Summary
Bleeding Assessment Tools (BATs) have been developed to aid in the standardized evaluation of bleeding symptoms. The Vicenza Bleeding Questionnaire (BQ), published in 2005, established a common framework and scoring key that has undergone subsequent modification over the years, culminating in the publication of the ISTH-BAT in 2010. Understanding the normal range of bleeding scores is critical when assessing the utility of a BAT. Within the context of The Merging Project, a bioinformatics system was created to facilitate the merging of legacy data derived from four different (but all Vicenza-based) BATs; the MCMDM1-VWD BQ, the Condensed MCMDM-1VWD BQ, the Pediatric Bleeding Questionnaire and the ISTH-BAT. Data from 1040 normal adults and 328 children were included in the final analysis, which showed that the normal range is 0–3 for adult males, 0–5 for adult females and 0–2 in children for both males and females. Therefore, the cut-off for a positive or abnormal BS is ≥4 in adult males, ≥6 in adult females and ≥3 in children. This information can now be used to objectively assess bleeding symptoms as normal or abnormal in future studies.
Keywords: bleeding questionnaire, bleeding score, ISTH-BAT, normal range
Introduction
Assessment and communication of bleeding symptom severity has long proved challenging because of the difficulty in reporting subjective haemorrhagic symptoms in a consistent format. This has led to the development of standardized, quantitative Bleeding Assessment Tools (BATs) that can translate the severities of a range of bleeding symptoms into a final, summative bleeding score (BS). Since the publication of the original Vicenza Bleeding Questionnaire (BQ) in 2005 [1], several consequent BATs have been developed based on the original, with modifications designed to improve the ease of evaluation and scoring accuracy.
Both the Vicenza BQ (which uses a 0 to +3 scoring system for each bleeding symptom) [1], and the subsequent MCMDM-1VWD BQ (which uses a −1 to +4 scoring system for each bleeding symptom) [2] were primarily used to assess bleeding symptoms in adults and each required approximately 40 min for administration. These BATs scored a range of mucocutaneous bleeding symptoms, such as epistaxis and cutaneous bleeding, as well as postsurgical and postpartum bleeding, based on the most severe instance of each of these symptoms. The tools assigned higher scores to episodes requiring medical consultation and medical interventions. The Vicenza BQ associated the VWD phenotype with a BS of greater than three in males and five in females [1]. The MCMDM-1VWD BQ, which introduced negative bleeding scores to adjust for the lack of bleeding in response to important haemostatic challenges such as surgery, childbirth or dental extraction, found an association between VWD and a BS >3 in both men and women [2]. In 2008, the Condensed MCMDM-1VWD BQ dramatically reduced questionnaire administration time to approximately 5–10 min, while maintaining much of the infrastructure of the previous tool. The normal range, which was determined from 100 healthy adults, was shown to be −3 to +3 [3]. Finally, in 2009, the Pediatric Bleeding Questionnaire (PBQ) added consideration of paediatric-specific bleeding symptoms, such as umbilical stump bleeding and bleeding after circumcision, to the existing Condensed MCMDM-1VWD BQ [4]. The PBQ facilitated the assessment of pediatric patients with an intermediate administration time (approximately 20 min). Investigations using the PBQ BAT identified a normal BS range of −1.5 to +2.5 [4].
In 2010, Rodeghiero et al. published the ISTH-BAT (International Society on Thrombosis and Hemostasis – Bleeding Assessment Tool) [5], which reflected a broad input from the individuals who developed the previous instruments, and was designed to optimize the BAT. It is intended for use in paediatric and adult patients and attempts to achieve greater accuracy by considering the frequency of bleeding episodes in addition to their severity. The most notable change in the scoring system used for the ISTH-BAT was the removal of the −1 scoring for a lack of excessive bleeding following dental extraction, surgery and delivery. Administration time is approximately 20 min.
As a first step in establishing the normal ranges of BS with the ISTH-BAT, we developed a bioinformatics system to allow for the merging of BS data collected using four different BATs, all based on the original Vicenza bleeding questionnaire (‘The Merging Project’). Importantly, there is a >90% overlap between the four BATs included in this project. We then used this data set to establish the normal BS ranges for the ISTH-BAT in both adult and paediatric subjects.
Patients, Materials and Methods
Bleeding score data (which were obtained for all subjects by expert-administration of the BAT) along with the von Willebrand factor (VWF) laboratory results, when available [VWF:Ag (VWF:antigen), VWF:RCo (VWF:ristocetin co-factor), FVIII:C (factor VIII coagulant)] and demographic data, were collected from 1422 normal subjects; adult data (n = 1079) were collected from individuals ≥18 years using the MCMDM-1VWD BQ (n = 294), Condensed MCMDM-1VWD BQ (n = 660), and ISTH-BAT (n = 125), while paediatric data (n = 343) were collected from individuals <18 years using the PBQ (n = 324) and ISTH-BAT (n = 19). A consistent definition of ‘normal’ was used across all studies. For adults and children, the specific wording was either individuals with no history of a known or previously diagnosed bleeding disorder or individuals with no known problem with bleeding or bruising, or both.
The data sets were merged using the Bleeding Phenotype Ontology (BPO), which was developed to explicitly represent the relationships among bleeding signs, symptoms, disorders, and treatments within the bleeding questionnaires [6]. The ontology is publicly available in the Bioportal ontology registry (http://bioportal.bioontology.org/ontologies/1166). Data elements (individual questions) from the four questionnaires were analysed to determine where they map to the BPO. From such analysis, a subset of common questions were identified and were utilized to compile data collected using different BATs for unified and standardized comparison. The aggregate data set was then stored on a MySQL database to facilitate data retrieval of various subgroups of patients. Data were separated into males and females for analysis. The mean and standard deviation (SD) were determined. Outlier values were defined as those above or below the mean ± 3 SDs. Once the outliers were removed, the middle 95th percentile was used to determine the normal range.
Results
The mean age of the adult population was 43 years and the mean age of the paediatric population was 9 years (Table 1). Among these, 40% of female adults and 45% of male adults had Type O blood, as well as 42% of female children and 38% of male children. VWF:Ag, VWF:RCo and FVIII:C results were consistently higher among adults as compared with children; there were no significant differences in these laboratory values between males and females in either age category.
Table 1.
Normal adult and paediatric data collected with four different BATs.
| Adults | Children | |||||
|---|---|---|---|---|---|---|
| Female (n = 705) | Male (n = 335) | P value | Female (n = 169) | Male (n = 159) | P value | |
| Mean age (years) (range) | 43 (18–88) | 43 (18–82) | 0.788 | 9 (0.4–17) | 9 (0.7–17) | 0.610 |
| Blood group O (%) | 188/469 (40) | 87/192 (45) | 0.861 | 27/65 (42) | 31/80 (39) | 0.714 |
| VWF:Ag (IU mL−1), mean (range) | 1.25 (n = 408) (0.52–3.99) | 1.29 (n = 188) (0.50–4.35) | 0.476 | 1.01 (n = 48) (0.50–3.97) | 0.91 (n = 53) (0.41–2.52) | 0.298 |
| VWF:RCo (IU mL−1), mean (range) | 1.12 (n = 307) (0.50–2.90) | 1.16 (n = 149) (0.50–4.24) | 0.439 | 0.92 (n = 23) (0.32–2.72) | 0.74 (n = 22) (0.35–1.22) | 0.147 |
| FVIII:C (IU mL−1), mean (range) | 1.21 (n = 387) (0.50–2.75) | 1.21 (n = 185) (0.52–3.12) | 0.814 | 1.17 (n = 41) (0.66–2.40) | 1.17 (n = 44) (0.57–2.08) | 0.989 |
| Median bleeding score (range) | 1 (0–6) | 0 (0–4) | <0.001 | 0 (0–2) | 0 (0–2) | 0.599 |
Mean values for the following laboratory results are shown: VWF:Ag (von Willebrand factor antigen, n = 697), VWF:RCo (von Willebrand factor:ristocetin co-factor, n = 501), and FVIII:C (factor VIII coagulant activity, n = 657). Shaded boxes show BS data. BS range in normal children is 0–2 for males and females (P = 0.599), 0–3 for adult males, and 0–5 for adult females (P < 0.001).
There was a wide range of BSs (0–14 in adults and 0–5 in children), but a large majority of scores were 0 (~60%). Thus, data were not normally distributed. We excluded outlier values [BS > 4.6 in adult males (n = 4), BS > 7.2 in adult females (n= 16) and >3.5 in children (n = 15)] in order to ensure that determination of the normal range was calculated on a population of healthy individuals. These adjustments led to final cohorts of 1040 adults and 328 children.
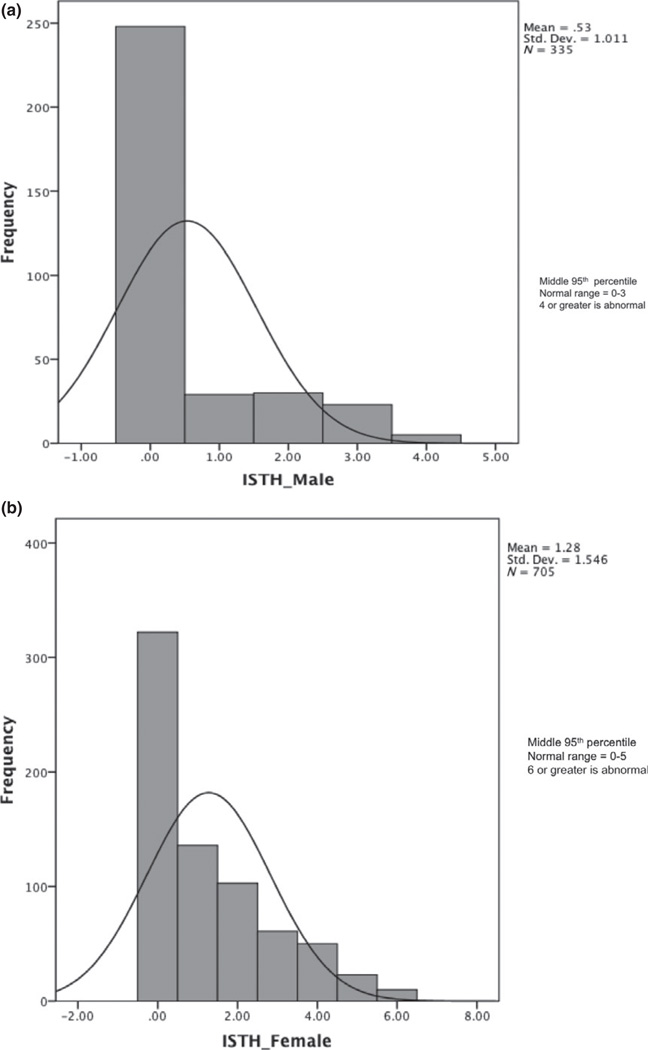
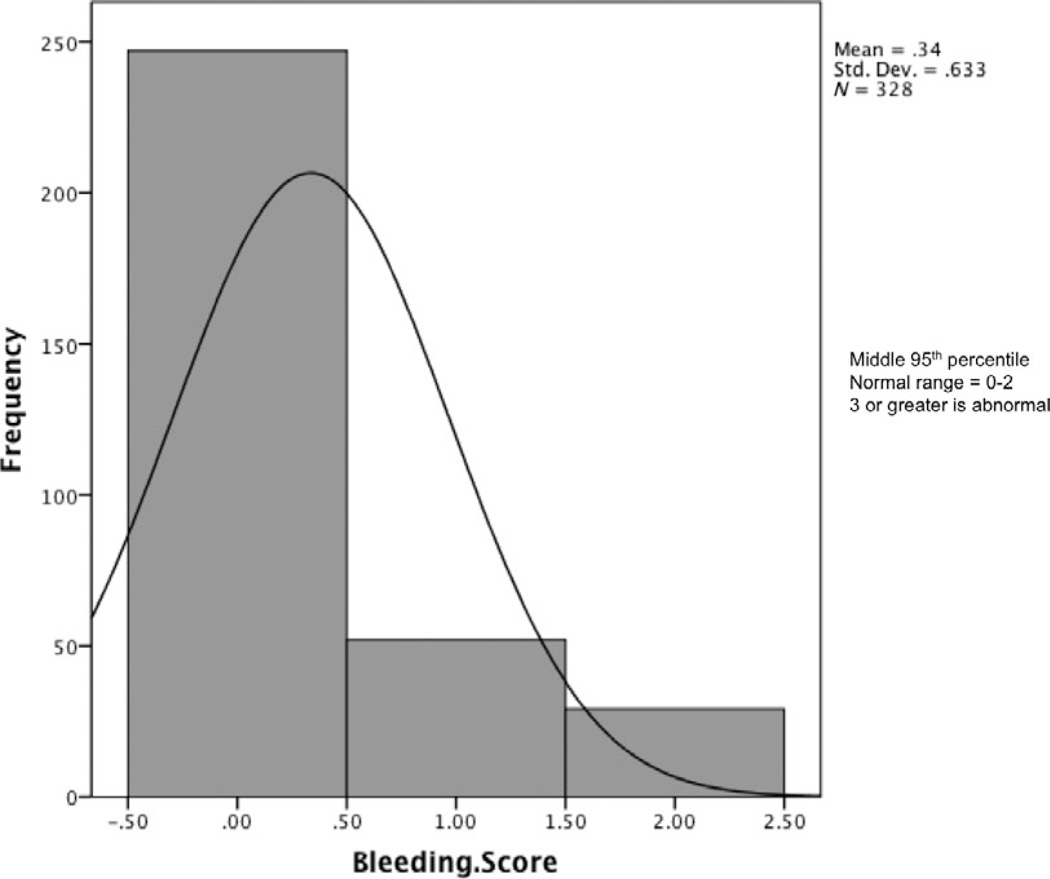
Using the methodology outlined above, the normal BS range was found to be 0–3 in adult males (Fig. 1a), 0–5 in adult females (Fig. 1b), and 0–2 in both male and female children (Fig. 2). The median BS was 0 in all groups except adult females, which had a median BS of 1. Adult female BSs were significantly higher than those of adult males (P < 0.001) (Table 1). When the gender-specific symptoms of menorrhagia and postpartum bleeding were removed from the analysis, the normal range in females was the same as in males (0–3). No correlation was seen between age and BS within the adult population.
Fig. 1.
(a) Bleeding Score data are shown for the final cohort of adult males that were included in the normal range determination. (b) Bleeding score data are shown for the final cohort of adult females who were included in the normal range determination.
Fig. 2.
Bleeding score data are shown for the final cohort of children who were included in the normal range determination. Given that there is no difference in the data when analysed by gender, the data are presented together.
Discussion
Bleeding Assessment Tools have proved useful tools in the assessment of patients with inherited bleeding disorders and multiple adaptations have improved accuracy, flexibility, and ease of use in both clinical and research applications. The ISTH-BAT was designed to optimize these qualities by accounting for bleeding frequency (in addition to severity) and allowing for use in children and adults while still maintaining a reasonable administration time. Establishing the normal range of the ISTH-BAT is a crucial first step in maximizing its utility. To jumpstart this process, we developed a bioinformatics mechanism, based on the ontology of bleeding symptoms used to structure the ISTH-BAT (and the Rockefeller University BQ), to merge legacy data from four different bleeding questionnaires, all developed from the same original BAT. After excluding outlier values, we determined the normal adult BS range to be 0–3 in males, 0–5 in females. Interestingly, the normal range is consistent with the results derived from the original Vicenza BQ, which also showed differences between males and females and does not assign −1 scores for any bleeding symptom. Additionally, when the gender-specific symptoms of menorrhagia and postpartum bleeding are removed, the normal range is the same between adult males and females; these results that are consistent with those of Mauer et al. in their assessment of 500 normals [7].
The newly established cutoffs for a positive (or abnormal) BS of ≥4 for adult males, ≥6 for adult females and ≥3 for children can now be used to objectively assess the affected status of individuals tested using ISTH-BAT in a standard fashion. Furthermore, the compilation of these data is an important start in the standardized unification of bleeding phenotype data, and the >90% overlap between the BATs included in this project makes the final data set reasonably homogeneous. In doing this, we aim to improve data accessibility in the long term by making these data available to investigators using the web-based ISTH-BAT system housed at Rockefeller University. Our goal is to expedite the correlation between bleeding phenotypes and genetic, molecular, and environmental data in order to improve diagnostic accuracy and provide insights into the factors that determine an individual’s tendency to bleed. By making the ISTH-BAT and its normal data available to investigators to conduct and analyse studies, we hope to expedite the development of new knowledge in the field.
Acknowledgements
This research was funded by a Canadian Hemophilia Society Research Grant and Summer Studentship; the Zimmerman Program for the Molecular and Clinical Biology of von Willebrand disease - National Institutes of Health Program Project Grant HL081588; Grant UL1 TR000043 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health and Translational Science Award (CTSA) and C17 Research Network.
Disclosures
PJ has received research funding from CSL Behring. RRM paid consulting fees from Baxter, Bayer, Biogen, CSL and Grifols.
Footnotes
Author contributions
ME, analysed data and wrote the paper; SM, JG, WH and SB analysed data; MD, AT, DC, ACM, MB, JR, PC and Zimmerman Program Investigators recruited subjects, generated and analysed data, RM, MLR, BC and PDJ supervised research, analysed data and wrote the paper.
References
- 1.Rodeghiero F, Castaman G, Tosetto A, et al. The discriminant power of bleeding history for the diagnosis of type 1 von Willebrand disease: an international, multicenter study. J Thromb Haemost. 2005;3:2619–2626. doi: 10.1111/j.1538-7836.2005.01663.x. [DOI] [PubMed] [Google Scholar]
- 2.Tosetto A, Rodeghiero F, Castaman G, et al. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM-1 VWD) J Thromb Haemost. 2006;4:766–773. doi: 10.1111/j.1538-7836.2006.01847.x. [DOI] [PubMed] [Google Scholar]
- 3.Bowman M, Mundell G, Grabell J, et al. Generation and validation of the Condensed MCMDM-1VWD Bleeding Questionnaire for von Willebrand disease. J Thromb Haemost. 2008;6:2062–2066. doi: 10.1111/j.1538-7836.2008.03182.x. [DOI] [PubMed] [Google Scholar]
- 4.Bowman M, Riddel J, Rand ML, Tosetto A, Silva M, James P. Evaluation of the diagnostic utility for von Willebrand disease of a pediatric bleeding questionnaire. J Thromb Haemost. 2009;7:1418–1421. doi: 10.1111/j.1538-7836.2009.03499.x. [DOI] [PubMed] [Google Scholar]
- 5.Rodeghiero F, Tosetto A, Abshire T, Arnold DM, Coller B, James P. ISTH/SSC bleeding assessment tool : a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010;8:2063–2065. doi: 10.1111/j.1538-7836.2010.03975.x. [DOI] [PubMed] [Google Scholar]
- 6.Mauer AC, Barbour EM, Khazanov NA, Levenkova N, Mollah SA, Coller BS. Creating an ontology-based human phenotyping system: The Rockefeller University bleeding history experience. Clin Transl Sci. 2009;2:382–385. doi: 10.1111/j.1752-8062.2009.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauer AC, Khazanov NA, Levenkova N, et al. Impact of sex, age, race, ethnicity and aspirin use on bleeding symptoms in healthy adults. J Thromb Haemost. 2011;1:100–108. doi: 10.1111/j.1538-7836.2010.04105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]