Abstract
Introduction
Severe injury can cause intestinal permeability through decreased expression of tight junction proteins, resulting in systemic inflammation. Activation of the parasympathetic nervous system after shock through vagal nerve stimulation is known to have potent anti-inflammatory effects; however, its effects on modulating intestinal barrier function are not fully understood. We postulated that vagal nerve stimulation improves intestinal barrier integrity after severe burn through an efferent signaling pathway, and is associated with improved expression and localization of the intestinal tight junction protein occludin.
Methods
Male balb/c mice underwent right cervical vagal nerve stimulation for 10 minutes immediately before 30% total body surface area, full-thickness steam burn. In a separate arm, animals underwent abdominal vagotomy at the gastroesophageal junction before vagal nerve stimulation and burn. Intestinal barrier injury was assessed by permeability to 4 kDa FITC-dextran, histology, and changes in occludin expression using immunoblotting and confocal microscopy.
Results
Cervical vagal nerve stimulation decreased burn-induced intestinal permeability to FITC-dextran, returning intestinal permeability to sham levels. Vagal nerve stimulation before burn also improved gut histology and prevented burn-induced changes in occludin protein expression and localization. Abdominal vagotomy abrogated the protective effects of cervical vagal nerve stimulation before burn, resulting in gut permeability, histology, and occludin protein expression similar to burn alone.
Conclusion
Vagal nerve stimulation performed before injury improves intestinal barrier integrity after severe burn through an efferent signaling pathway and is associated with improved tight junction protein expression.
Keywords: Burn, Tight junction, Gut, Intestinal barrier, Permeability, Confocal microscopy
The intestinal epithelium plays a key role in host defense by maintaining a barrier against harmful bacteria and toxic products present within the gut lumen. Specifically, the intestinal tight junction serves as the regulator of barrier function in an intact intestinal epithelium.1 Loss of tight junction integrity opens the paracellular space between gut epithelial cells allowing harmful luminal products to access normally protected layers of the intestinal wall. The subsequent intestinal inflammatory response can further exacerbate the breakdown of tight junction proteins leading to a systemic inflammatory response and serious sequelae.2
Occludin is an intestinal tight junction protein which contains several transmembrane domains that attach the apical portion of adjacent cells forming the tight junction.3 Increased paracellular permeability has been associated with either decreased occludin protein expression or altered localization of occludin away from areas of cell-cell contact.4 Decreased intestinal occludin expression has been associated with increased intestinal permeability in animal models of severe burn, traumatic brain injury, sepsis, and pancreatitis.5–8 Changes in intestinal occludin expression have also been seen clinically in patients with inflammatory bowel disease.9 Strategies aimed at maintaining normal expression and localization of occludin after injury may be important in preventing intestinal barrier loss.
Recent research suggests that the central nervous system may coordinate an anti-inflammatory response to injury. Tracey et al.10 have recently described the cholinergic anti-inflammatory pathway, proposing that efferent vagal nerve signaling regulates cytokine production. Vagal nerve stimulation has been shown to decrease proinflammatory cytokine levels and also decrease the occurrence of shock in animals given a lethal dose of endotoxin.11 Signaling from the efferent vagus nerve to the splenic nerve is responsible for the suppression of tumor necrosis factor-α seen in endotoxemic animals that underwent vagal nerve stimulation.12 The ability of vagus nerve stimulation to affect other cell types within the abdomen has yet to be fully elucidated.
The gastrointestinal tract is innervated by the enteric nervous system, which contains a complex network of enteric neurons that control many aspects of intestinal function. The central nervous system communicates with the enteric nervous system through both efferent and afferent pathways via the vagus nerve.13 Therefore, stimulation of the vagus nerve may alter intestinal function through modulation of the various cell types within the enteric nervous system; including intestinal epithelial cells, endothelial cells, or enteric glia.14
Strategies that prevent intestinal barrier breakdown after injury may be useful in improving outcomes in patients after severe trauma and burn. Activation of the parasympathetic nervous system after shock through vagal nerve stimulation is known to have potent anti-inflammatory effects. The ability of vagal nerve stimulation to improve intestinal barrier function is not fully understood. We postulated that vagal nerve stimulation improves intestinal barrier integrity following severe burn through an efferent signaling pathway and is associated with improved expression and localization of the intestinal tight junction protein occludin.
MATERIALS AND METHODS
Surgical Abdominal Vagotomy
Male balb/c mice weighing 24 g to 28 g were obtained from Jackson Laboratories (Sacramento, CA). Animals were anesthetized with inhaled isoflurane before the experimental protocol. In one cohort of animals, surgical abdominal vagotomy was performed immediately before vagal nerve stimulation and subsequent burn by making a midline laparotomy incision. The gastroesophageal junction was identified and the dorsal and ventral vagus nerves were visualized on the distal esophagus using an Olympus SZ61 stereo microscope (Leeds Precision Instruments, Minneapolis, MN). Both branches of the vagus nerve were isolated and transected. The abdomen was then closed using interrupted silk sutures.
Vagal Nerve Stimulation
A right cervical neck incision was performed and the right cervical vagus nerve exposed. Stimulation of the right cervical vagus nerve was performed using a VariStim III probe (Medtronic Xomed, Jacksonville, FL) at 2 mA for 10 minutes. After nerve stimulation, the incision was closed with interrupted silk suture and the animal was immediately subjected to burn injury as previously described. Sham animals underwent right cervical incision and exposure of the vagus nerve but did not receive electrical stimulation.
Thermal Injury Model
Animals underwent dorsal fur clipping with an electric clipper before 30% total body surface area (TBSA) dorsal steam burn for 7 seconds using a template designed to estimate 30% TBSA.15 Following burn, animals received a subcutaneous injection of normal saline containing buprenorphine (0.05 mg/kg) in a non-burned area for fluid resuscitation and pain control. Animals were recovered from anesthesia and returned to their cage where they were provided food and water ad libitum. All animal experiments were approved by the University of California San Diego Institutional Animal Care and Use Committee.
Intestinal Permeability Assay
An in vivo intestinal permeability assay was performed to assess intestinal barrier function (n ≥ 3 animals per group). Four hours after injury, animals were anesthetized with inhaled isoflurane. A midline laparotomy incision was performed, and a 5-cm segment of distal ileum was isolated between silk ties. A solution of 200 μL containing 4 kDa FITC-Dextran (25 mg/mL, Sigma, St. Louis, MO) diluted in phosphate buffered saline (PBS) was injected into the lumen of the isolated segment of intestine. The bowel was returned to the abdominal cavity and the abdomen closed. The animal was maintained under general anesthesia for 30 minutes, at which time systemic blood was drawn by cardiac puncture and placed in heparinized Eppendorf tubes on ice. Plasma was obtained by centrifuging the blood at 10,000 g for 10 minutes at −4°C. Plasma fluorescence was measured in a fluorescence spectrophotometer (SpectraMax M5, Molecular Devices, Sunnyvale, CA) and compared with a standard curve of known concentrations of FITC-Dextran diluted in mouse plasma.
Tissue Harvest
Animals were sacrificed 4 hours after injury and tissues harvested. Segments of distal small intestine were removed and immediately snap frozen in liquid nitrogen before storage at −80°C for analysis. Segments of distal small intestine were also harvested and preserved in 10% buffered formalin (Richard Allan Scientific, Pittsburgh, PA) or optimal cutting technique embedding media (OCT, Sakura Finetek, Torrance, CA) for histologic evaluation.
Histologic Evaluation
Segments of distal ileum were preserved in 10% buffered formalin, embedded in paraffin, and sectioned. Intestinal sections were stained with Hematoxylin and Eosin (Surgi-path, Richmond, IL). Sections were viewed using an Olympus IX70 light microscope (Melville, NY) and viewed with Q-imaging software (Surrey, BC, Canada). Sections were reviewed by a pathologist (Paul Wolf, MD) blinded to the experimental groups.
Occludin Expression
Distal small intestine harvested from animals at 4 hours after burn (n = 4–5 animals per group) were homogenized in ice-cold tissue protein extraction reagent, containing 1% protease and 1% phosphatase inhibitors (Pierce Biotechnology, Rockford, IL). The homogenized tissue was centrifuged at 10,000 g for 5 minutes, the supernatants collected, and the protein concentration of each sample measured using the bicinchoninic protein assay (Pierce). Protein was suspended in sodium dodecyl sulfate sample buffer and boiled for 5 minutes. Proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 8% to 16% Tris-glycine gradient gels (Invitrogen, Carlsbad, CA), then transferred to nitrocellulose membranes (Invitrogen). Membranes were blocked with 5% bovine serum albumin (BSA, Sigma) in Tris-buffered saline/Tween 20 for 1 hour. Membranes were then incubated overnight at 4°C in primary antibody for occludin (Zymed, Carlsbad, CA) prepared in a 1:500 concentration in 5% BSA/Tween 20. The membranes were then washed and incubated with a horseradish peroxidase-linked anti-rabbit IgG secondary antibody (Cell Signaling) before application of the Pierce Supersignal West Pico Chemiluminescent Kit for antibody detection. Luminescence was detected using the Xenogen IVIS Lumina (Caliper Life Science, Hopkinton, MA) imaging system. Mean pixel density of each sample was estimated using UN-SCAN-IT Gel Digitizing software (Silk Scientific, Orem, UT). The relative band density of each band was calculated by dividing the pixel density of each sample by the mean pixel density of the sham samples.
Confocal Microscopy
Segments of distal ileum (n = 3 animals per group) were embedded in optimal cutting technique media and stored at −80°C. Sections of intestine were cut 10 μm thick using a Reichert-Jung Cryocut 1800 at −20°C (Reichert Microscopes, Depew, NY). Sections were fixed onto glass slides with 3.7% paraformaldehyde (Electron Microscopy Series, Hatfield, PA) for 10 minutes, washed with PBS, then permeabilized with 0.01% Triton X-100 (Sigma) for 1 minute. Sections were washed once again in PBS before blocking for 1 hour in 3% BSA, Sigma. The sections were then incubated overnight in the occludin antibody. Sections were then treated with the secondary antibody Alexa Fluor 488 (Invitrogen) in 1% BSA for 1 hour. Prolong Fade (Invitrogen) was added upon placement of cover slips. Images were viewed using the Olympus Fluoview laser scanning confocal microscope with exposure matched settings (Advanced Software v1.6, Olympus) at 60× magnification.
Statistical Analysis
Values are expressed as the mean ± the SEM of n samples where n represents the number of animals in each experimental group. The statistical significance between groups was determined using analysis of variance with Bonferroni correction. Statistical analysis was performed using SPSS Statistics software version 11.5 (SPSS, Chicago, IL). A p value < 0.05 was considered statistically significant.
RESULTS
Vagal Nerve Stimulation Attenuates Intestinal Barrier Injury
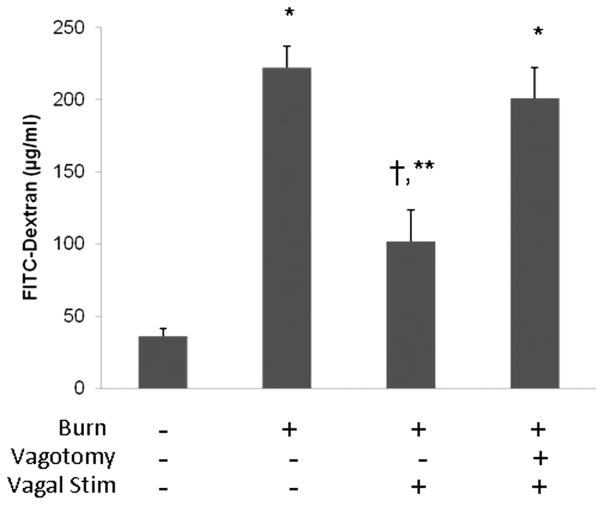
The physiologic effects of vagal nerve stimulation on intestinal barrier function following severe burn were assessed using an in vivo intestinal permeability assay to 4 kDa FITC-Dextran (Fig. 1). Severe burn injury alters gut barrier function resulting in a significant increase in permeability to FITC-Dextran. Performing cervical vagal nerve stimulation before 30% TBSA burn attenuated burn-induced intestinal permeability. There was no significant difference between sham and burned animals that underwent vagal nerve stimulation. Another group of animals underwent abdominal vagotomy at the gastroesophageal junction before cervical vagal nerve stimulation to confirm that the protective effects were due to efferent signaling down the vagus nerve. Intestinal permeability was elevated in burned animals that underwent abdominal vagotomy before vagal nerve stimulation, with systemic concentration of FITC-Dextran similar to animals subjected to burn alone.
Figure 1.
Vagal nerve stimulation attenuates burn-induced intestinal permeability. In vivo intestinal permeability to 4 kilodalton FITC-Dextran measured 4 hours after injury. Severe burn injury significantly increased gut permeability. Animals that underwent right cervical vagal nerve stimulation immediately before burn decreased intestinal permeability to sham levels. Performing an abdominal vagotomy abrogated the protective effects of vagal nerve stimulation, suggesting that stimulating the vagus nerve improves intestinal barrier function through efferent signaling down the vagus nerve. *p < 0.01 versus Sham, †p < 0.01 versus burn, **p < 0.05 versus vagotomy/vagal stimulation/burn.
We correlated the finding of the in vivo intestinal permeability assay by assessing changes in gut histology from sections harvested 4 hours after injury (Fig. 2). Vagal nerve stimulation improves the histologic appearance of the small intestine following burn, with improved villous height compared with animals that were subjected to burn alone. The protective effects of vagal nerve stimulation are diminished in specimens obtained from animals that underwent abdominal vagotomy before vagal stimulation and subsequent burn injury. This once again suggests that the vagal nerve stimulation decreases intestinal barrier injury through an efferent pathway from the cervical vagus nerve into the enteric nervous system.
Figure 2.
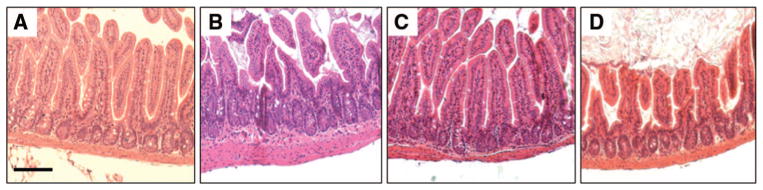
Vagal nerve stimulation limits histologic intestinal injury following severe burn. Sections of distal small intestine harvested 4 hours after injury, stained with hematoxylin and eosin, and viewed using light microscopy. (A) Normal appearing sections of intestine harvested from sham animals. (B) There is evidence of histologic gut injury from animals 4 hours following burn characterized by decreased villous height. (C) Gut specimens from animals that underwent cervical vagal nerve stimulation before burn have an appearance similar to sham. (D) Surgical abdominal vagotomy eliminates the protective effects of vagal nerve stimulation, with altered villous height noted from sections of small intestine. Size bar = 100 μm.
Stimulation of the Vagus Nerve Before Burn Improves Intestinal Occludin Expression
We correlated the changes in intestinal barrier function with changes in occludin protein expression from intestinal tissue harvested 4 hours after injury (Fig. 3). There was a significant decrease in occludin protein expression from intestinal tissue harvested from animals following 30% TBSA burn. Vagal nerve stimulation before injury prevented the burn-induced decrease in intestinal occludin protein expression. There was no difference in occludin expression between sham and animals undergoing vagal nerve stimulation before severe burn. Intestinal occludin protein levels were significantly decreased in animals that underwent surgical abdominal vagotomy before cervical vagal nerve stimulation and burn.
Figure 3.
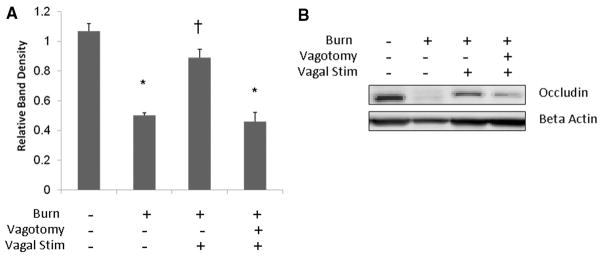
Stimulation of the vagus nerve before injury prevents the burn-induced decrease of intestinal occludin protein expression. Intestinal extracts were obtained from animals 4 hours following injury for measurement of occludin protein expression using Western blot. (A) Graph representing relative band densities from Western blots measuring intestinal occludin protein expression. Vagal nerve stimulation attenuates the loss of occludin protein seen following severe burn. Abdominal vagotomy abrogates the protective effects of vagal nerve stimulation, with occludin expression similar to burn alone. (B) Representative Western blot for intestinal occludin. *p < 0.001 versus Sham, †p < 0.001 versus burn and vagotomy/vagal stimulation/burn.
Vagal Nerve Stimulation Maintains Intestinal Occludin Localization After Burn Injury
Next, we assessed changes in intestinal occludin localization after burn, as altered tight junction protein localization has also been associated with increased intestinal barrier breakdown. Occludin is normally distributed at the periphery of the intestinal epithelial cell at areas of cell-cell contact, as seen in the sham animal (Fig. 4, A). This normal pattern of intestinal occludin localization is disturbed in animals 4 hours after severe burn, with loss of the normal pattern of staining at the cell periphery (Fig. 4, B). The pattern of occludin staining in sections of intestine harvested from animals that underwent stimulation of the vagus nerve before burn is similar to sham, with a continuous, orderly pattern of staining at the edge of the epithelial cells (Fig. 4, C). The localization of intestinal occludin is altered in animals subjected to abdominal vagotomy before vagal stimulation and burn. Similar to animals subjected to burn alone, there is loss of the continuous pattern of staining normally seen at points of cell-cell contact (Fig. 4, D).
Figure 4.
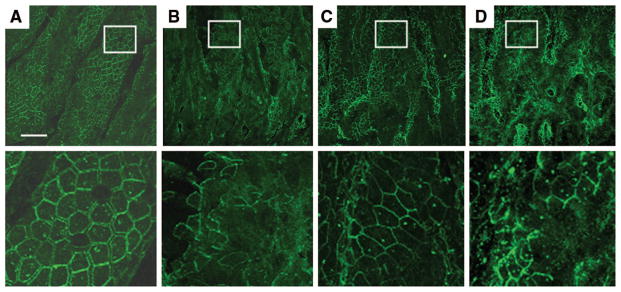
Vagal nerve stimulation limits the altered localization of intestinal occludin after severe burn injury. Sections of small intestine harvested 4 hours following injury stained for occludin (green) and viewed using a confocal microscope. Representative images from each group are seen in the top row, with a magnification of the inset area marked by the white box seen below. (A) Normal distribution of occludin seen in the sham animal with staining localizing to the periphery of the cell. (B) Altered localization of occludin seen from animals 4 hours following burn. There is loss of occludin staining at areas of cell-cell contact. (C) Localization of intestinal occludin is improved in animals that underwent vagal nerve stimulation before burn, with continuous staining seen at the periphery of the intestinal epithelial cells. (D) Surgical abdominal vagotomy eliminates the protective effects of vagal nerve stimulation. The appearance of occludin localization is similar to animals subjected to burn alone, with a discontinuous pattern of occludin staining. Size bar = 30 μm.
DISCUSSION
Intestinal barrier failure may represent an inciting event in the development of post-injury systemic inflammatory response syndrome and multiorgan failure. Therefore, therapies aimed at limiting intestinal barrier injury may decrease distant organ injury and the late deaths seen after severe injury. Intestinal tight junction proteins may represent an important therapeutic target, as they are a key regulator of intestinal permeability. In this series of experiments, we have shown that stimulation of the vagus nerve normalized expression of the tight junction protein occludin, which was associated with improved intestinal barrier function.
The intestinal tight junction is made up of several important molecules, which are responsible for maintaining a barrier in the paracellular space between epithelial cells. The tight junction protein occludin is a regulator of intestinal permeability with a decrease in occludin protein expression correlating with increased paracellular permeability.1 In vitro studies have shown that proinflammatory cytokines decrease occludin expression in intestinal cell lines.16,17 Modulation of intestinal occludin expression has been implicated as a cause of inflammatory bowel disease and necrotizing enterocolitis.18,19 Recently, we have shown that severe burn injury results in increased intestinal permeability which is associated with both decreased occludin protein expression and altered occludin localization using confocal microscopy.7
Regulation of occludin expression and its localization to the apical epithelial cell at the tight junction has been the subject of considerable investigation. The phosphorylation state of occludin has been shown to be important, with the more highly phosphorylated form localizing to the tight junction in an in vitro study.20 Endocytosis of tight junction proteins has also been implicated as a mechanism by which barrier function is disrupted. A study by Ivanov et al.21 suggests that the entire apical tight junction complex may undergo endocytosis at early time points after stress. This may account for the changes in occludin protein expression seen after burn injury in this study. However, the mechanism by which occludin expression and localization is improved in this study is unclear.
The vagus nerve innervates the enteric nervous system and alters numerous gastrointestinal functions including peristalsis and digestion.22 Recent research also suggests that efferent vagal nerve signaling may have important immunomodulatory properties and specifically serves as a modulator of intestinal inflammation.14,23 Luyer et al.24 has previously shown that efferent vagal nerve signaling stimulated by administration of high fat enteral nutrition attenuated hemorrhagic shock-induced intestinal permeability. The protective effects of high fat enteral nutrition on gut barrier integrity were abrogated in vagotomized animals. Subsequent studies have shown that efferent vagal nerve signaling after injury can increase expression of the tight junction protein zonula occludens protein-1.25
The mechanism by which efferent vagal nerve stimulation is able to improve intestinal barrier integrity and tight junction protein expression is unknown. Prior studies detailing the immunomodulatory effects of vagal nerve signaling have focused on the nicotinic Acetylcholine receptor. Specifically, the alpha 7 subunit of the nicotinic Acetylcholine receptor is known to be essential in preventing pro-inflammatory cytokine release.26 Ghia et al.27 has shown that nicotinic cholinergic signaling attenuates intestinal inflammation in a model of colitis. The role of cholinergic signaling in maintaining tight junction integrity is unclear. Previous studies have shown that cholinergic agonists can increase paracellular permeability in rat ileum, whereas another study in mouse small intestine demonstrated that cholinergic signaling did not affect paracellular permeability.28,29
Enteric glia cells may also play a role in modulating gut barrier integrity and intestinal tight junction protein expression. We have recently discovered that vagal nerve stimulation increase activation of enteric glia cells in the distal small intestine after severe burn injury (unpublished data). Enteric glia cells are similar to astrocytes of the central nervous system and play an important role in maintaining barrier integrity.30 Ablation of enteric glia cells in transgenic animals is fatal within 19 days due to hemorrhagic necrosis of the gut.31 Enteric glia cells release factors such as S-nitrosoglutathione, which has barrier inducing properties and has been shown to increase tight junction protein expression in vitro and in an in vivo model of inflammatory bowel disease.32 Studies investigating the role of vagal nerve stimulation on enteric glia cell activation and secretion of S-nitrosoglutathione are currently ongoing in our laboratory.
In this study, we performed stimulation of the vagus nerve immediately before severe burn injury. Further studies are needed to investigate the effects of vagal nerve stimulation after injury to define the “window of opportunity” for this therapeutic intervention. Similarly vagal nerve signaling will need to occur at very early time points after injury, as we have previously documented increased expression of the tight junction protein myosin light chain kinase by 2 hours after burn.33 By defining the mechanism by which efferent vagal nerve signaling improves intestinal tight junction protein expression and barrier function, we may be able to identify a pharmacologic approach to efferent vagal nerve signaling.
In summary, vagal nerve stimulation performed before severe burn injury maintained intestinal barrier integrity through an efferent signaling pathway, which was associated with improved expression and localization of the intestinal tight junction protein occludin. Limiting alterations in intestinal tight junction protein expression and subsequent gut barrier breakdown may limit the systemic inflammatory response syndrome response and improve outcomes in patients after severe injury.
Footnotes
Presented as an oral podium presentation at the 68th Annual Meeting of the American Association for the Surgery of Trauma, October 1–2, 2009, Pittsburgh, Pennsylvania.
References
- 1.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen L, Turner JR. Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: tight junction dynamics exposed. Am J Physiol Gastrointest Liver Physiol. 2006;290:G577–G582. doi: 10.1152/ajpgi.00439.2005. [DOI] [PubMed] [Google Scholar]
- 3.Furuse M, Hirase T, Itoh M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raimondi F, Santoro P, Barone MV, et al. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G906–G913. doi: 10.1152/ajpgi.00043.2007. [DOI] [PubMed] [Google Scholar]
- 5.Han X, Fink MP, Yang R, et al. Increased iNOS activity is essential for intestinal epithelial tight junction dysfunction in endotoxemic mice. Shock. 2004;21:261–270. doi: 10.1097/01.shk.0000112346.38599.10. [DOI] [PubMed] [Google Scholar]
- 6.Yasuda T, Takeyama Y, Ueda T, et al. Breakdown of intestinal mucosa via accelerated apoptosis increases intestinal permeability in experimental severe acute pancreatitis. J Surg Res. 2006;135:18–26. doi: 10.1016/j.jss.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 7.Costantini TW, Loomis WH, Putnam JG, et al. Burn-induced gut barrier injury is attenuated by phosphodiesterase inhibition: effects on tight junction structural proteins. Shock. 2009;31:416–422. doi: 10.1097/SHK.0b013e3181863080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bansal V, Costantini T, Kroll L, et al. Traumatic Brain Injury and Intestinal Dysfunction: Uncovering the Neuro-Enteric Axis. J Neurotrauma. 2009 doi: 10.1089/neu.2008.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kucharzik T, Walsh SV, Chen J, et al. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159:2001–2009. doi: 10.1016/S0002-9440(10)63051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 12.Rosas-Ballina M, Ochani M, Parrish WR, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105:11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackshaw LA, Brookes SJ, Grundy D, et al. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 14.Van Der Zanden EP, Boeckxstaens GE, de Jonge WJ. The vagus nerve as a modulator of intestinal inflammation. Neurogastroenterol Motil. 2009;21:6–17. doi: 10.1111/j.1365-2982.2008.01252.x. [DOI] [PubMed] [Google Scholar]
- 15.Walker HL, Mason AD., Jr A standard animal burn. J Trauma. 1968;8:1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Costantini TW, Deree J, Loomis W, et al. Phosphodiesterase inhibition attenuates alterations to the tight junction proteins occludin and ZO-1 in immunostimulated Caco-2 intestinal monolayers. Life Sci. 2009;84:18–22. doi: 10.1016/j.lfs.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–4649. doi: 10.4049/jimmunol.178.7.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark JA, Doelle SM, Halpern MD, et al. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol. 2006;291:G938–G949. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- 19.Fries W, Muja C, Crisafulli C, et al. Dynamics of enterocyte tight junctions: effect of experimental colitis and two different anti-TNF strategies. Am J Physiol Gastrointest Liver Physiol. 2008;294:G938–G947. doi: 10.1152/ajpgi.00469.2007. [DOI] [PubMed] [Google Scholar]
- 20.Wong V. Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am J Physiol. 1997;273:C1859–C1867. doi: 10.1152/ajpcell.1997.273.6.C1859. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The FO, Boeckxstaens GE, Snoek SA, et al. Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology. 2007;133:1219–1228. doi: 10.1053/j.gastro.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 24.Luyer MD, Greve JW, Hadfoune M, et al. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med. 2005;202:1023–1029. doi: 10.1084/jem.20042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luyer MD, Buurman WA, Hadfoune M, et al. Pretreatment with high-fat enteral nutrition reduces endotoxin and tumor necrosis factor-alpha and preserves gut barrier function early after hemorrhagic shock. Shock. 2004;21:65–71. doi: 10.1097/01.shk.0000101671.49265.cf. [DOI] [PubMed] [Google Scholar]
- 26.Gallowitsch-Puerta M, Tracey KJ. Immunologic role of the cholinergic anti-inflammatory pathway and the nicotinic acetylcholine alpha 7 receptor. Ann N Y Acad Sci. 2005;1062:209–219. doi: 10.1196/annals.1358.024. [DOI] [PubMed] [Google Scholar]
- 27.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, et al. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–1130. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Cameron HL, Perdue MH. Muscarinic acetylcholine receptor activation increases transcellular transport of macromolecules across mouse and human intestinal epithelium in vitro. Neurogastroenterol Motil. 2007;19:47–56. doi: 10.1111/j.1365-2982.2006.00845.x. [DOI] [PubMed] [Google Scholar]
- 29.Bijlsma PB, Kiliaan AJ, Scholten G, et al. Carbachol, but not forskolin, increases mucosal-to-serosal transport of intact protein in rat ileum in vitro. Am J Physiol. 1996;271:G147–G155. doi: 10.1152/ajpgi.1996.271.1.G147. [DOI] [PubMed] [Google Scholar]
- 30.Savidge TC, Sofroniew MV, Neunlist M. Starring roles for astroglia in barrier pathologies of gut and brain. Lab Invest. 2007;87:731–736. doi: 10.1038/labinvest.3700600. [DOI] [PubMed] [Google Scholar]
- 31.Bush TG, Savidge TC, Freeman TC, et al. Fulminant jejunoileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 32.Savidge TC, Newman P, Pothoulakis C, et al. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344–1358. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 33.Costantini TW, Loomis WH, Putnam JG, et al. Pentoxifylline modulates intestinal tight junction signaling after burn injury: effects on myosin light chain kinase. J Trauma. 2009;66:17–24. doi: 10.1097/TA.0b013e318191bb1f. [DOI] [PMC free article] [PubMed] [Google Scholar]