Abstract
Background
Severe injury results in intestinal barrier dysfunction that may be responsible for significant morbidity and mortality. We postulated that mining a peptide library that was displayed on phage would identify peptide sequences that bind and internalize into the gut epithelium following injury.
Methods
We utilized a severe full thickness burn in mice as a model of severe injury. Candidate peptides were identified by screening 1012 phage displaying unique peptide sequences. In vivo assessment was performed by injecting targeted phage into the lumen of a segment of distal ileum following burn injury, then analyzed for uptake of peptide sequence using quantitative polymerase chain reaction (PCR), DNA sequencing, and confocal microscopy of the peptide bound to quantum dots (Qdots).
Results
Phage screening identified the peptide sequence T18 (LTHPQDSPPASA) as an optimal candidate for in vivo testing. PCR of intestinal cells following injury showed a higher level of T18 sequence when compared to untargeted phage. Confocal microscopy of the peptide sequence bound to Qdots showed internalization into gut mucosa following injury.
Conclusion
We have identified a peptide sequence that targets the injured intestinal epithelium and may allow for the development of targeted therapies to attenuate inflammation, or other pathologic conditions of the small bowel.
An intact intestinal epithelial barrier is an essential defense mechanism against bacteria, endotoxin, and other cytotoxic elements carried in the lumen of the intestine. The gut barrier prevents the passage of intraluminal bacteria or bacterial products into normally protected layers of the intestinal wall. Intestinal injury is known to result from several clinical conditions including severe trauma,1 burn,2 inflammatory bowel disease,3 and necrotizing enterocolitis,4 resulting in significant morbidity and mortality. Intestinal barrier injury has been implicated in systemic injury following severe trauma and burn by inciting an intestinal inflammatory response causing production of pro-inflammatory cytokines.5,6 These pro-inflammatory mediators are then carried through the mesenteric lymph to the systemic circulation resulting in neutrophil activation and distant organ injury.7,8
Phage display is an extremely powerful molecular technique that is used to identify functional targeting ligands and their corresponding receptors. This approach allows diverse libraries of peptide sequences to be displayed by utilizing the bacteriophage M13. This technique allows a single peptide sequence to be displayed on a single phage and allows for in vivo biopanning of a large number of peptide sequences. Phage display has been used to identify cell targeting ligands by screening phage libraries both in vitro and in vivo, and can lead to the identification of organ-specific ligands.9–11 The ability to select peptide sequences that bind to, and are internalized into, the intestinal epithelial cell may prove useful for the delivery of biotherapeutics directly to the site of gut injury.12
We postulated that by utilizing in vivo phage display, we would identify peptide sequences that would internalize into the intestinal epithelial following severe injury. After the identification of candidate peptide sequences was achieved, we confirmed their uptake by the intestinal mucosa following injury using confocal microscopy imaging. This sequence may allow for targeted therapies designed to attenuate inflammation, or other pathologic conditions of the small bowel frequently seen in clinical practice such as inflammatory bowel disease and necrotizing enterocolitis.
MATERIALS AND METHODS
Animal model of severe injury
We used a 30% total body surface area (TBSA) steam burn in male balb/c mice (Jackson Laboratory, Sacramento, CA) weighing 20–25 grams (7–9 weeks old) as a model of severe injury. Briefly, mice were placed under general anesthesia using inhaled isoflurane. The dorsal fur was removed using an electronic clipper. A template was made to estimate a 30% TBSA burn. While under general anesthesia, the mice were placed in the template and subjected to a steam burn for 7 s. Immediately following the burn, animals received a subcutaneous injection of 1.5 ml of normal saline with buprenorphine in a non-burned area. Animals were then returned to their cage to recover from anesthesia. Sham animals were placed under anesthesia, underwent dorsal fur removal, and received an injection of buprenorphine, but were not burned. Following the procedure, animals were provided food and water ad libitum.
These experiments were approved by the University of California Animal Subjects Committee and are in accordance with guidelines established by the National Institutes of Health.
Preparation of phage library
Ph.D-12 Phage Display Peptide Library Kit (Cat. # E8110S; New England Biolabs, Inc., Cambridge, MA) was used in repeated rounds of bio-panning with isolated intestinal mucosal cells from mice 2h post-burn. Cells were washed with either Trypsin/EDTA or “acid wash” Glycine buffer pH 2.0. Phage were amplified and incubated with isolated intestinal mucosa from animals 2h following severe burn.
Screening for candidate peptide sequences
Peptide sequences were “blasted” for protein homology and tissue specificity using Peptide Blast on Pubmed. Candidate sequences were selected for further screening using these criteria. Six more sequences were chosen on the basis of similarity to the transmucosal transport (TMT) 7-peptide phage sequence (YPRLLTP) described by Duerr et al.13 The phage bearing the fusion peptide were amplified and titer determined by serial dilution plating on E. coli.
Ex vivo staining for peptide sequences
Distal ileum from animals 2 h post-burn was excised and mounted in optimal cutting technique (OCT; Sakura Finetek, Torrance, CA) media and immediately frozen at −80°C. Ten micron sections were cut and fixed to slides with 3.7% paraformaldehyde (Electron Microscopy Series, Hatfield, PA), blocked with 3% bovine serum albumin (BSA) and rinsed in PBS. Each of the candidate phage were diluted to 1 × 107/μl in 1% BSA and incubated at room temperature on the fixed tissue for 1 h. The tissue was rinsed with PBS and pre-incubated with goat anti mouse F(ab′), then for 1 h with anti-M13 (RL-ph1, SCBT sc-53004). After PBS rinse the anti-mouse Alexa Fluor 488 (Invitrogen, Carlsbad, CA) was used for secondary label and the tissue evaluated under an Olympus Fluoview FV1000 (Olympus, Melville, NY) laser scanning confocal microscope for the best set of candidates for in vivo screening.
In vivo screening of fusion peptides displayed on phage
Intestinal injection of peptide sequences
Two h following burn, animals were once again placed under general anesthesia using inhaled isoflurane. A midline laparotomy was performed. The distal small intestine was exposed. Four consecutive segments of small intestine were isolated using silk suture, with each segment of bowel measuring 2 cm. A 200 μl solution at 1 × 107 particles/μl of T18 phage (LTHPQDSPPASA), TMT phage (YPRLLTP), empty vector, or PBS was injected intraluminally into its own segment of bowel. The intestine was then returned to the abdominal cavity and the skin was sutured closed. The animal was maintained under general anesthesia until 30 min following intraluminal injection at which time the laparotomy incision was re-opened. Each segment of small intestine was removed and a PE-10 catheter was inserted into the lumen of the intestine and secured with a silk suture. The segment of bowel was then placed in an Eppendorf tube on ice for further analysis.
Washing tissue with Trypsin/EDTA
Bowel segment catheters attached to tubing were washed at 4°C using a peristaltic pump delivering 1.5–2 ml/min to each of the 4 segments for 10 min. The input solution was changed to Trypsin/EDTA (Gibco 25200) for 10 min longer and then switched back to PBS for a final 10 min. These tissue segments were then placed in Eppendorf tubes, quick frozen at −80°C, and the wet weight determined.
Purification of phage DNA from tissue
Tissue was ground for 30 s using a tissue grinder in 200 μl, 50 mM glucose, 25mM Tris-Cl, pH 8, 10mM EDTA, 400 μl 0.2M NaOH, 1% (w/v) sodium dodecyl sulfate, and mixed on a rotator at 4°C for 30 min. Reactions were neutralized with 3 mol/L potassium acetate, and 2 mol/L acetic acid, pH 4.0, and placed on the rotator for 15 min at 4°C. Samples were then spun at 10,000 × g for 20 min and the supernatant transferred to new tubes (750 μl). DNA was precipitated with 750 μl isopropyl alcohol and the pellet dried. The pellet was reprecipitated using sodium iodide and ethanol, dried, and resuspended in 20 μl tris-EDTA buffer or RNAse/DN-Ase free water.
Polymerase chain reaction
Amplification of the Phage DNA from tissue (0.5 μl) was amplified by relative polymerase chain reaction (Invitrogen Platinum Blue PCR Supermix) and by quantitative PCR (Bio-Rad iQ Sybr Green Supermix; 170-8880) to visualize and quantitate the number of phage particles per milligram tissue. Briefly, the acid precipitated DNA solution for each tissue was diluted serially 10-fold over 4 magnitudes and compared to the known titer for the TMT phage standard curve starting at 1 × 105. The number of particles per milligram protein was then calculated.
Peptide synthesis and conjugation to quantum dot (Qdot) nanospheres
The conjugation of the peptide sequence to Qdots was modified from the protocol described by Cai et al.14 Qdots (250 μl Qdot 705; Invitrogen) were mixed with 2 μmol 4-Maleimidobutyric acid N-succinimidyl ester in 10 mM Borate buffer at pH 8.5. The Qdot mixture was then incubated at room temperature for 1 h with gentle shaking. The mixture was then loaded into an NAP-10 column with PBS. The deepest colored fraction was removed and used for conjugation to the candidate peptide sequence by adding 0.5 μM of the T18 sequence. The T18-Qdot conjugates were then injected into the lumen of the intestine of animals following burn as previously described. Unconjugated Qdots were injected into segments of intestine following injury as a control.
Confocal microscopy of Qdot internalization
Thirty min following intestinal injection of the Qdot-peptide conjugate, segments of bowel were harvested and washed with Trypsin as previously described. Segments of intestine were placed in OCT media and stored at −80°C. Sections of intestine were cut 60μm thick using a Reichert-Jung Cryocut 1800 (Reichert Microscopes, Depew, NY) and placed on glass slides. Images were viewed using an Olympus Fluoview FV1000 laser scanning confocal microscope at 20 × magnification.
RESULTS
Identification of candidate peptide sequences
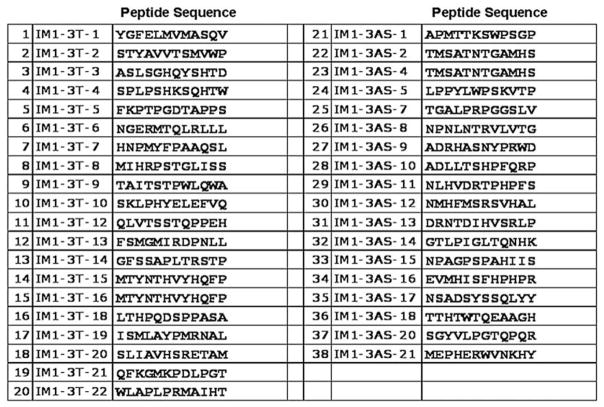
A total of 3 rounds of phage screening resulted in 32 candidate 12-amino acid peptide sequences (Fig 1). Our list of candidate sequences was further narrowed by evaluating protein homology and tissue specificity using Peptide Blast on Pubmed.
Fig 1.
Candidate peptide sequences from screened phage clones. A total of 32 unique candidate 12-amino acid sequences were identified.
Ex vivo staining for candidate peptide sequences
Candidate peptide sequences were further screened for gut specificity by performing ex vivo staining for the phage M13 coat protein on sections of distal ileum harvested 2 h following injury. Confocal microscopy images indicated that sequence T18 and AS8 had the strongest staining pattern, with the T18 sequence (LTHPQDSPPASA) showing the best gut specificity of the analyzed candidate sequences (Fig 2). The T18 protein sequence was therefore chosen for further in vivo screening.
Fig 2.
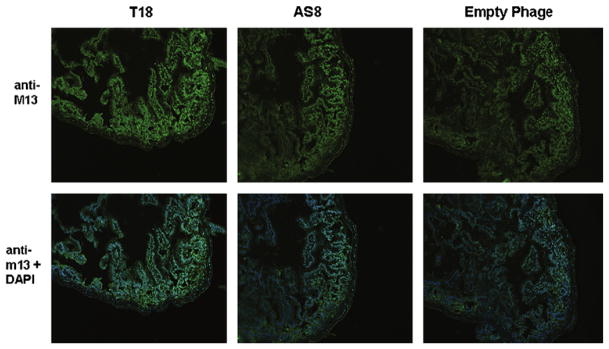
Ex vivo staining of intestinal sections to screen candidate peptide sequences. Candidate peptide sequences were incubated with fixed intestinal specimens from burned animals. Sections were then stained with anti-M13, which recognizes phage and DAPI. Positive staining was seen in candidate sequences T18 (LTHPQDSPPASA) and AS8 (NPNLNTRVLVTG). Empty phage bearing no peptide sequence was used as a control.
Polymerase chain reaction after in vivo screening
Quantitative PCR was performed on intestinal extracts following in vivo screening of peptide-phage internalization in animals 2 h following burn and sham. The T18-phage was internalized into the intestinal epithelium following injury, with 18,281 particles/mg tissue measured by quantitative PCR (Fig 3, A). There were 16,226 particles/mg of the TMT sequence (YPRLLTP) measured from the intestinal epithelium following burn. In sham animals, there were 38,591 particles of T18-phage/mg tissue compared to 109,178 particles of TMT-phage/mg tissue (Fig 3, B). There was minimal internalization of untargeted phage, which did not express any peptide sequence. This data is representative of in vivo phage screening using the highest dose of phage injected into the intestinal lumen following injury. There is increased uptake of the peptide bound phage in sham compared to burn, which is not surprising because the injured mucosa likely has decreased ability to internalize the peptide sequence due to mucosal injury. However, the T18-phage is internalized in large numbers into the injured gut mucosa and is a viable candidate for targeting the intestinal epithelium.
Fig 3.
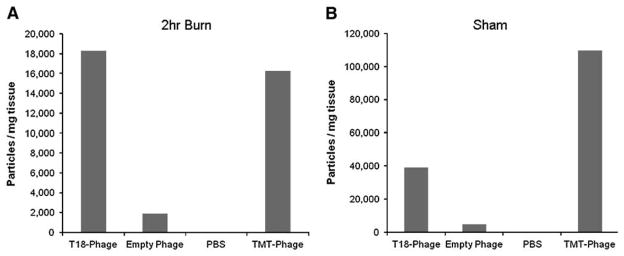
Quantitative PCR of intestinal extracts from in vivo phage screening. Quantitative PCR of the T18 sequence (LTHPQDSPPASA) was compared to empty phage bearing no peptide sequence, PBS, and the previously published 7-peptide TMT sequence (YPRLLTP). (A) Quantitative PCR of intestinal mucosa from animals 2 h following severe cutaneous burn. (B) Quantitative PCR of intestinal mucosa from sham animals.
DNA sequencing of internalized peptide sequence
In order to confirm that the T18-phage was recovered and measured by PCR, we performed DNA sequencing using the DNA extracts taken from intestinal tissue harvested following post-injury in vivo screening. DNA sequencing confirmed that the T18 peptide sequence targeted the intestinal mucosa, was internalized into the epithelial cell, and successfully harvested (Fig 4).
Fig 4.
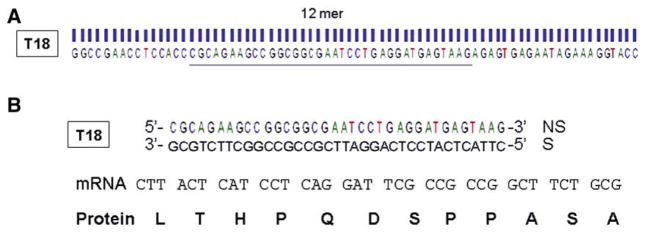
DNA sequencing of PCR product obtained from in vivo phage screening. (A) DNA sequence of the peptide-phage conjugate recovered from intestinal mucosa of animals 2 h following severe burn. (B) Translation of the DNA sequence obtained yields an mRNA sequence which would yield the T18 peptide sequence (LTHPQDSPPASA) confirming that the T18-phage was internalized into the intestinal mucosa and recovered.
Confocal microscopy of Qdot internalization
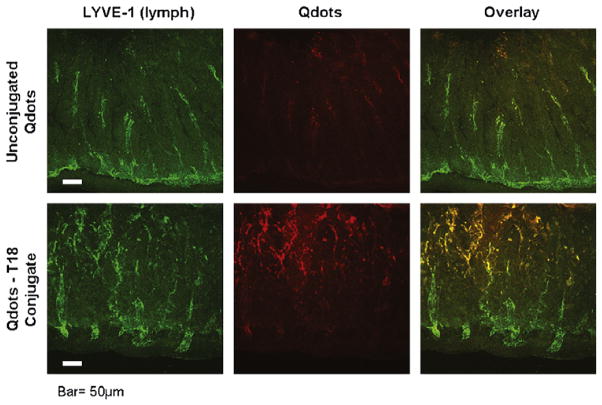
To better visualize internalization into the gut mucosa we utilized Qdot imaging with a confocal microscope. Qdots are nanoparticles that emit fluorescence and can be conjugated to various oligonucleotides. The T18 sequence was conjugated to Qdots and injected in vivo into the intestinal lumen of animals 2 h following severe burn. Thirty min after the injection of the T18-Qdot conjugate, the distal ileum was harvested, washed, and sectioned for confocal microscopy. Unconjugated Qdots were injected into the intestine of animals following burn as a control. Sections of gut were also stained with LYVE-1, an antibody specific for lymphatic endothelium, to evaluate for co-localization within the gut mucosa.
Confocal microscopy shows internalization of the T18-Qdot conjugates into the intestinal mucosa following severe burn (Fig 5). The T18-Qdot conjugates appear to localize near the tips of the intestinal villi. There is clear co-localization with the LYVE-1 antibody, suggesting that the T18 sequence may have some specificity for the intestinal lymphatics. Images of unconjugated Qdots show minimal fluorescence signal from within the gut mucosa.
Fig 5.
Confocal microscopy of candidate peptide sequence conjugated to Quantum Dots (Qdots). Sections of distal small intestine obtained from animals 2 h following severe cutaneous burn after in vivo exposure to intraluminal Qdot-T18 conjugates or Qdots alone. Fluorescence from Qdots viewed using a laser scanning confocal microscope. Intestinal sections also stained for LYVE-1, an antibody specific for lymphatic endothelium. The T18-Qdot conjugate allows for visualization of internalization of the T18 sequence into burn-injured intestinal mucosa. The T18 sequence co-localizes with the LYVE-1 antibody suggesting affinity for the intestinal lymphatics. Size bar = 50μm.
DISCUSSION
Gut injury following severe injury is believed to be the source of the systemic inflammatory response (SIRS), which can cause distant organ injury and multiple organ failure. Gut injury results in serious morbidity and mortality in medical conditions such as inflammatory bowel disease,15 necrotizing enterocolitis,16 severe burn,17 and colitis,18 due to tight junction breakdown and the resultant intestinal inflammatory response. Therefore, the ability to effectively target the intestinal mucosa to deliver biotherapies in an attempt to prevent gut injury or speed barrier healing could be of powerful clinical utility.
The question of how to prevent gut barrier breakdown or restore barrier integrity in a timely manner after injury is of paramount clinical significance. There is currently no capacity to target the gut barrier with either drugs or substances that would promote either rapid restoration of the damaged gut barrier or, if administered early after the initial insult, would attenuate gut dysfunction and subsequent inflammatory response. In order to be effective, biotherapeutics must be delivered to the cells of the intestinal wall in sufficient quantities to achieve the desired effect. This can be increasingly difficult during acute injury when intestinal perfusion is decreased, thus making delivery of systemically administered therapeutics challenging.
Phage display is a powerful combinatorial technique aimed at identifying target specific peptides that can bind to various cell types.19 Phage-based vectors can be used to identify peptides that can perform targeted delivery of genes, antibiotics, medications, or other biotherapeutic strategies.20 This phage display technique identifies receptor-ligand systems that offer the ability to deliver targeted therapies locally, possibly limiting the effects of clearance, compromised blood flow, and systemic side effects. Phage display has been used to identify peptide sequences that target internalization into various cell types and has been studied experimentally in the treatment of numerous medical conditions including lung cancer,21 leukemia,22 and neurologic disease.23 While phage display has been studied for targeting inflammatory intestinal mucosa in the setting of inflammatory bowel disease,24 little investigation has occurred in the setting of severe trauma and burn. Here, we target the injured intestinal epithelium 2 h following 30% TBSA burn, a time point at which we have previously shown changes in tight junction protein expression and increased permeability to small molecular weight (4 kilodalton) probes.17,25
In this study we identified a 12-amino acid sequence protein that binds to and is internalized into the injured epithelium after severe injury. Duerr et al13 have previously identified a 7-amino acid sequence protein, TMT, which also internalizes into healthy gut mucosa. The ability to target injured mucosa after serious injury has important clinical implications, because injured mucosa likely results in differential ability to recognize various amino acid sequences. We have not only demonstrated recovery of our T18 peptide sequence by PCR, but also confirmed its internalization by performing DNA sequencing of the mucosal extracts. The DNA sequencing confirmed that our T18 peptide was internalized into the mucosa and extracted for PCR analysis, and serves as a powerful confirmation of our analysis. We also demonstrated that we are able to bind a reporter to our T18 peptide sequence. This is important because the ultimate goal of this peptide sequence would be to deliver a payload, either a drug or growth factor, into the intestinal epithelial cell.
Fluorescent nanoparticles known as quantum dots (Qdots) were bound to the T18 sequence in order to image the localization of the peptide within the gut. These Qdot conjugates allowed us to visually track the location of the T18 sequence using a confocal microscope. We were able to clearly visualize the Qdot-T18 conjugate within the gut mucosa following burn injury. Interestingly, we noted that the Qdot-T18 conjugate co-localized with an antibody specific for lymphatic endothelium (LYVE-1). These images seem to indicate that the T18 sequence may have affinity for the intestinal lymphatics; however, further studies are needed to fully elucidate the characteristics of this gut targeting peptide.
Burn-induced gut injury has been linked to intestinal barrier injury and may be the source of SIRS and distant organ injury. While intestinal injury is common to many common medical conditions, our ability to directly target the gut with therapeutics is limited. Ideally, drugs or growth factors could be targeted directly to the intestinal mucosa, where these therapies could be delivered to specific cell types at the site of injury. In the present study, using a burn insult as a model of severe injury, we identified a 12-amino-acid peptide sequence that binds and internalizes into the intestinal mucosa. This sequence may allow for targeted therapies designed to attenuate intestinal dysfunction following severe injury, inflammation, or other pathologic conditions of the small bowel frequently seen in clinical practice such as inflammatory bowel disease and necrotizing enterocolitis.
Footnotes
Presented as an SUS oral presentation at the 4th Annual Academic Surgical Congress, Fort Myers, Florida, February 4–6, 2009.
References
- 1.Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg. 1999;178:449–53. doi: 10.1016/s0002-9610(99)00231-7. [DOI] [PubMed] [Google Scholar]
- 2.Costantini TW, Loomis H, Putnam JG, Drusinsky D, Deree J, Choi S, et al. Burn-induced gut barrier injury is attenuated by phosphodiesterase inhibition: Effects on tight junction structural proteins. Shock. 2009;31:416–22. doi: 10.1097/SHK.0b013e3181863080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarz BT, Wang F, Shen L, Clayburgh D, Su L, Wang Y, et al. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 2007;132:2383–94. doi: 10.1053/j.gastro.2007.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, et al. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol. 2006;291:G938–49. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- 5.Deitch EA. Bacterial translocation or lymphatic drainage of toxic products from the gut: what is important in human beings? Surgery. 2002;131:241–4. doi: 10.1067/msy.2002.116408. [DOI] [PubMed] [Google Scholar]
- 6.Deree J, de Campos T, Shenvi E, Loomis WH, Hoyt DB, Coimbra R. Hypertonic saline and pentoxifylline attenuates gut injury after hemorrhagic shock: the kinder, gentler resuscitation. J Trauma. 2007;62:818–28. doi: 10.1097/TA.0b013e31802d9745. [DOI] [PubMed] [Google Scholar]
- 7.Diebel LN, Liberati DM, Ledgerwood AM, Lucas CE. Systemic not just mesenteric lymph causes acute lung injury following hemorrhagic shock. Surgery. 2008;144:686–93. doi: 10.1016/j.surg.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez RJ, Moore EE, Ciesla DJ, Meng X, Biffl WL, Silliman CC. Post-hemorrhagic shock mesenteric lymph lipids prime neutrophils for enhanced cytotoxicity via phospholipase A2. Shock. 2001;16:218–22. doi: 10.1097/00024382-200116030-00008. [DOI] [PubMed] [Google Scholar]
- 9.Barry MA, Dower WJ, Johnston SA. Toward cell-targeting gene therapy vectors: selection of cell-binding peptides from random peptide-presenting phage libraries. Nat Med. 1996;2:299–305. doi: 10.1038/nm0396-299. [DOI] [PubMed] [Google Scholar]
- 10.Ludtke JJ, Sololoff AV, Wong SC, Zhang G, Wolff JA. In vivo selection and validation of liver-specific ligands using a new T7 phage peptide display system. Drug Deliv. 2007;14:357–69. doi: 10.1080/10717540601098765. [DOI] [PubMed] [Google Scholar]
- 11.Kolonin MG, Sun J, Do KA, Vidal CI, Ji Y, Baggerly KA, et al. Synchronous selection of homing peptides for multiple tissues by in vivo phage display. FASEB J. 2006;20:979–81. doi: 10.1096/fj.05-5186fje. [DOI] [PubMed] [Google Scholar]
- 12.Larocca D, Kassner PD, Witte A, Ladner RC, Pierce GF, Baird A. Gene transfer to mammalian cells using genetically targeted filamentous bacteriophage. FASEB J. 1999;13:727–34. doi: 10.1096/fasebj.13.6.727. [DOI] [PubMed] [Google Scholar]
- 13.Duerr DM, White SJ, Schluesener HJ. Identification of peptide sequences that induce the transport of phage across the gastrointestinal mucosal barrier. J Virol Methods. 2004;116:177–80. doi: 10.1016/j.jviromet.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Cai W, Chen X. Preparation of peptide-conjugated quantum dots for tumor vasculature-targeted imaging. Nat Protoc. 2008;3:89–96. doi: 10.1038/nprot.2007.478. [DOI] [PubMed] [Google Scholar]
- 15.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–9. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179:4808–20. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 17.Costantini TW, Loomis WH, Putnam JG, Kroll L, Eliceiri BP, Baird A, et al. Pentoxifylline modulates intestinal tight junction signaling after burn injury: effects on myosin light chain kinase. J Trauma. 2009;66:17–24. doi: 10.1097/TA.0b013e318191bb1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzon E, Cuzzocrea S. Absence of functional peroxisome proliferator-activated receptor-alpha enhanced ileum permeability during experimental colitis. Shock. 2007;28:192–201. doi: 10.1097/SHK.0b013e318033eb29. [DOI] [PubMed] [Google Scholar]
- 19.Larocca D, Baird A. Receptor-mediated gene transfer by phage-display vectors: applications in functional genomics and gene therapy. Drug Discov Today. 2001;6:793–801. doi: 10.1016/s1359-6446(01)01837-2. [DOI] [PubMed] [Google Scholar]
- 20.Burg MA, Jensen-Pergakes K, Gonzalez AM, Ravey P, Baird A, Larocca D. Enhanced phagemid particle gene transfer in camptothecin-treated carcinoma cells. Cancer Res. 2002;62:977–81. [PubMed] [Google Scholar]
- 21.Lee TY, Lin CT, Kuo SY, Chang DK, Wu HC. Peptide-mediated targeting to tumor blood vessels of lung cancer for drug delivery. Cancer Res. 2007;67:10958–65. doi: 10.1158/0008-5472.CAN-07-2233. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura S, Takahashi S, Kamikatahira H, Kuroki Y, Jaalouk DE, O’Brien S. Combinatorial targeting of the macropinocytotic pathway in leukemia and lymphoma cells. J Biol Chem. 2008;283:11752–62. doi: 10.1074/jbc.M708849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johanson CE, Duncan JA, Stopa EG, Baird A. Enhanced prospects for drug delivery and brain targeting by the choroid plexus-CSF route. Pharm Res. 2005;22:1011–37. doi: 10.1007/s11095-005-6039-0. [DOI] [PubMed] [Google Scholar]
- 24.Takagi T, Arisawa T, Yamamoto K, Hirata I, Nakano H, Sawada M. Identification of ligands binding specifically to inflammatory intestinal mucosa using phage display. Clin Exp Pharmacol Physiol. 2007;34:286–9. doi: 10.1111/j.1440-1681.2007.04563.x. [DOI] [PubMed] [Google Scholar]
- 25.Costantini TW, Peterson CY, Kroll LM, Loomis WH, Putnam JG, Eliceiri BP, et al. Role of p38 MAPK signaling in burn-induced intestinal barrier breakdown. J Surg Res. 2009 doi: 10.1016/j.jss.2009.03.066. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]