Abstract
Background
Intestinal barrier breakdown after severe burn can lead to intestinal inflammation, which may act as the source of the systemic inflammatory response. In vitro intestinal cell studies have shown that mitogen-activated protein kinase (MAPK) signaling is an important modulator of intestinal inflammation. We have previously observed that pentoxifylline (PTX) attenuates burn-induced intestinal permeability and tight junction breakdown. We hypothesized that PTX would limit intestinal barrier breakdown and attenuate inflammatory signaling via the MAPK pathway.
Methods
Male balb/c mice underwent 30% total body surface area full-thickness steam burn. Immediately after burn, animals received an intraperitoneal injection of PTX (12.5 mg/kg) in normal saline or normal saline alone. In vivo intestinal permeability to 4 kDa fluorescein isothiocyanate-dextran was measured. Intestinal extracts were obtained to measure interleukin-6 by enzyme-linked immunosorbent assay, and phosphorylated p38 MAPK, p38 MAPK, phosphorylated extracellular signal-related kinase ½ (ERK ½), and ERK ½ by immunoblotting. Acute lung injury was assessed by histology at 24 hours after burn.
Results
Administration of PTX immediately after injury attenuated burn-induced intestinal permeability. PTX also decreased the burn-induced phosphorylation of p38 MAPK and decreased phosphorylation of ERK ½ at 2 hours and 24 hours after injury. Animals given PTX had decreased intestinal interleukin-6 levels. A single dose of PTX also decreased histologic lung injury at 24 hours after burn.
Conclusion
PTX attenuates burn-induced intestinal permeability and subsequent intestinal inflammation. Use of PTX after burn was also associated with decreased acute lung injury. Because of its compelling anti-inflammatory effects, PTX may be an ideal candidate for use as an immunomodulatory adjunct to resuscitation fluid.
Keywords: Burn, Intestine, Intestinal permeability, MAPK, ERK, IL-6, Inflammation, Lung injury, Distant organ injury
The intestine has been recognized as a key source of proinflammatory mediators, which can ignite the systemic inflammatory response syndrome (SIRS). Several studies have shown that shock-induced intestinal inflammation can result in the generation of activated mesenteric lymph, containing these gut-derived inflammatory mediators, which are carried to the systemic circulation via the mesenteric lymphatics.1,2 Once in the systemic circulation, these inflamma-tory products can cause further activation of the inflammatory and endothelial cells, propagating the inflammatory response, and ultimately leading to distant organ injury and multiple organ failure (MOF).3–5 Therefore, the intestine is a key target for therapies directed at limiting SIRS, with the goal of improving outcomes after severe injury.
MOF is a significant cause of late deaths after severe trauma accounting for nearly two-thirds of deaths after 7 days.6 The use of various resuscitation fluids can impact the inflammatory response after severe injury.7 Racemic Ringer's Lactate, once the preferred resuscitation fluid, has lost favor after it was discovered that the D-isomer of lactate has proinflammatory effects.8 Clearly, choice or resuscitation fluid and timing of its delivery has effects on the immune response, which may ultimately affect morbidity and mortality. Although there is likely no “magic bullet,” the use of immunomodulatory resuscitation fluids may improve the outcomes in severely injured patients.
Pentoxifylline (1-{5-oxohexyl}-3,7-dimethylxanthine, PTX) is a nonspecific phosphodiesterase inhibitor, which can modulate intracellular signaling through its ability to increase cyclic AMP.9,10 PTX has been given to numerous patients as a treatment for intermittent claudication, because of its ability to improve microcirculation through increasing the deformability of red blood cells.11 Although PTX has proved to have limited utility in improving symptoms for patients with peripheral vascular disease, it has significant potential as an anti-inflammatory adjunct in critically ill patients. Although PTX has not been used clinically for this purpose in the United States, numerous clinical trials have shown that PTX can decrease systemic inflammation due to liver disease,12 after cardiac bypass surgery,13 and in neonatal sepsis.14 The utility of using a resuscitation fluid containing PTX clinically for patients after trauma and burn, however, has not been studied.
During the past decade, our laboratory has studied the potential of using PTX as an immunomodulatory adjunct to resuscitation in animal models of hemorrhagic shock, burn, sepsis, and pancreatitis. We have shown that PTX decreases inflammatory cell activation, limits the increase in proinflammatory cytokine levels, and decreases organ injury after shock.15–19 PTX has also been shown to attenuate inflammatory signaling via the mitogen-activated protein kinase (MAPK) and nuclear factor kappa-B (NF-κB) pathways.20,21 In recent studies, we have discovered that PTX may limit the intestinal barrier injury and subsequent intestinal inflammation by preventing the burn-induced modulation of the tight junction proteins, which normally maintain the barrier between adjacent intestinal epithelial cells and control paracellular permeability.22,23
The intestinal inflammatory response is mediated by several intracellular signaling molecules, including members of the MAPK pathway.24 Specifically, intestinal inflammation is associated with activation of p38 MAPK and extracellular signal-related kinase ½ (ERK ½), which have recently been shown to modulate intestinal barrier integrity.25,26 In this series of experiments, we hypothesized that injection of PTX immediately after injury would limit burn-induced intestinal barrier breakdown and attenuate inflammatory signaling via p38 MAPK and ERK ½. Ultimately, we hypothesized that limiting intestinal inflammation would decrease the distant organ injury that is known to occur after severe injury and may improve our understanding of the anti-inflammatory benefits of using PTX as part of an immunomodulatory resuscitation strategy.
MATERIALS AND METHODS
Thermal Injury Model
Male balb/c mice (Jackson Laboratory, Sacramento, CA) weighing 20 g to 25 g were anesthetized with inhaled isoflurane. While under general anesthesia, animals underwent dorsal fur clipping with an electric clipper before 30% total body surface area (TBSA) dorsal steam burn for 7 seconds. A template was designed to estimate 30% TBSA based on the Walker-Mason burn model.27 Immediately after burn, animals received an intraperitoneal injection of PTX (12.5 mg/kg, Sigma, St. Louis, MO) in 500 μL normal saline (NS) or NS alone. Animals also received a subcutaneous injection of 1.5 mL NS with buprenorphine in a nonburned area for pain control and fluid resuscitation. After injury, animals were recovered from anesthesia and returned to their cage where they were provided food and water. All animal experiments were approved by the University of California San Diego Institutional Animal Care and Use Committee.
Tissue Harvest
Animals were once again anesthetized using inhaled isoflurane at several time points after burn injury. Animals were exsanguinated via cardiac puncture, and the blood was collected in Eppendorf tube and placed on ice before being centrifuged at 10,000g for 10 minutes. The plasma was removed and stored at −70°C for later analysis. The samples of distal small intestine were removed, immediately snap frozen in liquid nitrogen, and stored at −80°C for later tissue protein extraction for immunoblotting and interleukin (IL)-6 determination. The lung and small intestine were also harvested and preserved in 10% formalin or optimal cutting technique embedding media (Sakura Finetek, Torrance, CA) for histologic evaluation.
Intestinal IL-6 Levels
Intestinal IL-6 levels were measured from intestinal extracts obtained 2 hours and 24 hours after severe burn injury (n ≥ 3 animals per group) using a commercially available enzyme-linked immunosorbent assay kit obtained from R&D Systems (Minneapolis, MN).
Intestinal Permeability Assay
Separate animals were used to assess in vivo intestinal permeability after severe burn (n = 4 animals per group). Animals were anesthetized with inhaled isoflurane 2 hours after burn. After a midline laparotomy incision, a 5-cm segment of distal ileum was isolated between silk ties. An intraluminal injection of 200 μL of 4 kDa fluorescein isothiocyanate (FITC)-Dextran (25 mg/mL, Sigma) in phosphate-buffered saline was performed, the bowel was returned to the abdominal cavity, and the abdomen was closed. The animal was maintained under general anesthesia for 30 minutes, at which time the systemic blood was drawn by cardiac puncture and placed in heparinized Eppendorf tubes on ice. The plasma was obtained by centrifuging the blood at 10,000g for 10 minutes at −4°C. The fluorescence of the plasma specimens was measured in a fluorescence spectrophotometer (SpectraMax M5, Molecular Devices, Sunnyvale, CA) and compared with a standard curve of known concentration FITC-dextran diluted in mouse serum.
MAPK Protein Expression
Distal small intestine harvested from animals at 2 hours and 24 hours after burn (n ≥ 3 animals per group) were homogenized in ice-cold tissue protein extraction reagent containing 1% protease and phosphatase inhibitors (Pierce Biotechnology, Rockford, IL). The homogenized tissue was centrifuged at 10,000g for 5 minutes, the supernatants were collected, and the protein concentration of each sample was measured using the bicinchoninic protein assay (Pierce). Protein was suspended in sodium dodecyl sulfate sample buffer and boiled for 5 minutes. Proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 8% to 16% Tris-glycine gradient gels (Invitrogen, Carlsbad, CA), then transferred to a nitrocellulose membrane (Invitrogen). Membranes were blocked with 5% bovine serum albumin (Sigma) in Tris-buffered saline/tween 20 for 1 hour. Membranes were then incubated overnight at 4°C in primary antibody for phosphorylated p38 MAPK, p38 MAPK, phosphorylated ERK, ERK, and β actin. All primary antibodies were obtained from Cell Signaling (Danvers, MA) and prepared in a 1:500 concentration in 5% bovine serum albumin/ tween 20. The membranes were then washed and incubated with a horseradish peroxidase-linked anti-rabbit IgG secondary antibody (Cell Signaling) before application of the Pierce Supersignal West Pico Chemiluminescent Kit for antibody detection. Luminescence was viewed using the Xenogen IVIS Lumina (Caliper Life Science, Hopkinton, MA) imaging system. Mean pixel density of each sample was estimated using UN-SCAN-IT Gel Digitizing software (Silk Scientific, Orem, UT). The relative band density of each band was calculated by dividing the pixel density of each sample by the mean pixel density of the sham samples.
Histopathologic Evaluation of Lung
The samples of lung (n ≥ 3 animals per group) were stored in formalin before embedding in paraffin block using an automated processor. Sections were cut, placed onto glass slides, and stained with hematoxylin-eosin. Images were obtained with an Olympus IX70 light microscope at 20× magnification using Q-imaging software (Q-imaging, Surrey, BC, Canada). Lung injury scores were obtained by grading three random fields from each section on a scale from 0 to 3 (0 = normal, 1 = mild, 2 = moderate, and 3 = severe). Separate ratings were estimated for inflammatory cell infiltration, congestion, intra-alveolar hemorrhage, and pulmonary edema. Total injury severity scores were graded on a scale from 0 to 12 (0 = normal, 12 = most severe). Lung injury scores were reviewed by a pathologist blinded to the experimental conditions.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics software (Chicago, IL). Values are expressed as the mean ± SEM of n samples. n represents the number of animals in each experimental group. The statistical significance between groups was determined using analysis of variance with Bonferroni correction. The Kruskall-Wallis test for nonparametric data followed by pairwise Mann-Whitney U test was used to for lung histology scoring. A p value <0.05 was considered statistically significant.
RESULTS
Intestinal IL-6 Levels
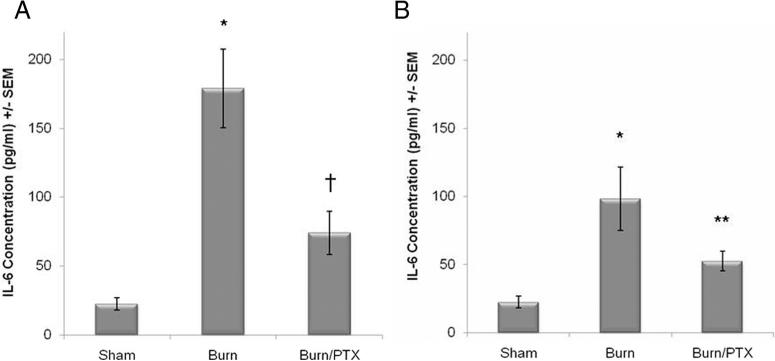
Severe cutaneous burn causes increased levels of intestinal IL-6 at 2 hours after injury compared with sham (178.9 pg/mL vs. 22.4 pg/mL, p < 0.0001). Injection of PTX immediately after injury attenuated the burn-induced increase in intestinal IL-6 (178.9 pg/mL vs. 74.0 pg/mL, p < 0.01). There was no significant difference between sham and burned animals treated with PTX (Fig. 1A). Intestinal IL-6 remained elevated at 24 hours after burn (98.1 pg/mL), which was significantly higher than animals injected with PTX immediately after burn (49.3 pg/mL, Fig. 1B).
Figure 1.
Effects on PTX on intestinal IL-6 levels after 30% TBSA full-thickness burn. IL-6 levels measured from intestinal extracts using enzyme-linked immunosorbent assay. (A) PTX limits the burn-induced increase in intestinal IL-6 concentration at 2 hours after burn. (B) Intestinal IL-6 remains elevated at 24 hours after burn, whereas animals given PTX immediately after burn have intestinal IL-6 levels similar to sham. *p < 0.001 versus sham, †p < 0.01 versus burn at 2 hours, and **p < 0.05 versus burn at 24 hours.
Intestinal Permeability to FITC-Dextran
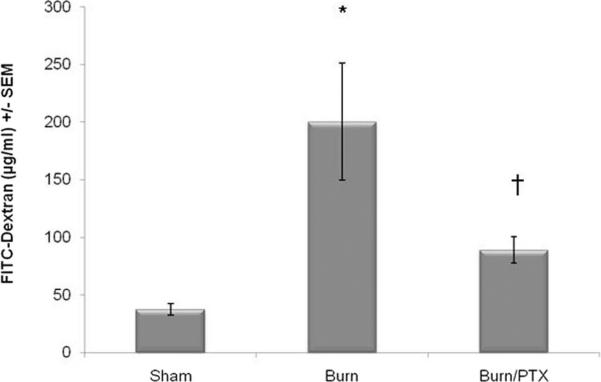
Intestinal permeability was assessed by measuring the movement of FITC-dextran from within the intestinal lumen into the systemic circulation (Fig. 2). Thirty percent TBSA steam burn results in increased intestinal permeability to 4 kDa FITC-dextran at 2 hours after injury compared with sham (200.3 μg/mL vs. 37.73 μg/mL, p < 0.01). Animals treated with an injection of PTX in addition to standard resuscitation had a significant decrease in intestinal permeability at 2 hours after burn (89.33 μg/mL). There was not a significant difference between sham and burned animals injected with PTX (Fig. 2).
Figure 2.
Pentoxifylline prevents burn-induced intestinal permeability. Intestinal permeability assay to intraluminal 4 kDa FITC-dextran measured 2 hours after severe burn (n ≥ 3 animals per group). Burn injury causes a significant increase in intestinal permeability. Injection with PTX (12.5 mg/kg) immediately after injury attenuates this burn-induced increased in intestinal permeability. *p < 0.01 versus sham and †p < 0.05 versus burn.
Intestinal p38 MAPK Protein Expression
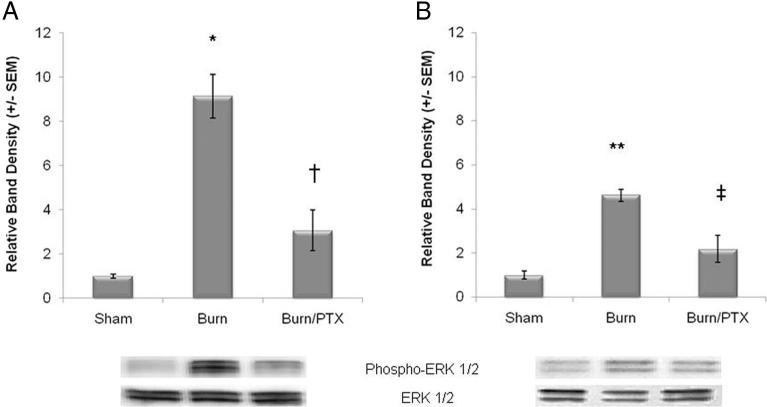
Severe burn resulted in an 8.1-fold increase in phosphorylated p38 MAPK compared with sham (p < 0.001) at 2 hours and remained elevated at 24 hours after injury (4.5-fold over sham, Fig. 3). Intraperitoneal injection of PTX immediately after burn resulted in phosphorylated p38 MAPK levels similar to sham at both 2 hours and 24 hours postinjury. There was no difference in total p38 MAPK expression in either group.
Figure 3.
Burn-induced phosphorylation of intestinal p38 MAPK is attenuated by PTX. p38 MAPK from intestinal extracts evaluated using Western blot (n ≥ 3 animals per group). (A) Burn injury results in an 8-fold increase in phosphorylated p38 MAPK at 2 hours after burn injury. Treatment with PTX after injury results in phosphorylated p38 MAPK levels similar to sham. (B) Phosphorylated p38 MAPK protein expression remains increased at 24 hours after burn. PTX continues to prevent the burn-induced phosphorylation of intestinal p38 MAPK. There is no difference in total p38 MAPK expression in either group. *p < 0.001 versus sham and †p < 0.001 versus burn at 2 hours. **p < 0.01 versus sham and ‡p < 0.01 versus burn at 24 hours.
Intestinal ERK Protein Expression
Thirty percent TBSA full-thickness burn increases phosphorylation of ERK ½ by 9.1-fold at 2 hours and 4.6-fold at 24 hours after injury compared with sham (Fig. 4). Treatment with PTX immediately after burn attenuated intestinal ERK ½ phosphorylation to levels similar to sham at 2 hours and 24 hours. There was no difference in total ERK expression in any group.
Figure 4.
Activation of intestinal ERK ½ after severe burn is decreased by PTX. Phosphorylated ERK ½ measured from intestinal extracts using Western blot (n ≥ 3 animals per group). (A) PTX prevents the burn-induced phosphorylation of intestinal ERK ½ at 2 hours after 30% TBSA burn. (B) Phosphorylated ERK ½ decreases somewhat by 24 hours after injury but remains significantly higher than sham. Treated with PTX continues to limit intestinal inflammation, with phosphorylated ERK ½ expression similar to sham. There is no difference in total ERK ½ protein expression in either group. *p < 0.01 versus sham and †p < 0.01 versus burn at 2 hours. **p < 0.001 versus sham and ‡p < 0.01 versus burn at 24 hours.
Distant Organ Injury
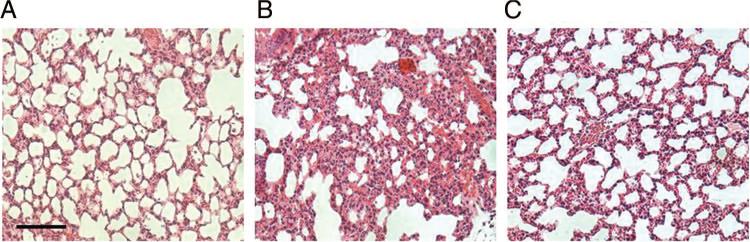
Lung specimens harvested from animals 24 hours after severe burn injury exhibit signs of histologic injury characterized by cellular infiltration, intra-alveolar hemorrhage, and hyaline membranes (Fig. 5). Treatment with PTX immediately after burn attenuated histologic lung injury. Lung specimens from PTX-treated animals have an appearance similar to sham with decreased cellular infiltration compared with burned animals receiving standard resuscitation. The lung specimens were scored with regards to inflammatory cell infiltration, congestion, intra-alveolar hemorrhage, and pulmonary edema. Total injury severity scores were graded on a scale from 0 to 12 (0 = normal, 12 = most severe). Lung injury scores were significantly elevated 24 hours after burn compared with sham (9.2 ± 1.0 vs. 1.0 ± 0.5, p < 0.05). Treatment with PTX decreased lung injury scores after burn (4.8 ± 1.0, p < 0.05 vs. burn).
Figure 5.
Pentoxifylline attenuates burn-induced distant organ injury. The lung sections harvested 24 hours after burn-injury stained with hematoxylin-eosin and viewed at ×20 magnification (n = 3 animals per group). (A) Normal appearance of the lung tissue from sham animal. (B) Evidence of acute lung injury in animals 24 hours after burn characterized by cellular infiltration, presence of hyaline membranes, and intra-alveolar hemorrhage. (C) Appearance of the lung tissue from animals given PTX immediately after burn is noticeably improved at 24 hours compared with burn with only minimal cellular infiltration. Bar = 100 μm.
DISCUSSION
Interest in developing alternative resuscitation strategies has increased since the discovery that certain resuscitation fluids have unwanted proinflammatory side effects. Research has now focused on studying fluids that can beneficially alter the cellular response to injury.28 Fluids such as hypertonic saline have been considered for its ability to both restore volume and decrease inflammation and tissue injury.29 Other strategies including the addition of anti-inflammatory medications and the use of targeted therapies are being investigated to deliver improved resuscitation strategies to injured patients. The ideal resuscitation fluid would be able to restore volume, while containing immunomodulatory capacity to decrease inflammatory cell activation, SIRS, and organ injury.
Intestinal barrier breakdown may be the inciting event, which leads to intestinal inflammation and subsequent SIRS. The intestinal tight junction forms the barrier between intestinal epithelial cells, which protects the intestinal tissue from the cytotoxic contents of the intestinal lumen.30 These tight junction proteins control the overall permeability of the intact intestinal barrier.31 We have documented in an in vitro model that PTX prevents loss of the intestinal tight junction proteins occludin and zonula occludens-1 (ZO-1) after exposure to the proinflammatory cytokines.32 We confirmed these findings using an in vivo model of severe burn, demonstrating that PTX attenuates burn-induced intestinal permeability after injury by modulating these intestinal tight junction proteins.22 PTX also prevented the burn-induced activation of myosin light chain kinase (MLCK), a protein that regulates contraction of the perijunctional actin cytoskeleton, and is a key determinant of tight junction permeability.23 This was associated with decreased signaling via the NF-κB pathway, resulting in decreased nuclear translocation of intestinal NF-κB p65.
The purpose of this current series of experiments was to investigate the effects of a single dose of PTX given with standard resuscitation fluid immediately after injury on intestinal inflammation. In this study, we document the ability of PTX to attenuate intestinal IL-6 production at 2 hours after injury. These anti-inflammatory effects continued to exist at 24 hours after injury. Intestinal IL-6 is not only a marker of inflammation but also plays a key role in modulating intestinal permeability. IL-6 knockout mice have been shown to be protected against shock-induced intestinal permeability.33 This inability to express intestinal IL-6 was also associated with improved expression and localization of the tight junction protein ZO-1 compared with wild type mice.34 This suggests a mechanism by which PTX may attenuate burn-induced intestinal permeability and gives insight into its ability to prevent the loss of ZO-1, which we have seen in previous studies.22
Here, we also show that PTX prevents phosphorylation of both intestinal p38 MAPK and ERK ½ after 30% TBSA burn. Signaling via the p38 MAPK pathway has been shown to regulate intestinal permeability in response to inflamma-tory cytokines, with pharmacologic inhibition of p38 MAPK phosphorylation limiting intestinal epithelial permeability.26 Signaling via p38 MAPK and ERK ½ may also regulate intestinal permeability through the transcriptional factor activator protein-1 (AP-1).35,36 Intestinal barrier integrity is modulated by the tight junction protein MLCK, which contains a promoter region for AP-1.37 Therefore, by preventing phosphorylation of p38 MAPK and ERK ½, PTX may prevent the binding of AP-1 to MLCK promoter, thus limiting the ability of MLCK to cause tight junction breakdown. This correlates with our previous results, showing that PTX prevents the burn-induced increase in intestinal MLCK protein expression.23 However, further studies are needed to definitively demonstrate whether PTX is able to decrease binding of AP-1 to the MLCK promoter.
This series of experiments also demonstrates that a single dose of PTX after burn has anti-inflammatory effects, which are still evident 24 hours after injury. Intestinal IL-6, phosphorylated p38 MAPK, and ERK ½ levels remain significantly lower in PTX-treated animals at 24 hours after burn. The ability of PTX to prevent loss of intestinal barrier integrity is likely central to its lasting anti-inflammatory effects. Intestinal permeability occurs early after injury leading to proinflammatory mediator synthesis, which can further perpetuate intestinal barrier loss, leading to an even greater intestinal inflammatory response. By maintaining intestinal barrier integrity at early time points after injury, PTX is likely able to limit the propagation of the intestinal inflammatory cascade.
The gut is believed to be the source of SIRS after severe injury, which leads to distant organ injury and MOF. Therefore, we were interested in evaluating the effects of PTX on acute lung injury at 24 hours after injury. Severe burn resulted in histologic lung injury, which was characterized by a significant cellular infiltration and the presence of hyaline membranes, characteristic signs of acute lung injury. Administration of PTX immediately after injury prevented burn-induced acute lung injury. The histologic appearance of the lung tissue from PTX-treated animals was markedly improved compared with burned animals that did not receive PTX. Limiting the gut inflammation after burn is associated with decreased distant organ injury and illustrates the immunomodulatory effects of PTX.
In this study, we chose the dose of PTX (12.5 mg/kg) based on our previous in vivo studies using this animal model of 30% TBSA burn injury. We have previously demonstrated that giving PTX alone to sham animals has no effect on intestinal permeability, and therefore, we did not repeat those experiments.22 We chose to administer PTX immediately after 30% TBSA burn. Clearly, this drug needs to be given early after injury as we see changes in inflammatory signaling and intestinal permeability by 2 hours. Further studies are needed to determine the therapeutic window during which PTX can be given while still exerting its anti-inflammatory effects. Defining this therapeutic window may have important clinical implications in determining the ideal strategy for delivering this drug to injured patients (prehospital vs. trauma bay).
The choice of resuscitation fluid administered to patients after severe trauma and burn may have important effects on the immune response to injury. We have demonstrated that PTX limits intestinal barrier injury and has both early and long-lasting effects on attenuating the intestinal inflammatory response and also limits distant organ injury. PTX has been given to patients for decades to treat other inflammatory conditions and is well tolerated with minimal side effects. Because of its compelling anti-inflammatory effects demonstrated in preclinical studies, PTX may be an ideal candidate for use as an immunomodulatory adjunct to resuscitation fluid with the goal of limiting the SIRS response and decreasing the late morbidity and mortality associated with severe injury.
Footnotes
Presented at the 39th Annual Meeting of the Western Trauma Association, February 22–28, 2009, Crested Butte, Colorado and winner of the Earl Young Resident Paper Competition.
REFERENCES
- 1.Magnotti LJ, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph: a link between burn and lung injury. Arch Surg. 1999;134:1333–1340. discussion 1340–1341. [PubMed] [Google Scholar]
- 2.Deitch EA, Xu D, Kaise VL. Role of the gut in the development of injury- and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci. 2006;11:520–528. doi: 10.2741/1816. [DOI] [PubMed] [Google Scholar]
- 3.Caruso JM, Feketeova E, Dayal SD, Hauser CJ, Deitch EA. Factors in intestinal lymph after shock increase neutrophil adhesion molecule expression and pulmonary leukosequestration. J Trauma. 2003;55:727–733. doi: 10.1097/01.TA.0000037410.85492.77. [DOI] [PubMed] [Google Scholar]
- 4.Damle SS, Moore EE, Nydam TL, et al. Postshock mesenteric lymph induces endothelial NF-kappaB activation. J Surg Res. 2007;143:136–140. doi: 10.1016/j.jss.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masuno T, Moore EE, Cheng AM, Sarin EL, Banerjee A. Bioactivity of postshock mesenteric lymph depends on the depth and duration of hemorrhagic shock. Shock. 2006;26:285–289. doi: 10.1097/01.shk.0000223132.72135.52. [DOI] [PubMed] [Google Scholar]
- 6.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Rhee P, Koustova E, Alam HB. Searching for the optimal resuscitation method: recommendations for the initial fluid resuscitation of combat casualties. J Trauma. 2003;54:S52–S62. doi: 10.1097/01.TA.0000064507.80390.10. [DOI] [PubMed] [Google Scholar]
- 8.Rhee P, Burris D, Kaufmann C, et al. Lactated Ringer's solution resuscitation causes neutrophil activation after hemorrhagic shock. J Trauma. 1998;44:313–319. doi: 10.1097/00005373-199802000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Bush TG, Savidge TC, Freeman TC, et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 10.Sinha B, Semmler J, Eisenhut T, Eigler A, Endres S. Enhanced tumor necrosis factor suppression and cyclic adenosine monophosphate accumulation by combination of phosphodiesterase inhibitors and prostanoids. Eur J Immunol. 1995;25:147–153. doi: 10.1002/eji.1830250125. [DOI] [PubMed] [Google Scholar]
- 11.Boldt J, Muller M, Heesen M, Martin K, Hempelmann G. The effects of pentoxifylline on circulating adhesion molecules in critically ill patients with acute renal failure treated by continuous veno-venous hemofiltration. Intensive Care Med. 1996;22:305–311. doi: 10.1007/BF01700451. [DOI] [PubMed] [Google Scholar]
- 12.Duman DG, Ozdemir F, Birben E, et al. Effects of pentoxifylline on TNF-alpha production by peripheral blood mononuclear cells in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2007;52:2520–2524. doi: 10.1007/s10620-006-9723-y. [DOI] [PubMed] [Google Scholar]
- 13.Heinze H, Rosemann C, Weber C, et al. A single prophylactic dose of pentoxifylline reduces high dependency unit time in cardiac surgery—a prospective randomized and controlled study. Eur J Cardiothorac Surg. 2007;32:83–89. doi: 10.1016/j.ejcts.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Haque K, Mohan P. Pentoxifylline for neonatal sepsis. Cochrane Database Syst Rev. 2003:CD004205. doi: 10.1002/14651858.CD004205. [DOI] [PubMed] [Google Scholar]
- 15.Coimbra R, Melbostad H, Loomis W, Tobar M, Hoyt DB. Phosphodiesterase inhibition decreases nuclear factor-kappaB activation and shifts the cytokine response toward anti-inflammatory activity in acute endotoxemia. J Trauma. 2005;59:575–582. [PubMed] [Google Scholar]
- 16.Coimbra R, Loomis W, Melbostad H, Tobar M, Porcides RD, Hoyt DB. LPS-stimulated PMN activation and proinflammatory mediator synthesis is downregulated by phosphodiesterase inhibition: role of pentoxifylline. J Trauma. 2004;57:1157–1163. doi: 10.1097/01.ta.0000151261.28640.f7. [DOI] [PubMed] [Google Scholar]
- 17.Deree J, de Campos T, Shenvi E, Loomis WH, Hoyt DB, Coimbra R. Hypertonic saline and pentoxifylline attenuates gut injury after hemorrhagic shock: the kinder, gentler resuscitation. J Trauma. 2007;62:818–827. doi: 10.1097/TA.0b013e31802d9745. [DOI] [PubMed] [Google Scholar]
- 18.Yada-Langui MM, Coimbra R, Lancellotti C, et al. Hypertonic saline and pentoxifylline prevent lung injury and bacterial translocation after hemorrhagic shock. Shock. 2000;14:594–598. doi: 10.1097/00024382-200014060-00004. [DOI] [PubMed] [Google Scholar]
- 19.de Campos T, Deree J, Martins JO, et al. Pentoxifylline attenuates pulmonary inflammation and neutrophil activation in experimental acute pancreatitis. Pancreas. 2008;37:42–49. doi: 10.1097/MPA.0b013e3181612d19. [DOI] [PubMed] [Google Scholar]
- 20.Deree J, Loomis WH, Wolf P, Coimbra R. Hepatic transcription factor activation and proinflammatory mediator production is attenuated by hypertonic saline and pentoxifylline resuscitation after hemorrhagic shock. J Trauma. 2008;64:1230–1238. doi: 10.1097/TA.0b013e31816a4391. discussion 1238–1239. [DOI] [PubMed] [Google Scholar]
- 21.Deree J, Melbostad H, Loomis WH, Putnam JG, Coimbra R. The effects of a novel resuscitation strategy combining pentoxifylline and hyper-tonic saline on neutrophil MAPK signaling. Surgery. 2007;142:276–283. doi: 10.1016/j.surg.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Costantini TW, Loomis WH, Putnam JG, et al. Burn-induced gut barrier injury is attenuated by phosphodiesterase inhibition: effects on tight junction structural proteins. Shock. 2009;31:416–422. doi: 10.1097/SHK.0b013e3181863080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costantini TW, Loomis WH, Putnam JG, et al. Pentoxifylline modulates intestinal tight junction signaling after burn injury: effects on myosin light chain kinase. J Trauma. 2009;66:17–24. doi: 10.1097/TA.0b013e318191bb1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grishin AV, Wang J, Potoka DA, et al. Lipopolysaccharide induces cyclooxygenase-2 in intestinal epithelium via a noncanonical p38 MAPK pathway. J Immunol. 2006;176:580–588. doi: 10.4049/jimmunol.176.1.580. [DOI] [PubMed] [Google Scholar]
- 25.Fyfe M, Bergström M, Aspengren S, Peterson A. PAR-2 activation in intestinal epithelial cells potentiates interleukin-1beta-induced chemokine secretion via MAP kinase signaling pathways. Cytokine. 2005;31:358–367. doi: 10.1016/j.cyto.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Guo XL, Wells-Byrum D, Noel G, Pritts TA, Ogle CK. Cytokine-induced epithelial permeability changes are regulated by the activation of the p38 mitogen-activated protein kinase pathway in cultured Caco-2 cells. Shock. 2008;29:531–537. doi: 10.1097/shk.0b013e318150737f. [DOI] [PubMed] [Google Scholar]
- 27.Walker HL, Mason AD., Jr A standard animal burn. J Trauma. 1968;8:1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Alam HB, Rhee P. New developments in fluid resuscitation. Surg Clin North Am. 2007;87:55–72. vi. doi: 10.1016/j.suc.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Hoyt DB. Fluid resuscitation: the target from an analysis of trauma systems and patient survival. J Trauma. 2003;54:S31–S35. doi: 10.1097/01.TA.0000047221.49816.0C. [DOI] [PubMed] [Google Scholar]
- 30.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–291. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- 31.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costantini TW, Deree J, Loomis W, et al. Phosphodiesterase inhibition attenuates alterations to the tight junction proteins occludin and ZO-1 in immunostimulated Caco-2 intestinal monolayers. Life Sci. 2009;84:18–22. doi: 10.1016/j.lfs.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Fang CH, Hasselgren PO. Intestinal permeability is reduced and IL-10 levels are increased in septic IL-6 knockout mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1013–R1023. doi: 10.1152/ajpregu.2001.281.3.R1013. [DOI] [PubMed] [Google Scholar]
- 34.Yang R, Han X, Uchiyama T, et al. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol. 2003;285:G621–G629. doi: 10.1152/ajpgi.00177.2003. [DOI] [PubMed] [Google Scholar]
- 35.Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell. 2004;116:491–497. doi: 10.1016/s0092-8674(04)00166-7. [DOI] [PubMed] [Google Scholar]
- 36.Choi EY, Park ZY, Choi EJ, et al. Transcriptional regulation of IL-8 by iron chelator in human epithelial cells is independent from NF-kappaB but involves ERK1/2- and p38 kinase-dependent activation of AP-1. J Cell Biochem. 2007;102:1442–1457. doi: 10.1002/jcb.21367. [DOI] [PubMed] [Google Scholar]
- 37.Graham WV, Wang F, Clayburgh DR, et al. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J Biol Chem. 2006;281:26205–26215. doi: 10.1074/jbc.M602164200. [DOI] [PubMed] [Google Scholar]