947. doi: 10.1021/jo010809+. [DOI] [PubMed] [Google Scholar]; (c) Gotor V. Org Process Res Dev. 2002;6:420. [Google Scholar]; (d) Palomo JM, Fernández-Lorente G, Mateo C, Fernández-Lafuente R, Guisan JM. Tetrahedron: Asymmetry. 2002;13:2375. [Google Scholar]; (e) Palomo JM, Fernández- Lorente G, Mateo C, Fuentes M, Guisan JM, Fernández-Lafuente R. Tetrahedron: Asymmetry. 2002;13:2653. [Google Scholar]; (f) de Gonzalo G, Brieva R, Sánchez VM, Bayod M, Gotor V. Tetrahedron: Asymmetry. 2003;14:1725. [Google Scholar]; (g) de Gonzalo G, Brieva R, Sánchez VM, Bayod M, Gotor V. J Org Chem. 2003;68:3333. doi: 10.1021/jo034120b. [DOI] [PubMed] [Google Scholar]; (h) Czibula L, Nemes A, Sebök F, Szántay C, Mák M. Eur J Org Chem. 2004:3336. [Google Scholar]
- 6.(a) Amat M, Hidalgo J, Bosch J. Tetrahedron: Asymmetry. 1996;7:1591. [Google Scholar]; (b) Amat M, Bosch J, Hidalgo J, Cantó M, Pérez M, Llor N, Molins E, Miravitlles C, Orozco M, Luque J. J Org Chem. 2000;65:3074. doi: 10.1021/jo991816p. [DOI] [PubMed] [Google Scholar]; (c) Liu LT, Hong PC, Huang HL, Chen SF, Wang CLJ, Wen YS. Tetrahedron: Asymmetry. 2001;12:419. [Google Scholar]; (d) Murthy KSK, Rey AW, Tjepkema M. Tetrahedron Lett. 2003;44:5355. [Google Scholar]; (e) Yamada S, Misono T, Tsuzuki S. J Am Chem Soc. 2004;126:9862. doi: 10.1021/ja0490119. [DOI] [PubMed] [Google Scholar]; (f) Yamada S, Jahan I. Tetrahedron Lett. 2005;46:8673. [Google Scholar]; (g) Escolano C, Amat M, Bosch J. Chem Eur J. 2006;12:8198. doi: 10.1002/chem.200600813. [DOI] [PubMed] [Google Scholar]
- 7.(a) Johnson TA, Curtis MD, Beak P. J Am Chem Soc. 2001;123:1004. doi: 10.1021/ja005748w. [DOI] [PubMed] [Google Scholar]; (b) Johnson TA, Jang DO, Slafer BW, Curtis MD, Beak P. J Am Chem Soc. 2002;124:11689. doi: 10.1021/ja0271375. [DOI] [PubMed] [Google Scholar]; (c) Greenhalgh DA, Simpkins NS. Synlett. 2002:2074. [Google Scholar]; (d) Gill CD, Greenhalgh DA, Simpkins NS. Tetrahedron. 2003;59:9213. [Google Scholar]
- 8.(a) Senda T, Ogasawara M, Hayashi T. J Org Chem. 2001;66:6852. doi: 10.1021/jo0103930. [DOI] [PubMed] [Google Scholar]; (b) Taylor MS, Jacobsen EN. J Am Chem Soc. 2003;125:11204. doi: 10.1021/ja037177o. [DOI] [PubMed] [Google Scholar]; (c) Hughes G, Kimura M, Buchwald SL. J Am Chem Soc. 2003;125:11253. doi: 10.1021/ja0351692. [DOI] [PubMed] [Google Scholar]; (d) Koech PK, Krische MJ. Tetrahedron. 2006;62:10594. [Google Scholar]; (e) Paraskar AS, Sudalai A. Tetrahedron. 2006;62:4907. [Google Scholar]; (f) Brandau S, Landa A, Franzén J, Marigo M, Jørgensen KA. Angew Chem, Int Ed. 2006;45:4305. doi: 10.1002/anie.200601025. [DOI] [PubMed] [Google Scholar]; (g) Ito M, Sakaguchi A, Kobayashi C, Ikariya T. J Am Chem Soc. 2007;129:290. doi: 10.1021/ja067777y. [DOI] [PubMed] [Google Scholar]; (h) Nemoto T, Sakamoto T, Fukuyama T, Hamada Y. Tetrahedron Lett. 2007;48:4977. [Google Scholar]; (i) Bower JF, Riis-Johannessen T, Szeto P, Whitehead AJ, Gallagher T. Chem Commun. 2007:728. doi: 10.1039/b617260a. [DOI] [PubMed] [Google Scholar]; (j) Hynes PS, Stupple PA, Dixon DJ. Org Lett. 2008;10:1389. doi: 10.1021/ol800108u. [DOI] [PubMed] [Google Scholar]; (k) Valero G, Schimer J, Cisarova I, Vesely J, Moyano A, Rios R. Tetrahedron Lett. 2009;50:1943. [Google Scholar]; (l) Kim MH, Park Y, Byeong-Seon J, Hyeung-guen P, Jew SS. Org Lett. 2010;12:2826. doi: 10.1021/ol100928v. [DOI] [PubMed] [Google Scholar]
- 9.White NA, DiRocco DA, Rovis T. J Am Chem Soc. 2013;135:8504. doi: 10.1021/ja403847e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For related NHC catalyzed additions of enals to nitroalkenes see: Nair V, Sinu CR, Babu BP, Varghese V, Jose A, Suresh E. Org Lett. 2009;11:5570. doi: 10.1021/ol901918x.Maji B, Ji L, Wang S, Vedachalam S, Rakesh G, Liu XW. Angew Chem Int Ed. 2012;51:8276. doi: 10.1002/anie.201203449.
- 11.DiRocco DA, Oberg KM, Dalton DM, Rovis T. J Am Chem Soc. 2009;131:10872. doi: 10.1021/ja904375q.For decomposition pathways of perfluorinated NHC catalysts, see: Zhao X, Glover GS, Oberg KM, Dalton DM, Rovis T. Synlett. 2013:1229. doi: 10.1055/s-0033-1338842.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.