Abstract
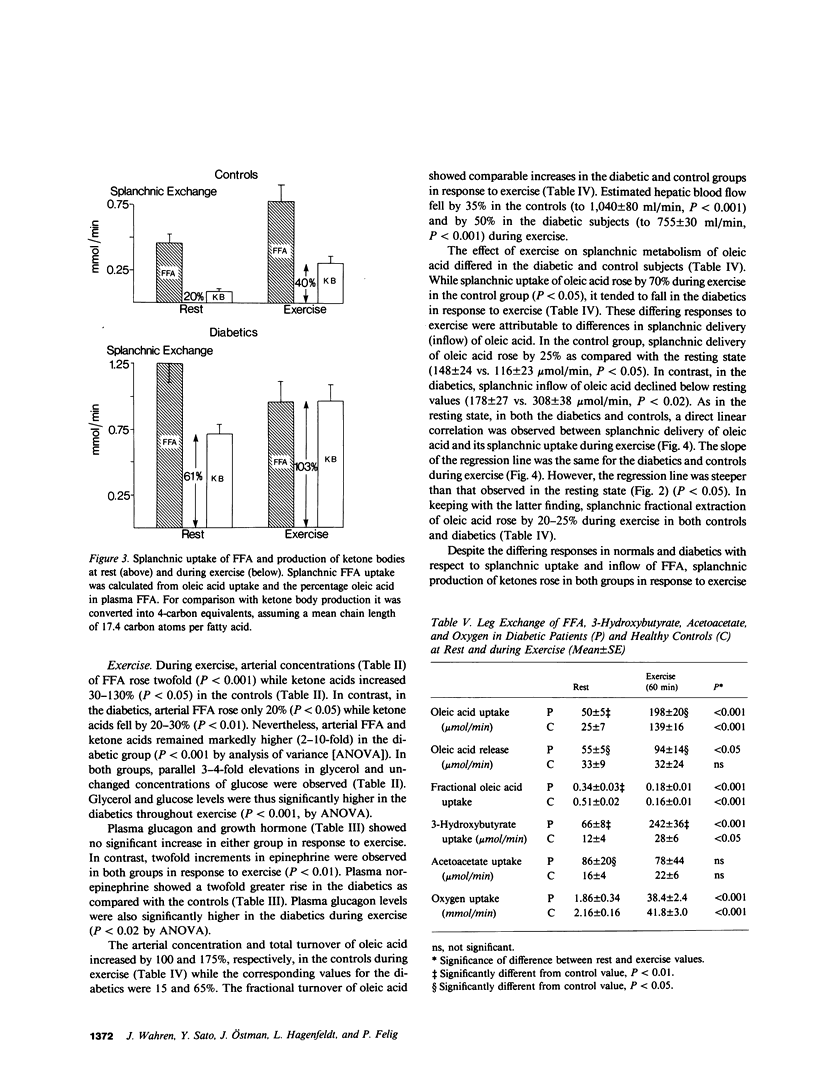
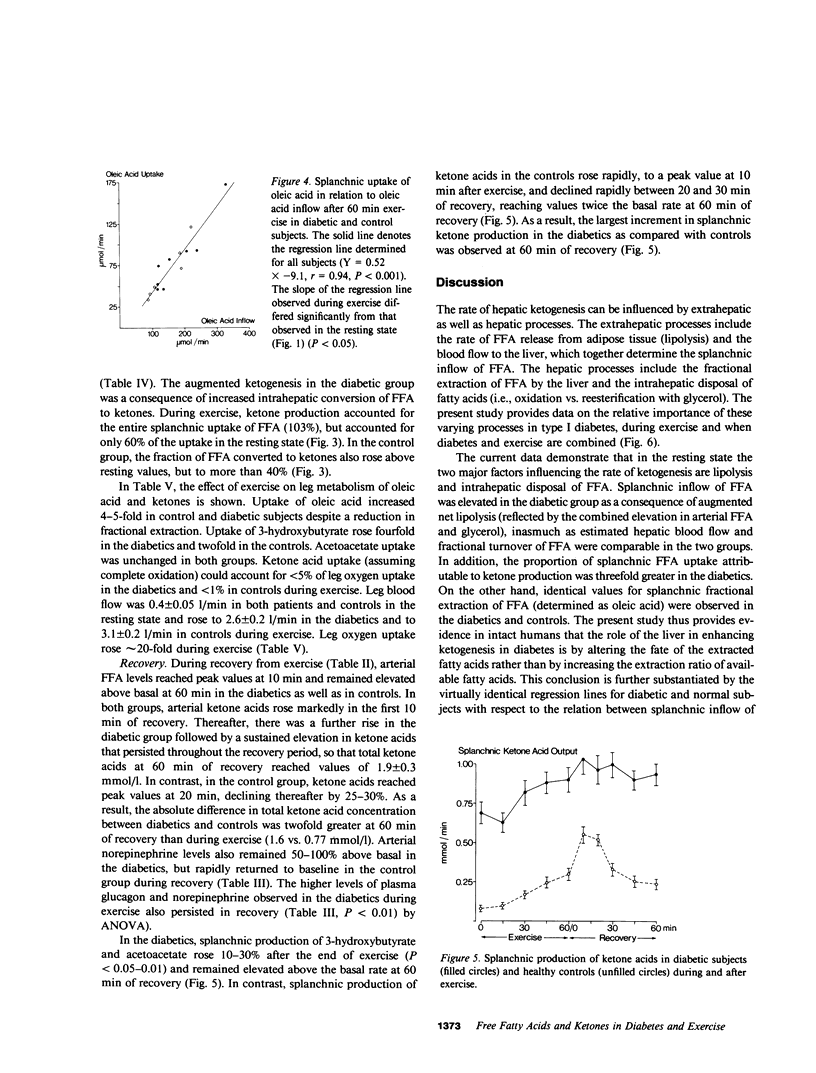
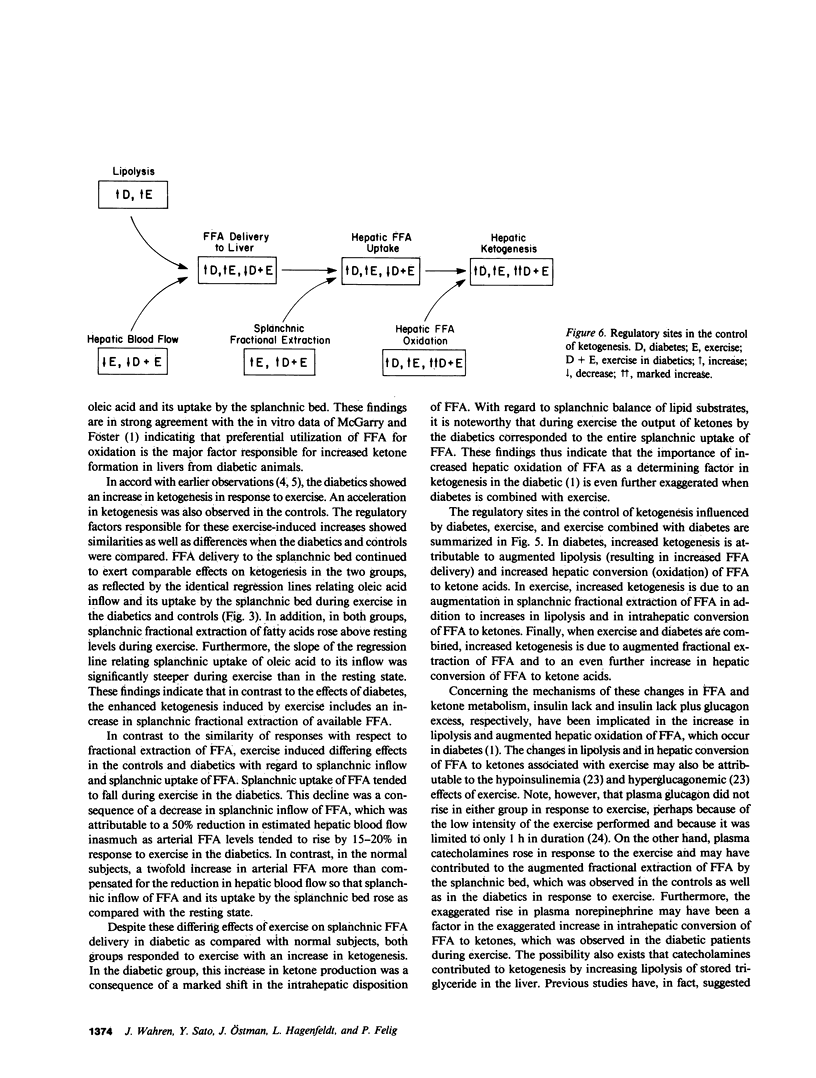
Nine insulin-dependent diabetics and six healthy controls were studied at rest, during, and after 60 min of bicycle exercise at a work load corresponding to 45% of their maximal oxygen intake. The catheter technique was employed to determine splanchnic and leg exchange of metabolites. FFA turnover and regional exchange was evaluated using [14C]oleate infusion. Basal glucose (13.8 +/- 1.1 mmol/l), ketone body (1.12 +/- 0.12 mmol/l), and FFA (967 +/- 110 mumol/l) concentrations were elevated in the diabetics in comparison with controls. In the resting state, splanchnic ketone acid production in the diabetics was 6-10-fold greater than in controls. Uptake of oleic acid by the splanchnic bed was increased 2-3-fold, and the proportion of splanchnic FFA uptake converted to ketones (61%) was threefold greater than in controls. In contrast, splanchnic fractional extraction of oleic acid was identical in diabetics and controls. A direct relationship was observed between splanchnic uptake and splanchnic inflow (plasma concentration X hepatic plasma flow) of oleic acid that could be described by the same regression line in the diabetic and control groups. During exercise, splanchnic ketone production rose in both groups. In the control group the increase in ketogenesis was associated with a rise in splanchnic inflow and in uptake of oleic acid, a rise in splanchnic fractional extraction of oleate, and an increase in the proportion of splanchnic FFA uptake converted to ketone acids from 20-40%. In the diabetic group, the increase in ketogenesis occurred in the absence of a rise in splanchnic inflow or uptake of oleic acid, but was associated with an increase in splanchnic fractional extraction of oleic acid and a marked increase in hepatic conversion of FFA to ketones, so that the entire uptake of FFA was accountable as ketone acid output. Splanchnic uptake of oleic acid correlated directly with splanchnic oleic acid inflow in both groups, but the slope of the regression line was steeper than in the resting state. Plasma glucagon levels were higher in the diabetic group at rest and during exercise, while plasma norepinephrine showed a twofold greater increment in response to exercise in the diabetic group (to 1,400-1,500 pg/ml). A net uptake of ketone acids by the leg was observed during exercise but could account for less than 5% of leg oxidative metabolism in the diabetics and less than 1% in controls. Despite the increase in ketogenesis during exercise, a rise in arterial ketone acid levels was not observed in the diabetics until postexercise recovery, during which sustained increments to values of 1.8-1.9 mmol/l and sustained increases in splanchnic ketone production were observed at 30-60 min. The largest increment in blood ketone acids and in splanchnic ketone production above values observed in controls thus occurred in the diabetics after 60 min of recovery from exercise. We concluded that: (a) In the resting state, increased ketogenesis in the diabetic is a consequence of augmented splanchnic inflow of FFA and increased intrahepatic conversion of FFA to ketones, but does not depend on augmented fractional extraction of circulating FFA by the splanchnic bed. (b) Exercise-induced increases in ketogenesis in normal subjects are due to augmented splanchnic inflow and fractional extraction of FFA as well as increased intrahepatic conversion of FFA to ketones. (c) When exercise and diabetes are combined, ketogenesis increases further despite the absence of a rise in splanchnic inflow of FFA. An increase in splanchnic fractional extraction of FFA and a marked increase intrahepatic conversion of FFA to ketones accounts for the exaggerated ketogenic response to exercise in the diabetic. (d) Elevated levels of plasma glucagon and/or norepinephrine may account for the increased hepatic ketogenic response to exercise in the diabetic. (e) Ketone utilization by muscle increases during exercise but constitutes a quantitatively minor oxidative fuel for muscle even in the diabetic. (f) The accelerated ketogenesis during exercise in the diabetic continues unabated during the recovery period, resulting in an exaggerated postexercise ketosis.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969 Jun;257(6):415–419. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- Ahlborg G., Felig P., Hagenfeldt L., Hendler R., Wahren J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest. 1974 Apr;53(4):1080–1090. doi: 10.1172/JCI107645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Berchtold P., Cüppers H. J., Drost H., Kley H. K., Müller W. A., Wiegelmann W., Zimmerman-Telschow H., Gries F. A., Krüskemper H. L. Metabolic and hormonal effects of muscular exercise in juvenile type diabetics. Diabetologia. 1977 Aug;13(4):355–365. doi: 10.1007/BF01223279. [DOI] [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Felig P., Wahren J. Fuel homeostasis in exercise. N Engl J Med. 1975 Nov 20;293(21):1078–1084. doi: 10.1056/NEJM197511202932107. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L. A gas chromatographic method for the determination of individual free fatty acids in plasma. Clin Chim Acta. 1966 Feb;13(2):266–268. doi: 10.1016/0009-8981(66)90304-4. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L., Wahren J. Human forearm muscle metabolism during exercise. 3. Uptake, release and oxidation of beta-hydroxybutyrate and observations on the beta-hydroxybutyrate/acetoacetate ratio. Scand J Clin Lab Invest. 1968;21(4):314–320. doi: 10.3109/00365516809076999. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L., Wahren J. Human forearm muscle metabolism during exercise. II. Uptake, release and oxidation of individual FFA and glycerol. Scand J Clin Lab Invest. 1968;21(3):263–276. doi: 10.3109/00365516809076994. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L., Wahren J. Human forearm muscle metabolism during exercise. VI. Substrate utilization in prolonged fasting. Scand J Clin Lab Invest. 1971 Jun;27(4):299–306. doi: 10.3109/00365517109080222. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L., Wennlung A., Felig P., Wahren J. Turnover and splanchnic metabolism of free fatty acids in hyperthyroid patients. J Clin Invest. 1981 Jun;67(6):1672–1677. doi: 10.1172/JCI110204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S. M. Review of energetics and blood flow in exercise. Diabetes. 1979 Jan;28 (Suppl 1):33–38. doi: 10.2337/diab.28.1.s33. [DOI] [PubMed] [Google Scholar]
- Johnson R. H., Walton J. L., Krebs H. A., Williamson D. H. Post-exercise ketosis. Lancet. 1969 Dec 27;2(7635):1383–1385. doi: 10.1016/s0140-6736(69)90931-3. [DOI] [PubMed] [Google Scholar]
- Jorfeldt L., Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971 Nov;41(5):459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Reichard G. A., Jr Human forearm metabolism during progressive starvation. J Clin Invest. 1971 Jul;50(7):1536–1545. doi: 10.1172/JCI106639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passon P. G., Peuler J. D. A simplified radiometric assay for plasma norepinephrine and epinephrine. Anal Biochem. 1973 Feb;51(2):618–631. doi: 10.1016/0003-2697(73)90517-4. [DOI] [PubMed] [Google Scholar]
- ROWELL L. B., BLACKMON J. R., BRUCE R. A. INDOCYANINE GREEN CLEARANCE AND ESTIMATED HEPATIC BLOOD FLOW DURING MILD TO MAXIMAL EXERCISE IN UPRIGHT MAN. J Clin Invest. 1964 Aug;43:1677–1690. doi: 10.1172/JCI105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosselin G., Assan R., Yalow R. S., Berson S. A. Separation of antibody-bound and unbound peptide hormones labelled with iodine-131 by talcum powder and precipitated silica. Nature. 1966 Oct 22;212(5060):355–357. doi: 10.1038/212355a0. [DOI] [PubMed] [Google Scholar]
- Schade D. S., Eaton R. P. The regulation of plasma ketone body concentration by counter-regulatory hormones in man. III. Effects of norepinephrine in normal man. Diabetes. 1979 Jan;28(1):5–10. doi: 10.2337/diab.28.1.5. [DOI] [PubMed] [Google Scholar]
- Sestoft L., Trap-Jensen J., Lyngsooe J., Clausen J. P., Holst J. J., Nielsen S. L., Rehfeld J. F., Schaffalitzky de Muckadell O. S. Regulation of gluconeogenesis and ketogenesis during rest and exercise in diabetic subjects and normal men. Clin Sci Mol Med. 1977 Nov;53(5):411–418. doi: 10.1042/cs0530411. [DOI] [PubMed] [Google Scholar]
- Tamborlane W. V., Sherwin R. S., Koivisto V., Hendler R., Genel M., Felig P. Normalization of the growth hormone and catecholamine response to exercise in juvenile-onset diabetic subjects treated with a portable insulin infusion pump. Diabetes. 1979 Aug;28(8):785–788. doi: 10.2337/diab.28.8.785. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Hagenfeldt L., Felig P. Splanchnic and leg exchange of glucose, amino acids, and free fatty acids during exercise in diabetes mellitus. J Clin Invest. 1975 Jun;55(6):1303–1314. doi: 10.1172/JCI108050. [DOI] [PMC free article] [PubMed] [Google Scholar]


