Abstract
Background
Measures of socioeconomic disadvantage may enable improved targeting of programs to prevent rehospitalizations, but obtaining such information directly from patients can be difficult. Measures of US neighborhood socioeconomic disadvantage are more readily available, although rarely employed clinically.
Objective
To evaluate the association between neighborhood socioeconomic disadvantage at the census block-group level, as measured by Singh’s validated Area Deprivation Index (ADI), and 30-day rehospitalization.
Design
Retrospective cohort study
Setting
United States
Patients
Random 5% national sample of fee-for-service Medicare patients discharged with congestive heart failure, pneumonia or myocardial infarction, 2004–2009 (N = 255,744)
Measurements
30-day rehospitalizations. Medicare data were linked to 2000 Census data to construct an ADI for each patient’s census block-group, which were then sorted into percentiles by increasing ADI. Relationships between neighborhood ADI grouping and rehospitalization were evaluated using multivariate logistic regression models, controlling for patient sociodemographics, comorbidities/severity, and index hospital characteristics.
Results
The 30-day rehospitalization rate did not vary significantly across the least disadvantaged 85% of neighborhoods, which had an average rehospitalization rate=21%. However, within the most disadvantaged 15% of neighborhoods, rehospitalization rates rose from 22% to 27% with worsening ADI. This relationship persisted after full adjustment, with the most disadvantaged neighborhoods having a rehospitalization risk (adjusted risk ratio = 1.09, confidence interval 1.05–1.12) similar to that of chronic pulmonary disease (1.06, 1.04–1.08) and greater than that of diabetes (0.95, 0.94–0.97).
Limitations
No direct markers of care quality, access
Conclusions
Residence within a disadvantaged US neighborhood is a rehospitalization predictor of magnitude similar to chronic pulmonary disease. Measures of neighborhood disadvantage, like the ADI, could potentially be used to inform policy and post-hospital care.
Primary Funding Source
National Institute on Aging
INTRODUCTION
Thirty-day rehospitalizations affect 1 in 5 hospitalized Medicare patients, cost over $17 billion annually, and result in hospital-based Medicare payment penalties for congestive heart failure, pneumonia and acute myocardial infarction rehospitalizations (1). Most believe that all hospitals can prevent at least some rehospitalizations by using a spectrum of programs to better support vulnerable patients during the high-risk post hospital period (1–3). Yet, the targeting of these programs has proven challenging, potentially because important factors contributing to rehospitalizations are not well measured—like socioeconomic disadvantage (4, 5).
Socioeconomic disadvantage is a complex theoretical concept, which describes the state of being challenged by low income, limited education and substandard living conditions for both the individual and their neighborhood or social network (6, 7). Detailed assessment of an individual patient’s socioeconomic status is a time-consuming and potentially uncomfortable task to add to a clinical encounter, and since such information is rarely available in the patient’s medical record, clinical teams often overlook socioeconomic factors when creating individualized post-hospital care plans (8). Alternatively, measures of neighborhood socioeconomic disadvantage, such as concentration of poverty in the neighborhood surrounding the patient’s residence, could be more easily accessed and assigned as a risk factor at the point of patient admission by using the patient’s address. However, the association between neighborhood disadvantage and rehospitalization risk has not yet been established.
It is plausible that neighborhood socioeconomic disadvantage would influence rehospitalization risk, because vulnerable patients depend on neighborhood supports for stability generally (9–12), and these needs are likely to be increased in the period after hospital discharge (3). US safety-net hospitals, which serve socioeconomically disadvantaged areas, are more apt to be financially penalized for their rehospitalization rates (13–16). Living in a socioeconomically disadvantaged neighborhood has been associated with health behaviors (17), access to food (18, 19) and safety (20), and with outcomes such as mortality (10, 12–17), birth weight (21), and rehospitalization risk for heart failure (22). Additionally, important health indicators improve with moving people to areas of less concentrated poverty (23, 24).
In 2003, Singh created a composite measure of neighborhood socioeconomic disadvantage for the US -- the Area Deprivation Index (ADI) -- based on similar measures used in many other countries for resource planning and health policy development (25–29). The ADI is a factor-based index which uses 17 US Census poverty, education, housing and employment indicators to characterize census-based regions (25, 27–29), and has been correlated with a number of health outcomes including all-cause, cardiovascular, cancer and childhood mortality, and cervical cancer prevalence (25, 27–32). Socioeconomic disadvantage based on neighborhood risk through a Zip code-linked ADI does not require a potentially lengthy and intrusive discussion with patients and families, and could be easily made available to clinical teams and policymakers.
Our objective was to determine whether or not neighborhood socioeconomic disadvantage could be useful to clinical planning by examining its relevance in a population likely to be targeted by clinical improvement activities designed to reduce readmission risk. We analyzed the association between ADI, defined at the census block group level, and 30-day rehospitalizations for patients discharged with congestive heart failure, pneumonia or acute myocardial infarction, the clinical conditions used for the current calculation of Medicare’s rehospitalization penalties.
METHODS
Data Sources, Study Population
We used 2004–2009 data from the Chronic Condition Data Warehouse (33), including Medicare claims and enrollment files pre-linked to annual Medicare provider of service files for a 5% random national sample of Medicare beneficiaries. Beneficiaries who received railroad retirement benefits or were in a health maintenance organization were excluded because these groups have incomplete data. We identified 307,827 patients >65 years of age hospitalized with congestive heart failure, acute myocardial infarction or pneumonia using Medicare readmission measure definitions (34–36). We used the Zip+4 code listed for the patient’s residence within Medicare data to link to the census block group with the same Zip+4 area in 2000 US Census data for the 50 US States and District of Columbia. Each census block group covers an area of 600–3,000 people, averaging 1,500 people (37). We excluded 52,083 patients without a Zip+4 code in their Medicare data (n=9,741) or whose documented Zip+4 code did not exist in the 2000 Census data (n=42,342). Patients in this latter category may include those who designate a post office box as their primary residence, or reside in post-year 2000 new Zip+4 areas, US Territories or institutions like prisons. Hand-checking of a small random sampling of these patients’ Zip+4 codes suggests that most were assigned to a post office box. The final sample size was 255,744 patients. These patients originated from 4,802 unique hospitals (average 53.3 patients per hospital; range 1–743). The University of Wisconsin (UW) Institutional Review Board approved this study.
Variables
Census Block Group-Level Variables
We calculated ADI scores for each US Census block group using Singh’s methodology (14, 16–18). This involved summing Singh’s 17 Census indicators weighted by Singh’s factor score coefficients for each indicator (25) (Table 1). See Appendix 1 for more detail on constructing the ADI. We examined the distribution of ADI values and sorted neighborhoods into percentiles by increasing ADI.
Table 1.
Census Data Block Group Components and Factor Score Coefficients in Singh’s Area Deprivation Index (ADI)*
| Census Block Group Components | Factor Score Coefficients |
|---|---|
| Percent of the block group’s population aged ≥ 25 years with < 9 years of education | 0.0849 |
| Percent aged ≥ 25 years with greater than or equal to a high school diploma | −0.0970 |
| Percent of employed persons ≥16 years of age in white-collar occupations | −0.0874 |
| Median family income | −0.0977 |
| Income disparity† | 0.0936 |
| Median home value | −0.0688 |
| Median gross rent | −0.0781 |
| Median monthly mortgage | −0.0770 |
| Percent owner-occupied housing units (home ownership rate) | −0.0615 |
| Percent of civilian labor force population ≥ 16 years of age unemployed (unemployment rate) | 0.0806 |
| Percent of families below the poverty level | 0.0977 |
| Percent of population below 150% of the poverty threshold | 0.1037 |
| Percent of single-parent households with children < 18 years of age | 0.0719 |
| Percent of occupied housing units without a motor vehicle | 0.0694 |
| Percent of occupied housing units without a telephone | 0.0877 |
| Percent of occupied housing units without complete plumbing (log) | 0.0510 |
| Percent of occupied housing units with more than one person per room (crowding) | 0.0556 |
Components and factor score coefficients drawn from reference 28. All coefficients are multiplied by −1 to ease interpretation (higher ADI = higher disadvantage).
Income disparity defined by Singh as the log of 100*ratio of number of households with <$10,000 income to number of households with $50,000+ income.
Patient-Level Variables
We constructed all-cause rehospitalization within 30 days of discharge from Medicare claims (34–36). Other variables drawn from Medicare files, included patient age, gender, race, Medicaid status, initial Medicare enrollment due to disability, index hospitalization length of stay and discharge to a skilled nursing facility. Race was categorized into ‘White’, ‘Black’, and ‘Other’ based upon the beneficiary race code. Each patient’s Centers for Medicare and Medicaid Services hierarchical condition category (HCC) score, calculated from all outpatient and inpatient claims over the 12 months prior to the index hospitalization, was included as a risk adjustment measure (38). Comorbid conditions were identified using Elixhauser methods, incorporating data from the index hospitalization and from all hospitalizations and physician claims during the year prior to the index hospitalization (39). Of the comorbidities identified using this approach, 17 had frequencies of greater than 5% in the sample and were included as indicators. Comorbidities occurring less often were compiled into an ‘other comorbidity’ indicator and included alcohol/drug abuse, rheumatoid arthritis/collagen vascular disease, chronic blood loss anemia, liver disease, lymphoma, metastatic cancer, solid tumor without metastases, paralysis, psychoses and peptic ulcer disease. We assessed rurality of each patient’s zip code of residence using the US Department of Agriculture’s Rural/Urban Commuting Area (RUCA) Codes, grouped into categories of “urban core areas,” “suburban areas,” “large town areas,” and “small town/isolated rural areas” (40, 41). Index hospital characteristics, including Medicare geographic region, for-profit status and medical school affiliation, were drawn from the Medicare provider of services file corresponding to the patient’s index hospitalization date (42). We estimated annual Medicare discharge volume for each hospital by multiplying the number of claims from each hospital in the 5% national sample, by 20. We then grouped hospitals into low, middle and high volume tertiles. About one percent of our sample was missing race data (n=291), and less than 3% were missing hospital medical school affiliation (n=777) and for-profit status (n=777). There were no missing data for other patient-level variables.
Statistical Analysis
We examined the unadjusted relationship between ADI percentile and 30-day rehospitalization, overall and by primary disease. Based upon the empiric ADI data, the most disadvantaged neighborhoods made up the top 15% of the distribution. To better assess for within-group differences, we divided this most disadvantaged 15% into three equally sized 5% groupings representing the third-most, the second-most and the most disadvantaged 5% of neighborhoods. The remainder of neighborhoods (85%) were grouped into a comparator category. We examined frequencies of patient and index hospital characteristics for each grouping.
We used logistic regression to assess the relationship between ADI grouping and 30-day rehospitalization. Next, to assess the full spectrum of ADI impact, we divided the distribution into 20 equally-sized neighborhood groupings of increasing ADI (5% each), and used logistic regression to assess the relationship between ADI grouping and rehospitalization. To investigate the within-hospital ADI effects (43), we employed conditional (44) and random effects logistic regression (45, 46). To assess for differences in disease grouping and rural-urban effects, the relationship was assessed using logistic regression models stratified by disease grouping and RUCA code. Patient numbers in stratified analyses were smaller, so we analyzed the most disadvantaged 15% of neighborhoods as a single group.
Control variables were drawn from theoretical models of rehospitalization (47) and included patient HCC score tertile, comorbidities, length of stay, discharge to skilled nursing facility, age, gender, race, Medicaid status, disability status and RUCA code of primary residence; and index hospital medical school affiliation, for-profit status and discharge volume tertile. We calculated adjusted risk ratios, predicted probabilities, and 95% confidence intervals from these models on the basis of marginal standardization, as per methods by Kleinman and Norton (48) and by Localio (49). All models were estimated twice—once accounting for hospital-level and patient-level clustering, and again using robust estimates of the variance. Since no differences were noted, we present the more conservative robust estimates. All analyses were performed using SAS 9.3 (SAS Institute. SAS Statistical Software. 9.3 ed. Cary, NC: SAS Institute; 2011) and STATA 12 (StataCorp. Stata Statistical Software. 12.0 ed. College Station, TX: StataCorp LP; 2011).
Role of the Funding Sources
This project was supported by a National Institute on Aging Beeson Career Development Award, the UW School of Medicine and Public Health’s Wisconsin Partnership Program and Health Innovation Program, and the UW NIH-Clinical and Translational Science Award. The UW Health Innovation Program provided assistance with Institutional Review Board application and data management. No other funding source had a role in the design or conduct; data collection, management, analysis or interpretation; or preparation, review or approval of the manuscript.
RESULTS
Neighborhood and Patient Characteristics by ADI Grouping
Patients in the most disadvantaged 15% of neighborhoods were more apt to be Black, on Medicaid, and to have higher rates of comorbidities, especially congestive heart failure, chronic pulmonary disease, and hypertension than patients from the other 85% of neighborhoods (Table 2). They were also more likely to have been hospitalized in a for-profit hospital. The majority of patients in the most disadvantaged 5% of neighborhoods lived in urban core areas. Those in the second- and third- most disadvantaged 5% groups were most likely to live in rural or large town areas.
Table 2.
Key Characteristics of Medicare Patients Discharged with Primary Diagnoses of Congestive Heart Failure, Acute Myocardial Infarction, and Pneumonia, Overall and by Area Deprivation Index (ADI) Grouping of the Patient’s Neighborhood of Residence, 2004–2009 (N=255,744)*
| Area Deprivation Index (ADI) Grouping of the Patient’s Neighborhood of Residence |
|||||
|---|---|---|---|---|---|
| Overall (N=255,744) |
Least Disadvantaged 85% (N= 12,813) |
Third-Most Disadvantaged 5% (N=12,798) |
Second-Most Disadvantaged 5% (N=12,779) |
Most Disadvantaged 5% (N=12,772) |
|
| Key Characteristics | % | % | % | % | % |
| Patient Characteristics | |||||
| Average Age in Years (SD) | 80.6 (8) | 80.8 (8) | 79.7 (8) | 79.3 (8) | 79.2 (8) |
| 65–69 years | 10 | 10 | 13 | 13 | 14 |
| 70–74 years | 15 | 14 | 17 | 18 | 18 |
| 75–79 years | 19 | 19 | 20 | 21 | 20 |
| 80–84 years | 22 | 23 | 21 | 21 | 21 |
| >=85 years | 34 | 34 | 30 | 28 | 28 |
| Gender: | |||||
| Male | 39 | 39 | 37 | 37 | 36 |
| Female | 61 | 61 | 63 | 63 | 64 |
| Race: | |||||
| White | 87 | 90 | 82 | 76 | 58 |
| Black | 9 | 7 | 15 | 20 | 33 |
| Other | 4 | 4 | 3 | 4 | 9 |
| Medicaid: | |||||
| No | 78 | 81 | 67 | 64 | 53 |
| Yes | 22 | 19 | 33 | 36 | 47 |
| Disabled: | |||||
| No | 98 | 98 | 98 | 97 | 97 |
| Yes | 2 | 2 | 2 | 3 | 3 |
| Rural Urban Commuting Area Code for Patient Residence: |
|||||
| Urban Core Area | 66 | 69 | 42 | 45 | 64 |
| Suburban Area | 8 | 9 | 6 | 4 | 2 |
| Large Town Area | 14 | 12 | 21 | 21 | 18 |
| Small Town and Isolated Rural Area |
12 | 9.3 | 31 | 30 | 17 |
| Average HCC Score Prior to Index Hospitalization Date (SD) |
2.89 (1.85) | 2.87 (1.85) | 2.86 (1.77) | 2.95 (1.84) | 3.13 (1.97) |
| Comorbidities: | |||||
| Hypertension | 57 | 56 | 59 | 61 | 62 |
| Congestive Heart Failure | 48 | 47 | 51 | 53 | 54 |
| Fluid and Electrolyte Disorders |
39 | 38 | 40 | 42 | 43 |
| Chronic Pulmonary Disease | 38 | 37 | 30 | 43 | 42 |
| Deficiency Anemias | 29 | 29 | 30 | 32 | 35 |
| Valvular Disease | 22 | 23 | 20 | 20 | 21 |
| Renal Failure | 21 | 20 | 21 | 24 | 25 |
| Diabetes, Uncomplicated | 17 | 17 | 19 | 19 | 20 |
| Hypothyroidism | 16 | 17 | 17 | 16 | 14 |
| Peripheral Vascular Disease | 16 | 16 | 17 | 19 | 19 |
| Depression | 12 | 12 | 14 | 13 | 12 |
| Other Neurological Disorders | 12 | 12 | 12 | 14 | 12 |
| Diabetes, Complicated | 11 | 11 | 12 | 15 | 15 |
| Pulmonary Circulation Disease |
9 | 9 | 9 | 10 | 10 |
| Obesity | 7 | 7 | 8 | 9 | 9 |
| Coagulopathy | 6 | 6 | 6 | 6 | 7 |
| Weight Loss | 6 | 6 | 6 | 8 | 8 |
| Other Comorbidity | 26 | 26 | 26 | 26 | 27 |
| Primary Diagnosis of Index Hospitalization: |
|||||
| Congestive Heart Failure | 44 | 43 | 44 | 46 | 49 |
| Acute Myocardial Infarction | 17 | 18 | 16 | 15 | 15 |
| Pneumonia | 39 | 39 | 40 | 39 | 36 |
| Average Length of Stay of Index Hospitalization in Days (SD) |
5.6 (4.8) | 5.6 (4.8) | 5.5 (4.6) | 5.5 (4.4) | 5.8 (5.5) |
| 2 Or Fewer Days | 18 | 19 | 18 | 17 | 17 |
| 3–4 Days | 33 | 33 | 34 | 35 | 32 |
| 5–6 Days | 21 | 21 | 21 | 21 | 21 |
| 7 Or More Days | 28 | 28 | 27 | 27 | 30 |
| Discharged to a Skilled Nursing Facility: |
|||||
| No | 77 | 76 | 78 | 78 | 80 |
| Yes | 23 | 24 | 22 | 22 | 20 |
| Index Hospital Characteristics | |||||
| Medical School Affiliation: | |||||
| None | 58 | 58 | 66 | 63 | 50 |
| Minor | 22 | 22 | 21 | 21 | 23 |
| Major | 20 | 20 | 14 | 16 | 27 |
| Hospital Type: | |||||
| Non-Profit/Public | 87 | 88 | 84 | 83 | 83 |
| For Profit | 13 | 12 | 16 | 17 | 17 |
| Average Annual Total Discharge Volume (SD) |
6920 (5423) | 7053 (5406) | 5677 (4967) | 5839 (5155) | 6984 (6092) |
| Highest Tertile (12,576 Average Annual Discharges ) |
35 | 36 | 27 | 28 | 33 |
| Middle Tertile (5,541 Average Annual Discharges) |
34 | 35 | 30 | 30 | 32 |
| Lowest Tertile (1,950 Average Annual Discharges) |
30 | 29 | 43 | 42 | 35 |
| 30 Day Patient Outcomes | |||||
| Rehospitalization | 21 | 21 | 22 | 23 | 24 |
| Death | 16 | 16 | 15 | 15 | 13 |
Values represent percentages unless otherwise specified
Abbreviation: SD= Standard Deviation; HCC=Hierarchical Condition Category Score created through the Centers for Medicare and Medicaid Services
30 Day Rehospitalization and Patient Neighborhood ADI
When compared to the other 85% of neighborhoods, residence within the most disadvantaged 15% of neighborhoods was associated with an increased risk of 30-day rehospitalization. The 30-day rehospitalization rate did not vary significantly across the least disadvantaged 85% of neighborhoods with an average rate of 21%. However, within the most disadvantaged 15% of neighborhoods, rehospitalization rates rose from 22% to 27% with worsening ADI (Figure 1). This pattern was maintained in all three primary diagnoses.
Figure 1. Unadjusted Relationship Between Area Deprivation Index (ADI) Percentile of a Medicare Patient's Neighborhood and 30 Day Rehospitalization.
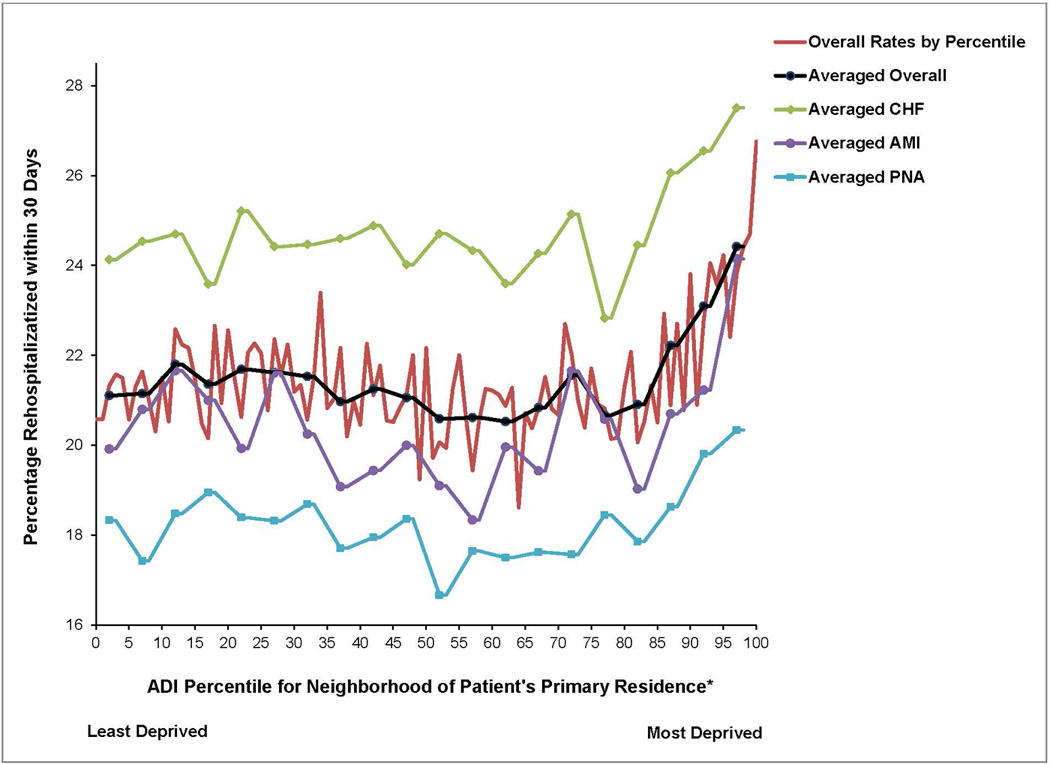
*On the ADI percentile range shown, 0 is the least socioeconomically disadvantaged group of neighborhoods ranging sequentially by equally sized neighborhood groupings up to 100 as the most disadvantaged group of neighborhoods. ‘Average’ lines represent the averaged relationship over each 5 ADI percentiles.
†Abbreviation: CHF = Congestive Heart Failure; AMI = Acute Myocardial Infarction; PNA = Pneumonia
After adjustment, residence within the most disadvantaged 15% of neighborhoods continued to be associated with increased rehospitalization risk, with the most disadvantaged 5% having the greatest risk (Table 3; Appendix Tables 1 and 2). The adjusted rehospitalization risk ratios associated with residence within the most disadvantaged 15% of neighborhoods were similar to those of chronic pulmonary disease and peripheral vascular disease, and greater than those associated with having diabetes or being on Medicaid (Appendix Table 1). This association was noted across all primary diagnoses (Appendix Table 3). Sensitivity analyses, including conditional logistic regression models with control for hospital, also suggest that when comparing two patients, otherwise the same, who differ by reason of neighborhood deprivation index and arrive at the same hospital, the association of deprivation and readmission remains (Appendix Table 4).
Table 3.
Risk of Rehospitalization Within 30 Days for Medicare Patients Discharged with Primary Diagnoses of Congestive Heart Failure, Acute Myocardial Infarction, and Pneumonia by Area Deprivation Index (ADI) Ranking of the Patient’s Neighborhood of Residence, 2004–2009 (N=255,744)*
| Characteristic | Unadjusted Risk Ratio (95% CI) |
P-Value | Adjusted† Risk Ratio (95% CI) |
P-Value | Predicted† Probability (95% CI) |
|---|---|---|---|---|---|
|
Area Deprivation Index (ADI) Grouping of the Patient’s Neighborhood of Residence |
|||||
| Least Disadvantaged 85% (Baseline Group), ADI Range = −52.63--113.44 |
1.00 | REF | 1.00 | REF | 0.21 (0.21, 0.21) |
| Third-Most Disadvantaged 5%, ADI Range = 113.45--115.12 |
1.05 (1.02, 1.09) | 0.003 | 1.05 (1.01, 1.08) | 0.01 | 0.22 (0.22, 0.23) |
| Second-Most Disadvantaged 5%, ADI Range = 115.13--117.46 |
1.09 (1.06, 1.13) | <0.001 | 1.07 (1.03, 1.1) | <0.001 | 0.23 (0.22, 0.23) |
| Most Disadvantaged 5%, ADI Range = 117.47--129.10 |
1.16 (1.12, 1.19) | <0.001 | 1.09 (1.05, 1.12) | <0.001 | 0.23 (0.22, 0.24) |
All models employ multivariate logistic regression methods to assess the relationship between ADI grouping and 30- day rehospitalization to produce risk ratios (using the methods of Kleinman and Norton (48)) and predicted probabilities.
All models adjusted for: Hierarchical Condition Category Score; indicator variables denoting the presence of comorbidities including hypertension, fluid and electrolyte disorders, congestive heart failure, chronic pulmonary disease, deficiency anemias, uncomplicated diabetes, complicated diabetes, valvular disease, hypothyroidism, peripheral vascular disease, coagulopathy, depression, other neurological disorders, obesity, pulmonary circulation disease, renal failure, weight loss and other comorbidity; length of stay of the index hospitalization; and an indicator variable for whether a patient was discharged to a skilled nursing facility; patient demographics including age, gender and race (White, Black or other), Medicaid status, disability status, Rural Urban Commuting Area (RUCA) for patient residence and index hospital characteristics including medical school affiliation, for-profit status and total discharge volume tertile. Race data were missing for 291 patients. Index hospital medical school affiliation and for-profit status were missing for 777 patients.
Appendix Table 1.
Risk of Rehospitalization Within 30 Days for Medicare Patients Discharged with Primary Diagnoses of Congestive Heart Failure, Acute Myocardial Infarction, and Pneumonia by Area Deprivation Index (ADI) Ranking of the Patient’s Neighborhood of Residence, 2004–2009 (N=255,744)*‡
| Characteristic | Unadjusted Risk Ratio (95% CI) |
P-Value | Adjusted† Risk Ratio (95% CI) |
P-Value | Predicted† Probability (95% CI) |
|---|---|---|---|---|---|
|
Area Deprivation Index (ADI) Grouping of the Patient’s Neighborhood of Residence |
|||||
| Least Disadvantaged 85% (Baseline Group), ADI Range = −52.63--113.44 |
1.00 | REF | 1.00 | REF | 0.21 (0.21, 0.21) |
| Third-Most Disadvantaged 5%, ADI Range = 113.45--115.12 |
1.05 (1.02, 1.09) | 0.003 | 1.05 (1.01, 1.08) | 0.01 | 0.22 (0.22, 0.23) |
| Second-Most Disadvantaged 5%, ADI Range = 115.13--117.46 |
1.09 (1.06, 1.13) | <0.001 | 1.07 (1.03, 1.1) | <0.001 | 0.23 (0.22, 0.23) |
| Most Disadvantaged 5%, ADI Range = 117.47--129.10 |
1.16 (1.12, 1.19) | <0.001 | 1.09 (1.05, 1.12) | <0.001 | 0.23 (0.22, 0.24) |
| Patient Comorbidity/Severity | |||||
| HCC Score Prior to Index Hospitalization Date |
1.12 (1.11, 1.13) | <0.001 | |||
| Comorbidities: | |||||
| Hypertension | 0.92 (0.9, 0.93) | <0.001 | |||
| Fluid and Electrolyte Disorders | 1.08 (1.06, 1.1) | <0.001 | |||
| Congestive Heart Failure | 1.16 (1.14, 1.18) | <0.001 | |||
| Chronic Pulmonary Disease | 1.06 (1.04, 1.08) | <0.001 | |||
| Deficiency Anemias | 1.09 (1.07, 1.11) | <0.001 | |||
| Diabetes, Uncomplicated | 0.95 (0.94, 0.97) | <0.001 | |||
| Diabetes, Complicated | 1.03 (1, 1.05) | 0.02 | |||
| Valvular Disease | 1.08 (1.06, 1.1) | <0.001 | |||
| Hypothyroidism | 1.03 (1.01, 1.05) | 0.01 | |||
| Peripheral Vascular Disease | 1.07 (1.05, 1.09) | <0.001 | |||
| Coagulopathy | 1 (0.97, 1.03) | 0.85 | |||
| Depression | 1.05 (1.02, 1.07) | <0.001 | |||
| Other Neurological Disorders | 0.98 (0.96, 1) | 0.09 | |||
| Obesity | 1.02 (0.99, 1.05) | 0.22 | |||
| Pulmonary Circulation Disease | 1.05 (1.02, 1.07) | <0.001 | |||
| Renal Failure | 1.11 (1.09, 1.14) | <0.001 | |||
| Weight Loss | 0.96 (0.93, 0.99) | 0.01 | |||
| Other Comorbidity | 1.03 (1.01, 1.05) | <0.001 | |||
| Length of Stay of Index Hospitalization: | |||||
| 2 Or Fewer Days | Ref | ||||
| 3–4 Days | 1.07 (1.04, 1.09) | <0.001 | |||
| 5–6 Days | 1.16 (1.13, 1.19) | <0.001 | |||
| 7 Or More Days | 1.34 (1.31, 1.37) | <0.001 | |||
| Discharged to a Skilled Nursing Facility: | |||||
| No | Ref | ||||
| Yes | 1.13 (1.11, 1.15) | <0.001 | |||
| Patient Demographics | |||||
| Age: | |||||
| 65–69 years | Ref | ||||
| 70–74 years | 1.01 (0.98, 1.05) | 0.38 | |||
| 75–79 years | 1.01 (0.98, 1.04) | 0.50 | |||
| 80–84 years | 1.01 (0.98, 1.05) | 0.36 | |||
| >=85 years | 0.99 (0.96, 1.02) | 0.42 | |||
| Gender: | |||||
| Male | Ref | ||||
| Female | 0.99 (0.97, 1) | 0.11 | |||
| Race: | |||||
| White | Ref | ||||
| Black | 1.06 (1.04, 1.09) | <0.001 | |||
| Other | 1 (0.96, 1.04) | 0.99 | |||
| Medicaid: | |||||
| No | Ref | ||||
| Yes | 1 (0.98, 1.02) | 0.82 | |||
| Disability: | |||||
| No | Ref | ||||
| Yes | 1.06 (1, 1.12) | 0.03 | |||
| Rural Urban Commuting Area (RUCA) for Patient Residence: |
|||||
| Urban Core Area | Ref | ||||
| Suburban Area | 1 (0.97, 1.03) | 0.91 | |||
| Large Town Area | 0.96 (0.94, 0.99) | 0.00 | |||
| Small Town and Isolated Rural Area | 1 (0.97, 1.03) | 0.97 | |||
| Index Hospital Characteristics | |||||
| Medical School Affiliation: | |||||
| None | Ref | ||||
| Minor | 1 (0.98, 1.02) | 0.73 | |||
| Major | 1.02 (1, 1.05) | 0.02 | |||
| Hospital Type: | |||||
| Non-Profit/Public | Ref | ||||
| For Profit | 1.01 (0.99, 1.03) | 0.34 | |||
| Average Annual Total Discharge Volume: | |||||
| Highest Tertile (12,576 Average Annual Discharges) |
Ref | ||||
| Middle Tertile (5,541 Average Annual Discharges) |
0.99 (0.97, 1.01) | 0.16 | |||
| Lowest Tertile (1,950 Average Annual Discharges) |
0.97 (0.95, 0.99) | 0.00 |
All models employ multivariate logistic regression methods to assess the relationship between ADI grouping and 30- day rehospitalization to produce risk ratios (using the methods of Kleinman and Norton (48)) and predicted probabilities.
Adjusted for all variables listed.
Race data were missing for 291 patients. Index hospital medical school affiliation and for-profit status were missing for 777 patients.
Appendix Table 2.
Risk of Rehospitalization Within 30 Days for Medicare Patients Discharged with Primary Diagnoses of Congestive Heart Failure, Acute Myocardial Infarction, and Pneumonia by Area Deprivation Index (ADI) Ranking of the Patient’s Neighborhood of Residence, 2004–2009 (N=255,744) *
| Characteristic | Unadjusted Risk Ratio (95% CI) |
P-Value | Adjusted† Risk Ratio (95% CI) |
P-Value | Predicted† Probability (95% CI) |
|---|---|---|---|---|---|
|
Area Deprivation Index (ADI) 5% of the Patient’s Neighborhood of Residence |
|||||
| Group 1, ADI Range = −52.63--69.49 (Least Disadvantaged 5%) |
1.00 | REF | 1.00 | REF | 0.21 (0.21, 0.22) |
| Group 2, ADI Range = 69.50--81.36 | 1 (0.95, 1.05) | 0.93 | 0.99 (0.94, 1.03) | 0.59 | 0.21 (0.2, 0.22) |
| Group 3, ADI Range = 81.37--87.69 | 1.03 (0.98, 1.08) | 0.18 | 1.02 (0.97, 1.06) | 0.52 | 0.22 (0.21, 0.22) |
| Group 4, ADI Range = 87.00--91.75 | 1.01 (0.96, 1.06) | 0.63 | 1 (0.95, 1.04) | 0.91 | 0.21 (0.2, 0.22) |
| Group 5, ADI Range = 91.76--94.81 | 1.03 (0.98, 1.08) | 0.26 | 1.02 (0.97, 1.06) | 0.48 | 0.22 (0.21, 0.22) |
| Group 6, ADI Range = 94.82--97.16 | 1.02 (0.98, 1.07) | 0.32 | 1.01 (0.97, 1.06) | 0.59 | 0.22 (0.21, 0.22) |
| Group 7, ADI Range = 97.17--99.31 | 1.02 (0.97, 1.07) | 0.42 | 1.02 (0.97, 1.07) | 0.44 | 0.22 (0.21, 0.22) |
| Group 8, ADI Range = 99.32--101.18 | 0.99 (0.95, 1.04) | 0.78 | 1 (0.95, 1.05) | 0.96 | 0.21 (0.21, 0.22) |
| Group 9, ADI Range = 101.19--102.91 | 1.01 (0.96, 1.05) | 0.79 | 1.01 (0.96, 1.06) | 0.70 | 0.21 (0.21, 0.22) |
| Group 10, ADI Range = 102.92--104.46 | 1 (0.95, 1.05) | 0.93 | 1.01 (0.96, 1.05) | 0.80 | 0.21 (0.21, 0.22) |
| Group 11, ADI Range = 104.47--105.86 | 0.98 (0.93, 1.02) | 0.31 | 0.98 (0.94, 1.03) | 0.51 | 0.21 (0.2, 0.22) |
| Group 12, ADI Range = 105.87--107.15 | 0.98 (0.93, 1.02) | 0.34 | 0.98 (0.93, 1.03) | 0.37 | 0.21 (0.2, 0.22) |
| Group 13, ADI Range = 107.16--108.36 | 0.97 (0.93, 1.02) | 0.25 | 0.98 (0.93, 1.02) | 0.31 | 0.21 (0.2, 0.21) |
| Group 14, ADI Range = 108.37--109.55 | 0.99 (0.94, 1.03) | 0.60 | 0.99 (0.94, 1.04) | 0.68 | 0.21 (0.2, 0.22) |
| Group 15, ADI Range = 109.56--110.75 | 1.02 (0.97, 1.07) | 0.38 | 1.02 (0.97, 1.07) | 0.46 | 0.22 (0.21, 0.22) |
| Group 16, ADI Range = 110.76--112.02 | 0.98 (0.93, 1.03) | 0.39 | 0.99 (0.94, 1.04) | 0.61 | 0.21 (0.2, 0.22) |
| Group 17, ADI Range = 112.03--113.44 | 0.99 (0.94, 1.04) | 0.69 | 0.99 (0.94, 1.04) | 0.74 | 0.21 (0.2, 0.22) |
| Group 18, ADI Range = 113.45--115.12 (Third-Most Disadvantaged 5%) |
1.05 (1, 1.1) | 0.03 | 1.05 (1, 1.1) | 0.06 | 0.22 (0.22, 0.23) |
| Group 19, ADI Range = 115.13--117.46 (Second-Most Disadvantaged 5%) |
1.09 (1.04, 1.14) | <0.001 | 1.06 (1.01, 1.11) | 0.01 | 0.23 (0.22, 0.23) |
| Group 20, ADI Range = 117.47--129.10 (Most Disadvantaged 5%) |
1.16 (1.1, 1.21) | <0.001 | 1.08 (1.03, 1.13) | 0.001 | 0.23 (0.22, 0.24) |
All models employ multivariate logistic regression methods to assess the relationship between ADI grouping and 30- day rehospitalization to produce risk ratios (using the methods of Kleinman and Norton (48)) and predicted probabilities.
All models adjusted for: Hierarchical Condition Category Score; indicator variables denoting the presence of comorbidities including hypertension, fluid and electrolyte disorders, congestive heart failure, chronic pulmonary disease, deficiency anemias, uncomplicated diabetes, complicated diabetes, valvular disease, hypothyroidism, peripheral vascular disease, coagulopathy, depression, other neurological disorders, obesity, pulmonary circulation disease, renal failure, weight loss and other comorbidity; length of stay of the index hospitalization; and an indicator variable for whether a patient was discharged to a skilled nursing facility; patient demographics including age, gender and race (White, Black or other), Medicaid status, disability status, Rural Urban Commuting Area (RUCA) for patient residence and index hospital characteristics including medical school affiliation, for-profit status and total discharge volume tertile. Race data were missing for 291 patients. Index hospital medical school affiliation and for-profit status were missing for 777 patients.
Appendix Table 3.
Risk of Rehospitalization Within 30 Days for Medicare Patients Discharged with Primary Diagnoses of Congestive Heart Failure, Acute Myocardial Infarction, and Pneumonia, 2004–2009, by Area Deprivation Index (ADI) Ranking of the Patient’s Neighborhood of Residence and Primary Discharge Diagnosis (N=255,744)*
| Congestive Heart Failure (N=112,192) |
Acute Myocardial Infarction (N=44,582) |
Pneumonia (N=98,970) | ||||
|---|---|---|---|---|---|---|
| Characteristic | Adjusted† Risk Ratio (95% CI) |
P-Value | Adjusted† Risk Ratio (95% CI) |
P-Value | Adjusted† Risk Ratio (95% CI) |
P-Value |
|
Area Deprivation Index (ADI) Grouping of the Patient’s Neighborhood of Residence |
||||||
| Least Disadvantaged 85% (Baseline Group), ADI Range = −52.63--113.44 |
1.00 | REF | 1.00 | REF | 1.00 | REF |
| Third-Most Disadvantaged 5%, ADI Range = 113.45--115.12 |
1.06 (1.02, 1.11) | 0.008 | 1.02 (0.94, 1.11) | 0.595 | 1.03 (0.97, 1.09) | 0.310 |
| Second-Most Disadvantaged 5%, ADI Range = 115.13--117.46 |
1.06 (1.01, 1.11) | 0.011 | 1.03 (0.94, 1.12) | 0.569 | 1.09 (1.02, 1.15) | 0.006 |
| Most Disadvantaged 5%, ADI Range = 117.47--129.10 |
1.08 (1.03, 1.13) | 0.001 | 1.09 (1, 1.19) | 0.046 | 1.08 (1.01, 1.14) | 0.020 |
All models employ multivariate logistic regression methods to assess the relationship between ADI grouping and 30- day rehospitalization to produce adjusted risk ratios for each diagnosis by using the methods of Kleinman and Norton (48).
All models adjusted for: Hierarchical Condition Category Score; indicator variables denoting the presence of comorbidities including hypertension, fluid and electrolyte disorders, congestive heart failure, chronic pulmonary disease, deficiency anemias, uncomplicated diabetes, complicated diabetes, valvular disease, hypothyroidism, peripheral vascular disease, coagulopathy, depression, other neurological disorders, obesity, pulmonary circulation disease, renal failure, weight loss and other comorbidity; length of stay of the index hospitalization; and an indicator variable for whether a patient was discharged to a skilled nursing facility; patient demographics including age, gender and race (White, Black or other), Medicaid status, disability status, Rural Urban Commuting Area (RUCA) for patient residence and index hospital characteristics including medical school affiliation, for-profit status and total discharge volume tertile. Race data were missing for 291 patients. Index hospital medical school affiliation and for-profit status were missing for 777 patients.
Appendix Table 4.
Unadjusted and Adjusted Results from Conditional Logistic Regression (N=252,155)†
| Characteristic | Unadjusted Odds Ratio (95% CI) |
P-Value | Adjusted* Odds Ratio (95% CI) |
P-Value |
|---|---|---|---|---|
|
Area Deprivation Index (ADI) Grouping of the Patient’s Neighborhood of Residence |
||||
| Least Disadvantaged 85% (Baseline Group), ADI Range = −52.63--113.44 |
1.00 | REF | 1.00 | REF |
| Third-Most Disadvantaged 5%, ADI Range = 113.45--115.12 |
1.06 (1.01, 1.11) | 0.011 | 1.04 (0.99, 1.09) | 0.131 |
| Second-Most Disadvantaged 5%, ADI Range = 115.13--117.46 |
1.14 (1.08, 1.19) | <0.001 | 1.09 (1.04, 1.14) | 0.001 |
| Most Disadvantaged 5%, ADI Range = 117.47--129.10 |
1.14 (1.09, 1.19) | <0.001 | 1.08 (1.03, 1.14) | 0.001 |
All models adjusted for: Hierarchical Condition Category Score; indicator variables denoting the presence of comorbidities including hypertension, fluid and electrolyte disorders, congestive heart failure, chronic pulmonary disease, deficiency anemias, uncomplicated diabetes, complicated diabetes, valvular disease, hypothyroidism, peripheral vascular disease, coagulopathy, depression, other neurological disorders, obesity, pulmonary circulation disease, renal failure, weight loss and other comorbidity; length of stay of the index hospitalization; and an indicator variable for whether a patient was discharged to a skilled nursing facility; patient demographics including age, gender and race (White, Black or other), Medicaid status, disability status, Rural Urban Commuting Area (RUCA) for patient residence and index hospital characteristics including medical school affiliation, for-profit status and total discharge volume tertile. Race data were missing for 291 patients. Index hospital medical school affiliation and for-profit status were missing for 777 patients.
2523 patients (642 hospitals) dropped from analysis because of all positive or all negative outcomes within group.
Geographic Distribution
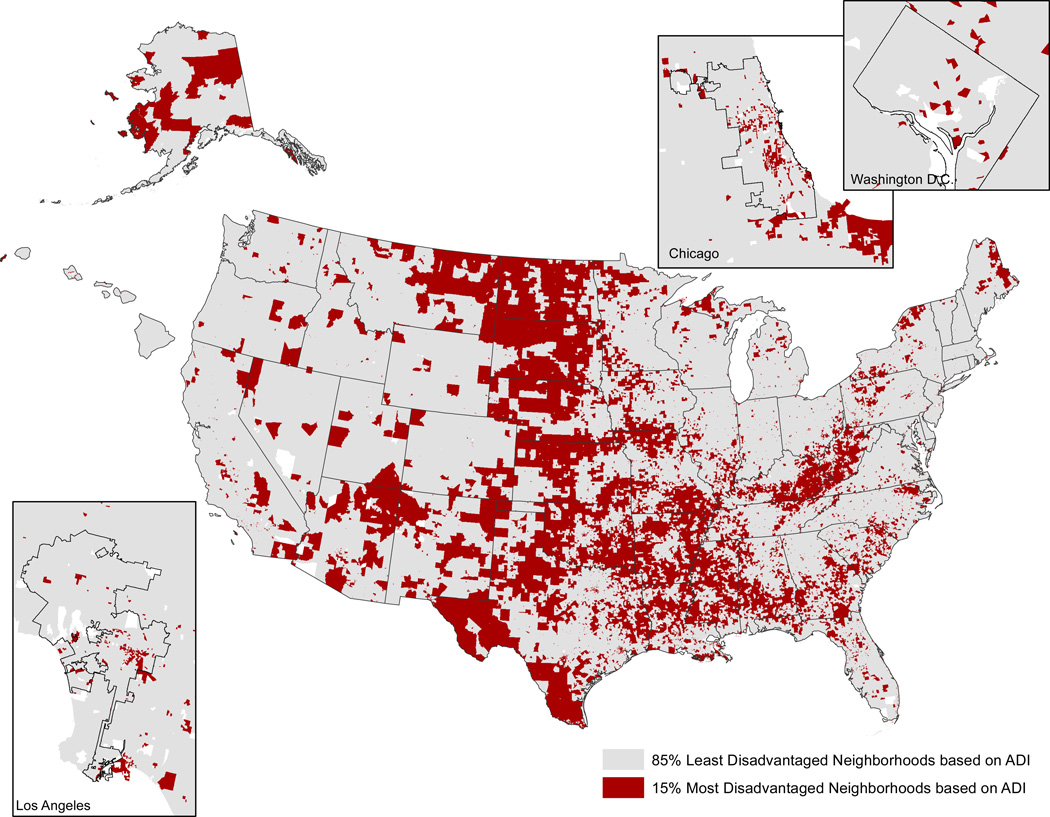
The prevalence of the most disadvantaged neighborhoods varied by Medicare geographic region (Table 4). Certain regions, like the Dallas, Atlanta, Chicago and Philadelphia regions, had a higher proportion of Medicare patients with the penalty-eligible conditions of congestive heart failure, pneumonia or acute myocardial infarction residing in the most disadvantaged US neighborhoods, than other regions. Some regions, like the Seattle region, had less than 5% of all eligible patients living in such neighborhoods. Figure 2 shows the locations of the most disadvantaged US patient neighborhoods (i.e., census block groups) in this study.
Table 4.
Medicare Patients Discharged with Primary Diagnoses of Congestive Heart Failure, Acute Myocardial Infarction, and Pneumonia, 2004–2009, and Residing in the 15% Most Disadvantaged Neighborhoods, by Medicare Region of Index Hospital (N=255,744)
| Centers for Medicare and Medicaid Services Region |
Number of Eligible Congestive Heart Failure, Acute Myocardial Infarction and Pneumonia Medicare Patients |
Eligible Patients Residing in the 15% Most Disadvantaged Neighborhoods by ADI %(N) |
|---|---|---|
| Boston Region (I) | 15566 | 4 (584) |
| New York Region (II) | 26362 | 10 (2744) |
| Philadelphia Region (III) | 26557 | 13 (3530) |
| Atlanta Region (IV) | 54867 | 18 (9846) |
| Chicago Region (V) | 54748 | 11 (6273) |
| Dallas Region (VI) | 28559 | 35 (9904) |
| Kansas City Region (VII) | 15395 | 23 (3486) |
| Denver Region (VIII) | 5676 | 13 (754) |
| San Francisco Region (IX) | 20937 | 5 (943) |
| Seattle Region (X) | 6301 | 3 (212) |
Figure 2. Locations of the 15% Most Disadvantaged Neighborhoods Based on Census Block Group Area Deprivation Index (ADI) Score.
*Urban block groups/neighborhoods must be viewed at higher magnification within this figure, because they comprise smaller geographic areas than their rural counterparts. Enlargements of sample urban areas are offered to demonstrate.
The distribution of the most disadvantaged neighborhoods also varied by rural-urban status. Nearly one-third of eligible patients residing in rural areas lived in neighborhoods that were among the most disadvantaged (Appendix Table 5). However, residence in the 15% most disadvantaged neighborhoods was a rehospitalization risk regardless of rural-urban area type.
Appendix Table 5.
Medicare Patients Discharged with Primary Diagnoses of Congestive Heart Failure, Acute Myocardial Infarction, and Pneumonia, 2004–2009, and Residing in the 15% Most Disadvantaged Neighborhoods, by Rural Urban Commuting Area (RUCA) Code of Patient Residence (N=255,744)†
| Risk of Rehospitalization within 30 Days for Patients Residing in the 15% Most Disadvantaged Neighborhoods Stratified by RUCA Code* |
||||||
|---|---|---|---|---|---|---|
| Rural Urban Commuting Area (RUCA) of Patient Residence |
Number of Eligible Congestive Heart Failure, Acute Myocardial Infarction and Pneumonia Medicare Patients |
Eligible Patients Residing in the 15% Most Disadvantaged Neighborhoods by ADI %(N) |
Unadjusted Risk Ratio (95% CI) |
P-Value | Adjusted‡ Risk Ratio (95% CI) |
P-Value |
| Urban Core Area | 195323 | 12 (23895) | 1.11 (1.08, 1.13) | <0.001 | 1.05 (1.03, 1.08) | <0.001 |
| Sub-Urban Area | 6839 | 8 (534) | 1.17 (0.99, 1.35) | 0.052 | 1.1 (0.92, 1.28) | 0.27 |
| Large Town Area | 33723 | 23 (7770) | 1.11 (1.06, 1.17) | <0.001 | 1.06 (1.01, 1.11) | 0.03 |
| Small Town and Isolated Rural Area | 19042 | 32 (6071) | 1.16 (1.1, 1.23) | <0.001 | 1.14 (1.07, 1.21) | <0.001 |
As compared to eligible patients not residing in the 15% most disadvantaged neighborhoods
All models employ multivariate logistic regression methods to assess the relationship between ADI grouping and 30- day rehospitalization to produce adjusted risk ratios for each diagnosis by using the methods of Kleinman and Norton (48).
All models adjusted for: Hierarchical Condition Category Score; indicator variables denoting the presence of comorbidities including hypertension, fluid and electrolyte disorders, congestive heart failure, chronic pulmonary disease, deficiency anemias, uncomplicated diabetes, complicated diabetes, valvular disease, hypothyroidism, peripheral vascular disease, coagulopathy, depression, other neurological disorders, obesity, pulmonary circulation disease, renal failure, weight loss and other comorbidity; length of stay of the index hospitalization; and an indicator variable for whether a patient was discharged to a skilled nursing facility; patient demographics including age, gender and race (White, Black or other), Medicaid status, disability status, and index hospital characteristics including medical school affiliation, for-profit status and total discharge volume tertile. Race data were missing for 291 patients. Index hospital medical school affiliation and for-profit status were missing for 777 patients.
DISCUSSION
Living in a severely disadvantaged neighborhood predicts rehospitalization as powerfully as does the presence of illnesses such as peripheral vascular disease or chronic pulmonary disease, and more powerfully than being on Medicaid or having diabetes. This effect holds after accounting for other patient- and hospital-level factors known to influence risk of rehospitalization, including race. Overall, patients from disadvantaged neighborhoods are at higher risk for rehospitalization regardless of their treating hospital.
Our findings suggest that neighborhood disadvantage is associated with a threshold effect, with strong and increasing risk of rehospitalization for residents of the most disadvantaged 15%. This threshold effect conforms with fundamental theories of social disadvantage (50) which indicate that there is generally some point beyond which individuals can no longer compensate and additional disadvantage leads to increasingly adverse outcomes (51). A wealth of social science research demonstrates that ‘areas of concentrated poverty’ (52, 53) place additional burdens on poor families that live within them, beyond the effect of the families’ individual circumstances (54). It is clear that social support and a patient’s environment can influence clinical outcomes, including rehospitalizations.
Although most clinicians would agree with our findings, in practice issues of socioeconomic disadvantage are often overlooked (8) for three reasons: 1) we do not agree on how to measure disadvantage, 2) we lack time and hesitate to ask for highly personal data, and 3) we do not always know what to do about disadvantage when we find it. These barriers have diminished recently. The ADI, which is widely studied and predicts rehospitalization, provides a useful measure that is usable right now, although better measures may be developed in the future. Because the ADI relies entirely on publicly-available census data, and (as of publication) will be available free on-line through the University of Wisconsin Health Innovation Program (www.HIPxChange.org) in the form of a Zip+4 code-ADI file as well as an individual look-up tool requiring only the patient’s Zip+4 code to query, it avoids both the time burden and the intrusiveness of collecting sensitive data from the patient.
Based on patient address alone, clinicians and health systems could, at the point of first contact, use the ADI to screen for patients returning to the most challenging environments; supporting early targeting of more intensive transitional care services, prompting discussion of socioeconomic environment and need, and activating additional community resources for these patients. Transitional care interventions decrease rehospitalizations by employing a series of interactions designed to empower patients, monitor for early signs of disease worsening, and ensure medical plans and follow-up are in place. The targeting of transitional care programs can sometimes be challenging, especially in low-resource health settings. We offer the ADI as a potential way to refine such targeting. Placing a look-up table in a hospital’s admission-processing system to supply this high-risk screener to the clinical team should be a very modest technical challenge.
Some European countries use composite measures of neighborhood socioeconomic disadvantage similar to the ADI to monitor population health and to allocate services and funding to ensure increased support in high-risk regions (25, 26). It could be used similarly in the US to refine characterizations of hospital service regions. Health systems and other health-related institutions could also use the ADI to identify neighborhoods that would most benefit from additional outreach and services. Policymakers could test innovative strategies for improving living conditions for older adults in severely disadvantaged areas (55). Finally, ADI scores could be used to direct funding towards community-based initiatives designed to lower unwanted rehospitalizations (56, 57).
Medicare hospital readmissions penalties fall more heavily on hospitals serving disadvantaged neighborhoods than on other hospitals (13–16). Adjusting for the socioeconomic status of individuals served (34–36) might level the playing field, but so far the debate has centered on the role of personal socioeconomic disadvantage in readmission risk, which remains unclear (4), and evidence that personal indicators of disadvantage are not ideally reliable or valid for elderly populations (58). Using the ADI to identify patients from the most severely disadvantaged neighborhoods could be explored as an adjuster for the current Medicare readmissions measures; one that might avoid the limitations of personal socioeconomic indicators and better screen for readmission risk.
A number of factors should be considered when interpreting these findings. To be included in our analyses a patient had to have a zip code of residence included within 2000 Census data. Therefore, the results of this analysis may not apply to patients without zip codes, such as the homeless, and those with zip codes absent in 2000 Census data. Although many of these latter patients list a post office box with Medicare, hospitals would have ample opportunity to gather residential Zip+4 codes directly. Census data collected in 2000 may not fully reflect neighborhood characteristics in the between-Census years of 2004–2009 used in this study. Next, patient-level analyses of any geographic-based measure, including the ADI, can introduce an ecological fallacy in which a region’s aggregate traits are inappropriately attributed to a particular individual. However, our suggested use of the ADI as a clinical screener, which could trigger clinical teams to more fully assess for post-discharge need, should avoid this problem. Finally, the administrative data on which we relied does not contain direct markers of care quality or access that may affect rehospitalization risk. It is possible that hospitals that serve predominantly disadvantaged neighborhoods provide different quality of care than hospitals that serve predominantly non-disadvantaged neighborhoods and this had an influence on our findings (43). The available data do not allow for a definitive conclusion in this regard but there is no clear evidence that safety-net hospitals, in general, differ from non-safety-net hospitals in their quality of care (59). Our analytic models provide evidence that the patients’ neighborhood remains a strong predictor of rehospitalization regardless of other hospital-level factors. More robust data should be utilized to study these across-hospital effects in the future.
The effects associated with neighborhood disadvantage may result from person-level socioeconomic disadvantage for which community data are a proxy (10–12). In studies of child health and mental health outcomes, neighborhood disadvantage has been demonstrated to be an additional risk factor beyond personal disadvantage, with worse health and social outcomes for individuals who live in both poor families and poor neighborhoods than for individuals living in poor families in less poor neighborhoods (23, 60). Our main aim was to produce a meaningful estimate of disadvantage that could be easily used by clinicians and discharge planners. The relative importance of individual and community disadvantage cannot be determined from our data. Clarifying these associations deserves further study.
We chose the ADI for this analysis because it is a well-established US census-based measure which provides a composite view of socioeconomic disadvantage for all areas of the US and which can be used to reliably ‘drill-down’ to a relatively small geographic region (25). Others have explored using single income-related component measures as socioeconomic markers (5, 61, 62), but single construct approaches likely miss issues critical to post-hospital planning, such as education and living conditions. This may be why income alone shows mixed results as a rehospitalization predictor in studies to date (61, 62).
In conclusion, residence within a disadvantaged US neighborhood is a rehospitalization predictor of magnitude similar to important chronic diseases. Measures of neighborhood disadvantage, like the ADI, are easily created using data already routinely collected by the US government and freely available to the public, and may be useful in targeting patient- and community-based initiatives designed to lower unwanted rehospitalization.
ACKNOWLEDGEMENTS
Financial Support: This project was supported by a National Institute on Aging Beeson Career Development Award (K23AG034551 [PI Kind], National Institute on Aging, The American Federation for Aging Research, The John A. Hartford Foundation, The Atlantic Philanthropies and The Starr Foundation) and by the Madison VA Geriatrics Research, Education and Clinical Center (GRECC-Manuscript #2013–13). Dr. Kind’s time was also partially supported by the University of Wisconsin School of Medicine and Public Health from the Wisconsin Partnership Program. Additional support was provided by the University of Wisconsin School Of Medicine and Public Health’s Health Innovation Program (HIP), and the Community-Academic Partnerships core of the University of Wisconsin Institute for Clinical and Translational Research (UW ICTR), grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health. Jane Brock’s time was partially supported by The Integrating Care for Populations & Communities National Coordinating Center at CFMC, under contract with the Centers for Medicare & Medicaid Services (CMS), an agency of the U.S. Department of Health and Human Services. The contents presented do not reflect CMS policy. PM-4010–242 CO 2013.No funding source had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
Dr. Kind reported receiving institutional grant support from the National Institutes of Health-NIA and the John Hartford Foundation. Dr. Jencks reported receiving consulting fees from Inovalon, Reinforced Care, Delmarva Foundation, Maryland Health Services Cost Review Commission, and Institute for Healthcare Improvement; fees for board membership from CareCentrix and Affymax; payment for speaking and education activities from Curaspan and Health Services Advisory Group; and payment for assisting in proposal preparation from Connecticut Peer Review Organization. Dr. Brock reported receiving consulting fees from the Administration on Aging; payment for lectures from Ramona VNA and Hospice, The Lewin Group, Montana Hospital Association, National Association for Public Hospitals, and DFWHC Foundation; and payment for travel and accommodations from Insignia Health, Commonwealth Fund, Institute for Healthcare Improvement, National Health Policy Forum, American College of Physicians, The Remington Report, American Association of Medical Society Executives, Robert Woods Johnson Foundation, Communities Joined in Action, and Health Industry Group Purchasing Association.
Additional Acknowledgements: The authors would like to thank Peggy Munson for Institutional Review Board assistance, Katie Ronk for data management assistance, Bill Buckingham for map creation, and Brock Polnaszek, Jacquelyn Porter, Melissa Hovanes and Colleen Brown for help with manuscript formatting.
Appendix 1.
CALCULATING THE SINGH AREA DEPRIVATION INDEX (ADI)
Introduction
In their analysis and monitoring of health, Great Britain, Sweden, Australia, New Zealand and many other nations use area-based composite deprivation indices; scores created by compiling measures of socioeconomic resources within a particular geographic area (25, 26). In 2003, Singh created a similar Area Deprivation Index (ADI) for the US (25, 27–29).
The ADI is a validated, factor-based deprivation index which uses 17 poverty, education, housing and employment indicators drawn from US Census data to create a measure of socioeconomic context for a particular census-based region (25, 27–29). The ADI has previously been used to document a number of socioeconomic-health associations, including the direct relationship between area deprivation and all-cause, cardiovascular, cancer and childhood mortality, and between area deprivation and cervical cancer prevalence (25, 27–32).
In the manuscript associated with this appendix, we calculated Area Deprivation Index (ADI) scores for each block group/neighborhood using methods proposed by Singh (25, 27–29) as a way to assess the socioeconomic context of a patient’s neighborhood. This appendix provides interested readers with a more detailed account of how we calculated the ADI using Singh’s methods.
Detailed Methods for Creating the ADI
Singh’s ADI uses 17 US Census variables in its construction. We calculated these for each geographic unit, in this case a census-block group, using publically available 2000 US Census data. The US Census variables are as follows:
Percent of population aged >= 25 years with < 9 years of education
Percent of population aged >= 25 years with < a high school diploma
Percent of employed persons >=16 years of age in white-collar occupations
Median family income
Income disparity (Defined by Singh as the log of 100 * the ratio of the number of households with <$10,000 in income to the number of households with $50,000 or more in income.) (25)
Median home value
Median gross rent
Median monthly mortgage
Percent owner-occupied housing units (home ownership rate)
Percent of civilian labor force population >= 16 years of age unemployed (unemployment rate)
Percent of families below the poverty level
Percent of population below 150% of the poverty threshold
Percent of single-parent households with children < 18 years of age
Percent of households without a motor vehicle
Percent of households without a telephone
Percent of occupied housing units without complete plumbing
Percent of households with more than one person per room (crowding).
Using Singh’s methods, these 17 indicators are weighted using factor score coefficients (see Table 1 of the accompanying manuscript). Using these Singh factors score coefficients, poverty, income and education have the largest relative weights amongst all of the 17 variables. These 17 US Census variables are multiplied by their factor weights and then summed for each geographic unit. The result is then transformed into a standardized index (the ADI) by arbitrarily setting the index mean at 100 and standard deviation at 20 (25). Using this approach, neighborhoods with higher ADI scores have higher levels of deprivation (25).
Typically, the ADI has been used to break geographic units into quintiles, deciles or other relatively ranked groupings by ADI score. To our knowledge, it has not been used as a predictor in its continuous, indexed form. For this study, we initially examined the distribution of ADI values and sorted neighborhoods into percentiles by increasing ADI.
Footnotes
This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
Author Contributions: Dr. Kind had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kind, Jencks, Brock
Acquisition of data: Kind, Bartels, Smith
Analysis and interpretation of data: Kind, Jencks, Brock, Yu, Bartels, Ehlenbach, Greenberg, Smith
Drafting of the manuscript: Kind, Jencks, Brock
Critical revision of the manuscript for important intellectual content: Kind, Jencks, Brock, Yu, Bartels, Ehlenbach, Greenberg
Statistical analysis: Kind, Yu
Obtained funding: Kind, Bartels, Smith
Administrative, technical or material support: Kind, Bartels, Ehlenbach, Greenberg, Smith
Study supervision: Kind
Conflict of Interest Disclosure: No other disclosures are reported.
Previous Presentation: None
REFERENCES
- 1.Patient Protection and Affordable Care Act. United States of America. 2010:290–294. [Google Scholar]
- 2.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 3.Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kansagara D, Englander H, Salanitro A, Kagen D, Theobald C, Freeman M, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joynt KE, Jha AK. A Path Forward on Medicare Readmissions. N Engl J Med. 2013;368:1175–1177. doi: 10.1056/NEJMp1300122. [DOI] [PubMed] [Google Scholar]
- 6.Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc. 2007;99:1013. [PMC free article] [PubMed] [Google Scholar]
- 7.Cederberg M, Hartsmar N, Lingärde S. Thematic report: Socioeconomic Disadvantage. Report from the EPASI (Educational Policies that Address Social Inequality) project supported by the European Commission’s department of Education & Culture. SOCRATES programme. 2009;2 [Google Scholar]
- 8.Fleegler EW, Lieu TA, Wise PH, Muret-Wagstaff S. Families' health-related social problems and missed referral opportunities. Pediatrics. 2007;119:e1332–e1341. doi: 10.1542/peds.2006-1505. [DOI] [PubMed] [Google Scholar]
- 9.Arbaje AI, Wolff JL, Yu Q, Powe NR, Anderson GF, Boult C. Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community-dwelling Medicare beneficiaries. Gerontologist. 2008;48:495–504. doi: 10.1093/geront/48.4.495. [DOI] [PubMed] [Google Scholar]
- 10.Robert SA. Socioeconomic position and health: the independent contribution of community socioeconomic context. Annual Review of Sociology. 1999:489–516. [Google Scholar]
- 11.House JS, Kessler RC, Herzog AR. Age, socioeconomic status, and health. The Milbank Quarterly. 1990;68:383–411. [PubMed] [Google Scholar]
- 12.House JS, Lepkowski JM, Kinney AM, Mero RP, Kessler RC, Herzog AR. The social stratification of aging and health. Journal of Health and Social Behavior. 1994;35:213–234. [PubMed] [Google Scholar]
- 13.Berenson J, Shih A. Higher readmissions at safety-net hospitals and potential policy solutions. Commonwealth Fund. 2012;34:1–16. [PubMed] [Google Scholar]
- 14.Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. J Am Med Assoc. 2011;305:675–681. doi: 10.1001/jama.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joynt KE, Jha AK. Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309:342–343. doi: 10.1001/jama.2012.94856. [DOI] [PubMed] [Google Scholar]
- 16.Rau J. Hospitals treating the poor hardest hit by readmission penalties. Kaiser Health News. Washington, DC: Henry J. Kaiser Family Foundation; 2012. [Google Scholar]
- 17.Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality. J Am Med Assoc. 1998;279:1703–1708. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- 18.Franco M, Diez Roux AV, Glass TA, Caballero B, Brancati FL. Neighborhood characteristics and availability of healthy foods in Baltimore. Am J Prev Med. 2008;35:561–567. doi: 10.1016/j.amepre.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore LV, Diez Roux AV. Associations of neighborhood characteristics with the location and type of food stores. Am J Public Health. 2006;96:325–331. doi: 10.2105/AJPH.2004.058040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh C-C, Pugh MD. Poverty, income inequality, and violent crime: a meta-analysis of recent aggregate data studies. Criminal Justice Review. 1993;18:182–202. [Google Scholar]
- 21.Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic disparities in adverse birth outcomes: a systematic review. Am J Prev Med. 2010;39:263–272. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Foraker RE, Rose KM, Suchindran CM, Chang PP, McNeill AM, Rosamond WD. Socioeconomic Status, Medicaid Coverage, Clinical Comorbidity, and Rehospitalization or Death After an Incident Heart Failure Hospitalization Atherosclerosis Risk in Communities Cohort (1987 to 2004) Circulation: Heart Failure. 2011;4:308–316. doi: 10.1161/CIRCHEARTFAILURE.110.959031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludwig J, Duncan GJ, Gennetian LA, Katz LF, Kessler RC, Kling JR, et al. Neighborhood effects on the long-term well-being of low-income adults. Science. 2012;337:1505–1510. doi: 10.1126/science.1224648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwig J, Sanbonmatsu L, Gennetian L, Adam E, Duncan GJ, Katz LF, et al. Neighborhoods, obesity, and diabetes--a randomized social experiment. N Engl J Med. 2011;365:1509–1519. doi: 10.1056/NEJMsa1103216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93:1137–1143. doi: 10.2105/ajph.93.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundquist K, Malmstrom M, Johansson SE, Sundquist J. Care Need Index, a useful tool for the distribution of primary health care resources. J Epidemiol Community Health. 2003;57:347–352. doi: 10.1136/jech.57.5.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh GK, Miller BA, Hankey BF. Changing area socioeconomic patterns in U.S cancer mortality, 1950–1998: Part II--Lung and colorectal cancers. J Natl Cancer Inst. 2002;94:916–925. doi: 10.1093/jnci/94.12.916. [DOI] [PubMed] [Google Scholar]
- 28.Singh GK, Siahpush M. Increasing inequalities in all-cause and cardiovascular mortality among US adults aged 25–64 years by area socioeconomic status, 1969–1998. Int J Epidemiol. 2002;31:600–613. doi: 10.1093/ije/31.3.600. [DOI] [PubMed] [Google Scholar]
- 29.Singh GK, Siahpush M. Widening socioeconomic inequalities in US life expectancy, 1980–2000. Int J Epidemiol. 2006;35:969–979. doi: 10.1093/ije/dyl083. [DOI] [PubMed] [Google Scholar]
- 30.Singh GK, Azuine RE, Siahpush M, Kogan MD. All-cause and cause-specific mortality among US youth: Socioeconomic and rural-urban disparities and international patterns. J Urban Health. 2012 doi: 10.1007/s11524-012-9744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socioeconomic disparities in U.S incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer. 2004;101:1051–1057. doi: 10.1002/cncr.20467. [DOI] [PubMed] [Google Scholar]
- 32.Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: Part I-all cancers and lung cancer and Part II-colorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi: 10.1155/2011/107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Medicare & Medicaid Services. Chronic Condition Data Warehouse (CCW) home page. West Des Moines, IA: Buccaneer Computer Systems and Service, Inc; 2009. [March 9, 2009]. Accessed at http://ccwdata.org. [Google Scholar]
- 34.Krumholz HM, Normand ST, Keenan PS, Lin Z, Drye EBK, Wang Y, et al. Hospital 30-Day Heart Failure Readmission Measure Methodology. 2008 [Google Scholar]
- 35.Krumholz HM, Normand ST, Keenan PS, Desai MM, Lin ZDEE, Bhat KR, et al. Hospital 30-Day Pneumonia Readmission Measure Methodology. 2008 [Google Scholar]
- 36.Krumholz HM, Normand ST, Keenan PS, Desai MM, Lin Z, Drye EE, et al. Hospital 30-Day Acute Myocardial Infarction Readmission Measure Methodology. 2008 [Google Scholar]
- 37.United States Census Bureau Geograph Divison. Census block groups cartographic boundary files descriptions and metadata. Washington, D.C: United States Department of Commerce; 2012. [March 7, 2013]. Accessed at http://www.census.gov/geo/www/cob/bg_metadata.html. [Google Scholar]
- 38.Pope GC, Kautter J, Ellis RP, Ash AS, Ayanian JZ, Lezzoni LI, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25:119–141. [PMC free article] [PubMed] [Google Scholar]
- 39.Elixhauser A, Steiner C, Harris DR, Coffey RN. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Economic Research Service. Rural Classifications Overview. Washington, D.C: United States Department of Agriculture; [March 10, 2014]. Accessed at http://www.ers.usda.gov/topics/rural-economy-population/rural-classifications.aspx. [Google Scholar]
- 41.Washington State Department of Health. A four-tier consolidation of the RUCA system at the sub-county level. Olympia, WA: Washington State Department of Health; 2009. [March 10, 2014]. Accessed at http://www.doh.wa.gov/Portals/1/Documents/5500/RuralUrbGuide.pdf. [Google Scholar]
- 42.Sharma G, Fletcher KE, Zhang D, Kuo Y-F, Freeman JL, Goodwin JS. Continuity of Outpatient and Inpatient Care by Primary Care Physicians for Hospitalized Older Adults. J Am Med Assoc. 2009;301:1671–1680. doi: 10.1001/jama.2009.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Begg MD, Parides MK. Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Stat Med. 2003;22:2591–2602. doi: 10.1002/sim.1524. [DOI] [PubMed] [Google Scholar]
- 44.Breslow NE, Day NE. Statistical methods in cancer research. Volume I - The analysis of case-control studies. IARC Sci Publ. 1980:5–338. [PubMed] [Google Scholar]
- 45.Berlin JA, Kimmel SE, Ten Have TR, Sammel MD. An empirical comparison of several clustered data approaches under confounding due to cluster effects in the analysis of complications of coronary angioplasty. Biometrics. 1999;55:470–476. doi: 10.1111/j.0006-341x.1999.00470.x. [DOI] [PubMed] [Google Scholar]
- 46.Neuhaus JM, Kalbfleisch JD. Between- and within-cluster covariate effects in the analysis of clustered data. Biometrics. 1998;54:638–645. [PubMed] [Google Scholar]
- 47.Kangovi S, Grande D. Hospital readmissions-not just a measure of quality. JAMA. 2011;306:1796–1797. doi: 10.1001/jama.2011.1562. [DOI] [PubMed] [Google Scholar]
- 48.Kleinman LC, Norton EC. What's the risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res. 2009;44:288–302. doi: 10.1111/j.1475-6773.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Localio AR, Margolis DJ, Berlin JA. Relative risks and confidence intervals were easily computed indirectly from multivariable logistic regression. J Clin Epidemiol. 2007;60:874–882. doi: 10.1016/j.jclinepi.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 50.Spicker P. The welfare state: a general theory SAGE Publications Limited. 2000 [Google Scholar]
- 51.Gordon D, Pantazis C. Breadline Britain in the 1990s Ashgate Publishing. 1997 [Google Scholar]
- 52.United States Census Bureau. Poverty Definitions. Washington D.C: U.S. Department of Commerce; [March 10, 2014]. Accessed at http://www.census.gov/hhes/www/poverty/methods/definitions.html. [Google Scholar]
- 53.Kneebone E, Nadeau C, Berube A. The Re-Emergence of Concentrated Poverty: Metropolitan Trends in the 2000s. Metropolitan Opportunity Series. 2011 [Google Scholar]
- 54.Federal Reserve System, The Brookings Institution. The Enduring Challenge of Concentrated Poverty in America: Case Studies from Communities Across the U.S. 2008 [Google Scholar]
- 55.U.S. Department of Housing and Urban Development. Expanding Housing Choices for HUD-Assisted Families. Washington, D.C: 1996. [Google Scholar]
- 56.Brock J, Mitchell J, Irby K, Stevens B, Archibald T, Goroski A, et al. Association Between Quality Improvement for Care Transitions in Communities and Rehospitalizations Among Medicare BeneficiariesQuality Improvement in Care Transitions. JAMA. 2013;309:381–391. doi: 10.1001/jama.2012.216607. [DOI] [PubMed] [Google Scholar]
- 57.Centers for Medicare & Medicaid Services. Details for Community Based Care Transition Program. Baltimore, MD: Centers for Medicare & Medicaid Services; 2011. [on 02/09, 2012]. Accessed at http://www.cms.gov/DemoProjectsEvalRpts/MD/itemdetail.asp?itemID=CMS1239313. [Google Scholar]
- 58.National Quality Forum. Patient Outcomes: All- Cause Readmissions Expedited Review. 2011:2012. [Google Scholar]
- 59.Ross JS, Bernheim SM, Lin Z, Drye EE, Chen J, Normand S-LT, et al. Based On Key Measures, Care Quality For Medicare Enrollees At Safety-Net And Non-Safety-Net Hospitals Was Almost Equal. Health Aff. 2012;31:1739–1748. doi: 10.1377/hlthaff.2011.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Acevedo-Garcia D, Osypuk TL, McArdle N, Williams DR. Toward a policy-relevant analysis of geographic and racial/ethnic disparities in child health. Health Aff (Millwood) 2008;27:321–333. doi: 10.1377/hlthaff.27.2.321. [DOI] [PubMed] [Google Scholar]
- 61.van Walraven C, Wong J, Forster AJ. Influence of neighborhood household income on early death or urgent hospital readmission. J Hosp Med. 2013;8:261–266. doi: 10.1002/jhm.2025. [DOI] [PubMed] [Google Scholar]
- 62.Philbin EF, Dec GW, Jenkins PL, DiSalvo TG. Socioeconomic status as an independent risk factor for hospital readmission for heart failure. Am J Cardiol. 2001;87:1367–1371. doi: 10.1016/s0002-9149(01)01554-5. [DOI] [PubMed] [Google Scholar]