Abstract
Gout is a common inflammatory arthritis triggered by the crystallization of uric acid within the joints. Gout affects millions worldwide and has an increasing prevalence. Recent research has been carried out to better qualify and quantify the risk factors predisposing individuals to gout. These can largely be broken into non-modifiable risk factors such as sex, age, race, and genetics, and modifiable risk factors such as diet and lifestyle. Increasing knowledge of factors predisposing certain individuals to gout could potentially lead to improved preventive practices. This review summarizes the non-modifiable and modifiable risk factors associated with development of gout.
Keywords: Gout, risk factors, race, sex, genetics, diet
Introduction
Gout is one of the most common inflammatory arthritides, caused by hyperuricemia, with an increasing prevalence. Hyperuricemia is often a consequence of renal under-excretion of uric acid as more than 70% of urate is excreted via the kidney primarily through the proximal tubule (1). Hyperuricemia is well proven to be positively associated with incident gout in a dose dependent manner as seen in both the Normative Aging and Framingham Heart Studies (2-4). According to data from 2007-2008, approximately 8.3 million US adults were affected by gout, reflecting a 1.2% increase in prevalence from data around 20 years prior (2, 5). Gout is associated with high economic burden, resulting in five more absence days from work and over $3,000 in additional annual cost compared to patients without gout (6). Given the burden of gout on society, factors that predispose to hyperuricemia and gout have been of keen interest, but there is a paucity of clinical trials for the primary prevention of gout (7).
Non-modifiable risk factors, including sex, age and race or ethnicity, have been under investigation for potential roles in gout development (4, 8, 9). More recently genome-wide association studies (GWAS) have revealed genetic variants primarily involving renal urate transport that may explain certain individuals' propensity for developing hyperuricemia and gout (10, 11). In addition to these non-modifiable risk factors, modifiable or lifestyle factors play a significant role in reducing or increasing the risk of gout (2, 12).
This review focuses on the non-modifiable and modifiable risk factors of gout. With the increasing prevalence of gout, a strong knowledge of these risk factors for preclinical gout and hyperuricemia is important so that at-risk individuals can be identified and appropriately counseled. The linkage between gout and co-morbidities including cardiovascular disease and metabolic syndrome, as well as the role of medications, is beyond the scope of this review.
Demographic Factors
Sex
In the population under 65 years of age, males have a fourfold higher prevalence of gout than do females; however, this ratio reduces to 3:1 male to female over 65 years (8). For females as for males, higher levels of uric acid confer an increase in risk of gout. Prospective cohort data suggest the incidence of gout in females increases with serum uric acid levels but a lower rate of this increase, such that a female with a uric acid level >5mg/dl has a significantly lower risk of gout than her male counterpart (4).
The mean age of gout onset is approximately 10 year older in females than males (13-15). This delayed onset has been attributed to estrogen's enhancement of renal tubular urate excretion leading to the reduced risk of hyperuricemia and gout in pre-menopausal females (16). Prior work reports increased risk of hyperuricemia and incident gout in both natural and surgical (removal of ovaries prior to cessation of menses) menopause after adjusting for age, body mass index (BMI), smoking, hypertension and diet, but decreased uric acid levels and risk of incident gout in post-menopausal females taking hormone therapy (16, 17). Interestingly the risk of incident gout was higher among females with surgical menopause and premature menopause (age<45 years) in comparison to those with natural and average age of menopause (17).
Mechanistic data for this association were provided via research on ovariectomized mice with and without hormone replacement. Estrogen and progesterone decreased posttranslational expression of the urate reabsorption system including urate transporter 1 (URAT1), glucose transporter 9 (GLUT9), sodium-coupled monocarboxylate transporter 1 (Smct1), and urate efflux transporter ATP-binding cassette sub-family G member 2 (ABCG2), thus reducing renal urate reabsorption (18). A second potential mechanism for the increased risk in post-menopausal females when compared to pre-menopausal females arises from the increased prevalence of insulin resistance in the post-menopausal population (14). Elevated insulin levels are known to reduce renal urate excretion, this effect is more pronounced in females than males and is likely mediated through sex hormones (14, 19).
Age
Increasing age is strongly associated with an increased risk of hyperuricemia and gout. Cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) and a claims database demonstrated increasing prevalence of gout or serum uric acid with increasing age groups (8, 20). Prevalence of other factors associated with gout such as hypertension, diabetes and diuretic use also increases with age (21). Indeed evidence from the Framingham Heart Study demonstrates increased risk of incident gout with age, obesity, alcohol, diuretic use, and hypertension, with age being the only variable to have a different strength of association between females and males (4). In both the Normative Aging Study and a Japanese population, age was a predictor for incident gout or hyperuricemia along with BMI, hypertension, cholesterol and alcohol (3, 22). Table 1 outlines charactersitcs of the selected studiess on the effect of sex and age on gout.
Table 1. Study design and populations from selected citations pertaining to age and sex in gout.
| Study | Analysis | Population | Outcome |
|---|---|---|---|
| Campion, 1987 (3) | Prospective cohort | The Normative Aging Study, United States 2,046 males | Incident gout predicted by BMI (p<0.01), age (p<0.01), hypertension (p<0.05), and cholesterol level (p<0.05) |
| Puig, 1991 (15) | Cross-sectional | Chart review of Spanish patients 45 females and 220 males | Mean age at time of gout diagnosis 60.9 for females and 51.2 years for males (p<0.001) |
| Fang, 2000 (20) | Cross-sectional | First National Health and Nutrition Examination Survey 5926 females and males | Increase in uric acid from 5.3 mg/dl in patients <45 years to 5.7 mg/dl in those >65 years (p<0.001) |
| Kuzuya, 2002 (22) | Prospective cohort | Japanese health center data 30,349 females and 50,157 males | Serum uric acid increases with age. Association with age persists after controlling for BMI and alcohol |
| Wallace, 2004 (8) | Cross-sectional | Claims database for 8,000,000 patients in Unites States from 1990-1999 | Increase in gout or hyperuricemia with increasing age groups 45-64 (14 per 1,000 persons), 65-74 (31 per 1,000 persons), and 75+ (41 per 1,000 persons) |
| Harrold, 2006 (120) | Cross-sectional | 7 managed care plans, United States 1158 females and 4975 males | Females with gout have a mean age of 70 years versus 58 years for males (p<0.001). Females more likely to have hypertension, dyslipidemia, coronary heart disease, diabetes mellitus, peripheral arterial disease, renal insufficiency, renal failure then males. Females received diuretics more often than males 77% versus 40% (p<0.001) |
| Hak, 2008 (16) | Cross-sectional | Third National Health and Nutrition Examination Survey, United States 7662 females | Serum uric acid levels in females with natural and surgical menopause were higher than premenopausal females by 0.34 mg/dl (95% CI, 0.19-0.49) and 0.36 mg/dl (95% CI, 0.14-0.57). In comparison to untreated postmenopausal females, postmenopausal hormone use associated with a lower serum uric acid level 0.24 mg/dl (95% CI, 0.11-0.36). |
| Bhole, 2010 (4) | Prospective cohort | Framingham Heart Study, United States 2,476 females and 1,951 males | Increasing age, obesity, alcohol, diuretic use, and hypertension increases incident gout in females (all p <0.05). Magnitude of associations differed from males for age only (p for interaction = 0.02) |
| Hak, 2010 (17) | Prospective cohort | The Nurses' Health Study, United States 92,535 females | Postmenopausal females have increased risk of incident gout RR=1.26 (95% CI, 1.03-1.55) compared to premenopausal females. In comparison to untreated postmenopausal females, postmenopausal hormone users have a reduced risk of gout RR=0.82 (95% CI, 0.70- |
| Chen, 2012 (14) | Prospective cohort | Outcome database from Taiwan's National Health Insurance 265 females and 1,341 males | Mean age for diagnosis of gout 62.2 years in females and 54 years for males (p<0.001). |
| Öztürk, 2013 (13) | Cross-sectional | Multicenter population in Turkey 55 females and 257 males | Mean age for symptoms of gout 60.4 years in females and 50.6 years in males (p<0.001) |
BMI: Body mass index (kg/m2), CI: Confidence interval, RR: relative risk
Race/Ethnicity
The risk of developing hyperuricemia and gout varies across populations according to race and ethnicity. A comparison of African American and Caucasian male physicians revealed that African American's had a twofold increased risk of gout with a respective incidence rate of 3.11 and 1.82 per 1,000 person-years, which was partially attributed to increased incidence of hypertension in African Americans (23). Further longitudinal evidence from the Atherosclerosis Risk in Communities (ARIC) demonstrated an increased risk of incident gout in African Americans with hazard ratio (HR) of 1.62 (95% CI: 1.24-2.12) for females, and HR 1.49 (95% CI: 1.11-2.00) for males, which persisted after adjustment for uric acid level, BMI, diet, diabetes, hypertension and diuretic use (9). In contrast, evidence from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort showed that young African American females and males (mean age 24) had lower uric acid levels than Caucasians after adjusting for BMI, glomerular filtration rate, medications, and diet (24). Over the 20 years of follow up, African American and Caucasian males had similar risk of incident hyperuricemia (HR 1.12, 95% CI, 0.88-1.40), but African American females had 2.3 times the risk of hyperuricemia (95% CI, 1.34-3.99) in comparison to Caucasian females (24). This study supports previous data from NHANES, which found that Caucasian adolescents of both sexes had higher uric acid levels than both African Americans and Hispanics (25). Factors including sugar intake, BMI, onset of puberty and glomerular filtration rate did not seem to account for the noted variance leading both investigators to consider genetics as a potential contributor to the different UA levels by race(24, 25).
The Maori population in New Zealand has been the focus of several population-based gout studies (26). Work from Klemp et al noted hyperuricemia was much more common in the Maori than Europeans -- 27.1% versus 9.4% in males and 26.6% versus 10.5% in females, respectively. Being Maori also conferred a higher prevalence (6.4%) of gout compared to Europeans (2.9%) (26). The high prevalence of elevated uric acid and gout has been confirmed in subsequent studies and is attributed to the high prevalence of co-morbidities such as obesity, diabetes and hypertension in the Maori potentially stemming from underlying genetic predisposition exacerbated by European introduction of alcohol and protein rich diet (27-30).
The Hmong, a group originating in southern China, have also displayed a propensity for gout with a prevalence of 6.1% in the Minnesota Hmong males versus 2.5% in the non-Hmong males (31). The unique, abrupt introduction of this population to a purine heavy western diet and alcohol after the Vietnam conflict was felt to have some responsibility for the results (31). In a retrospective study of Hmong versus Caucasian patients in Minnesota, the Hmong were found to be significantly younger at gout onset (37.4 vs. 55 years) and have higher uric acid levels at disease onset, yet had fewer co-morbid diseases including hypertension, chronic kidney disease, obesity, and diuretic or heavy alcohol use, also leading to speculation on genetic predisposition (32).
A recent review of gout in the Filipino population reported that Filipino's are also at a higher risk of elevated uric acid levels and gout with mean serum urate levels among US Filipinos around 1 mg/dl higher than other US races and Filipinos in the Philippines (33). The possibility that the purine heavy western diet was at play was considered, but discrepancy persisted even after controlling for diet, furthermore, Filipinos in the US continued to have lower purine diets that their non-Filipino counterparts (33). Ultimately the variation was thought to be secondary to a potential combination of comorbidities including obesity, hypertension, diabetes, renal impairment and heart disease as well as genetic co-factors (33).
Genetic Factors
Heritability
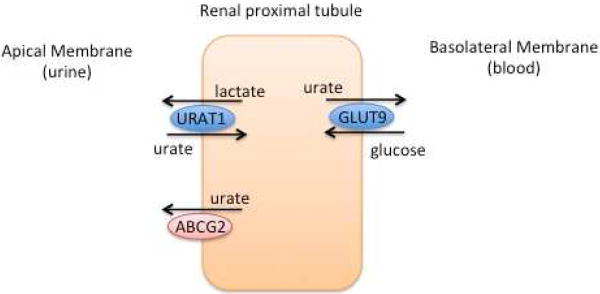
In the early 1990s Emmerson et al sought to explain a genetic predisposition for gout through renal urate clearance (34). In studying 37 pairs of normouricemic twins, they found that monozygotic twins had more similar values of urate clearance and fractional excretion of urate than dizygotes. They calculated the heritability of renal urate clearance to be 60%, while the heritability of the fractional excretion of urate was found to be 87% (34). Further investigation through segregation analysis on serum uric acid found a significant correlation in between siblings (r=0.19) and parent-offspring (r=0.22) (35). However, the segregation analysis was not consistent with a major Mendelian gene, leading the authors to conclude that uric acid levels were likely multifactorial with contribution from several genetic as well as environmental factors such as diet and menopausal status (35). The first GWAS for potential genes associated with uric acid utilized a cohort from the Framingham Heart Study. Evidence of linkage to uric acid was found on chromosome 15 and suggested on chromosomes 2 and 8 (36). Further interest in defining a role for genetics in gout has since culminated in many GWAS, which have identified genetic polymorphisms associated with uric acid and gout (10). Many of the uncovered genes code for proteins essential to renal urate reabsorption and secretion as discussed below (1) (Figure 1).
Figure 1. Visual representation of uric acid transport in the renal proximal tubule.
URAT1 (SLC22A12) and GLUT9 (SLC2A9) function to reabsorb uric acid while ABCG2 acts to secrete uric acid.
Adapted from Lipkowitz MS. Regulation of uric acid excretion by the kidney. Curr Rheumatol Rep 2012;14(2):179-88 and Reginato AM, Mount DB, Yang I, Choi HK. The genetics of hyperuricaemia and gout. Nat Rev Rheumatol 2012;8(10):610-21; with permission.
Specific Genetic Factors
SLC22A12
SLC22A12 encodes urate anion transporter 1 (URAT1), which is located on the brush border of the proximal tubules and is integral in urate absorption. URAT1 was first identified in 2002 by Enomoto et al and was pivotal from a clinical standpoint as analysis of a Japanese patient with idiopathic renal hypouricemia showed mutations in SLC22A12 (37). Furthermore, oocytes with mutated URAT1 cRNA had abolished urate transport ability (37). SCL22A12 variants have since been observed in additional patients with low serum urate levels, and low susceptibility to gout (38, 39). SCL22A12 alleles have also been associated with hyperuricemia and decreased fractional excretion of uric acid (FEUA) in Chinese and German populations respectively (40, 41). Further work in a cohort of 69 patients with gout showed that 23% had mutations in the SLC22A12 gene (42). Differing polymorphisms in SLC22A12 have been shown to have various effects on renal urate excretion through URAT1 and therefore influence serum urate levels and corresponding gout risk. A summary of studies and outcomes can be found in Table 2.
Table 2.
Studies pertaining to SLC22A12 polymorphisms and effect on serum uric acid and gout.
| Study | Population | Polymorphism | Outcome |
|---|---|---|---|
| Iwai, 2004 (39) | Japanese | Multiple missense, nonsense and deletion mutations | Mutations associated with decreased SUA |
| Taniguchi, 2005 (38) | Japanese | Mutation in exon 4 | Alleles associated with decreased SUA and protection from gout |
| Graessler, 2006 (40) | German | rs3825016 rs7932775 rs11231825 rs11602903 |
Alleles associated with SUA levels and varying risk for reduced FEUA |
| Shima, 2006 (121) | Japanese | rs893006 | Alleles associated with varying range of SUA levels |
| Vazquez-Mellado, 2007 (42) | Mexican | Mutations in exon 4 and 5 | Mutations found in patients with gout |
| Jang, 2008 (122) | Korean | rs1529909 | Alleles associated with decreased SUA and increased FEUA * |
| Guan, 2009 (123) | Chinese | rs893006 | Alleles associated with varying range of SUA levels |
| Kolz, 2009 (49) | European | rs505802 | Alleles associated with decreased SUA |
| Li, 2010 (41) | Chinese | rs382507 rs57606 rs7932775 rs11231825 rs11602903 |
Alleles associated with increased SUA |
| Tu, 2010 (124) | Chinese, Solomon Islanders | rs475688 rs7932775 |
Alleles associated with increased SUA, gout risk and tophi |
| Tin, 2011 (59) | African American | rs12800450 | Alleles associated with decreased SUA and gout risk |
| Karns, 2012 (50) | Croatian | rs17300741 | Alleles associated with increased SUA |
SUA: serum uric acid FEUA: fractional excretion of uric acid
significant association in males only
SLC2A9
SLC2A9 encodes glucose transporter type 9 (GLUT9), a urate uniporter, initially thought to be a glucose or fructose transporter located on the basolateral membrane of the proximal tubule. However, data from GWAS have elucidated its role as a urate transporter and demonstrated its inhibition with known uricosuric agents such as probenecid, losartan, and benzbromarone (10, 43-45). GLUT9 is responsible for a large portion of urate reabsorption, such that complete loss of GLUT9 leads to dramatic urate excretion more so than seen with URAT1 (1). As with SLC22A12, mutations in SLC2A9 were initially described in patients with idiopathic renal hypouricemia without mutation in SLC22A12 (44, 46). Many studies have correlated polymorphisms in SLC2A9 with uric acid levels and gout as listed in Table 3. Of the genetic loci, SLC2A9 has perhaps the strongest association with uric acid levels and also appears to exert a greater effect in females as discerned by several studies (45, 47-51). SLC2A9 is estimated to explain 3.4–8.8% of serum uric acid variance in females and 0.5–2.0% in males (10). Certain SLC2A9 alleles have been linked to tophaceous gout (52). This is notable as SLC2A9 is found on chondrocytes (53). Since evidence is lacking for why certain patients develop tophi, SLC2A9, with is presence on chondrocytes becomes an enticing target (52).
Table 3.
Studies pertaining to SLC2A9 polymorphisms and effect on serum uric acid and gout.
| Study | Population | Polymorphism | Outcome |
|---|---|---|---|
| Li, 2007 (125) | Sardinian, Chianti | rs6855911 rs7442295 |
Alleles associated with varying range of SUA levels* |
| Brandstätter, 2008 (51) | American, Italian | rs6449213 rs6855911 rs7442295 rs12510549 |
Alleles associated with decreased SUA* |
| Dehghan, 2008 (55) | American, Dutch | rs6449213 rs16890979 |
Alleles associated with SUA and gout risk* |
| Doring, 2008 (47) | German, Austrian, Polish | rs6449213 rs7442295 rs6855911 rs12510549 |
Alleles associated with varying SUA levels and gout * |
| McArdle, 2008 (126) | Amish American | rs10489070 rs16890979 |
Alleles associated with decreased SUA* |
| Stark, 2008 (127) | German | rs6449213 rs6855911 rs7442295, rs12510549 |
Alleles associated with predisposition to and protection from gout ** |
| Wallace, 2008 (48) | European | rs6449213 rs7442295 |
Alleles associated with increased SUA |
| Hollis-Moffatt, 2009 (128) | New Zealand: Maori, Pacific Island, Caucasian | rs5028843 rs11942223 rs12510549 rs16890979 |
Alleles associated with predisposition to and protection from gout |
| Kolz, 2009 (49) | European | rs734553 rs12498742 |
Alleles associated with decreased SUA* |
| Vitart, 2008 (45) | Croatian, Scottish, German | rs6449213 rs737267 rs1014290 |
Alleles associated with SUA, FEUA and gout * |
| Tu, 2010 (129) | Chinese, Solomon Islanders | rs3733589 rs3733591 rs1014290 rs10939650 |
Alleles associated with increased SUA, gout risk and tophi |
| Urano, 2010 (130) | Japanese | rs1014290 rs3733591 |
Alleles associated with predisposition to and protection from gout |
| Yang, 2010 (131) | American, Dutch, Icelandic | rs13129697 | Alleles associated with decreased SUA and gout risk |
| Charles, 2011 (132) | African American | rs3775948 rs6449213 rs6856396 rs7663032 |
Alleles associated with SUA* |
| Hamajima, 2011 (133) | Japanese | rs11722228 | Alleles associated with varying range of SUA levels * |
| Hollis-Moffatt, 2011 (52) | New Zealand: Maori, Pacific Island, Caucasian | rs3733591 | Allele associated with tophi in Maori but not gout in populations studied |
| Sulem, 2011 (134) | Icelandic | rs734553 | Alleles associated with SUA and gout risk |
| Tin, 2011 (59) | African American | rs13129697 rs7663032 |
Alleles associated with increased SUA and gout risk |
| Li, 2012 (135) | Chinese | rs6850166 rs13124007 |
Alleles associated with predisposition to and protection from gout and risk of tophi |
| Karns, 2012 (50) | Croatian | rs737267 rs3775948 rs6855911 rs717615 rs7442295 rs13129697 rs16890979 |
Alleles associated with decreased SUA* |
| Voruganti, 2013 (136) | Mexican American | rs737267 rs6449213 rs6832439 rs13131257 |
Alleles associated with decreased SUA |
| Voruganti, 2013 (137) | American Indian | rs737267 rs6449213 rs6832439 rs10805346 rs12498956 rs13131257 rs16890979 |
Alleles associated with varying SUA levels |
SUA: serum uric acid FEUA: fractional excretion of uric acid
association stronger in females then males
association stronger in males then females
ABCG2
ABCG2 encodes the adenosine triphosphate (ATP)-binding cassette transporter 2 (ABCG2), a multidrug resistance transporter that mediates urate secretion across the apical membrane of the proximal tubule. ABCG2 was identified through GWAS and its functionality proven by demonstrating a 53% reduction in urate transport rate with mutation (54). Population studies confirmed a linkage with both serum uric acid and gout, though the association was stronger in males than females (54, 55). Variants in ABCG2 are thought to be responsible for 10% of gout in whites, in concordance another finding that 10% of Japanese gout patients had genotypes causing a 75% reduction in ABCG2 function (54, 56). Patients with severe ABCG2 dysfunction were found to have symptom onset 6.5 years earlier than those with normal function providing further clinical relevance to these polymorphisms (57). ABCG2 may have a role in gout severity, a recent study found a variant of ABCG2 to be associated with a 50% increase in tophaceous gout compared to non-tophaceous gout (58). Moreover these investigators discovered a gene-environment interaction reporting an additive effect of alcohol and the Q141K (rs2231142) variant, patients with Q141K and current alcohol use had an OR for tophaceous gout of 12.69 (95% CI, 2.88–55.86) in comparison to an OR of 6.21 (95% CI, 2.47–15.64) for Q141K alone (58). Studies pertaining to ABCG2 are listed in Table 4.
Table 4. Studies pertaining to ABCG2 polymorphisms and effect on serum uric acid and gout.
| Study | Population | Polymorphism | Outcome |
|---|---|---|---|
| Dehghan, 2008 (55) | American, Dutch | rs2231142 | Alleles associated with increased SUA and gout risk* |
| Kolz, 2009 (49) | European | rs2231142 rs2199936 |
Alleles associated with increased SUA* |
| Matsuo, 2009 (56) | Japanese | rs2231142, rs72552713 |
Alleles associated with increased SUA and gout risk |
| Woodward, 2009 (54) | American | rs2231142 | Alleles Associated with increased SUA levels and gout risk* |
| Phipps-Green, 2010 (138) | New Zealand: Maori, Pacific Island, Caucasian | rs2231142 | Associated with increased risk of gout. Association not seen in Maori |
| Wang, 2010 (139) | Chinese | rs2231142 | Associated with increased risk of gout ** |
| Yamagishi, 2010 (140) | Japanese | rs2231142 | Alleles associated with increased SUA and gout risk |
| Yang, 2010 (131) | American, Dutch, Icelandic | rs2199936 | Alleles associated with increased SUA and gout risk |
| Matsuo, 2011 (141) | Japanese | rs2231142, rs72552713 |
Alleles associated with increased SUA and gout risk |
| Matsuo, 2011 (142) | Japanese | rs2231142, rs72552713 |
Alleles associated with increased SUA and gout risk |
| Sulem, 2011 (134) | Icelandic | rs2231142 | Alleles associated with SUA and gout |
| Karns, 2012 (50) | Croatian | rs2231142 rs2199936 |
Alleles associated with increased SUA* |
| Matsuo, 2013 (57) | Japanese | rs22311423 rs72552713 |
Associated with early onset gout |
| Tu, 2014 (58) | Taiwanese | rs2231137 rs2231142 rs72552713 |
Alleles associated with tophaceous gout. Alcohol consumption exerts an additive risk in patients with rs2231142 |
SUA: serum uric acid
association stronger in males then females
non-significant association with elevated SUA
Evident in this genetic work are both the homogeneity and heterogeneity among populations. Certain polymorphisms have a level of concordance: for instance, of the 11 loci associated with uric acid in Europeans, 10 of these were replicated in African Americans (11, 59). However, other alleles appear to be population-specific as the SLC2A9 variant associated with gout in Chinese and Japanese populations could not be replicated in New Zealanders (52). As Dehghan et al concluded, though the risk of individual genetic variants may be unimpressive, allelic combinations and the prevalence of some alleles in a population may compound into a larger effect on uric acid and gout. The overall genetic risk score from this study indicated a 40fold risk of incident gout, which is purportedly higher than the risk conferred by environmental factors (55). Studies have continued to define new reproducible variants and confirm those previously discovered. (49, 60). A recent study by Kottgen found 28 loci associated with uric acid concentrations, 18 of these loci were novel. Notably, the novel loci appeared to be associated with carbohydrate metabolism but not urate transport (60). Deeper understanding of genetic polymorphisms and their role in gout may shed light on individuals' susceptibility to gout and lead to more targeted therapy.
Dietary Factors
Alcohol
Since antiquity, alcohol consumption has been linked to gout. More formal research from the 1960s demonstrated that alcohol administration caused decreased uric acid excretion and hyperuricemia (61). Ethanol ingestion increases serum lactate levels which inhibit uric acid excretion at the renal tubule; however, this has not been confirmed in subsequent studies (62). In terms of the production theory, ethanol prompts adenosine triphosphate (ATP) consumption leading to purine degradation, yielding an increase in plasma oxypurines and uric acid (62, 63). Longitudinal studies in beer consumption confirmed increased plasma uric acid levels and attributed this to production via ethanol but failed to find increased plasma levels of oxypurines suggesting this was a short-term consequence (64, 65). Some have instead posited that the guanosine purine load in beer specifically may cause increase in uric acid synthesis (66)
This spurred further consideration as to whether the purine components of beer augmented the hyperuricemic effects of ethanol. A comparison of alcoholic beer to nonalcoholic beer found that plasma uric acid levels increased 6.5% and 4.4% (p<0.05), respectively suggesting that purine load alone had a significant effect on uric acid (67). With regard to hyperuricemia, beer poises the greatest threat with the combine effects of ethanol and purine. A cross-sectional NHANES study showed serum uric acid levels significantly increased with greater beer and liquor intake after adjustment for age (68). Uric acid increased per serving per day 0.46 mg/dl (95% CI, 0.32-0.60) with beer and 0.29 mg/dl (95% CI, 0.14-0.45) with liquor. The effect was lessened but remained significant after adjustment for diet, diuretics, hypertension, BMI and creatinine. No association was seen with wine (68). A subsequent cross-sectional analysis using the CARDIA cohort found high uric acid levels with increased beer intake. The effect was strongest in females with uric acid increase of 0.03 mg/dl per each additional weekly serving of beer (69). In terms of incident gout, a prospective cohort study of males found that two or more beers per day increased the risk of gout by 2.5 with a multivariate RR per 12-oz serving per day of 1.49 (95% CI, 1.32-1.70). A similar amount of spirits increased risk by 1.6 in comparison to none with a RR per drink or shot per day of 1.15 (95% CI, 1.04-1.28) (70). Less common, but certainly of interest, is moonshine, in addition to an ethanol load its distillation often leads to lead contamination. Chronic moonshine imbibers are at risk for lead toxicity and concomitant saturnine gout due to lead-induced renal dysfunction, decreased urate excretion, and potentially an increase in urate production (71, 72). The associations of other alcoholic beverage such as liquor and wine have also been investigated as outlined in Table 4. Though the definitive verdict on the contribution of wine to gout risk is still unclear, the evidence above confirms longstanding suspicion for alcohol, especially beer, as a gout risk factor. A meta-analysis of 17 observational studies did in fact show an increased risk of gout associated with alcohol consumption and recommended reducing alcohol intake for the primary prevention of gout (73).
Purine Rich Food
Food rich in purines including meats, seafood, some vegetables, and animal protein have been theorized to lead to gout, as uric acid is the end product of purine degradation. Skepticism existed as protein can have a uricosuric effect which would actually lower urate levels (74). Recent NHANES data demonstrated increased uric acid levels in association with greater meat and seafood consumption, but not with total protein intake (75). The elevation in uric acid levels between the 1st and 5th quintiles of meat intake was 0.11 mg/dl (95% CI, 0.01,-0.22), and 0.10 mg/dl for seafood (95% CI, 0.02-0.18) after adjustment for age, sex, BMI, creatinine, medication, hypertension, and diet (75). Another prospective study by Choi et al found that each additional daily serving of meat increased the incident risk of gout by 21% with a multivariate RR of 1.41 (95% CI, 1.07-1.86) between the lowest and highest quintiles of meat intake. Each additional weekly serving of seafood increased risk by 7% with a RR of 1.51 (95% CI, 1.17-1.95) between the highest and lowest consumers (76). No effect was seen with purine-rich vegetables; however, those in the highest quintile of vegetable protein intake had a RR of 0.73 (95% CI, 0.56-0.96) in comparison to the lowest quintile. These differences were thought to be secondary to the differing types, quantity, and bioavailability of purines found in various foods (76). Despite moderate purine content in soy, soy has not been shown to be associated with gout and may be inversely associated with hyperuricemia (77-79). An absolute low purine diet may not be necessary in the primary prevention of gout, as many purine-containing foods do not contribute to hyperuricemia or gout and may in fact be protective (80).
Fructose/Sugar-Sweetened Beverages
As diets have come to include increasing quantities of fructose and sugar-sweetened beverages (main sweetener being fructose), these additives have come under investigation for their contribution to gout. Initial studies on these sweeteners found increased plasma uric acid and lactate levels probably driven either by purine nucleotide degradation or de novo purine synthesis (81-83). Fructose is the only known carbohydrate to increase uric acid levels, which is felt to be secondary to degradation of ATP. Since fructose phosphorylation depletes phosphate, a path towards uric acid formation is favored instead of regeneration of ATP. Additionally, de novo purine synthesis from these beverages is still considered to have a role in increased uric acid. Lastly, fructose may increase the risk of insulin resistance and subsequent hyperinsulinemia, decreasing uric acid excretion further promoting hyperuricemia (19, 84-86). Interestingly, several groups have linked the handling of fructose and sugar-sweetened beverages to genetic polymorphisms in SLCA9 and ABCG2. Data suggests that different alleles influence serum urate responses to a fructose or sucrose load (87-90).
Cross-sectional NHANES data showed an increase in uric acid of 0.33 mg/dl (95% CI,0.11-0.73) in participants drinking 1-3.9 sugar-sweetened servings per day in comparison to those drinking none after adjustment for diet including total energy intake, age, sex, medications, hypertension and glomerular filtration rate (84). In these moderate utilizers, the odds ratio for hyperuricemia (defined as serum uric acid level >7.0 mg/dl in males and >5.7 mg/dl in females) was 1.51 (p=0.003 for trend) in comparison to no intake (84). This was in accordance with prior NHANES data showing that males had increased uric acid levels with increased added sugar or sugar-sweetened beverages in their diet (91). Prospective cohort studies have also shown that those consuming two or more sugar-sweetened beverages per day had an increased risk of incident gout (RR 1.85, 95% CI,1.08-3.16) in comparison to those drinking less than one sugar-sweetened beverage a month (85). Males in the highest 5th of fructose ingestion incurred double the risk of gout than those in the lowest (RR 2.02, 95% CI, 1.49-2.75) after adjustment for total carbohydrate intake (85). These associations were confirmed in a prospective cohort study of females showing that one serving of sugar-sweetened beverage per day increased the risk of incident gout (RR 1.74, 95% CI, 1.19-2.25) when compared to less than 1 serving per day. An increased gout risk was also found with fructose intake with a RR for the highest quintile of 1.62 (95% CI, 1.20-2.19) in comparison to the lowest after adjustment for total carbohydrate intake (86).
The role of fructose in gout has been contested in the literature. A meta analysis of controlled fructose feeding trials and uric acid levels among diabetic and non-diabetics reported that isocaloric fructose intake did not alter uric acid levels; only the hypercaloric (fructose levels double the 95th percentile for fructose intake) increased uric acid. It is not clear that all previous observational studies had appropriately adjusted for total energy and carbohydrate intake (92). Several studies did not find an association with fructose and hyperuricemia, including one cross-sectional analysis finding and association with sugar-sweetened beverages but not with fructose (93, 94). Whether or not fructose itself is the responsible component for hyperuricemic effects of sugar-sweetened beverages is debatable. However, current data suggest that heavy utilization of these food items is not advisable for those at risk of hyperuricemia and gout.
Dairy Products
Initial studies demonstrated decrease in serum uric acid levels after milk protein (casein and lactalbumin) ingestion secondary to a presumed uricosuric effect of the protein load (95). In a cross-sectional NHANES study, Choi et al found an inverse association for uric acid and dairy with a decrease in uric acid of 0.21 mg/dl (95% CI, -0.37 to -0.04) between the highest and lowest total dairy intake quintiles. This effect remained significant after adjustment for covariates (75). Prospective data on incident gout showed that males in the highest quintile of dairy intake had a RR of 0.56 (95 % CI, 0.42-0.74) when compared to the lowest quintile (76). This inverse correlation of dairy and uric acid has been observed in the Scottish and Korean populations as well (94, 96). A randomized controlled trial (RCT) found that milk consumption lead to an acute 10% decrease in serum uric acid (p<0.0001) and an increase in FEUA (97). Additional work in both cellular and murine acute gout models showed that the dairy fractions glycomacropeptide (GMP) and G600 milk fat extract had anti-inflammatory effects causing a decrease in interleukin-1β(IL-1β) expression thus providing a second protective mechanism for dairy (98). This knowledge was applied to a second RCT investigating skim mild enriched with GMP and G600 in patients with gout. Though all patients received milk products and had a decrease in gout flares, those in the group supplemented with GMP and G600 had a greater decrement in number of flares and increase in FEUA (99). Dairy products have been shown to be protective in terms of gout from a urate lowering and potentially anti-inflammatory standpoint.
Coffee
Due to caffeine-induced diuresis and the hypothesis that uric acid excretion would increase with increased renal blood flow, coffee versus green tea consumption was studied in Japanese males. Uric acid decreased as coffee intake rose, a correlation that was not seen with green tea (100). A second study in Japanese males and females confirmed this association, and found it to be stronger in males than females (101). Cross-sectional studies in a US population also document an inverse association with uric acid and coffee consumption, but not with tea or total caffeine (102). Subjects drinking 4-5 cups of coffee a day had a significant uric acid decrement of 0.26 mg/dl (95% CI, -0.41 to -0.11) in comparison to those not drinking coffee after adjustment for age and sex. A moderate inverse correlation was seen with decaffeinated coffee leading to the conclusion that the effect was due to factors outside of caffeine (102). A prospective cohort study found that the risk of incident gout was inversely associated with daily intake of 4-5 cups of coffee (RR 0.60, 95% CI, 0.41-0.87) and 4 or more cups of decaffeinated coffee (RR 0.73, 95% CI 0.46–1.17) but not with total caffeine (RR 0.83, 95% CI 0.64–1.08) (103). Similar results regarding coffee consumption and incident gout were noted in the Nurses' Health Study (104). It was postulated that the anti-oxidant properties including those of phenol chlorogenic acid might increase insulin sensitivity and decrease serum insulin, as discussed above insulin levels have a positive correlation with uric acid due to decreased renal excretion. Furthermore, xanthines, either in caffeine or in coffee itself, could inhibit xanthine oxidase acting in a manner similar to allopurinol (101-104). Coffee represents another potentially protective beverage for those at risk for gout.
Vitamin C
Vitamin C has been touted as protective against gout, ingestion of ascorbic acid was found to increase the fractional clearance of uric acid resulting in a reduction of serum uric acid (105). Supplementation with 500 mg/day of vitamin C significantly reduced serum uric acid levels in an RCT with a mean uric acid reduction of 0.5 mg/dl (95% CI, -0.6 to -0.3) (106). Observational data in males demonstrated an inverse relationship for vitamin C doses and serum uric acid with levels, which remained significant after adjustment for covariates including BMI, blood pressure, medications, and diet (107). A prospective cohort study of male health professionals reported a decreased risk of incident gout in patients taking 1500 mg/day of vitamin C (RR 0.55, 95% CI, 0.38-0.80) compared to those with intake less than 250 mg/day (108). A meta-analysis of 13 RCTs on vitamin C and uric acid in patients without gout confirmed the inverse association and suggested that the combined effect demonstrated a serum uric acid reduction of 0.35 mg/dl (95% CI, -0.66 to -0.03) (109). The uricosuric effects of vitamin C have been explained by its ability to compete with uric acid for reabsorption at the proximal tubule. Evidence suggests this competitive inhibition may be occurring at URAT1 and a sodium-dependent anion co-transporter. It has also been postulated that vitamin C improves renal function further augmenting the uricosuric effect and functions as an antioxidant reducing inflammation (107, 108).
Cherries
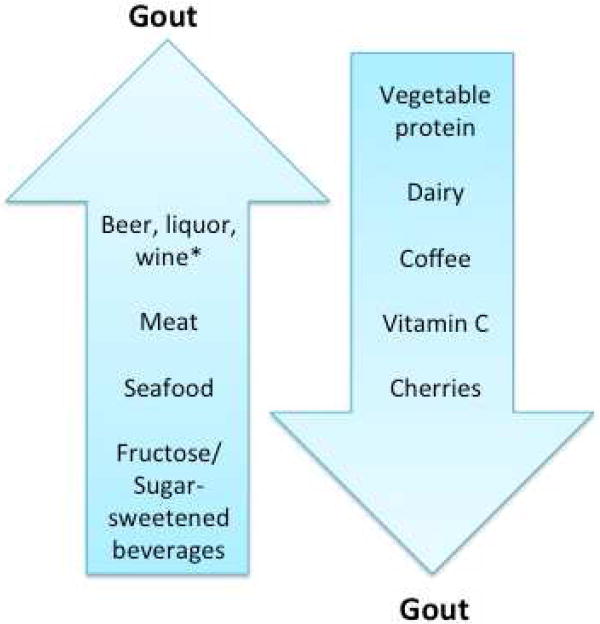
A few studies have addressed the role of cherries in gout. The first by Jacob et al sought to follow up on prior reports from the 1950s in light of antioxidant and anti-inflammatory effects of polyphenols including anthocyanins, and vitamin C found in the fruit (110). Cherry consumption was found to acutely lower serum uric acid in healthy females ages 22-40 years old (110). To date, there are no studies on the risk of incident gout related to cherry consumption, but cherry consumption was found to lower recurrent gout attacks by 35% in a case-cross over study (111). The effect of cherries on glomerular filtration rate, xanthine oxidase, as well as their antioxidant effects were all taken into consideration as reasoning for the beneficial outcome on gout (111). A prospective RCT of cherry juice, available regardless of season, found a significant reduction in gout flares after 4 months with 55% being free of attack (112). Although the effects on pre-clinical gout have yet to be elucidated, cherries may lower uric acid levels and decrease the risk of incident gout. There is an ongoing RCT that determines the effect of blueberries on uric acid in hyperuricemic patients who are not on pharmacotherapy (ClinicalTrials.gov Identifier: NCT01532622). Figure 2 Summarizes the effects of diet on gout.
Figure 2. Summary of the effects of diet on risk of gout.
* Discrepancy surrounding effect
Other Lifestyle Factors
Few studies examined the role of physical activity and body weight on the risk of gout. A study of male runners found that males who ran over 4 kilometers/day or faster than 4.0 meters/second had a lower incidence of gout, though this was partially attributable to their leaner frames and BMI (113). In a prospective cohort of males, Choi et al found that greater BMI correlated with increased age-adjusted RR of incident gout, BMI of 25 to 29.9 (RR 1.95, 95% CI, 1.44-2.65), BMI of 30 to 34.9 (RR 2.33, 95% CI, 1.62-3.36), and BMI of 35 or greater (RR 2.97, 95% CI, 1.73-5.10) compared to males with a BMI of 21 to 22.9 (114). Additionally, males who gained 30 lbs. or greater since 21 years of age had a RR of 1.99 (95% CI, 1.49-2.66) versus males who had stable weight within 4 lbs. The RR was attenuated but remained significant for after adjustment for diet, hypertension, and chronic renal failure (114). As many turn to surgical means of weight loss, it is encouraging that a recent prospective study of 60 patients with type 2 diabetes and BMI ≥35 kg/m2 found clinically significant reductions in serum uric acid levels one year after bariatric surgery (115). Interestingly, uric acid levels and gout attacks initially increase peri-operatively secondary in part to the catabolic effect of surgery, rapid weight loss, and renal impairment(115, 116). However, patients with weight loss post bariatric surgery ultimately benefit from reduced inflammation and show decreased production of interleukins including IL-1β, IL-6 and IL-8 in response to MSU crystals (117). Weight reduction is encouraged for many disease processes and may help stave off the risk of incident gout as well.
Relatively little investigation has been done on smoking and risk for gout. A study by Hanna et al actually reported lower serum uric acid levels in chronic smokers, though data was not adjusted for potential confounders such as BMI (116, 118). This confirmed findings from a prior cross-over study reporting decreased uric acid levels after smoking a cigarette (119). As uric acid is a potent antioxidant, the reduction was thought to be a testament to be oxidative stress caused by cigarette use (118). From a health standpoint, smoking is clearly not a viable mechanism for uric acid reduction. However, it does raise the question as to whether susceptible individuals who cease smoking are at risk for increasing uric acid levels and subsequently incident gout.
Conclusion
There is robust evidence for non-modifiable and modifiable risk factors and their contribution to incident gout. While a patient's individual risk likely represents a complex interplay between factors outside of their control, such as age, sex, race and genetics and modifiable factors such as diet and lifestyle, patients at risk for hyperuricemia or gout should be educated on modifiable factors to reduce the risk. With the current knowledge in the literature, it is conceivable that a risk score could be developed to better predict an individual's risk for developing gout. Despite a large body of data for gout that we have summarized in this review, further work in risk stratification and primary prevention of gout is needed.
Table 5. Summary of the effects of beer, liquor and wine on SUA or gout by study.
| Study | Design | Population | Beer | Liquor | Wine | |||
|---|---|---|---|---|---|---|---|---|
| SUA | Gout | SUA | Gout | SUA | Gout | |||
| Eastmond, 1995 (143) | Pre-post comparison | 4 gout patients | ↑ | - | ↔ | - | - | - |
| van der Gaag, 2000 (144) | Randomized cross-over trial | 11 males | ↑ | - | ↑ | - | ↑ | - |
| Choi, 2004 (70) | Prospective cohort | 47,150 males without historyof gout | - | ↑ | - | ↑ | - | ↔ |
| Choi, 2004 (68) | Cross-sectional | 14,809 males and females | ↑ | - | ↑** | - | ↔ | - |
| Zhang, 2006 (145) | Case-crossover | 197 gout patients | - | ↑ | - | ↑ | - | ↑ |
| Yu, 2008 (78) | Cross-sectional | 2176 males and females | ↑* | - | - | - | - | - |
| Gaffo, 2010 (69) | Cross-sectional | 3123 males and females | ↑** | - | ↑* | - | ↔ | - |
| Neogi, 2014 (146) | Case-crossover | 724 gout patients | - | ↑ | - | ↑ | - | ↑ |
SUA: serum uric acid
effect noted only in males
effect stronger in females
Key points.
The prevalence of gout and hyperuricemia increases with age; females tend to be affected by gout at an older age than their male counterparts.
Genome-wide association studies have identified several genetic polymorphisms, mainly affecting renal urate excretion, which alter serum uric acid levels and subsequently the risk of developing gout.
Alcohol, purines from meat and seafood, and fructose or sugar sweetened beverages have been associated with increased risk of incident gout, whereas dairy products, coffee, vitamin C and cherries may protect patients from developing hyperuricemia and gout.
Obesity and weight gain of 30 pounds or greater is associated with risk of incident gout.
Acknowledgments
Kim is supported by the NIH grant K23 AR059677. She received a research grant from Pfizer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lipkowitz MS. Regulation of uric acid excretion by the kidney. Curr Rheumatol Rep. 2012;14(2):179–88. doi: 10.1007/s11926-012-0240-z. [DOI] [PubMed] [Google Scholar]
- 2.Roddy E, Choi HK. Epidemiology of Gout. Rheum Dis Clin North Am. 2014;40(2):155–175. doi: 10.1016/j.rdc.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82(3):421–6. doi: 10.1016/0002-9343(87)90441-4. [DOI] [PubMed] [Google Scholar]
- 4.Bhole V, de Vera M, Rahman MM, Krishnan E, Choi H. Epidemiology of gout in women: Fifty-two-year followup of a prospective cohort. Arthritis Rheum. 2010;62(4):1069–76. doi: 10.1002/art.27338. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136–41. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 6.Wertheimer A, Morlock R, Becker MA. A revised estimate of the burden of illness of gout. Curr Ther Res Clin Exp. 2013;75:1–4. doi: 10.1016/j.curtheres.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh JA, Reddy SG, Kundukulam J. Risk factors for gout and prevention: a systematic review of the literature. Curr Opin Rheumatol. 2011;23(2):192–202. doi: 10.1097/BOR.0b013e3283438e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31(8):1582–7. [PubMed] [Google Scholar]
- 9.Maynard JW, McAdams-Demarco MA, Law A, Kao L, Gelber AC, Coresh J, et al. Racial Differences in Gout Incidence in a Population-Based Cohort: Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2013 doi: 10.1093/aje/kwt299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reginato AM, Mount DB, Yang I, Choi HK. The genetics of hyperuricaemia and gout. Nat Rev Rheumatol. 2012;8(10):610–21. doi: 10.1038/nrrheum.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merriman TR, Choi HK, Dalbeth N. The Genetic Basis of Gout. Rheum Dis Clin North Am. 2014;40(2):279–290. doi: 10.1016/j.rdc.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med. 2005;143(7):499–516. doi: 10.7326/0003-4819-143-7-200510040-00009. [DOI] [PubMed] [Google Scholar]
- 13.Ozturk MA, Kaya A, Senel S, Donmez S, Balkarli A, Cobankara V, et al. Demographic and clinical features of gout patients in Turkey: a multicenter study. Rheumatol Int. 2013;33(4):847–52. doi: 10.1007/s00296-012-2442-0. [DOI] [PubMed] [Google Scholar]
- 14.Chen JH, Yeh WT, Chuang SY, Wu YY, Pan WH. Gender-specific risk factors for incident gout: a prospective cohort study. Clin Rheumatol. 2012;31(2):239–45. doi: 10.1007/s10067-011-1802-6. [DOI] [PubMed] [Google Scholar]
- 15.Puig JG, Michan AD, Jimenez ML, Perez de Ayala C, Mateos FA, Capitan CF, et al. Female gout. Clinical spectrum and uric acid metabolism. Arch Intern Med. 1991;151(4):726–32. doi: 10.1001/archinte.151.4.726. [DOI] [PubMed] [Google Scholar]
- 16.Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women--the Third National Health and Nutrition Examination Survey. Arthritis Res Ther. 2008;10(5):R116. doi: 10.1186/ar2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hak AE, Curhan GC, Grodstein F, Choi HK. Menopause, postmenopausal hormone use and risk of incident gout. Ann Rheum Dis. 2010;69(7):1305–9. doi: 10.1136/ard.2009.109884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takiue Y, Hosoyamada M, Kimura M, Saito H. The effect of female hormones upon urate transport systems in the mouse kidney. Nucleosides Nucleotides Nucleic Acids. 2011;30(2):113–9. doi: 10.1080/15257770.2010.551645. [DOI] [PubMed] [Google Scholar]
- 19.Choi HK, Ford ES. Haemoglobin A1c, fasting glucose, serum C-peptide and insulin resistance in relation to serum uric acid levels--the Third National Health and Nutrition Examination Survey. Rheumatology (Oxford) 2008;47(5):713–7. doi: 10.1093/rheumatology/ken066. [DOI] [PubMed] [Google Scholar]
- 20.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA. 2000;283(18):2404–10. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 21.Saag KG, Choi H. Epidemiology, risk factors, and lifestyle modifications for gout. Arthritis Res Ther. 2006;8(Suppl 1):S2. doi: 10.1186/ar1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuzuya M, Ando F, Iguchi A, Shimokata H. Effect of aging on serum uric acid levels: longitudinal changes in a large Japanese population group. J Gerontol A Biol Sci Med Sci. 2002;57(10):M660–4. doi: 10.1093/gerona/57.10.m660. [DOI] [PubMed] [Google Scholar]
- 23.Hochberg MC, Thomas J, Thomas DJ, Mead L, Levine DM, Klag MJ. Racial differences in the incidence of gout. The role of hypertension. Arthritis Rheum. 1995;38(5):628–32. doi: 10.1002/art.1780380508. [DOI] [PubMed] [Google Scholar]
- 24.Gaffo AL, Jacobs DR, Jr, Lewis CE, Mikuls TR, Saag KG. Association between being African-American, serum urate levels and the risk of developing hyperuricemia: findings from the Coronary Artery Risk Development in Young Adults cohort. Arthritis Res Ther. 2012;14(1):R4. doi: 10.1186/ar3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeBoer MD, Dong L, Gurka MJ. Racial/ethnic and sex differences in the relationship between uric acid and metabolic syndrome in adolescents: an analysis of National Health and Nutrition Survey 1999-2006. Metabolism. 2012;61(4):554–61. doi: 10.1016/j.metabol.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klemp P, Stansfield SA, Castle B, Robertson MC. Gout is on the increase in New Zealand. Ann Rheum Dis. 1997;56(1):22–6. doi: 10.1136/ard.56.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose BS. Gout in Maoris. Semin Arthritis Rheum. 1975;5(2):121–45. doi: 10.1016/0049-0172(75)90002-5. [DOI] [PubMed] [Google Scholar]
- 28.Singh JA. Racial and gender disparities among patients with gout. Curr Rheumatol Rep. 2013;15(2):307. doi: 10.1007/s11926-012-0307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winnard D, Wright C, Taylor WJ, Jackson G, Te Karu L, Gow PJ, et al. National prevalence of gout derived from administrative health data in Aotearoa New Zealand. Rheumatology (Oxford) 2012;51(5):901–9. doi: 10.1093/rheumatology/ker361. [DOI] [PubMed] [Google Scholar]
- 30.Stamp LK, Wells JE, Pitama S, Faatoese A, Doughty RN, Whalley G, et al. Hyperuricaemia and gout in New Zealand rural and urban Maori and non-Maori communities. Intern Med J. 2013;43(6):678–84. doi: 10.1111/imj.12062. [DOI] [PubMed] [Google Scholar]
- 31.Portis AJ, Laliberte M, Tatman P, Moua M, Culhane-Pera K, Maalouf NM, et al. High prevalence of gouty arthritis among the Hmong population in Minnesota. Arthritis Care Res (Hoboken) 2010;62(10):1386–91. doi: 10.1002/acr.20232. [DOI] [PubMed] [Google Scholar]
- 32.Wahedduddin S, Singh JA, Culhane-Pera KA, Gertner E. Gout in the Hmong in the United States. J Clin Rheumatol. 2010;16(6):262–6. doi: 10.1097/RHU.0b013e3181eeb487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prasad P, Krishnan E. The Filipino Gout- A Review. Arthritis Care Res (Hoboken) 2013 doi: 10.1002/acr.22118. [DOI] [PubMed] [Google Scholar]
- 34.Emmerson BT, Nagel SL, Duffy DL, Martin NG. Genetic control of the renal clearance of urate: a study of twins. Ann Rheum Dis. 1992;51(3):375–7. doi: 10.1136/ard.51.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilk JB, Djousse L, Borecki I, Atwood LD, Hunt SC, Rich SS, et al. Segregation analysis of serum uric acid in the NHLBI Family Heart Study. Hum Genet. 2000;106(3):355–9. doi: 10.1007/s004390000243. [DOI] [PubMed] [Google Scholar]
- 36.Yang Q, Guo CY, Cupples LA, Levy D, Wilson PW, Fox CS. Genome-wide search for genes affecting serum uric acid levels: the Framingham Heart Study. Metabolism. 2005;54(11):1435–41. doi: 10.1016/j.metabol.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417(6887):447–52. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 38.Taniguchi A, Urano W, Yamanaka M, Yamanaka H, Hosoyamada M, Endou H, et al. A common mutation in an organic anion transporter gene, SLC22A12, is a suppressing factor for the development of gout. Arthritis Rheum. 2005;52(8):2576–7. doi: 10.1002/art.21242. [DOI] [PubMed] [Google Scholar]
- 39.Iwai N, Mino Y, Hosoyamada M, Tago N, Kokubo Y, Endou H. A high prevalence of renal hypouricemia caused by inactive SLC22A12 in Japanese. Kidney Int. 2004;66(3):935–44. doi: 10.1111/j.1523-1755.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- 40.Graessler J, Graessler A, Unger S, Kopprasch S, Tausche AK, Kuhlisch E, et al. Association of the human urate transporter 1 with reduced renal uric acid excretion and hyperuricemia in a German Caucasian population. Arthritis Rheum. 2006;54(1):292–300. doi: 10.1002/art.21499. [DOI] [PubMed] [Google Scholar]
- 41.Li C, Han L, Levin AM, Song H, Yan S, Wang Y, et al. Multiple single nucleotide polymorphisms in the human urate transporter 1 (hURAT1) gene are associated with hyperuricaemia in Han Chinese. J Med Genet. 2010;47(3):204–10. doi: 10.1136/jmg.2009.068619. [DOI] [PubMed] [Google Scholar]
- 42.Vazquez-Mellado J, Jimenez-Vaca AL, Cuevas-Covarrubias S, Alvarado-Romano V, Pozo-Molina G, Burgos-Vargas R. Molecular analysis of the SLC22A12 (URAT1) gene in patients with primary gout. Rheumatology (Oxford) 2007;46(2):215–9. doi: 10.1093/rheumatology/kel205. [DOI] [PubMed] [Google Scholar]
- 43.Caulfield MJ, Munroe PB, O'Neill D, Witkowska K, Charchar FJ, Doblado M, et al. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med. 2008;5(10):e197. doi: 10.1371/journal.pmed.0050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anzai N, Ichida K, Jutabha P, Kimura T, Babu E, Jin CJ, et al. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem. 2008;283(40):26834–8. doi: 10.1074/jbc.C800156200. [DOI] [PubMed] [Google Scholar]
- 45.Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008;40(4):437–42. doi: 10.1038/ng.106. [DOI] [PubMed] [Google Scholar]
- 46.Matsuo H, Chiba T, Nagamori S, Nakayama A, Domoto H, Phetdee K, et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet. 2008;83(6):744–51. doi: 10.1016/j.ajhg.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doring A, Gieger C, Mehta D, Gohlke H, Prokisch H, Coassin S, et al. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet. 2008;40(4):430–6. doi: 10.1038/ng.107. [DOI] [PubMed] [Google Scholar]
- 48.Wallace C, Newhouse SJ, Braund P, Zhang F, Tobin M, Falchi M, et al. Genome-wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. Am J Hum Genet. 2008;82(1):139–49. doi: 10.1016/j.ajhg.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5(6):e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karns R, Zhang G, Sun G, Rao Indugula S, Cheng H, Havas-Augustin D, et al. Genome-wide association of serum uric acid concentration: replication of sequence variants in an island population of the Adriatic coast of Croatia. Ann Hum Genet. 2012;76(2):121–7. doi: 10.1111/j.1469-1809.2011.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brandstatter A, Kiechl S, Kollerits B, Hunt SC, Heid IM, Coassin S, et al. Sex-specific association of the putative fructose transporter SLC2A9 variants with uric acid levels is modified by BMI. Diabetes Care. 2008;31(8):1662–7. doi: 10.2337/dc08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hollis-Moffatt JE, Gow PJ, Harrison AA, Highton J, Jones PB, Stamp LK, et al. The SLC2A9 nonsynonymous Arg265His variant and gout: evidence for a population-specific effect on severity. Arthritis Res Ther. 2011;13(3):R85. doi: 10.1186/ar3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mobasheri A, Neama G, Bell S, Richardson S, Carter SD. Human articular chondrocytes express three facilitative glucose transporter isoforms: GLUT1, GLUT3 and GLUT9. Cell Biol Int. 2002;26(3):297–300. doi: 10.1006/cbir.2001.0850. [DOI] [PubMed] [Google Scholar]
- 54.Woodward OM, Kottgen A, Coresh J, Boerwinkle E, Guggino WB, Kottgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A. 2009;106(25):10338–42. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dehghan A, Kottgen A, Yang Q, Hwang SJ, Kao WL, Rivadeneira F, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372(9654):1953–61. doi: 10.1016/S0140-6736(08)61343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuo H, Takada T, Ichida K, Nakamura T, Nakayama A, Ikebuchi Y, et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci Transl Med. 2009;1(5):5ra11. doi: 10.1126/scitranslmed.3000237. [DOI] [PubMed] [Google Scholar]
- 57.Matsuo H, Ichida K, Takada T, Nakayama A, Nakashima H, Nakamura T, et al. Common dysfunctional variants in ABCG2 are a major cause of early-onset gout. Sci Rep. 2013;3:2014. doi: 10.1038/srep02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tu HP, Ko AM, Chiang SL, Lee SS, Lai HM, Chung CM, et al. Joint effects of alcohol consumption and ABCG2 Q141K on chronic tophaceous gout risk. J Rheumatol. 2014;41(4):749–58. doi: 10.3899/jrheum.130870. [DOI] [PubMed] [Google Scholar]
- 59.Tin A, Woodward OM, Kao WH, Liu CT, Lu X, Nalls MA, et al. Genome-wide association study for serum urate concentrations and gout among African Americans identifies genomic risk loci and a novel URAT1 loss-of-function allele. Hum Mol Genet. 2011;20(20):4056–68. doi: 10.1093/hmg/ddr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kottgen A, Albrecht E, Teumer A, Vitart V, Krumsiek J, Hundertmark C, et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 2013;45(2):145–54. doi: 10.1038/ng.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maclachlan MJ, Rodnan GP. Effect of food, fast and alcohol on serum uric acid and acute attacks of gout. Am J Med. 1967;42(1):38–57. doi: 10.1016/0002-9343(67)90005-8. [DOI] [PubMed] [Google Scholar]
- 62.Faller J, Fox IH. Ethanol-induced hyperuricemia: evidence for increased urate production by activation of adenine nucleotide turnover. N Engl J Med. 1982;307(26):1598–602. doi: 10.1056/NEJM198212233072602. [DOI] [PubMed] [Google Scholar]
- 63.Faller J, Fox IH. Ethanol induced alterations of uric acid metabolism. Adv Exp Med Biol. 1984;165(Pt A):457–62. doi: 10.1007/978-1-4684-4553-4_90. [DOI] [PubMed] [Google Scholar]
- 64.Moriwaki Y, Ka T, Takahashi S, Tsutsumi Z, Yamamoto T. Effect of beer ingestion on the plasma concentrations and urinary excretion of purine bases: one-month study. Nucleosides Nucleotides Nucleic Acids. 2006;25(9-11):1083–5. doi: 10.1080/15257770600893990. [DOI] [PubMed] [Google Scholar]
- 65.Ka T, Moriwaki Y, Takahashi S, Yamamoto A, Tsutsumi Z, Inokuchi T, et al. Effects of long-term beer ingestion on plasma concentrations and urinary excretion of purine bases. Horm Metab Res. 2005;37(10):641–5. doi: 10.1055/s-2005-870540. [DOI] [PubMed] [Google Scholar]
- 66.Gibson T, Rodgers AV, Simmonds HA, Toseland P. Beer drinking and its effect on uric acid. Br J Rheumatol. 1984;23(3):203–9. doi: 10.1093/rheumatology/23.3.203. [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto T, Moriwaki Y, Takahashi S, Tsutsumi Z, Ka T, Fukuchi M, et al. Effect of beer on the plasma concentrations of uridine and purine bases. Metabolism. 2002;51(10):1317–23. doi: 10.1053/meta.2002.34041. [DOI] [PubMed] [Google Scholar]
- 68.Choi HK, Curhan G. Beer, liquor, and wine consumption and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2004;51(6):1023–9. doi: 10.1002/art.20821. [DOI] [PubMed] [Google Scholar]
- 69.Gaffo AL, Roseman JM, Jacobs DR, Jr, Lewis CE, Shikany JM, Mikuls TR, et al. Serum urate and its relationship with alcoholic beverage intake in men and women: findings from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort. Ann Rheum Dis. 2010;69(11):1965–70. doi: 10.1136/ard.2010.129429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363(9417):1277–81. doi: 10.1016/S0140-6736(04)16000-5. [DOI] [PubMed] [Google Scholar]
- 71.Dalvi SR, Pillinger MH. Saturnine gout, redux: a review. Am J Med. 2013;126(5):450e1–8. doi: 10.1016/j.amjmed.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 72.Krishnan E, Lingala B, Bhalla V. Low-level lead exposure and the prevalence of gout: an observational study. Ann Intern Med. 2012;157(4):233–41. doi: 10.7326/0003-4819-157-4-201208210-00003. [DOI] [PubMed] [Google Scholar]
- 73.Wang M, Jiang X, Wu W, Zhang D. A meta-analysis of alcohol consumption and the risk of gout. Clin Rheumatol. 2013;32(11):1641–8. doi: 10.1007/s10067-013-2319-y. [DOI] [PubMed] [Google Scholar]
- 74.Matzkies F, Berg G, Madl H. The uricosuric action of protein in man. Adv Exp Med Biol. 1980;122A:227–31. doi: 10.1007/978-1-4615-9140-5_36. [DOI] [PubMed] [Google Scholar]
- 75.Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005;52(1):283–9. doi: 10.1002/art.20761. [DOI] [PubMed] [Google Scholar]
- 76.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093–103. doi: 10.1056/NEJMoa035700. [DOI] [PubMed] [Google Scholar]
- 77.Villegas R, Xiang YB, Elasy T, Xu WH, Cai H, Cai Q, et al. Purine-rich foods, protein intake, and the prevalence of hyperuricemia: the Shanghai Men's Health Study. Nutr Metab Cardiovasc Dis. 2012;22(5):409–16. doi: 10.1016/j.numecd.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu KH, See LC, Huang YC, Yang CH, Sun JH. Dietary factors associated with hyperuricemia in adults. Semin Arthritis Rheum. 2008;37(4):243–50. doi: 10.1016/j.semarthrit.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 79.Messina M, Messina VL, Chan P. Soyfoods, hyperuricemia and gout: a review of the epidemiologic and clinical data. Asia Pac J Clin Nutr. 2011;20(3):347–58. [PubMed] [Google Scholar]
- 80.Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol. 2010;22(2):165–72. doi: 10.1097/BOR.0b013e328335ef38. [DOI] [PubMed] [Google Scholar]
- 81.Fox IH, Kelley WN. Studies on the mechanism of fructose-induced hyperuricemia in man. Metabolism. 1972;21(8):713–21. doi: 10.1016/0026-0495(72)90120-5. [DOI] [PubMed] [Google Scholar]
- 82.Raivio KO, Becker A, Meyer LJ, Greene ML, Nuki G, Seegmiller JE. Stimulation of human purine synthesis de novo by fructose infusion. Metabolism. 1975;24(7):861–9. doi: 10.1016/0026-0495(75)90133-x. [DOI] [PubMed] [Google Scholar]
- 83.Emmerson BT. Effect of oral fructose on urate production. Ann Rheum Dis. 1974;33(3):276–80. doi: 10.1136/ard.33.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008;59(1):109–16. doi: 10.1002/art.23245. [DOI] [PubMed] [Google Scholar]
- 85.Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336(7639):309–12. doi: 10.1136/bmj.39449.819271.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choi HK, Willett W, Curhan G. Fructose-rich beverages and risk of gout in women. JAMA. 2010;304(20):2270–8. doi: 10.1001/jama.2010.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dalbeth N, House ME, Gamble GD, Horne A, Pool B, Purvis L, et al. Population-specific influence of SLC2A9 genotype on the acute hyperuricaemic response to a fructose load. Ann Rheum Dis. 2013;72(11):1868–73. doi: 10.1136/annrheumdis-2012-202732. [DOI] [PubMed] [Google Scholar]
- 88.Dalbeth N, House ME, Gamble GD, Pool B, Horne A, Purvis L, et al. Influence of the ABCG2 gout risk 141 K allele on urate metabolism during a fructose challenge. Arthritis Res Ther. 2014;16(1):R34. doi: 10.1186/ar4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Batt C, Phipps-Green AJ, Black MA, Cadzow M, Merriman ME, Topless R, et al. Sugar-sweetened beverage consumption: a risk factor for prevalent gout with SLC2A9 genotype-specific effects on serum urate and risk of gout. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jeroncic I, Mulic R, Klismanic Z, Rudan D, Boban M, Zgaga L. Interactions between genetic variants in glucose transporter type 9 (SLC2A9) and dietary habits in serum uric acid regulation. Croat Med J. 2010;51(1):40–7. doi: 10.3325/cmj.2010.51.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao X, Qi L, Qiao N, Choi HK, Curhan G, Tucker KL, et al. Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in US men and women. Hypertension. 2007;50(2):306–12. doi: 10.1161/HYPERTENSIONAHA.107.091041. [DOI] [PubMed] [Google Scholar]
- 92.Wang DD, Sievenpiper JL, de Souza RJ, Chiavaroli L, Ha V, Cozma AI, et al. The effects of fructose intake on serum uric acid vary among controlled dietary trials. J Nutr. 2012;142(5):916–23. doi: 10.3945/jn.111.151951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun SZ, Flickinger BD, Williamson-Hughes PS, Empie MW. Lack of association between dietary fructose and hyperuricemia risk in adults. Nutr Metab (Lond) 2010;7:16. doi: 10.1186/1743-7075-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zgaga L, Theodoratou E, Kyle J, Farrington SM, Agakov F, Tenesa A, et al. The association of dietary intake of purine-rich vegetables, sugar-sweetened beverages and dairy with plasma urate, in a cross-sectional study. PLoS One. 2012;7(6):e38123. doi: 10.1371/journal.pone.0038123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garrel DR, Verdy M, PetitClerc C, Martin C, Brule D, Hamet P. Milk- and soy-protein ingestion: acute effect on serum uric acid concentration. Am J Clin Nutr. 1991;53(3):665–9. doi: 10.1093/ajcn/53.3.665. [DOI] [PubMed] [Google Scholar]
- 96.Ryu KA, Kang HH, Kim SY, Yoo MK, Kim JS, Lee CH, et al. Comparison of nutrient intake and diet quality between hyperuricemia subjects and controls in Korea. Clin Nutr Res. 2014;3(1):56–63. doi: 10.7762/cnr.2014.3.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dalbeth N, Wong S, Gamble GD, Horne A, Mason B, Pool B, et al. Acute effect of milk on serum urate concentrations: a randomised controlled crossover trial. Ann Rheum Dis. 2010;69(9):1677–82. doi: 10.1136/ard.2009.124230. [DOI] [PubMed] [Google Scholar]
- 98.Dalbeth N, Gracey E, Pool B, Callon K, McQueen FM, Cornish J, et al. Identification of dairy fractions with anti-inflammatory properties in models of acute gout. Ann Rheum Dis. 2010;69(4):766–9. doi: 10.1136/ard.2009.113290. [DOI] [PubMed] [Google Scholar]
- 99.Dalbeth N, Ames R, Gamble GD, Horne A, Wong S, Kuhn-Sherlock B, et al. Effects of skim milk powder enriched with glycomacropeptide and G600 milk fat extract on frequency of gout flares: a proof-of-concept randomised controlled trial. Ann Rheum Dis. 2012;71(6):929–34. doi: 10.1136/annrheumdis-2011-200156. [DOI] [PubMed] [Google Scholar]
- 100.Kiyohara C, Kono S, Honjo S, Todoroki I, Sakurai Y, Nishiwaki M, et al. Inverse association between coffee drinking and serum uric acid concentrations in middle-aged Japanese males. Br J Nutr. 1999;82(2):125–30. [PubMed] [Google Scholar]
- 101.Pham NM, Yoshida D, Morita M, Yin G, Toyomura K, Ohnaka K, et al. The relation of coffee consumption to serum uric Acid in Japanese men and women aged 49-76 years. J Nutr Metab. 2010 doi: 10.1155/2010/930757. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Choi HK, Curhan G. Coffee, tea, and caffeine consumption and serum uric acid level: the third national health and nutrition examination survey. Arthritis Rheum. 2007;57(5):816–21. doi: 10.1002/art.22762. [DOI] [PubMed] [Google Scholar]
- 103.Choi HK, Willett W, Curhan G. Coffee consumption and risk of incident gout in men: a prospective study. Arthritis Rheum. 2007;56(6):2049–55. doi: 10.1002/art.22712. [DOI] [PubMed] [Google Scholar]
- 104.Choi HK, Curhan G. Coffee consumption and risk of incident gout in women: the Nurses' Health Study. Am J Clin Nutr. 2010;92(4):922–7. doi: 10.3945/ajcn.2010.29565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stein HB, Hasan A, Fox IH. Ascorbic acid-induced uricosuria. A consequency of megavitamin therapy. Ann Intern Med. 1976;84(4):385–8. doi: 10.7326/0003-4819-84-4-385. [DOI] [PubMed] [Google Scholar]
- 106.Huang HY, Appel LJ, Choi MJ, Gelber AC, Charleston J, Norkus EP, et al. The effects of vitamin C supplementation on serum concentrations of uric acid: results of a randomized controlled trial. Arthritis Rheum. 2005;52(6):1843–7. doi: 10.1002/art.21105. [DOI] [PubMed] [Google Scholar]
- 107.Gao X, Curhan G, Forman JP, Ascherio A, Choi HK. Vitamin C intake and serum uric acid concentration in men. J Rheumatol. 2008;35(9):1853–8. [PMC free article] [PubMed] [Google Scholar]
- 108.Choi HK, Gao X, Curhan G. Vitamin C intake and the risk of gout in men: a prospective study. Arch Intern Med. 2009;169(5):502–7. doi: 10.1001/archinternmed.2008.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Juraschek SP, Miller ER, 3rd, Gelber AC. Effect of oral vitamin C supplementation on serum uric acid: a meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken) 2011;63(9):1295–306. doi: 10.1002/acr.20519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jacob RA, Spinozzi GM, Simon VA, Kelley DS, Prior RL, Hess-Pierce B, et al. Consumption of cherries lowers plasma urate in healthy women. J Nutr. 2003;133(6):1826–9. doi: 10.1093/jn/133.6.1826. [DOI] [PubMed] [Google Scholar]
- 111.Zhang Y, Neogi T, Chen C, Chaisson C, Hunter DJ, Choi HK. Cherry consumption and decreased risk of recurrent gout attacks. Arthritis Rheum. 2012;64(12):4004–11. doi: 10.1002/art.34677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schlesinger N, Schlesinger M. Previously reported prior studies of cherry juice concentrate for gout flare prophylaxis: comment on the article by Zhang et al. Arthritis Rheum. 2013;65(4):1135–6. doi: 10.1002/art.37864. [DOI] [PubMed] [Google Scholar]
- 113.Williams PT. Effects of diet, physical activity and performance, and body weight on incident gout in ostensibly healthy, vigorously active men. Am J Clin Nutr. 2008;87(5):1480–7. doi: 10.1093/ajcn/87.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165(7):742–8. doi: 10.1001/archinte.165.7.742. [DOI] [PubMed] [Google Scholar]
- 115.Dalbeth N, Chen P, White M, Gamble GD, Barratt-Boyes C, Gow PJ, et al. Impact of bariatric surgery on serum urate targets in people with morbid obesity and diabetes: a prospective longitudinal study. Ann Rheum Dis. 2014;73(5):797–802. doi: 10.1136/annrheumdis-2013-203970. [DOI] [PubMed] [Google Scholar]
- 116.Antozzi P, Soto F, Arias F, Carrodeguas L, Ropos T, Zundel N, et al. Development of acute gouty attack in the morbidly obese population after bariatric surgery. Obes Surg. 2005;15(3):405–7. doi: 10.1381/0960892053576802. [DOI] [PubMed] [Google Scholar]
- 117.Dalbeth N, Pool B, Yip S, Cornish J, Murphy R. Effect of bariatric surgery on the inflammatory response to monosodium urate crystals: a prospective study. Ann Rheum Dis. 2013;72(9):1583–4. doi: 10.1136/annrheumdis-2013-203545. [DOI] [PubMed] [Google Scholar]
- 118.Hanna BE, Hamed JM, Touhala LM. Serum uric Acid in smokers. Oman Med J. 2008;23(4):269–74. [PMC free article] [PubMed] [Google Scholar]
- 119.Tsuchiya M, Asada A, Kasahara E, Sato EF, Shindo M, Inoue M. Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasma. Circulation. 2002;105(10):1155–7. doi: 10.1161/hc1002.105935. [DOI] [PubMed] [Google Scholar]
- 120.Harrold LR, Yood RA, Mikuls TR, Andrade SE, Davis J, Fuller J, et al. Sex differences in gout epidemiology: evaluation and treatment. Ann Rheum Dis. 2006;65(10):1368–72. doi: 10.1136/ard.2006.051649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shima Y, Teruya K, Ohta H. Association between intronic SNP in urate-anion exchanger gene, SLC22A12, and serum uric acid levels in Japanese. Life Sci. 2006;79(23):2234–7. doi: 10.1016/j.lfs.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 122.Jang WC, Nam YH, Park SM, Ahn YC, Park SH, Choe JY, et al. T6092C polymorphism of SLC22A12 gene is associated with serum uric acid concentrations in Korean male subjects. Clin Chim Acta. 2008;398(1-2):140–4. doi: 10.1016/j.cca.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 123.Guan M, Zhang J, Chen Y, Liu W, Kong N, Zou H. High-resolution melting analysis for the rapid detection of an intronic single nucleotide polymorphism in SLC22A12 in male patients with primary gout in China. Scand J Rheumatol. 2009;38(4):276–81. doi: 10.1080/03009740802572483. [DOI] [PubMed] [Google Scholar]
- 124.Tu HP, Chen CJ, Lee CH, Tovosia S, Ko AM, Wang SJ, et al. The SLC22A12 gene is associated with gout in Han Chinese and Solomon Islanders. Ann Rheum Dis. 2010;69(6):1252–4. doi: 10.1136/ard.2009.114504. [DOI] [PubMed] [Google Scholar]
- 125.Li S, Sanna S, Maschio A, Busonero F, Usala G, Mulas A, et al. The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet. 2007;3(11):e194. doi: 10.1371/journal.pgen.0030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McArdle PF, Parsa A, Chang YP, Weir MR, O'Connell JR, Mitchell BD, et al. Association of a common nonsynonymous variant in GLUT9 with serum uric acid levels in old order amish. Arthritis Rheum. 2008;58(9):2874–81. doi: 10.1002/art.23752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stark K, Reinhard W, Neureuther K, Wiedmann S, Sedlacek K, Baessler A, et al. Association of common polymorphisms in GLUT9 gene with gout but not with coronary artery disease in a large case-control study. PLoS One. 2008;3(4):e1948. doi: 10.1371/journal.pone.0001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hollis-Moffatt JE, Xu X, Dalbeth N, Merriman ME, Topless R, Waddell C, et al. Role of the urate transporter SLC2A9 gene in susceptibility to gout in New Zealand Maori, Pacific Island, and Caucasian case-control sample sets. Arthritis Rheum. 2009;60(11):3485–92. doi: 10.1002/art.24938. [DOI] [PubMed] [Google Scholar]
- 129.Tu HP, Chen CJ, Tovosia S, Ko AM, Lee CH, Ou TT, et al. Associations of a non-synonymous variant in SLC2A9 with gouty arthritis and uric acid levels in Han Chinese subjects and Solomon Islanders. Ann Rheum Dis. 2010;69(5):887–90. doi: 10.1136/ard.2009.113357. [DOI] [PubMed] [Google Scholar]
- 130.Urano W, Taniguchi A, Anzai N, Inoue E, Sekita C, Endou H, et al. Association between GLUT9 and gout in Japanese men. Ann Rheum Dis. 2010;69(5):932–3. doi: 10.1136/ard.2009.111096. [DOI] [PubMed] [Google Scholar]
- 131.Yang Q, Kottgen A, Dehghan A, Smith AV, Glazer NL, Chen MH, et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet. 2010;3(6):523–30. doi: 10.1161/CIRCGENETICS.109.934455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Charles BA, Shriner D, Doumatey A, Chen G, Zhou J, Huang H, et al. A genome-wide association study of serum uric acid in African Americans. BMC Med Genomics. 2011;4:17. doi: 10.1186/1755-8794-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hamajima N, Okada R, Kawai S, Hishida A, Morita E, Yin G, et al. Significant association of serum uric acid levels with SLC2A9 rs11722228 among a Japanese population. Mol Genet Metab. 2011;103(4):378–82. doi: 10.1016/j.ymgme.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 134.Sulem P, Gudbjartsson DF, Walters GB, Helgadottir HT, Helgason A, Gudjonsson SA, et al. Identification of low-frequency variants associated with gout and serum uric acid levels. Nat Genet. 2011;43(11):1127–30. doi: 10.1038/ng.972. [DOI] [PubMed] [Google Scholar]
- 135.Li C, Chu N, Wang B, Wang J, Luan J, Han L, et al. Polymorphisms in the presumptive promoter region of the SLC2A9 gene are associated with gout in a Chinese male population. PLoS One. 2012;7(2):e24561. doi: 10.1371/journal.pone.0024561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Voruganti VS, Kent JW, Jr, Debnath S, Cole SA, Haack K, Goring HH, et al. Genome-wide association analysis confirms and extends the association of SLC2A9 with serum uric acid levels to Mexican Americans. Front Genet. 2013;4:279. doi: 10.3389/fgene.2013.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Voruganti VS, Franceschini N, Haack K, Laston S, Maccluer JW, Umans JG, et al. Replication of the effect of SLC2A9 genetic variation on serum uric acid levels in American Indians. Eur J Hum Genet. 2013 doi: 10.1038/ejhg.2013.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Phipps-Green AJ, Hollis-Moffatt JE, Dalbeth N, Merriman ME, Topless R, Gow PJ, et al. A strong role for the ABCG2 gene in susceptibility to gout in New Zealand Pacific Island and Caucasian, but not Maori, case and control sample sets. Hum Mol Genet. 2010;19(24):4813–9. doi: 10.1093/hmg/ddq412. [DOI] [PubMed] [Google Scholar]
- 139.Wang B, Miao Z, Liu S, Wang J, Zhou S, Han L, et al. Genetic analysis of ABCG2 gene C421A polymorphism with gout disease in Chinese Han male population. Hum Genet. 2010;127(2):245–6. doi: 10.1007/s00439-009-0760-4. [DOI] [PubMed] [Google Scholar]
- 140.Yamagishi K, Tanigawa T, Kitamura A, Kottgen A, Folsom AR, Iso H. The rs2231142 variant of the ABCG2 gene is associated with uric acid levels and gout among Japanese people. Rheumatology (Oxford) 2010;49(8):1461–5. doi: 10.1093/rheumatology/keq096. [DOI] [PubMed] [Google Scholar]
- 141.Matsuo H, Takada T, Ichida K, Nakamura T, Nakayama A, Takada Y, et al. Identification of ABCG2 dysfunction as a major factor contributing to gout. Nucleosides Nucleotides Nucleic Acids. 2011;30(12):1098–104. doi: 10.1080/15257770.2011.627902. [DOI] [PubMed] [Google Scholar]
- 142.Matsuo H, Takada T, Ichida K, Nakamura T, Nakayama A, Suzuki H, et al. ABCG2/BCRP dysfunction as a major cause of gout. Nucleosides Nucleotides Nucleic Acids. 2011;30(12):1117–28. doi: 10.1080/15257770.2011.633954. [DOI] [PubMed] [Google Scholar]
- 143.Eastmond CJ, Garton M, Robins S, Riddoch S. The effects of alcoholic beverages on urate metabolism in gout sufferers. Br J Rheumatol. 1995;34(8):756–9. doi: 10.1093/rheumatology/34.8.756. [DOI] [PubMed] [Google Scholar]
- 144.van der Gaag MS, van den Berg R, van den Berg H, Schaafsma G, Hendriks HF. Moderate consumption of beer, red wine and spirits has counteracting effects on plasma antioxidants in middle-aged men. Eur J Clin Nutr. 2000;54(7):586–91. doi: 10.1038/sj.ejcn.1601061. [DOI] [PubMed] [Google Scholar]
- 145.Zhang Y, Woods R, Chaisson CE, Neogi T, Niu J, McAlindon TE, et al. Alcohol consumption as a trigger of recurrent gout attacks. Am J Med. 2006;119(9):800, e13–8. doi: 10.1016/j.amjmed.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 146.Neogi T, Chen C, Niu J, Chaisson C, Hunter DJ, Zhang Y. Alcohol quantity and type on risk of recurrent gout attacks: An internet-based case-crossover study. Am J Med. 2014 doi: 10.1016/j.amjmed.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]