Abstract
Background
Transcatheter aortic valve replacement (TAVR) has emerged as a less invasive option for valve replacement for patients with severe aortic stenosis. Although it has been recommended that TAVR not be offered to patients who will not improve functionally or derive meaningful survival benefit from the procedure, no guidance exists on how best to identify such patients. The first step in this process is to define a poor outcome that can then be used as a foundation for subsequent case identification. We sought to evaluate potential definitions of a poor outcome after TAVR that combine both mortality and quality of life (QoL) components.
Methods and Results
Using data from 463 patients who underwent TAVR as part of the Placement of AoRTic TraNscathetER Valve (PARTNER) Trial, we evaluated 6-month mortality and QoL outcomes using the Kansas City Cardiomyopathy Questionnaire (KCCQ) to explore potential definitions of a poor outcome. We then compared the strengths and weaknesses of each potential definition by examining the relationship between baseline and 6-month KCCQ scores for each patient. Based on these analyses, we argue that the most appropriate definition of a poor outcome after TAVR is either (1) death, (2) KCCQ overall summary score <45, or (3) KCCQ decrease of ≥ 10 points, which best reflects a failure to achieve the therapeutic goals of TAVR.
Conclusions
Using empiric data on a large number of patients enrolled in the PARTNER trial, we propose a definition for poor outcome after TAVR that combines both mortality and QoL measures into a single composite endpoint. Use of this endpoint (or other similar endpoints) in future studies can facilitate development of predictive models that may be useful to identify patients who are poor candidates for TAVR and to provide such patients and their families with appropriate expectations of functional recovery after TAVR.
Keywords: aortic valve stenosis, quality of life, transcatheter aortic valve, valve
Transcatheter aortic valve replacement (TAVR) has recently emerged as a less invasive treatment option for patients with severe aortic stenosis, and the enthusiasm for the implementation and expansion of this technology has been overwhelming.1–2 Currently approved for use in multiple countries and for limited use in the U.S., this technology expands the pool of patients eligible for aortic valve replacement and has been embraced by cardiologists eager to offer a less invasive treatment option to their very elderly patients with critical aortic stenosis. Clinical trials have thus far demonstrated a 20% absolute reduction in 1-year mortality and a substantial improvement in quality of life (QoL) for inoperable patients undergoing TAVR, as compared with medical therapy3–4 and non-inferior outcomes compared with surgical valve replacement among patients at high risk for surgical complications.5–6 However, clinicians, regulators, and payers remain concerned about the number of patients undergoing TAVR that have poor outcomes.7–10 Often termed “Cohort C” patients, these individuals have cardiovascular or comorbid conditions that are so severe that they have limited potential to derive functional improvement or live longer following TAVR.8–9 Despite the obvious desire to avoid the risks and expense of treatment in such patients, there has been no guidance about to how to prospectively identify these patients for whom valve replacement may not have a positive impact on their quantity and quality of life.
Although several studies have begun to explore methods to identify patients at high-risk for poor outcomes after TAVR, to date these studies have focused almost exclusively on mortality.11–15 Given the age and underlying burden of comorbidity among patients currently considered for TAVR, improved health status and QoL may be even more important treatment goals than extending life.16–17 Consequently, integrating QoL outcomes into the definition of a poor (and conversely, an acceptable) outcome is particularly relevant in these challenging and complex patients.10 The first step in examining this issue must be to rigorously define what constitutes a “poor outcome” of TAVR. Although some might consider these definitions to be self-evident, there is actually a large range of definitions that could be considered—each of which may have different implications for patient selection and shared decision-making. In an effort to explore these issues, we used data from the PARTNER trial to examine the strengths and weaknesses of alternative definitions of a poor outcome after TAVR.
METHODS
Study Population and Protocol
Patients for our study were drawn from the Placement of AoRTic TraNscathetER Valve (PARTNER) trial of patients with severe symptomatic aortic stenosis who were considered potential candidates for TAVR. Details of the study inclusion and exclusion criteria have been described previously.3, 5 Briefly, enrolled patients were required to have severe aortic stenosis (aortic-valve area of <0.8 cm2 with either a mean aortic-valve gradient of ≥40 mmHg or a peak aortic-jet velocity of ≥4.0 m/s); New York Heart Association (NYHA) class II or greater heart failure symptoms; and high surgical risk based on the Society for Thoracic Surgeons (STS) risk score and other factors. Eligible patients were classified as either high risk but suitable for surgical aortic valve replacement (cohort A)5 or ineligible for cardiac surgery due to coexisting medical conditions associated with a predicted probability of death or permanent disability ≥50% (Cohort B).3 Cohort A patients were randomized to either TAVR or surgical AVR, whereas Cohort B patients were randomized to TAVR vs. medical therapy. For the purposes of this study, only patients who were randomized to TAVR and who actually underwent the procedure were included in the study population.
Patients were assessed for clinical factors and health status at baseline and at 1, 6, and 12 months after randomization. The baseline health status questionnaires were administered before randomization, and follow-up questionnaires were administered during in-person visits to the enrolling centers or by mail. An independent clinical events committee adjudicated all serious adverse events. The institutional review boards at each participating site approved the study, and all patients provided written informed consent prior to participation.
Assessment of Health Status
Although patients with severe aortic stenosis may experience angina or syncope as their major presenting symptom, heart failure symptoms predominate among patients presenting for aortic valve replacement.18–19 As such, use of a heart-failure-specific instrument has been suggested as a potentially useful approach for monitoring symptoms and quality of life in this population.20 While overall health status would also be expected to improve among highly symptomatic patients undergoing TAVR, generic health status measures are less sensitive to change and do not specifically assess symptoms and functional status along the disease pathway of aortic stenosis. As such, they would not be as sensitive or specific as a disease-specific measure for determining whether a patient has had an acceptable outcome after TAVR.
For this study, disease-specific health status, which includes symptoms, functional status, and quality of life, was assessed by means of the Kansas City Cardiomyopathy Questionnaire (KCCQ),21 a 23-item self-administered questionnaire that addresses specific health domains pertaining to heart failure: physical limitation, symptoms, QoL, social limitation, symptom stability, and self-efficacy. The first 4 of these domains are combined into an overall summary scale, which was the primary health status outcome for our study. Values for each domain (including the summary scale) range from 0 to 100 with higher scores indicating lower symptom burden and better QoL. Linguistically and culturally validated translations of the KCCQ were provided to non-English speakers.
Based on previous work, a KCCQ summary score >75 corresponds roughly to NYHA functional class I, and scores of 60–75, 45–60, and <45 correspond to NYHA functional classes II, III, and IV, respectively.22 Among outpatients with heart failure, small, moderate, or large clinical improvements as rated by treating physicians corresponded with changes in the KCCQ summary score of approximately 5, 10, and 20 points, respectively. The KCCQ has undergone extensive reliability and validity testing in various heart failure populations21, 23–24 as well as in patients with severe aortic stenosis,25 and it has been shown to predict mortality, readmission25–26 and costs.27
Conceptual Framework
A poor outcome of an intervention is, by definition, one in which there is a failure to achieve the expected treatment goals of that intervention. In order to determine the most appropriate definition for poor outcome after TAVR, it is important to clarify the treatment goals of TAVR, particularly from patients’ perspectives. In patients with severe symptomatic aortic stenosis, TAVR has 2 important potential benefits: improved survival and reduced symptoms. While most patients likely choose TAVR for some combination of these potential benefits, the relative importance of these benefits likely vary by patients’ characteristics prior to treatment. Clinical logic would suggest that most patients who have minimal symptoms are choosing to undergo TAVR primarily to achieve an expected improvement in survival. Conversely, patients with substantial symptoms and functional limitation from their aortic stenosis most likely choose to undergo TAVR for an expected improvement in symptoms
Analytic Approach
For the purposes of this study, we used various combinations of mortality and health status outcomes 6 months after TAVR to define clinical success or failure. Although this time frame is somewhat arbitrary, given the advanced age of the TAVR population, we felt that even relatively brief improvements in health and survival would be important. We did not consider a 1-month timeframe to be appropriate for this evaluation, however, since some patients might not have fully recovered from the procedure and would require more time to improve their functioning and symptom control to establish a new ‘steady state’.
For each conceptual definition of clinical success or failure, we considered a poor outcome to be either death or failure to achieve a specific level of QoL 6 months after TAVR. We then plotted 6-month vs. baseline KCCQ scores among those who were alive at 6 months, and we characterized the cohort of patients who would be considered to have had a poor outcome according to each of the 4 potential definitions. As there is no gold standard for a “poor outcome” after TAVR, we considered the strengths and weaknesses of each of the definitions qualitatively using the conceptual framework outlined above. All statistical analyses were performed with the use of SAS software, version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Patient Population
A total of 527 patients underwent initial TAVR as part of the PARTNER trial (348 in Cohort A, 179 in Cohort B) via either the transfemoral (n=423) or transapical route (n=104). Of these patients, 89 died within 6 months of TAVR. Among the 438 who survived 6 months, KCCQ data were available for 374 (85%). As such, our analytic population included 463 patients who underwent TAVR and who were either dead or completed the KCCQ at 6 months after their procedure. Patients who were alive but missing 6-month data (n=64) had similar demographic and clinical characteristics to those with 6-month KCCQ data, except that patients with missing data were more likely to be classified as NYHA functional class III or IV at baseline (100% vs. 92%, p=0.013). Baseline KCCQ scores were similar between the 2 groups, however (missing vs. not missing: 40.6 vs. 39.1, p=0.662).
The baseline characteristics of the study population are shown in Table 1. The mean age of the population was 84 years, half were female, and 73% had a history of coronary artery disease. Mean aortic valve gradient was 43 mmHg and >90% had NYHA class III or IV symptoms. Eighty percent of patients were treated via the transfemoral route, and the average estimated risk of operative mortality for the patients was nearly 12%.
Table 1.
Baseline Demographic and Clinical Characteristics of the Analytic Population
| Characteristic | N=463 |
|---|---|
| Age (y) | 83.5±7.4 |
| Female | 46.6% |
| Caucasian | 93.2% |
| Coronary Artery Disease (%) | 73.2% |
| Peripheral Vascular Disease (%) | 38.6% |
| Diabetes Mellitus (%) | 38.2% |
| Oxygen-Dependent Lung Disease (%) | 13.8% |
| Creatinine (mg/dL) | 1.30±0.47 |
| Mini Mental State Exam Score | 26.7±3.5 |
| NYHA Class | |
| II | 6.3% |
| III | 44.5% |
| IV | 49.2% |
| Mean Aortic Valve Gradient (mmHg) | 43.3±14.9 |
| Ejection Fraction (%) | 52.9±13.2 |
| Cohort A (vs. B) | 66.2% |
| Transfemoral Approach (%) | 80.1% |
| STS Mortality Risk Score (%) | 11.7±4.4 |
STS, Society of Thoracic Surgeons
Potential Definitions
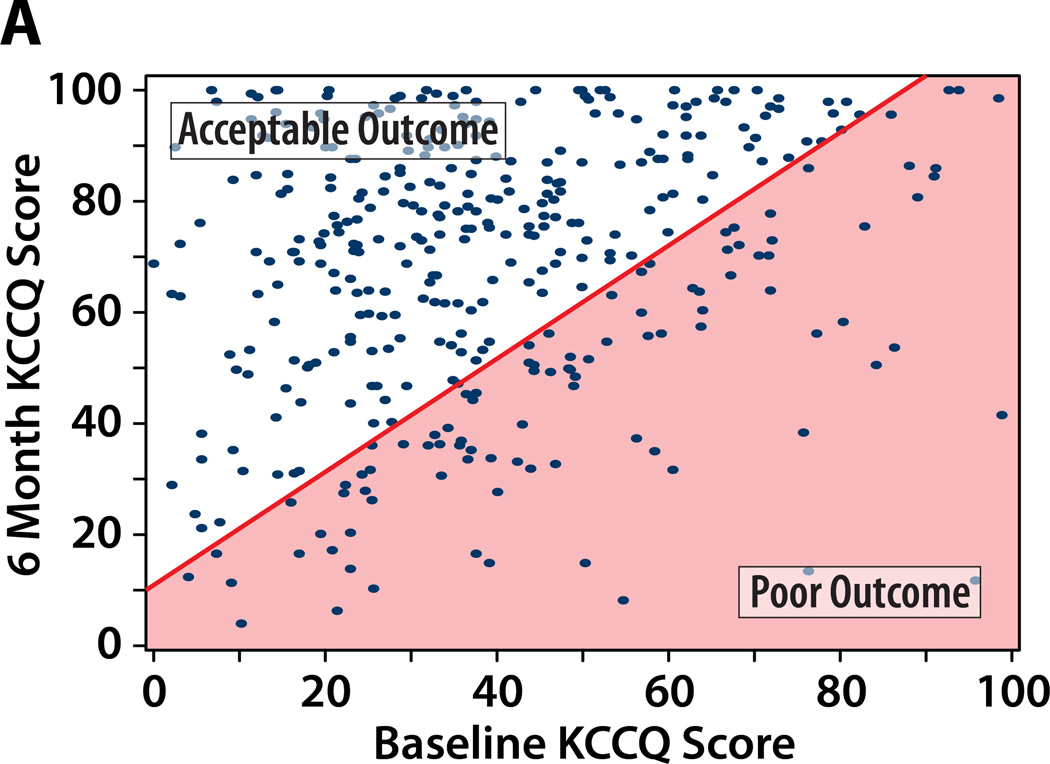
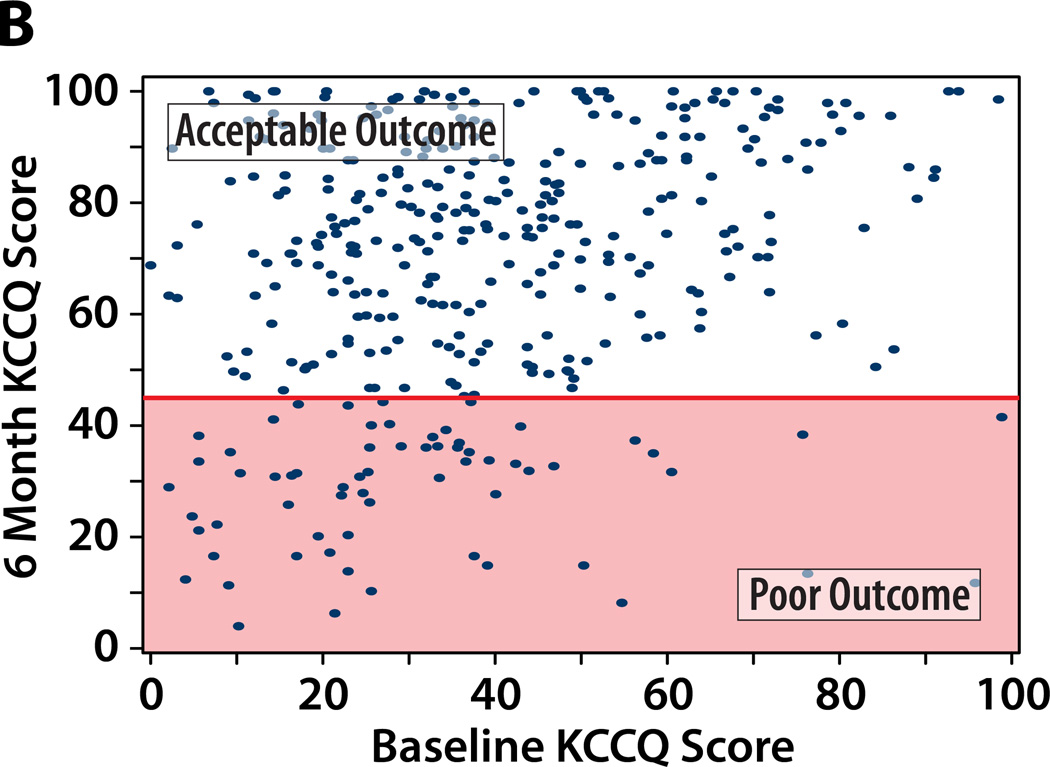
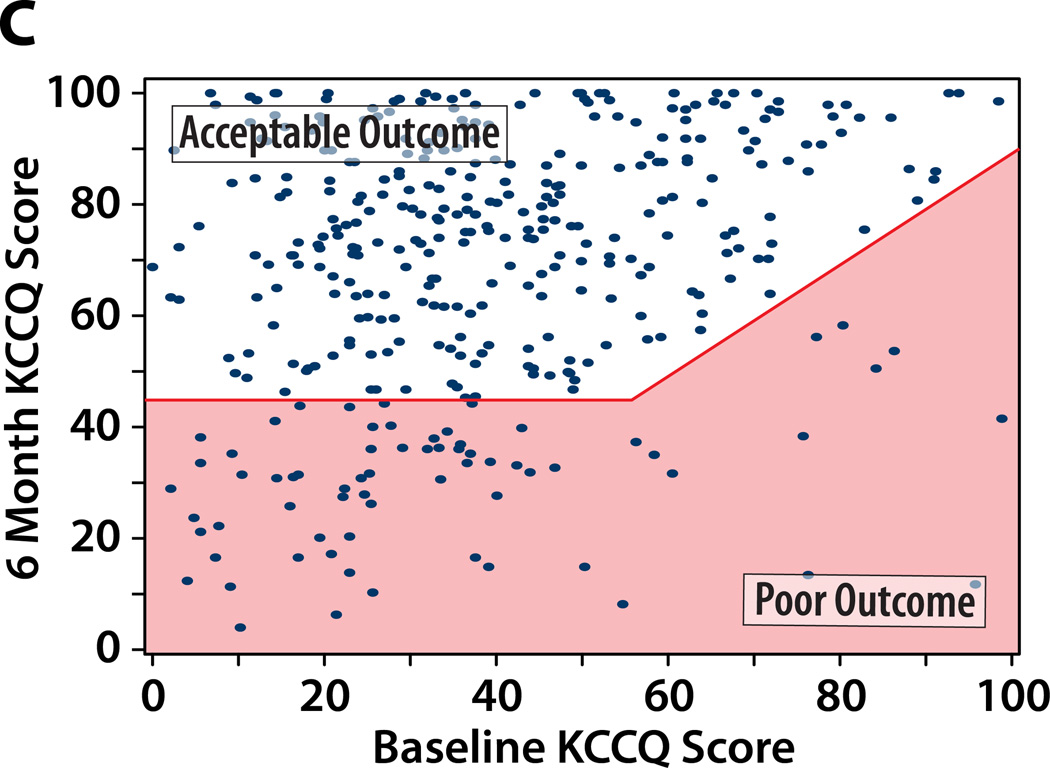
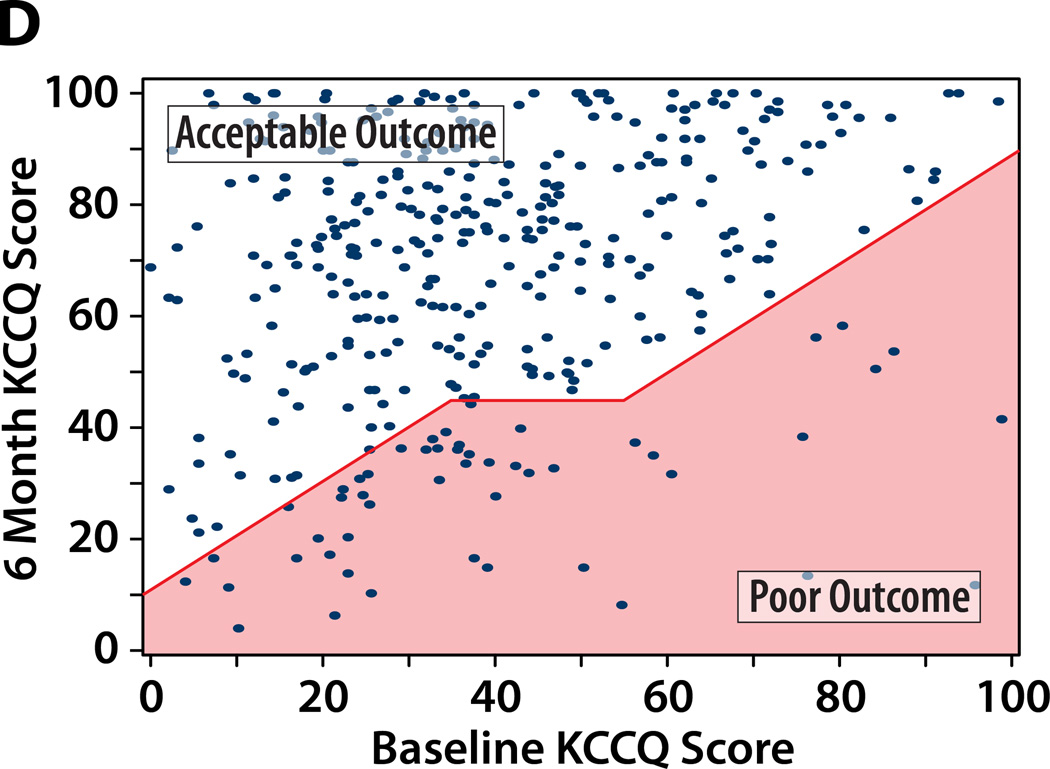
We considered 4 potential definitions of a poor outcome after TAVR, each of which includes death as a poor outcome. Figure 1 illustrates the paired baseline and 6-month KCCQ scores within the study population classified as having a poor or acceptable outcomes at 6 months after TAVR according to each of the potential definitions, and Table 2 summarizes the percentage of patients that fall into each outcome category by each definition.
Figure 1. A–D. Patients categorized as having a poor QoL outcome after TAVR by each definition.
Poor QoL Outcome indicated by the shaded area. An additional 89 patients who died by 6 months would be considered as having a poor outcome (not shown on the graph).
A. Definition #1: Poor QoL Outcome = KCCQ increase (improvement) of <10 points. B: Definition #2: Poor QoL Outcome = KCCQ <45 points. C: Definition #3: Poor QoL Outcome = KCCQ <45 or decrease (worsening) of ≥10 points. Definition #4: Poor QoL Outcome = KCCQ <45 (unless KCCQ increase ≥10 points) or decrease (worsening) of ≥10 points.
Table 2.
Frequency of Poor Outcome After TAVR by Each Definition
| Death | Poor QoL | Poor Outcome (total) |
Acceptable Outcome |
|
|---|---|---|---|---|
| Definition #1 | 89 (19%) | 90 (20%) | 179 (39%) | 280 (61%) |
| Death or KCCQ increase <10 points | ||||
| Definition #2 | 89 (19%) | 68 (14%) | 157 (33%) | 321 (67%) |
| Death or KCCQ <45 points | ||||
| Definition #3 | 89 (19%) | 72 (16%) | 161 (35%) | 302 (65%) |
| Death or KCCQ <45 or KCCQ decrease ≥10 points | ||||
| Definition #4 | 89 (19%) | 53 (11%) | 142 (31%) | 321 (69%) |
| Death or KCCQ decrease ≥10 points or KCCQ <45 (unless KCCQ increases ≥10 points) | ||||
Sample sizes among definitions may differ slightly due to the amount of QoL data needed to establish the definition of poor outcome
Definition #1 of Poor Outcome: Death or KCCQ improvement of <10 points
Under this definition, patients who either die or fail to achieve at least a moderate improvement in health status22 at 6 months would be considered to have had a poor outcome after TAVR. In the PARTNER population, this definition would have identified 39% of patients as having had a poor outcome of TAVR (19% death, 20% limited QoL benefit; Table 2). As this definition requires patients to realize both a survival and a QoL benefit from TAVR, it has face validity among a group of highly symptomatic patients with aortic stenosis. However, it is evident from Figure 1a that patients who started with lower KCCQ scores were more likely to achieve a 10 point improvement after TAVR. In addition, any patient with a baseline KCCQ score of >90 would be categorized as having a poor outcome regardless of the KCCQ score at 6 months. Thus, by using this definition for poor outcome, we would preferentially identify patients who started at the higher end of the range of baseline KCCQ values as not deriving a QoL benefit from the procedure. It is not clear that this represents treatment failure, however, since this subgroup was not particularly symptomatic prior to TAVR and thus were most likely to be undergoing the procedure for its survival benefit. Therefore, it does not seem appropriate to classify this outcome as poor for all TAVR candidates.
Definition #2 of Poor Outcome: Death or KCCQ <45
Since a KCCQ overall summary score <45 generally corresponds to NYHA class IV, this definition would classify patients as having a poor outcome if at 6 months after TAVR they were either dead or had very poor self-reported health status. One-third of PARTNER TAVR patients would be classified as having had a poor outcome after TAVR according to this definition (19% death, 14% poor QoL; Table 2). This definition has face validity in that a patient who continues to have NYHA Class IV heart failure symptoms (and thus a very poor functional capacity after TAVR) would not be considered to have had an acceptable outcome from the procedure.. As shown in Figure 1b, this definition preferentially identifies patients with lower baseline KCCQ scores as more likely to have a poor outcome. This performance characteristic is intuitively appealing, since patients with very poor QoL pre-TAVR are more likely to have chosen the intervention for its expected benefits on symptoms and QoL, as opposed to its survival benefit. However, this definition ignores patients with relatively high pre-TAVR KCCQ scores whose health status declines after treatment (e.g., a patient whose KCCQ overall summary score declines from 80 pre-TAVR to 50 at 6-months after TAVR would still be considered an acceptable outcome).
Definition #3 of Poor Outcome: Death or KCCQ <45 or KCCQ decrease of ≥10 points
This definition seeks to overcome the limitations of Definition #2 by requiring that the patient achieve a minimum QoL level (i.e., KCCQ ≥45 ≈ NYHA Class III symptoms or better) and not experience a clinically-meaningful decline in QoL post procedure (KCCQ decrease of ≥10 points). Approximately 35% of the PARTNER population would be categorized as having had a poor outcome of TAVR according to this definition (19% death, 16% poor QoL; Table 2 and Figure 1c). A potential criticism of this definition is that patients who begin with very low KCCQ scores and who improve after TAVR but not to the threshold of 45 would still be considered to have experienced a poor outcome (e.g., a patient whose KCCQ overall summary score increases from 10 to 25).
Definition of Poor Outcome #4: Death or KCCQ decrease of ≥10 points or KCCQ <45 (unless KCCQ increases by ≥10 points)
This definition addresses the potential criticism of Definition #3, by categorizing as acceptable any patient who is alive with at least a modest improvement in health status at 6 months after TAVR, regardless of his or her final level of QoL. Relative to Definition #3, Definition #4 recategorizes an additional 4% of PARTNER TAVR patients as having had an acceptable outcome, such that 31% of the population would be considered to have had a poor outcome (19% death, 11% poor QoL; Table 2; Figure 1d). However, the patient that is reclassified as having an acceptable outcome by this definition still has a very poor QoL with marked symptom burden and function limitation; thus, while modestly improved, this may still not an acceptable outcome of TAVR. Of note, this approach adds a level of complexity above Definition #3 that results in only minimal reclassification.
DISCUSSION
Aortic valve replacement, either with surgery or TAVR, is effective at relieving the hemodynamic obstruction of aortic stenosis and can lead to excellent outcomes in good operative candidates. However, many patients with severe aortic stenosis are less than ideal candidates for surgical valve replacement. Advanced age, multiple comorbidities, deconditioning, and frailty can all contribute to an inability to recover from surgery. While some patients may clearly be very poor candidates for surgical valve replacement, with the emergence of TAVR as a less invasive treatment modality, many of these patients can now be considered for definitive treatment of their aortic stenosis. Nonetheless, some of these potential TAVR candidates do not appear to derive a mortality or QoL benefit from the procedure, despite successful relief of the hemodynamic obstruction.3–6 Avoiding an unnecessary, expensive, and somewhat risky procedure for such patients would therefore seem to be a worthwhile goal. In order to identify patients in whom valve replacement is unlikely to provide benefit, the first step is to establish a definition for a poor outcome after TAVR. For a procedure such as TAVR that is used to treat a condition that results in both reduced life expectancy and impaired QoL, this definition must include both a mortality and a QoL component. However, combining these two endpoints into a single definition of a poor outcome can be challenging. To address this issue, we have explored several alternative definitions of a poor outcome after TAVR and the relative strengths and weaknesses of each approach. We believe that this is the first attempt to rigorously and objectively define a poor outcome for TAVR and, as such, an important step along the path to defining patients for whom the procedure might be considered “inappropriate” or, at a minimum, ill-advised due to the likelihood of poor outcome after the treatment.
Since TAVR provides both quality and quantity of life benefits, patients may choose TAVR for a combination of these two benefits, but the priority of the benefits is likely to differ across individuals. For patients with a good QoL prior to TAVR, the most important benefit is likely to be a reduction in the risk of mortality, since they already have minimal symptoms or QoL impairment from their aortic stenosis. In these cases, even if there were no QoL improvement after TAVR, the patient would likely consider this to be an acceptable outcome, since their pre-procedure QoL was reasonable and they would expect to receive a substantial survival benefit from the procedure. For such patients, the result would be considered acceptable as long as their QoL is not worsened by TAVR.
On the other hand, for patients who have a very poor QoL prior to TAVR, we suspect, based on both our clinical experience as well as prior studies of patients with severe heart failure16–17, that the primary motivation for undergoing TAVR is for its QoL benefit. Thus, if a patient’s QoL failed to improve to a minimum acceptable level after TAVR, this would not be considered an acceptable result. Due to these differing priorities of survival and QoL for patients with differing clinical manifestations of aortic stenosis, we favor Definitions #3 or #4 as providing a reasonably balanced and nuanced summary of the overall success (or failure) of TAVR. In our experience, these definitions seem to most commonly encompass the values and goals of the patients who are considering TAVR. Ultimately, we favor Definition #3 since Definition #4 adds substantial complexity to the definition with minimal net reclassification. Moreover, in our experience, patients who continue to have very poor QoL after TAVR are unlikely to view the procedure as having been successful, even if their QoL is improved from baseline. Future prospective evaluations of patients’ perspectives of the benefits of TAVR are needed to confirm our assessment.
Insights from Other Patient Populations
A unique consideration in evaluating TAVR is that there is a range of patients who are candidates for TAVR who may prioritize the QoL and survival benefits differently. It is reasonable to assume that highly symptomatic patients are choosing the procedure for symptom relief, while those without significant functional impairment are choosing the procedure to prolong their lives (while maintaining their current health status). Few other procedures in cardiovascular medicine have such a balance of survival and QoL motivations and therefore need to consider both factors in any definition of a poor outcome.
One example of a procedure that provides both survival and QoL benefits is the use of left ventricular assist devices (LVAD) as destination therapy for advanced heart failure. Similar to TAVR candidates, patients who are considered for LVADs are highly symptomatic and would be expected to have a markedly reduced life expectancy without intervention. In the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial of patients with end-stage heart failure ineligible for cardiac transplantation, there was a 27% absolute reduction in 1-year mortality with LVAD implantation vs. medical therapy.28 Patients who received LVADs also had markedly improved QoL. However, roughly half of the patients treated with LVADs died within the first 1 year, and only 1 in 4 survived 2 years—results similar (albeit even more extreme) to those observed in the inoperable cohort of the PARTNER trial (Cohort B).3 While some of the deaths observed in REMATCH were due to device-related complications from the LVAD, other patients were simply too ill to survive the operation and recovery period. Even with improvements in device technology and surgical experience, more recent results among patients undergoing LVADs for destination therapy demonstrated that one quarter of patients died in the hospital after device implantation and half were dead at a year.29 In that study, the authors constructed a risk score for poor 1-year outcome and identified poor nutrition, hematological abnormalities, and markers of end-organ or right ventricular dysfunction as factors associated with greater mortality; however, this analysis was based solely on mortality. Given the importance of QoL improvement as a therapeutic goal for these patients, consideration of a combined endpoint similar to ours may be appropriate for future studies on the value of LVADs in patients with end-stage heart failure.
Implications for Future Studies
Defining a poor (or acceptable) outcome is an essential first step in identifying patients who are at high-risk for a poor outcome—a critical goal in defining patient expectations when procedures involve significant risk and a prolonged recovery period. While many prior analyses of TAVR and of other similar interventions have focused on identifying patients at high-risk for mortality, we believe that focusing only on mortality does not properly reflect the range of treatment goals that are important to patients and provides patients with only a limited view of the potential outcomes after the procedure. While each endpoint could be analyzed separately, these types of models are often more difficult to interpret—particularly if the predictors of mortality and poor QoL (among survivors) differ. On the other hand, if it were possible to prospectively identify patients at high-risk for either death or poor QoL, this information can be provided to patients and their families prior to their undergoing TAVR to both inform their expectations and help guide treatment decisions.
It is important to note that our study population included some patients who experienced major procedural complications during TAVR (such as major vascular injury, cardiac perforation, or stroke). Although these complications may be difficult to predict, a priori they can certainly affect patient’s subsequent life expectancy and QoL. As such, factors that predict these complications are likely to be distinct from those that are associated with poor survival or limited QoL benefit among patients who do not experience procedural complications. Given the unpredictable nature of these complications, it may be reasonable to exclude such patients from future analyses that seek to identify predictors of a poor outcome after TAVR and from calculations of the proportion of patients in whom TAVR might be considered to have been “inappropriate” or “futile”. However, for the purposes of this study, we chose to include such individuals in both the numerator and denominator of our calculations, since we believe that an early death or poor QoL after TAVR, even if primarily due to a procedural complication, remains a poor outcome from the patient’s perspective.
Limitations
There are potential limitations to our study that merit further discussion. Most importantly, individual patients may disagree with our definition(s) of a poor outcome and value the benefits of TAVR in a unique manner, which is an inherent challenge in defining any adverse outcome or measure of net clinical benefit for a population. However, this information can still be used to guide expectations and support informed discussion with patients prior to deciding on a treatment plan. In addition, further qualitative research may help us understand how patients with severe aortic stenosis considering TAVR would define a poor (or acceptable) outcome. Ultimately, the ability to tailor risk predictions to an individual’s own unique concept of a successful and poor outcome would be ideal. Second, our definition for a poor outcome was derived from 2 clinical trials of very sick and highly symptomatic patients. As TAVR outcomes improve with device modifications and greater experience and the procedure expands into the population of patients who are less sick, this definition may need to be refined. Nonetheless, even as it expands into lower-risk patients, TAVR is likely to continue to be offered to challenging patients at high surgical risk for whom our proposed categorization of a poor outcome would still be relevant. Furthermore, our systematic approach could be used to re-evaluate what constitutes an acceptable outcome after TAVR as the patient population changes.
Conclusions
Although clinical trials have demonstrated that TAVR is associated with significant QoL and survival benefits for patients at high surgical risk, not all patients derive these benefits and many never fully recover from the procedure. We propose a specific definition for poor outcome after TAVR, which combines these two domains of health into a single composite endpoint. According to this definition, a patient would be considered as having a poor outcome after TAVR if he or she is either dead, has a very poor QoL, or had a substantial decline in his or her QoL from baseline to 6 months. Establishing this definition is a first step in a research agenda aimed at determining what patient characteristics are associated with a high risk of a poor outcome, so that patients can be provided with appropriate expectations of functional recovery after TAVR and clinicians can target therapy to those most likely to derive meaningful long-term benefit.
What is Known.
It has been recommended that transcatheter aortic valve replacement (TAVR) not be offered to patients who have limited potential to derive functional improvement or live longer following TAVR; however, there has been no guidance about to how to prospectively identify these patients.
In order to predict which patients are high risk for a poor outcome after TAVR, we must first define what outcome (encompassing both a mortality and a quality of life component) constitutes a poor vs. acceptable outcome.
TAVR offers the possibility of both a quality and quantity of life benefit, and patients may choose to undergo TAVR for one or both of these two potential benefits depending on how symptomatic they are from their aortic stenosis prior to the procedure.
What this Article Adds
Using mortality and quality of life data from the PARTNER trial of patients undergoing TAVR, this paper considers 4 potential definitions for a poor outcome after TAVR that combine mortality and quality of life endpoints and examines the advantages and disadvantages of each definition.
The authors suggest defining a poor outcome at 6 months after TAVR as either (1) death, (2) a very poor quality of life, or (3) a substantial worsening of quality of life and provide quantitative definitions for the second and third criteria; this endpoint can then be used to facilitate development of predictive models that may be useful to identify patients who are poor candidates for TAVR.
Acknowledgments
Spertus: owns the copyright to the KCCQ
Thourani: research support from Edwards Lifesciences, Sorin Medical; consulting income from DirectFlow, St. Jude Medical, Sorin Medical; royalties/intellectual property rights from Apica
Herrmann: research support from Abbott Vascular, Boston Scientific, Edwards Lifesciences, Medtronic, Siemens, W.L. Gore & Associates; consulting income from Paeion, W.L. Gore & Associates
Leon: travel reimbursements from Edwards Lifesciences for activities related to his position on the Executive Committee of the PARTNER Trial
Cohen: research support from Edwards Lifesciences, Medtronic, Boston Scientific, Abbott Vascular, MedRad, Merck/Schering-Plough, and Eli Lilly-Daiichi Sankyo; consulting income from Schering-Plough, Eli Lilly, Medtronic, and Cordis; and speaking honoraria from Eli Lilly, The Medicines Company, and St. Jude Medical.
Funding Source:
The PARTNER trial was sponsored by Edwards Lifesciences. This current study was self-funded, and the funding organization for the trial did not play a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest Disclosures:
The other authors report no potential conflicts.
REFERENCES
- 1.Van Brabandt H, Neyt M, Hulstaert F. Transcatheter aortic valve implantation (tavi): Risky and costly. BMJ. 2012;345:e4710. doi: 10.1136/bmj.e4710. [DOI] [PubMed] [Google Scholar]
- 2.Desai CS, Bonow RO. Transcatheter valve replacement for aortic stenosis: Balancing benefits, risks, and expectations. JAMA. 2012;308:573–574. doi: 10.1001/jama.2012.9427. [DOI] [PubMed] [Google Scholar]
- 3.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds MR, Magnuson EA, Lei Y, Leon MB, Smith CR, Svensson LG, Webb JG, Babaliaros VC, Bowers BS, Fearon WF, Herrmann HC, Kapadia S, Kodali SK, Makkar RR, Pichard AD, Cohen DJ. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124:1964–1972. doi: 10.1161/CIRCULATIONAHA.111.040022. [DOI] [PubMed] [Google Scholar]
- 5.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds MR, Magnuson EA, Wang K, Thourani VH, Williams M, Zajarias A, Rihal CS, Brown DL, Smith CR, Leon MB, Cohen DJ. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: Results from the partner (placement of aortic transcatheter valve) trial (cohort a) J Am Coll Cardiol. 2012;60:548–558. doi: 10.1016/j.jacc.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 7.Holmes DR, Jr, Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, Calhoon JH, Carabello BA, Desai MY, Edwards FH, Francis GS, Gardner TJ, Kappetein AP, Linderbaum JA, Mukherjee C, Mukherjee D, Otto CM, Ruiz CE, Sacco RL, Smith D, Thomas JD. 2012 accf/aats/scai/sts expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200–1254. doi: 10.1016/j.jacc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration. Fda executive summary: Edwards sapien™ transcatheter heart valve. 2011 Jul 20; Www.Fda.Gov/downloads/.../ucm262930.Pdf.
- 9.Centers for Medicare & Medicaid Services. Decision memo for transcatheter aortic valve replacement (tavr) (cag-00430n) 2012 May 5; [Google Scholar]
- 10.Lazar HL. Transcatheter aortic valves-where do we go from here? N Engl J Med. 2010;363:1667–1668. doi: 10.1056/NEJMe1009405. [DOI] [PubMed] [Google Scholar]
- 11.Seiffert M, Schnabel R, Conradi L, Diemert P, Schirmer J, Koschyk D, Linder M, Kersten JF, Grosser A, Wilde S, Blankenberg S, Reichenspurner H, Baldus S, Treede H. Predictors and outcomes after transcatheter aortic valve implantation using different approaches according to the valve academic research consortium definitions. Catheter Cardiovasc Interv. 2012 Nov 21; doi: 10.1002/ccd.24751. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Pilgrim T, Kalesan B, Wenaweser P, Huber C, Stortecky S, Buellesfeld L, Khattab AA, Eberle B, Gloekler S, Gsponer T, Meier B, Juni P, Carrel T, Windecker S. Predictors of clinical outcomes in patients with severe aortic stenosis undergoing tavi: A multistate analysis. Circ Cardiovasc Interv. 2012;5:856–861. doi: 10.1161/CIRCINTERVENTIONS.112.974899. [DOI] [PubMed] [Google Scholar]
- 13.Rodes-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Osten M, Feindel CM, Natarajan MK, Velianou JL, Martucci G, Devarennes B, Chisholm R, Peterson M, Thompson CR, Wood D, Toggweiler S, Gurvitch R, Lichtenstein SV, Doyle D, Delarochelliere R, Teoh K, Chu V, Bainey K, Lachapelle K, Cheema A, Latter D, Dumesnil JG, Pibarot P, Horlick E. Long-term outcomes after transcatheter aortic valve implantation: Insights on prognostic factors and valve durability from the canadian multicenter experience. J Am Coll Cardiol. 2012;60:1864–1875. doi: 10.1016/j.jacc.2012.08.960. [DOI] [PubMed] [Google Scholar]
- 14.Moat NE, Ludman P, de Belder MA, Bridgewater B, Cunningham AD, Young CP, Thomas M, Kovac J, Spyt T, MacCarthy PA, Wendler O, Hildick-Smith D, Davies SW, Trivedi U, Blackman DJ, Levy RD, Brecker SJ, Baumbach A, Daniel T, Gray H, Mullen MJ. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: The u.K. Tavi (united kingdom transcatheter aortic valve implantation) registry. J Am Coll Cardiol. 2011;58:2130–2138. doi: 10.1016/j.jacc.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 15.Wenaweser P, Pilgrim T, Kadner A, Huber C, Stortecky S, Buellesfeld L, Khattab AA, Meuli F, Roth N, Eberle B, Erdos G, Brinks H, Kalesan B, Meier B, Juni P, Carrel T, Windecker S. Clinical outcomes of patients with severe aortic stenosis at increased surgical risk according to treatment modality. J Am Coll Cardiol. 2011;58:2151–2162. doi: 10.1016/j.jacc.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 16.Tsevat J, Dawson NV, Wu AW, Lynn J, Soukup JR, Cook EF, Vidaillet H, Phillips RS. Health values of hospitalized patients 80 years or older. Help investigators. Hospitalized elderly longitudinal project. JAMA. 1998;279:371–375. doi: 10.1001/jama.279.5.371. [DOI] [PubMed] [Google Scholar]
- 17.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20:1016–1024. doi: 10.1016/s1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 18.Straumann E, Kiowski W, Langer I, Gradel E, Stulz P, Burckhardt D, Pfisterer M, Burkart F. Aortic valve replacement in elderly patients with aortic stenosis. Br Heart J. 1994;71:449–453. doi: 10.1136/hrt.71.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Descoutures F, Himbert D, Lepage L, Iung B, Detaint D, Tchetche D, Brochet E, Castier Y, Depoix JP, Nataf P, Vahanian A. Contemporary surgical or percutaneous management of severe aortic stenosis in the elderly. Eur Heart J. 2008;29:1410–1417. doi: 10.1093/eurheartj/ehn081. [DOI] [PubMed] [Google Scholar]
- 20.Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, Krucoff MW, Mack M, Mehran R, Miller C, Morel MA, Petersen J, Popma JJ, Takkenberg JJ, Vahanian A, van Es GA, Vranckx P, Webb JG, Windecker S, Serruys PW. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: A consensus report from the valve academic research consortium. J Am Coll Cardiol. 2011;57:253–269. doi: 10.1016/j.jacc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the kansas city cardiomyopathy questionnaire: A new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 22.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS. Monitoring clinical changes in patients with heart failure: A comparison of methods. Am Heart J. 2005;150:707–715. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Pettersen KI, Reikvam A, Rollag A, Stavem K. Reliability and validity of the kansas city cardiomyopathy questionnaire in patients with previous myocardial infarction. Eur J Heart Fail. 2005;7:235–242. doi: 10.1016/j.ejheart.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Spertus JA, Jones PG, Kim J, Globe D. Validity, reliability, and responsiveness of the kansas city cardiomyopathy questionnaire in anemic heart failure patients. Qual Life Res. 2008;17:291–298. doi: 10.1007/s11136-007-9302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold SV, Spertus JA, Lei Y, Allen KB, Chhatriwalla AK, Leon MB, Smith CR, Reynolds MR, Webb JG, Svensson LG, Cohen DJ. Use of the kansas city cardiomyopathy questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6:61–67. doi: 10.1161/CIRCHEARTFAILURE.112.970053. [DOI] [PubMed] [Google Scholar]
- 26.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation. 2004;110:546–551. doi: 10.1161/01.CIR.0000136991.85540.A9. [DOI] [PubMed] [Google Scholar]
- 27.Chan PS, Soto G, Jones PG, Nallamothu BK, Zhang Z, Weintraub WS, Spertus JA. Patient health status and costs in heart failure: Insights from the eplerenone post-acute myocardial infarction heart failure efficacy and survival study (ephesus) Circulation. 2009;119:398–407. doi: 10.1161/CIRCULATIONAHA.108.820472. [DOI] [PubMed] [Google Scholar]
- 28.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 29.Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, Rogers JG, Naka Y, Mancini D, Miller LW. Outcomes of left ventricular assist device implantation as destination therapy in the post-rematch era: Implications for patient selection. Circulation. 2007;116:497–505. doi: 10.1161/CIRCULATIONAHA.107.691972. [DOI] [PubMed] [Google Scholar]