Abstract
Highly resilient synthetic hydrogels were synthesized by using the efficient thiol-norbornene chemistry to cross-link hydrophilic poly(ethylene glycol) (PEG) and hydrophobic polydimethylsiloxane (PDMS) polymer chains. The swelling and mechanical properties of the hydrogels were well-controlled by the relative amounts of PEG and PDMS. In addition, the mechanical energy storage efficiency (resilience) was more than 97% at strains up to 300%. This is comparable with one of the most resilient materials known: natural resilin, an elastic protein found in many insects, such as in the tendons of fleas and the wings of dragonflies. The high resilience of these hydrogels can be attributed to the well-defined network structure provided by the versatile chemistry, low cross-link density, and lack of secondary structure in the polymer chains.
Keywords: Hydrogels, Resilience, Thiol-Ene, Resilin
Introduction
Synthetic hydrogels, cross-linked materials that typically consist of more than 50% water,1 are notoriously brittle and have poor mechanical properties, including low strain to break and toughness and high stress-strain hysteresis.2 In contrast, biological materials often have robust mechanical properties in the hydrated state, such as the rubber-like proteins that can be deformed to high degrees of strain without failing.3, 4 The proteins elastin and resilin are both water swollen (40–60%), yet have the remarkable ability to undergo significant reversible deformation with no energy loss, also known as having high resilience.3 Nature appears to exploit this property for mechanical energy storage that facilitates movement.
Resilin, which is even more resilient than elastin, hence its name, serves a variety of purposes, from being involved in the jumping mechanism in fleas, to the flight system of dragonflies and the sound producing organs of the locust.5 First investigated by Weis-Fogh6 in the 1960s in the form of the dragonfly tendon, it was shown to be 92–97% resilient, which is greater than polybutadiene rubber (80%), perhaps the most prototypical synthetic elastomer.7 Studies on the structure of resilin have shown that the cross-linking chemistry is highly specific, occurring only through the tyrosine units, with approximately 40 to 60 amino acid residues (~4 to 7 kDa) between junctions.8 In addition, resilin is an amorphous material, with no stable secondary structures within the cross-linked primary chains.8, 9 It is this uniform network (narrowly-defined molecular weight between cross-links as well as robust cross-linking chemistry), low cross-link density, and absence of secondary structure within the primary chains that are thought to be responsible for the remarkable elastomeric properties of resilin.9
In recent years, recombinant protein methods have been used to prepare elastic protein materials similar to elastin and resilin with reasonable success.7, 10 Cross-linked recombinant rec1-resilin, with an equilibrium water content of approximately 80%, was found to have a modulus of 2.5 kPa and could be stretched to 300% of its original length with negligible hysteresis upon removal of the load (resilience of 97%).7 Resilin-like polypeptide (RLP)-based elastomers with biologically active domains were also synthesized through a recombinant modular approach by Charati and coworkers.11 When hydrated, these materials had a water content of 85% and a Young’s modulus (in tension) of 30–60 kPa. Further work on these hydrogels demonstrated that their properties could be tuned while maintaining a high resilience (>90%), even when extended to 200% strain.10, 11
Nevertheless, these materials are protein-based. At the same time, a number of approaches have been employed to improve the mechanical properties of non protein-based synthetic hydrogels, often in an effort to match the performance of natural tissues.12, 13 However, there has been significantly less progress toward the creation of materials with resilience similar to resilin. One reason for this is that many conventional cross-linking methods create inhomogeneous network structures with defects such as loops and dangling chains.14 Efforts to improve the mechanical performance of hydrogels have involved the manipulation of network microstructures, as seen in nanocomposite, block copolymer, and double network (DN) gels, and the use of amphiphilic systems, where the hydrophobic component influenced the water content and mechanical properties.1, 15–22 Studies on DN gels composed of a rigid highly cross-linked poly(2-acrylamido-2-methylpropanesulfonic acid) (PAMPS) first network and a loosely cross-linked polyacrylamide (PAAm) second network have shown that these gels have a high tensile strength and toughness, but exhibit considerable hysteresis after just one loading cycle.18 Similar large hysteresis loops have been observed for other gels, such as triblock copolymer gels,23 hybrid hydrogels,24 and hydrophobically functionalized polyelectrolyte hydrogels.25 Thus, many current synthetic hydrogels with seemingly attractive mechanical properties are of limited practical use when resilience is required, as one instance of loading to high strain results in permanent deformation or fracture and irreversibly changes the material properties. Other efforts have included end-linking reactions, such as Michael-type additions and click chemistry, to synthesize well-defined network structures.14, 26, 27 However, the mechanical performance of such networks has not been systematically investigated. In 2008, Sakai and coworkers reported a homogeneous gel formed by cross-linking symmetrical tetrahedron-like poly(ethylene glycol) (PEG) macromonomers that were designed to minimize entanglements.28 This design strategy was an effective way to improve the elastic properties of the materials; however, to date, this chemistry has been limited to PEG.29, 30
In this paper, a versatile synthetic platform capable of reproducing resilin’s essential structural features was pursued that yields resilient hydrogels with tunable properties including water content, stiffness, and fracture toughness. The networks described herein are based on the fast, photoinitiated cross-linking reaction of telechelic norbornene end-functionalized polymer chains with a tetra-functional thiol cross-linker. This combination of telechelic polymer chains coupled with the highly efficient thiol-ene cross-linking chemistry enables the formation of homogeneous networks with uniformly spaced cross-links, low cross-link densities, and high water content, similar to resilin. We demonstrate this concept using a hydrophilic PEG-based network, and tune the water content and mechanical properties by incorporating a hydrophobic component, polydimethylsiloxane (PDMS). This method is exceptionally simple, involving only the mixing of all of the components before photoinitiated network formation, and provides many options for tailoring the hydrogel properties using the same synthetic platform.
Results and Discussion
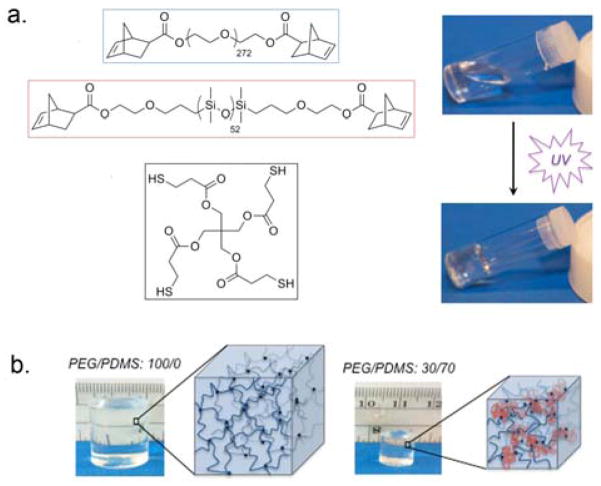
The networks reported here consist of PEG and PDMS precursor polymer chains functionalized with norbornene end-groups, as shown in Figure 1a [see Supporting Information (SI) for experimental details; 1H NMRs in SI Figure S1]. They were cross-linked with the tetra-functional cross-linker pentaerythritol tetrakis(3-mercaptopropionate) (PETMP) in an organic solvent, so that the PEG and PDMS were homogeneously mixed in the precursor solution during network formation. The gels were washed and then swollen to equilibrium in deionized water. By simply varying the molar ratio of PEG to PDMS, the water content and mechanical properties of the hydrogels were controlled, while maintaining high resilience. Robust mechanical analysis demonstrates that these are a new class of materials that remains highly resilient across all strains (up to 300%) examined to date, with exquisitely tunable mechanical properties and water content.
Figure 1.
a. Chemical structures of the norbornene end-functionalized PEG and PDMS polymer precursors and the PETMP cross-linker (left), as well as photographs before and after UV exposure (right). b. Photographs of 100:0 and 30:70 PEG/PDMS hydrogels swollen to equilibrium in water, along with the corresponding schematics illustrating the PEG (blue) and PDMS (red) polymer chains that constitute the networks.
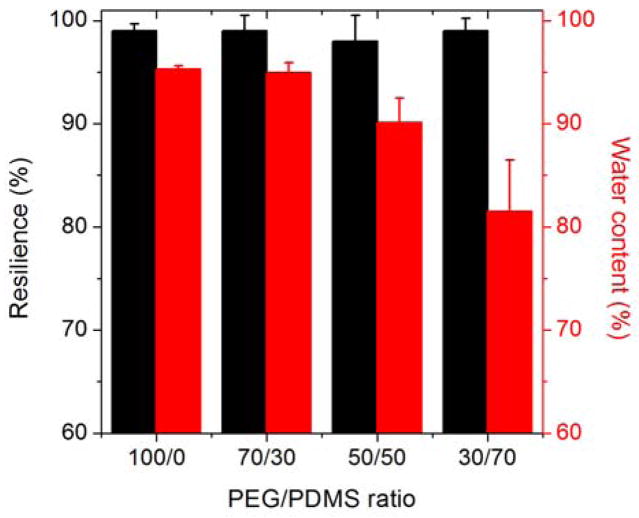
A series of hydrogels were prepared with molar ratios of PEG to PDMS ranging from 100:0 to 30:70 (details in SI Table S1). As the PDMS content increased, the equilibrium water content decreased. This difference in swelling is visible in the representative photographs in Figure 1b comparing the 100:0 and 30:70 PEG/PDMS hydrogels swollen to equilibrium in water. The equilibrium water contents are shown in Figure 2, going from over 95% water in the 100:0 PEG hydrogel (equilibrium mass swelling ratio Q = 21) to 82% water in the 30:70 hydrogel (Q = 6). While this is higher than the water content of natural resilin (50–60%), it is comparable to reported values for recombinant RLP-based materials (80–90%) that show similar resilience.7, 10
Figure 2.
Plot of the resilience and water content of the PEG/PDMS hydrogels as a function of the molar ratio of PEG:PDMS. Error bars indicate one standard deviation, with n≥3.
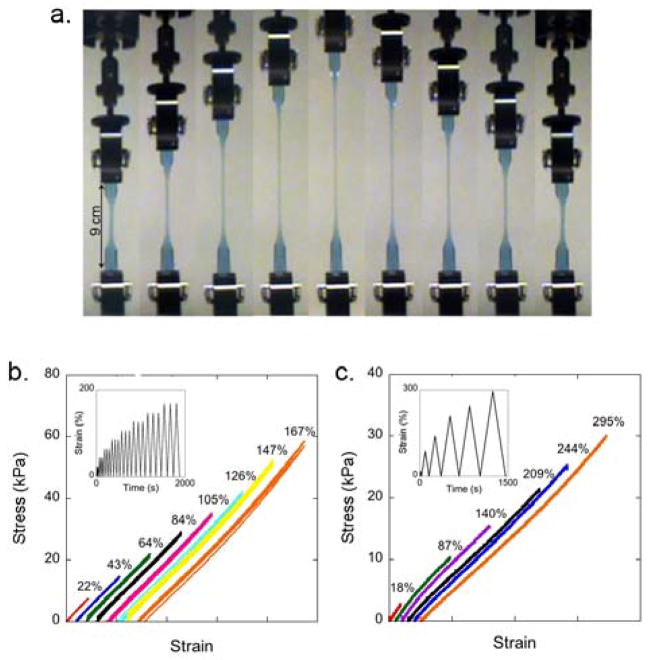
To investigate the resilience of the PEG and PEG/PDMS hydrogels, tensile measurements were carried out with a cyclic loading profile for two of the compositions (PEG:PDMS = 100:0 and 30:70) (Figure 3). Resilience, defined as the energy recovered after removal of the stress divided by the total energy of deformation, measures the ability of a material to deform reversibly (elastically) without loss of energy. As shown in Figures 3b and 3c, both the PEG and PEG/PDMS hydrogels showed negligible hysteresis across the entire measured range of strains. In addition, during consecutive loading cycles for each strain, the loading and unloading stress-strain curves were identical, demonstrating that there was no permanent network damage to these highly swollen, synthetic materials. This data also suggests that the Mullins effect, the stress softening phenomenon under loading, did not occur,31, 32 which is commonly observed and difficult to prevent in filled rubbers and the DN hydrogels.18
Figure 3.
a. Photographs of a 100:0 PEG hydrogel during tensile testing over one strain cycle, reaching a maximum strain of 300%. b and c. Representative stress-strain curves for the 30:70 and 100:0 PEG/PDMS hydrogels, respectively. For clarity, the curves are shifted on the strain axis, and the final strains are given on the plots. The tick marks on the x-axis represent 50% strain in b and 100% strain in c. The insets show the corresponding strain profiles as a function of time. Note in b that each strain % shows three cycles, demonstrating the reversibility of the stress-strain curves of these hydrogels.
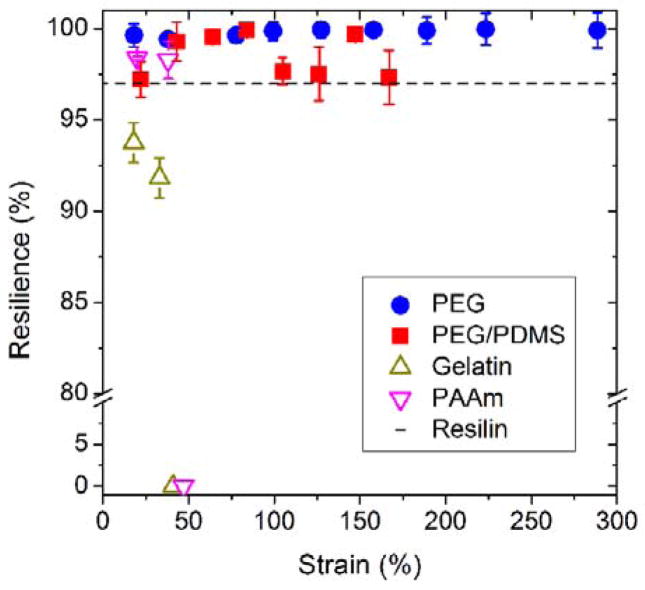
These studies demonstrate that the resilience of these hydrogels (≥97%) is comparable with that of the elastic proteins found in nature. Resilin, in the form of a dragonfly tendon, is reported to have a resilience of up to 97%, and elastin, a protein found in human skin and arteries, has a resilience of approximately 90% in the fully hydrated state.33 An in vitro study, however, found that the water content had a significant impact on the resilience of both native resilin and elastin.3 For example, reducing the water content by as little as 5% dramatically decreased the resilience, making the proteins brittle and glassy.34 In contrast, for these new hydrogels, the water content, controlled by the amount of PDMS in the hydrogels, had little influence on the resilience. As shown in Figures 2 and 4, both the PEG and PEG/PDMS hydrogels maintained a resilience of more than 97% across the range of measured strains (up to 300%), even though the water content varied from 95% to 82%.
Figure 4.
Resilience as a function of strain for the 100:0 and 30:70 PEG/PDMS hydrogels, PAAm, gelatin, and resilin. The resilience was calculated from cyclic stress-strain curves in tension, and the error bars represent one standard deviation from three cycles for each measured strain. Zero resilience represents that fracture occurs at the corresponding strain. Resilin shows a high resilience at both small and large strains. (Data taken from M. Jensen and T. Weis-Fogh, Phil. Trans. Roy. Soc. Lond. B Biol. Sci., 1962 and C. Elvin et al. Nature, 2005)
Two commonly used prototypical hydrogels, with fundamentally different cross-linked network structures, were also synthesized and their resilience characterized for comparison. PAAm, a chemically cross-linked material, and gelatin, a physically cross-linked material, were studied (SI Table S1). For both hydrogels, the water content was around 90%, and the Young’s modulus (in tension) was approximately 20 kPa, which was similar to these PEG and PEG/PDMS hydrogels (16 and 34 kPa, respectively). Resilience was measured using the same cyclic loading profile, and the results are shown in the form of resilience as a function of strain in Figure 4. At low strains (<20%), resilience of more than 90% was observed for both systems. This was expected, as resilience is typically high for all materials at small deformations. However, fracture occurred at small strains (~40%) for both the PAAm and gelatin hydrogels, demonstrating their known relatively weak and brittle mechanical performance.
As mentioned previously, DN hydrogels exhibit significant hysteresis in the first loading cycle,18 and consequently demonstrate poor resilience. Furthermore, the resilience decreases with increasing strain, suggesting more permanent damage at larger strains. This is due to the permanent breakage of the covalent bonds in the randomly cross-linked PAMPS network, formed by free radical polymerization. The DN hydrogels, in addition to many other hydrogel systems in the literature, have been studied under compression, as this is easier to perform experimentally in terms of both sample preparation and testing. Therefore, to enable direct comparison, the hysteresis behavior of the PEG and PEG/PDMS hydrogels was also examined in compression. While the overall swelling and moduli of these two materials were different, the resilience was greater than 90% for all of the measured strains, and the energy loss was likely due to the inevitable friction between the hydrogel and the loading plates (SI Figures S2 and S3).23
When considering the PEG and PEG/PDMS hydrogels as desirable materials, other mechanical properties in addition to resilience, such as the modulus and maximum strain, are important. The properties of several representative water-swollen materials are summarized in Table 1, including the water content (ϕwater), Young’s modulus (E), maximum tensile strain (εmax), critical strain energy release rate (Gc), and resilience at low and high strain. The PAAm, gelatin, and DN hydrogels shared the same water content (ϕwater ≈ 0.90), which was in the range of the hydrogels reported here (ϕwater= 0.82–0.95). The stiffness, which was characterized by the Young’s modulus in tension and scales inversely with the water content, was similar among all the hydrogels, while the stiffness of resilin and elastin is much higher due to the significantly higher protein content (ϕwater= 0.50–0.60). Comparing the PEG and PEG/PDMS materials shows that increasing the PDMS content controlled the stiffness. For maximum tensile strain and toughness (related to Gc), the PEG and PEG/PDMS hydrogels were less than those of the DN hydrogels but an order of magnitude higher than those of the commonly used PAAm and gelatin hydrogels. Moreover, high resilience was achieved across the entire range of strains and water content for these new materials, in contrast to other purely organic hydrogels.
Although the underlying reasons for the extraordinary resilience of these synthetic hydrogels will require more investigation, some answers may be found in the network structure. The design strategy was based on the fundamental network elements of resilin that result in its unique elastic properties. The thiol-ene reaction between primary thiols and the norbornene double bond is known to be fast, highly efficient, and very selective.38, 39 This provided the hydrogels with well-defined cross-linked network structures, in which network defects, such as dangling chains and loops, were largely diminished. This is in sharp contrast to conventional radical polymerization, which is commonly used in gel synthesis. The use of telechelic polymer chains provided a precise molecular weight between cross-links, and the PEG provided an unstructured, hydrophilic chain that allowed the materials to swell significantly in water. The PDMS volume fraction controlled the overall water content of the hydrogels as .
Support for the uniform structure of these novel networks was obtained by small-angle neutron scattering (SANS). The SANS spectra show a monotonic decrease in scattering intensity over the q-range of 0.005 to 0.5 Å−1, and do not display the broad peak in scattering intensity previously observed for similarly end-functionalized, yet free radically cross-linked PEG-dimethacrylate (PEGDM) hydrogels (SI Figure S4).40 Fitting these data to common models for scattering from polymer solutions and gels41, 42 shows only one mesh size of 28.6 ± 0.2 Å. In addition, the Kratky plot shows no maximum, in contrast to the strong peak observed from the randomly cross-linked PEGDM hydrogels,40 illustrating that the networks reported here have an extremely homogeneous structure compared to conventional randomly cross-linked hydrogels (SI Figure S5).
This uniform network structure is reflected in the mechanical performance of the PEG and PEG/PDMS hydrogels. During stretching of these hydrogels, the applied strain energy is distributed uniformly due to the limited number of defects, allowing the network to approach theoretical maximum strain limits (SI, Gc calculation). Moreover, structural defects would typically induce rearrangement of the network, resulting in irreversible energy loss during cyclic loading, especially at large strain, reflected by a decrease in resilience. However, this phenomenon was not observed in these hydrogels, which exhibited near constant resilience until fracture (Figure 4).
Conclusions
In summary, the combination of high resilience and excellent mechanical properties seen in water-swollen natural materials like resilin and elastin has been difficult to reproduce in purely synthetic hydrogels to date. Here, the fundamental network elements of resilin, including a uniform network structure and unstructured primary chains, were built into simple synthetic polymer systems to form new networks using novel, robust, yet scalable synthetic polymer chemistry. This resulted in a unique hydrogel system with tunable properties. Cyclic tensile testing on the 100:0 and 30:70 PEG/PDMS hydrogels (95% and 82% water, respectively) demonstrated that they maintain a resilience greater than 97% across all measured strains (up to 300%). In addition, these synthetic systems demonstrated similar extensibility and resilience as resilin. This work highlights the importance of understanding the structure-property relationships of natural materials, and how this knowledge can be applied in the development of synthetic systems.
Supplementary Material
Table 1.
Summary of the mechanical properties of a series of elastic materials, both synthetic and natural, including the water content (ϕwater), Young’s modulus in tension (E), maximum tensile strain (εmax), critical strain energy release rate (Gc), and resilience at low and high degrees of strain.
| Materials | ϕwater | E [kPa] | εmax | Gc [J/m2] | Resilience* [%]
|
|
|---|---|---|---|---|---|---|
| ε < 0.5 | ε > 0.5 | |||||
| PEG | 0.95 | 16 | 3.0 | 10 | 98–99 | 99 |
| PEG/PDMS | 0.8 | 34 | 1.7 | 80 | 97 | 97 |
| PAAm | 0.9 | 29 | 0.5 | 1–10 | 98 | - |
| DN18, 35 | 0.9 | 100 | 10–20 | 102–103 | 77 | 40 |
| Gelatin | 0.9 | 17 | 0.4 | 2–100 | 90 | - |
| Elastin34, 36 | 0.4–0.45 | 1,200 | 1–2 | ND | 90 | ND |
| Resilin9, 37 | 0.5–0.6 | 2,000 | 1.9 | ND | 96–97 | 97 |
The resilience data were taken from the first cycle of the stress-strain curve.
Acknowledgments
We acknowledge NSF PIRE (NSF-0730243), DMR-0820506, and CMMI-0531171 for funding. Partial support was provided from ARO W911NF-09-1-0373 and ONR N00014-10-1-0348. This work utilized facilities supported in part by the National Science Foundation under Agreement No. DMR-0944772. We acknowledge the support of the National Institute of Standards and Technology, U.S. Department of Commerce, in providing the neutron research facilities used in this work. The authors thank Mr. Hitesh Thaker and Dr. Ke Zhang for their assistance in preparing the manuscript.
Footnotes
Supporting Information Available. Detailed experimental procedures, Figures S1–S5, Table S1, the Gc calculation, and Supplementary Movies 1 and 2. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Alfred J. Crosby, Email: crosby@mail.pse.umass.edu.
Gregory N. Tew, Email: tew@mail.pse.umass.edu.
References
- 1.Tanaka Y, Gong JP, Osada Y. Prog Polym Sci. 2005;30(1):1–9. [Google Scholar]
- 2.Calvert P. Adv Mater. 2009;21(7):743–756. [Google Scholar]
- 3.Gosline J, Lillie M, Carrington E, Guerette P, Ortlepp C, Savage K. Philos Trans R Soc, B. 2002;357(1418):121–132. doi: 10.1098/rstb.2001.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munch E, Launey ME, Alsem DH, Saiz E, Tomsia AP, Ritchie RO. Science. 2008;322(5907):1516–1520. doi: 10.1126/science.1164865. [DOI] [PubMed] [Google Scholar]
- 5.Gosline JM. Rubber Chem Technol. 1987;60(3):417–438. [Google Scholar]
- 6.Weis-Fogh T. J Exp Biol. 1960;37(4):889–907. [Google Scholar]
- 7.Elvin CM, Carr AG, Huson MG, Maxwell JM, Pearson RD, Vuocolo T, Liyou NE, Wong DCC, Merritt DJ, Dixon NE. Nature. 2005;437(7061):999–1002. doi: 10.1038/nature04085. [DOI] [PubMed] [Google Scholar]
- 8.Elliott GF, Huxley AF, Weis-Fogh T. J Mol Biol. 1965;13(3):791–795. [Google Scholar]
- 9.Weis-Fogh T. J Mol Biol. 1961;3(5):648–667. [Google Scholar]
- 10.Li LQ, Teller S, Clifton RJ, Jia XQ, Kiick KL. Biomacromolecules. 2011;12(6):2302–2310. doi: 10.1021/bm200373p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charati MB, Ifkovits JL, Burdick JA, Linhardt JG, Kiick KL. Soft Matter. 2009;5(18):3412–3416. doi: 10.1039/b910980c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan ZB, Roland JT, Bai JZ, Ma SX, McIntire TM, Nguyen M. J Am Chem Soc. 2004;126(7):2058–2065. doi: 10.1021/ja039127p. [DOI] [PubMed] [Google Scholar]
- 13.Chen YL, Guan ZB. J Am Chem Soc. 2010;132(13):4577–4579. doi: 10.1021/ja9104446. [DOI] [PubMed] [Google Scholar]
- 14.Malkoch M, Vestberg R, Gupta N, Mespouille L, Dubois P, Mason AF, Hedrick JL, Liao Q, Frank CW, Kingsbury K, Hawker CJ. Chem Commun. 2006;(26):2774–2776. doi: 10.1039/b603438a. [DOI] [PubMed] [Google Scholar]
- 15.Xiao L, Liu C, Zhu J, Pochan DJ, Jia X. Soft Matter. 6(21):5293–5297. doi: 10.1039/C0SM00511H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson JA, Turro NJ, Koberstein JT, Mark JE. Prog Polym Sci. 2010;35(3):332–337. [Google Scholar]
- 17.Zhu MF, Liu Y, Sun B, Zhang W, Liu XL, Yu H, Zhang Y, Kuckling D, Adler HJP. Macromol Rapid Commun. 2006;27(13):1023–1028. [Google Scholar]
- 18.Webber RE, Creton C, Brown HR, Gong JP. Macromolecules. 2007;40(8):2919–2927. [Google Scholar]
- 19.Hou Y, Schoener CA, Regan KR, Munoz-Pinto D, Hahn MS, Grunlan MA. Biomacromolecules. 11(3):648–56. doi: 10.1021/bm9012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo C, Bailey TS. Soft Matter. 2010;6(19):4807–4818. [Google Scholar]
- 21.Mespouille L, Hedrick JL, Dubois P. Soft Matter. 2009;5(24):4878–4892. [Google Scholar]
- 22.Erdodi G, Kennedy JP. Prog Polym Sci. 2006;31(1):1–18. [Google Scholar]
- 23.Seitz ME, Martina D, Baumberger T, Krishnan VR, Hui CY, Shull KR. Soft Matter. 2009;5(2):447–456. [Google Scholar]
- 24.Lin WC, Fan W, Marcellan A, Hourdet D, Creton C. Macromolecules. 2010;43(5):2554–2563. [Google Scholar]
- 25.Miquelard-Garnier G, Creton C, Hourdet D. Soft Matter. 2008;4(5):1011–1023. doi: 10.1039/b717460h. [DOI] [PubMed] [Google Scholar]
- 26.Lutolf MP, Hubbell JA. Biomacromolecules. 2003;4(3):713–722. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- 27.Gupta N, Lin BF, Campos LM, Dimitriou MD, Hikita ST, Treat ND, Tirrell MV, Clegg DO, Kramer EJ, Hawker CJ. Nat Chem. 2010;2(2):138–45. doi: 10.1038/nchem.478. [DOI] [PubMed] [Google Scholar]
- 28.Sakai T, Matsunaga T, Yamamoto Y, Ito C, Yoshida R, Suzuki S, Sasaki N, Shibayama M, Chung UI. Macromolecules. 2008;41(14):5379–5384. [Google Scholar]
- 29.Sakai T, Akagi Y, Matsunaga T, Kurakazu M, Chung UI, Shibayama M. Macromol Rapid Commun. 2010;31(22):1954–9. doi: 10.1002/marc.201000286. [DOI] [PubMed] [Google Scholar]
- 30.Fukasawa M, Sakai T, Chung UI, Haraguchi K. Macromolecules. 2010;43(9):4370–4378. [Google Scholar]
- 31.Mullins L, Tobin RN. Rubber Chem Technol. 1957;30:555–571. [Google Scholar]
- 32.Diani J, Fayolle B, Gilormini P. Eur Polym J. 2009;45(3):601–612. [Google Scholar]
- 33.Society for Experimental Biology (Great Britain) The Mechanical properties of biological materials. Published for the Society for Experimental Biology [by] Cambridge University Press; Cambridge; New York: 1980. [Google Scholar]
- 34.Gosline JM. Biopolymers. 1978;17(3):697–707. doi: 10.1002/bip.1978.360170312. [DOI] [PubMed] [Google Scholar]
- 35.Gong JP, Katsuyama Y, Kurokawa T, Osada Y. Adv Mater. 2003;15(14):1155–1158. [Google Scholar]
- 36.Aaron BB, Gosline JM. Biopolymers. 1981;20(6):1247–1260. [Google Scholar]
- 37.Jensen M, Weis-Fogh T. Philos Trans R Soc, B. 1962;245(721):137–169. [Google Scholar]
- 38.Hoyle CE, Lee TY, Roper T. J Polym Sci, Part A: Polym Chem. 2004;42(21):5301–5338. [Google Scholar]
- 39.Fairbanks BD, Schwartz MP, Halevi AE, Nuttelman CR, Bowman CN, Anseth KS. Adv Mater. 2009;21(48):5005–5010. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin-Gibson S, Jones RL, Washburn NR, Horkay F. Macromolecules. 2005;38(7):2897–2902. [Google Scholar]
- 41.Kline SR. J Appl Crystallogr. 2006;39:895–900. [Google Scholar]
- 42.Hammouda B, Ho DL, Kline S. Macromolecules. 2004;37(18):6932–6937. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.