INTRODUCTION
Oxytocin (OT)-containing neurons originating in supraoptic (SON) and paraventricular (PVN) nuclei of the hypothalamus were for years thought to be supplied exclusively to the posterior lobe of the pituitary gland and concerned primarily with milk letdown, parturition, and osmoregulation. Recently, new OT-containing fiber systems have been identified in the median eminence1–4 and have been implicated in the control of prolactin (PRL) and adrenocorticotropin (ACTH) secretion from the anterior lobe of the pituitary gland.5–12 This paper summarizes experimental findings that evaluate the hypophysiotropic function of median eminence OT by characterizing neuropeptide secretion into the blood of pituitary portal vessels under physiological conditions when the secretion of PRL is known to be altered.
OXYTOCIN ACTION ON PROLACTIN SECRETION FROM THE ANTERIOR PITUITARY GLAND
Exogenous OT administration is known to stimulate PRL secretion both in vivo and in vitro,5–9 Oxytocin receptors have been identified in the anterior pituitary gland. Two pharmacologically distinct types of OT receptors have been characterized in this gland. One, resembling uterine-type OT receptors in its pharmacological specificity, has been proposed to mediate the OT action on PRL secretion.13 The other apparently binds with vasopressin and mediates OT action of ACTH release.14 Estrogen, which is a potent PRL stimulator,15–17 has been shown to modulate uterine-type OT receptors in the anterior pituitary.13 The intracellular mechanism of OT action on PRL release has not been characterized.
OXYTOCINERGIC NEURONAL PROJECTION TO THE MEDIAN EMINENCE: SOURCE OF THE PITUITARY PORTAL PLASMA OXYTOCIN
Oxytocinergic nerve fibers have been identified both in the external and internal layers of the median eminence; however, the majority of the fibers is found in the internal layer of the median eminence.1–4,18–20 The neurites in the external zone are axon terminals that originate in the parvocellular cell bodies in the PVN. These oxytocinergic terminals are localized in the immediate vicinity of primary capillary plexus of the pituitary portal vascular system and appear to be a major source of the portal OT. The fibers in the internal layer of the median eminence are preterminal axons of magnocellular neurons from the PVN and SON in passage to the posterior pituitary gland. Recently published observations suggest that a proportion of portal OT may also be derived from these preterminal magnocellular axons in the internal layer of the median eminence.21,22
CONCENTRATION OF OXYTOCIN IN PITUITARY PORTAL PLASMA: METHODOLOGICAL CONSIDERATION
A number of experimental paradigms are available for studying the hypothalamic regulation of PRL secretion from the lactotropes in the anterior pituitary gland. In view of the now-apparent multifactorial control of PRL release,23,24 measurement of hypothalamic hormones in pituitary portal blood appeared to be best suited for unraveling the complexities of neural regulation of PRL secretion. The major limitation of this approach in rats is that it requires acute anesthesia and surgical trauma. However, comparing pituitary portal plasma concentrations of hypothalamic hormones in rats under acute preparation with anesthesia with those of conscious sheep prepared with chronic cannulae, showed both preparations to have similar qualitative characteristics of the central regulatory process.25–32 Thus, the measurement of hypothalamic hormone secretion using the portal preparation approach in rats appeared to provide physiologically relevant information on the central regulatory processes.
The choice of anesthetic has been shown to have impact on the hypothalamic hormone release into the blood of pituitary portal vessels. Alphaxalone (Saffan; Glaxo) anesthesia appeared to be best suited for studying the majority of neurohormone secretions.31,33–38 This anesthetic had very little effect on the elevated secretion of anterior pituitary luteinizing hormone (LH) and PRL before ovulation in rats.33,35 Under this anesthetic, a surge release of LH-releasing hormone (LHRH) into portal blood was observed before preovulatory gonadotropin release in female rats.33–34 In contrast, sodium pentobarbitone and urethane anesthesia blocked both gonadotropin and LHRH surges.34 The level of thyrotropic-releasing hormone in pituitary portal plasma was higher under saffan than under either urethane or sodium pentobarbitone.39 Urethane anesthesia appeared to abolish the circadian rhythm of ACTH, but not of corticosterone.40 The level of somatostatin-14 in pituitary portal plasma was higher under urethane than under sodium pentobarbitone or Saffan.41
Our investigation on pituitary portal plasma concentrations of OT began with the determination of the anesthesia effect on the basal secretion of median eminence OT in ovariectomized rats. We used the transpharengeal approach to expose the pituitary stalk31,42 and then collected pituitary portal plasma samples from these rats hourly for a period of two hours. The basal secretion of OT into the blood of pituitary portal vessels was similar in animals anesthetized with Saffan, urethane, or sodium pentobarbitone (Table 1). This study also revealed that OT is present in rat pituitary portal plasma in amounts much greater than those in peripheral plasma of ovariectomized rats. Using the transpharengeal or parapharengeal approach, several groups of investigators have also obtained 20- to 50-fold higher concentrations of OT in pituitary portal plasma than in peripheral plasma in male and female rats.22,40,43–45 It is unlikely that a significant amount of OT in pituitary portal plasma is due to backflow from the posterior pituitary gland, since the level of pituitary portal OT did not change following removal of the pituitary gland (Table 1). It has been shown that a significant amount of OT is present in the cerebrospinal fluid (CSF) and in tanycytes lining the floor of the third ventricle.46–48 However, OT levels in the CSF are too low to significantly contribute to the relatively high OT levels in pituitary portal blood. Horn et al. have recently presented evidence that OT from the cut axons of the supraoptico-hypophysial tract does not significantly contribute to the high neuropeptide level in pituitary portal plasma following sectioning of the pituitary stalk.44 Thus, the OT concentration in pituitary portal blood reflects the secretory activity of OT neurons in the median eminence.
TABLE 1.
Effect of Anesthetics and Hypophysectomy on Mean (± SEM) Oxytocin (OT) Concentration in Pituitary Portal Plasma and Peripheral Plasma of Ovariectomized Female Ratsa
| Treatment | Portal OT (ng/ml) |
Peripheral OT (ng/ml) |
|---|---|---|
| Saffan (Pituitary intact) | 1.37 ± 0.32 | 0.04 ± 0.01b |
| Saffan (Pituitary removed) | 1.17 ± 0.27 | — |
| Urethane (Pituitary removed) | 1.41 ± 0.32 | — |
| Sodium pentobarbital (Pituitary removed) | 1.48 ± 0.46 | — |
n = 4–6.
p < 0.001
CHANGES OF OXYTOCIN CONCENTRATION IN THE PITUITARY PORTAL PLASMA
Oxytocin Release during Reproductive Cycle
Gonadal steroids have long been known to influence OT levels in the hypothalamus and pituitary gland.49–57 Furthermore, recent studies have provided evidence of a gonadal steroid modulatory role on OT synthesis in the hypothalamus and of gonadal steroid induction of OT receptors in the anterior pituitary (see chapters by Brooks, Johnson, Pfaff et al., and Schumacher et al.).13 An important consideration is whether or not the OT secretory activity in the median eminence alters with the dynamic changes of the plasma level of gonadal steroids during the reproductive cycle. To address this issue, we determined the concentration of OT in pituitary portal plasma during the estrous cycle in female rats under Saffan anesthesia. On each afternoon of the four-day estrous cycle, animals were anesthetized with Saffan, pituitary stalk was exposed, pituitary was removed, and pituitary portal blood samples were collected at one-hour intervals. The OT concentration in pituitary portal plasma collected between 1400 and 1600 hr of proestrus (1079 ± 185 pg/ml; n = 12) was significantly higher than that of any other time studied.58 The mean OT concentration between 1600 and 1800 hr of proestrus was 494 ± 107 pg/ml (n = 10). There were no appreciable differences between the mean OT levels in portal blood obtained on the afternoon (1500–1700 hr) of estrus (198 ± 29 pg/ml; n = 6), diestrus-1 (283 ± 55 pg/ml; n = 5), or diestrus-2 (266 ± 49 pg/ml; n = 6). The OT concentration in peripheral plasma was low (58 ± 3 pg/ml; n = 4) throughout the cycle. Comparison of the pituitary portal plasma OT with peripheral plasma PRL in Saffan-anesthetized rats58 indicated a positive correlation (r = 0.828; p < 0.05) between these hormones during the estrous cycle.
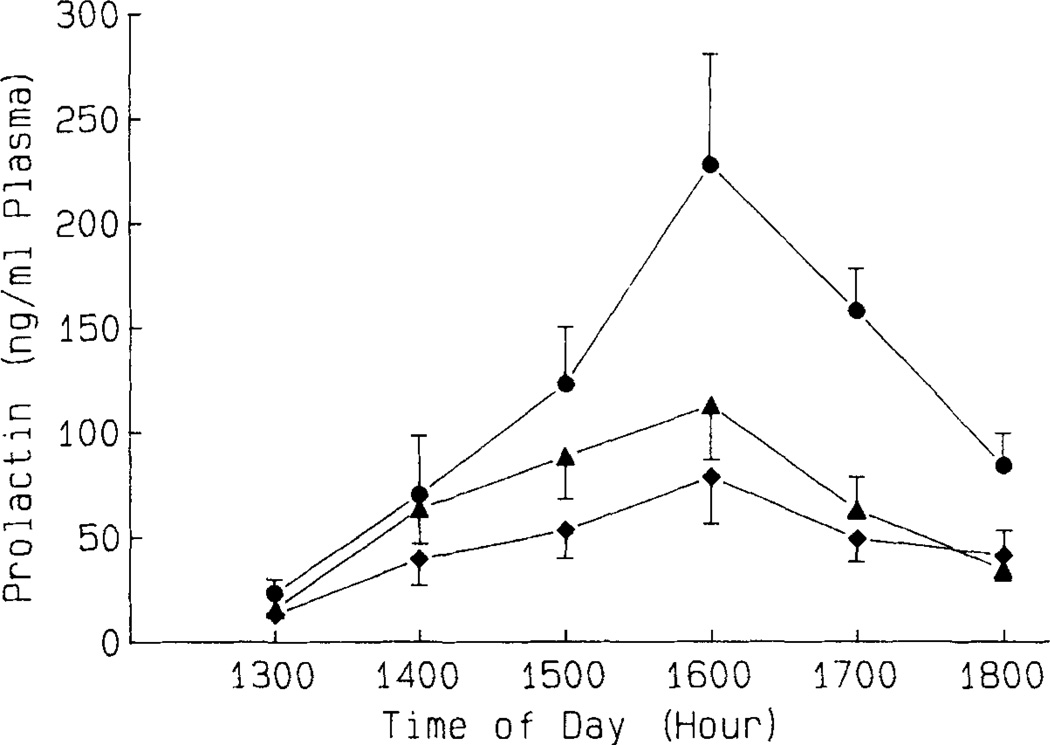
The elevated median eminence OT secretion into the blood of pituitary portal vessels immediately before the PRL surge during the afternoon of proestrus suggests a possible involvement of OT in preovulatory PRL release. This possibility is experimentally verified by studying the effect of immunoneutralization of endogenous OT with anti-OT serum in cyclic female rats (see also below).59 A specific antiserum for OT was administered in female rats on the early afternoon (1330–1400 hr) of proestrus, and pituitary portal plasma samples were collected at one-hour intervals between 1400 and 1700 hr. Portal plasma samples were assayed for OT by radioimmunoassay after preincubation with S. aureus cells to adsorb IgG. This was followed by removal of this S. aureus cell-attached IgG from the pituitary portal samples using ODS silica disposable extraction columns. The OT level in pituitary portal plasma following OT antiserum treatment was very low (56–85 pg/ml) and was 15- to 20-fold lower than those after the treatment with normal rabbit serum (NRS). It appeared, therefore, that the antiserum effectively neutralized the endogenous levels of OT in pituitary portal plasma during the afternoon of proestrus. Animals treated with OT antiserum showed attenuated elevation in systemic plasma PRL concentrations between 1500 and 1800 hr when compared to those in NRS-treated controls (Fig. 1). These data are consistent with the findings that passive immunoneutralization of endogenous OT significantly reduced, but did not completely abolish, the estrogen-induced PRL surge in ovariectomized rats.9
FIGURE 1.
Effect of an i.v. injection of normal rabbit serum (NRS; 0.25 ml; ●—●), NRS (0.15 ml) + anti-OT serum (AOTS; 0.01 ml; ▲ — ▲) or AOTS (0.25 ml; ◆ — ◆) on plasma PRL during the afternoon of proestrus in cyclic female rats. Values are mean ± SEM of five to seven rats. (Reprinted from the original manuscript [Sarkar59] with the permission of the S. Karger AG.)
These results also agree with the finding that OT antagonist analogues prevented the preovulatory PRL surge in cyclic female rats.60 Together, these data provide strong evidence for a physiological role of hypothalamic OT in the cyclic PRL secretion. However, it should be noted that passive immunoneutralization of endogenous OT failed to completely abolish PRL secretion. It is therefore possible that a multiplicity of factors is responsible for the integrated control of preovulatory PRL release. For example, a reduction of dopamine inhibition of PRL release may play a role, in part, in the rise in PRL secretion during proestrus.61,62 In addition to dopamine withdrawal, an increased secretion of hypothalamic vasoactive intestinal peptide (VIP) by elevated estrogen may also contribute to the preovulatory increase in PRL release.63 Therefore, OT, dopamine (DA), and VIP appeared to be the major factors involved in multifactorial regulation of PRL release at the anterior pituitary level during the reproductive cycle.
Oxytocin Release during Lactation
The relative importance of the multiple releasing and inhibiting factors that modulate PRL secretion appears to vary according to the animal’s reproductive status.23,24 Although it has been postulated that a putative releasing factor from the posterior pituitary may be essential for regulation of PRL secretion during lactation,64–68 hypothalamic PRL-releasing factors (PRFs) have also been implicated in modulation of suckling-induced PRL release.9,63 Since hypothalamic OT appeared to be a potential candidate of PRFs involved in suckling-induced PRL release, the OT level changes in pituitary portal plasma during lactation were determined. Portal plasma concentrations of VIP were also measured in order to compare the relative contributions of VIP and OT in the regulation of PRL release during lactation. A physiological role for hypothalamic VIP in suckling-induced PRL release has been proposed.63
Pituitary portal plasma samples were obtained from 5 to 8-day postpartum lactating rats under urethane anesthesia. This anesthetic was chosen because, unlike Saffan and sodium pentobarbital anesthesia, urethane does not block suckling-induced PRL release (unpublished observation). Ten milliliters of 0.9% saline was injected before urethane anesthesia, since this treatment is known to prevent dehydration associated with urethane. The experiment was begun three hours after induction of anesthesia. All but one pup were removed from lactating mother rats six to twelve hours before experiment. In one set of rats, atrial catheters were implanted, and blood samples were collected at 15-min intervals for three hours. Six pups were reintroduced to the mothers 45 min after the beginning of blood sampling, and the pups were allowed to suckle for 135 min. Suckling was confirmed by increased pup weight. Control mothers did not receive pups during the blood collection period. The plasma samples were measured for PRL.
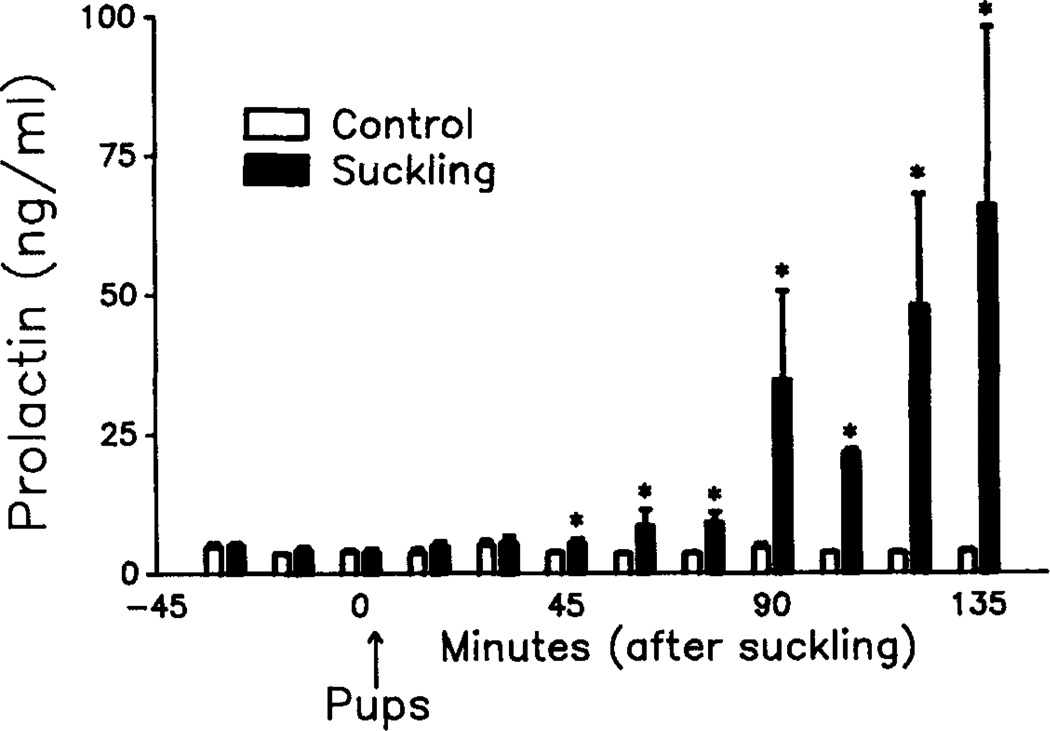
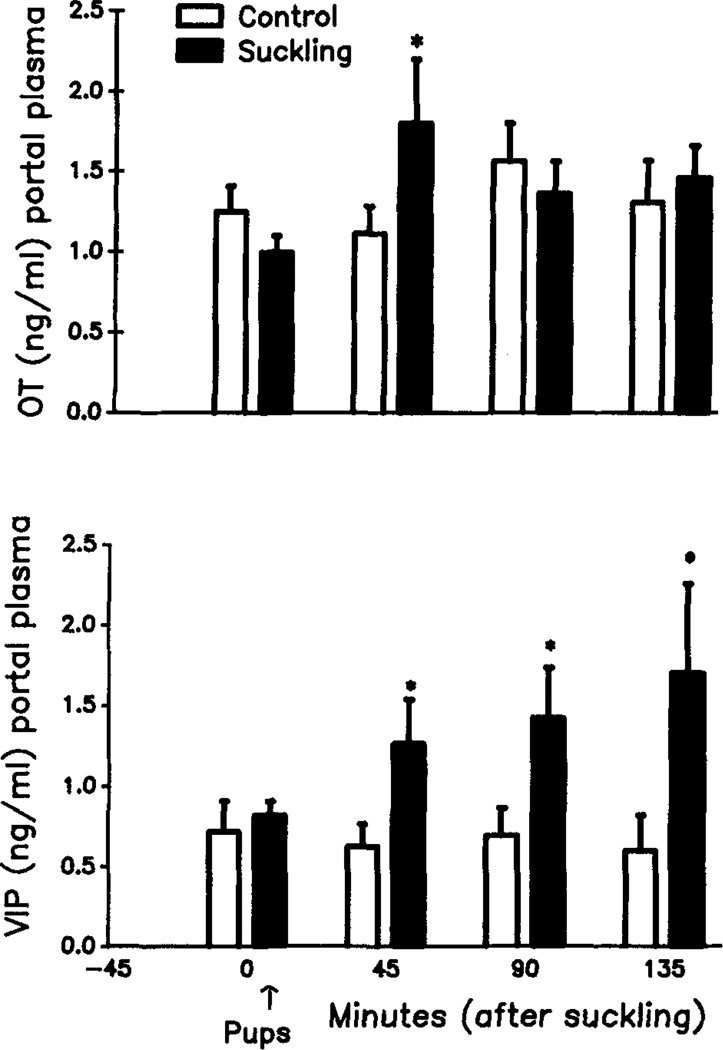
In another set of animals, the pituitary stalk was surgically exposed, animals hypophysectomized, and pituitary portal plasma samples were collected at 45-min intervals. After the initial control sample, the response to suckling or control was examined, and samples were measured for OT and VIP. At the end of systemic or pituitary portal blood collection, suckled and control mothers were sacrificed, and each animal’s mediobasal hypothalamus (MBH) was removed and measured for OT and VIP. Results indicated that PRL level rose significantly at 45 min after initiation of suckling (Fig. 2). Thereafter, levels continued to rise throughout the collection period and reached the peak at 135 min after pup introduction. However, in control animals, levels of PRL remained low throughout the collection period. Portal OT concentration was significantly increased during 0–45 min of suckling (Fig. 3), but the OT level did not change significantly after this period. The VIP level in pituitary portal plasma, however, gradually increased throughout the suckling period.
FIGURE 2.
Effect of suckling on PRL secretion in urethane (1 g/kg) anesthetized lactating rats. Values are the mean ± SEM of five to six rats. *, p < 0.05.
FIGURE 3.
Effect of suckling on the concentrations of pituitary portal plasma OT (upper panel) and VIP (lower panel) in lactating rats under urethane (1 g/kg) anesthesia. Values are the mean ± SEM of eight to ten rats. *, p < 0.05.
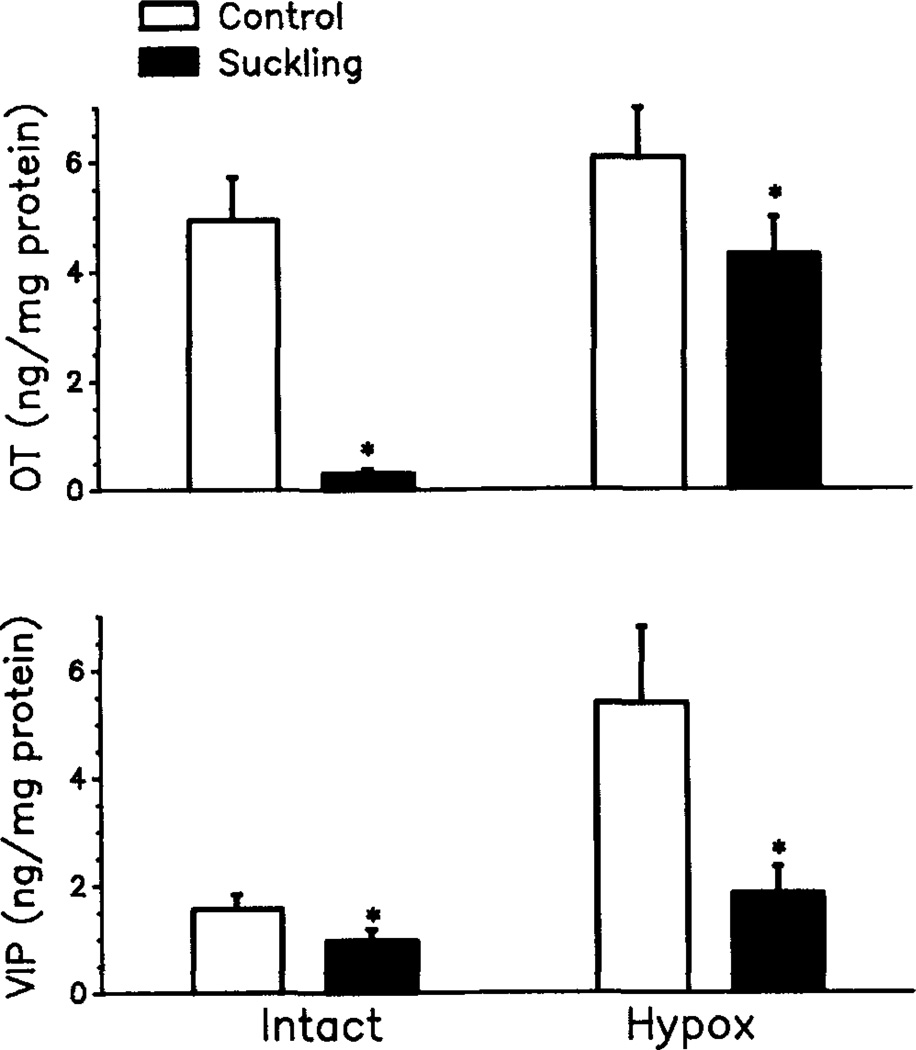
There was also a positive correlation between pituitary portal plasma VIP and systemic plasma PRL during suckling. OT and VIP concentrations in the MBH also responded to suckling. Hypothalamic OT decreased markedly in response to suckling in animals with intact pituitaries and decreased moderately in response to suckling in animals without the in situ pituitaries, (Fig. 4). Hypothalamic VIP also reduced in response to suckling in both pituitary intact and hypophysectomized mothers. Interestingly, however, the MBH levels of VIP in hypophysectomized suckled and nonsuckled mothers were significantly higher than those in suckled and nonsuckled mothers with in situ pituitaries. The positive correlation between VIP and PRL secretion during suckling and depletion of MBH VIP in the suckled mothers suggests that hypothalamic VIP can be a significant PRF during suckling-induced PRL release. This concept is further supported by the observation that passive immunoneutralization of VIP, with a specific antiserum for VIP, significantly blunted the suckling-induced PRL release.63 It should be noted that MBH concentrations of VIP in both suckled and nonsuckled mothers were increased after removal of the in situ pituitaries, suggesting that an inhibitory pituitary factor is involved in the control of VIP secretion from the hypothalamus. These findings are in agreement with our recent data demonstrating the existence of a negative short-loop feedback action of PRL on hypothalamic VIP release (see below).69
FIGURE 4.
Mean ± SEM concentrations of OT (upper panel) and VIP (lower panel) in the mediobasal hypothalamus following suckling for 135 min in urethane-anesthetized lactating rats with intact in situ pituitary (Intact) or with pituitary removed (Hypox). Control intact rats and hypox rats were anesthetized with urethane and were deprived with pups: n = 5–10. *, p < 0.05.
The brief increase in pituitary portal plasma OT levels, which also coincide with the initial rise of PRL, along with the decrease in MBH OT concentrations support the hypothesis that OT may modulate PRL secretion during lactation. Recently, Samson et al. have shown that infusion of OT antiserum into lactating female rats before pup reinstatement reduced subsequent PRL surges.9 These data are in agreement with the above view. However, this hypothesis should be viewed with caution because surgical removal of pituitary neurointermediate lobes (which would contain OT neuronal terminals) reduces the magnitude of the suckling-induced PRL surge66 and because the possibility remains that the effect of OT antiserum on PRL release is the consequence of its effect on intermediate lobe OT.
It is noteworthy that the MBH concentration of OT responded less to the suckling stimulus in the hypophysectomized rats than in the rat with in situ pituitaries. This is perhaps indicative that a positive-feedback action of PRL (see below)69 may also be involved in the interaction between MBH OT and pituitary PRL during lactation.
Oxytocin Release during Hyperprolactinemia
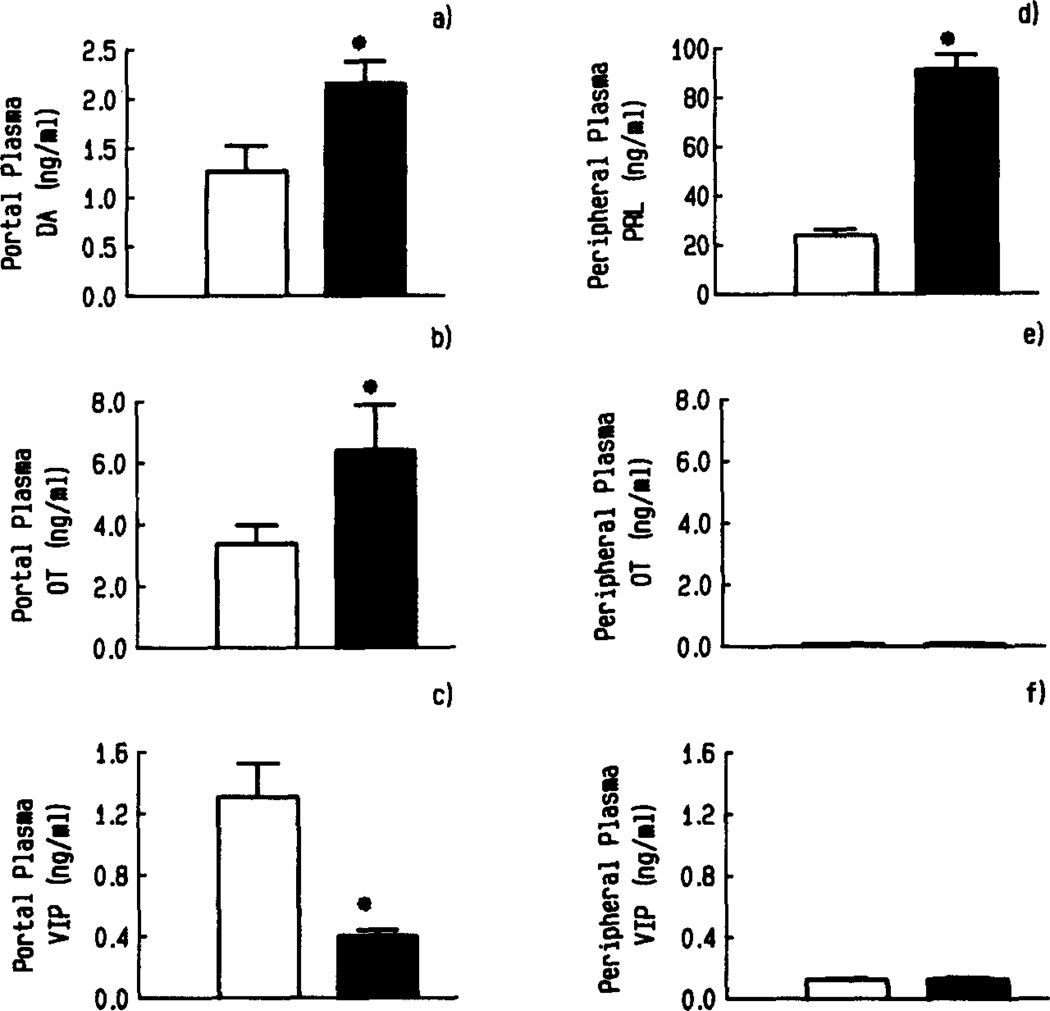
As noted above, there is a possibility of a bidirectional interaction between MBH OT and pituitary PRL in the regulation of PRL secretion. In order to further explore this possibility, we have determined the effect of hyperprolactinemia on OT secretion into the blood of pituitary portal vessels. Hyperprolactinemia was induced in ovariectomized rats by transplanting four anterior pituitaries to the kidney capsule for two to three weeks. Pituitary transplants induced hyperprolactinemia in these rats, since pituitary-transplanted rats showed elevated plasma PRL levels (3.8-fold) and decreased in situ pituitary weight (0.62-fold) and PRL concentrations (0.63-fold) as compared to the sham-transplanted rats.69 Hyperprolactinemic rats showed a significant increase in the OT level in pituitary portal plasma (Fig. 5), indicating a stimulatory action of PRL on OT release from the hypothalamus. This stimulatory action of PRL on OT appeared to be unique and was not evident on the other hypothalamic regulators of PRL (dopamine and VIP). Hyperprolactinemic rats showed reduced DA and VIP levels in pituitary portal plasma, as compared to controls (Fig. 5). The observed stimulatory action of PRL on pituitary portal OT is consistent with our findings that PRL acutely increased OT release from the fetal hypothalamic neurons in primary cultures.69 These results provide correlative evidence for the involvement of multiple mediators in the short-loop feedback action of PRL on PRL release. The increase in the secretion of OT following hyperprolactinemia is somewhat surprising in view of the PRL-releasing activity of OT (see above).
FIGURE 5.
Effect of hyperprolactinemia on the concentration of DA (a), OT (b), and VIP (c), in pituitary portal plasma and on the level of PRL (d), OT (e), and VIP (f) in peripheral plasma of ovariectomized rats. Hyperprolactinemia was induced by transplanting four anterior pituitaries (APs) under the kidney capsule (■). Control rats were sham AP-transplanted (□). n = 7–10; *, p < 0.05, compared to control. (Reprinted from the original manuscript [Sarkar69] with the permission of the S. Karger AG.)
A positive-feedback system for OT has been demonstrated during parturition where cervical stretch by the fetus provides a feedforward system for further OT release to stimulate additional uterine contraction.70 Hence, it may be possible that PRL secretion represents the operation of yet another feedforward system for OT secretion in order to further release PRL during lactation. Such a mechanism would not only facilitate OT-induced milk let-down, but would stimulate further PRL secretion to increase milk synthesis in preparation for the next feeding. The stimulatory influence of PRL on OT secretion may also explain the increased, possibly OT-mediated, maternal behavior during pregnancy and lactation.71,72
SUMMARY
The median eminence receives fibers from both parvocellular and magnocellular OT neurons in the hypothalamus. The OT neuronal terminal in the median eminence is secretory and is the major source of the neuropeptide in the blood of pituitary portal vessels. The OT secretion into pituitary portal plasma increases by ovarian steroids, PRL, and the suckling stimulus. The OT secretion into the blood of pituitary portal vessels changes in parallel with the altered secretion of PRL from the pituitary. Because of correlative association between pituitary portal plasma OT and systemic plasma PRL and much abundant evidence for a direct stimulatory action of OT on PRL release, we propose that the pituitary portal vascular system serves as the window for the central OT neurotransmission to the pituitary lactotropes.
REFERENCES
- 1.Vandesande F, Dierickx K. Identification of the vasopressin producing and of the oxytocin producing neurons in the hypothalamic magnocellular neurosecretory system of the rat. Cell Tissue Res. 1975;164:153–162. doi: 10.1007/BF00218970. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman EA, Nilaver G, Itou-Yi A, Silverman AJ. Vasopressinergic and oxytocinergic pathways in the central nervous system. Fed. Proc. 1984;43:91–96. [PubMed] [Google Scholar]
- 3.Vandersande F, Dierickx K. Immuno-cytochemical demonstration of the inability of the homozygous Brattleboro rat to synthesize vasopressin-associated neurophysin. Cell Tissue Res. 1976;165:307–316. doi: 10.1007/BF00222435. [DOI] [PubMed] [Google Scholar]
- 4.Sawchenko PE, Swanson LW. Relationship of oxytocin pathways to the control of neuroendocrine and autonomic function. In: Amico JA, Robinson AG, editors. Oxytocin. Clinical and Laboratory Studies. International Congress Series 666. Amsterdam: Elsevier; 1985. pp. 87–103. [Google Scholar]
- 5.Forsling ML, Reinhard V, Himmler V. Neurophysial hormones and prolactin release. J. Endocrinol. 1974;63:579–580. doi: 10.1677/joe.0.0630579. [DOI] [PubMed] [Google Scholar]
- 6.Vaughan MH, Blask DE, Johnson LY. Effect of subcutaneous injections of melatonin, arginine vasotocin and related peptides on pituitary and plasma levels of luteinizing hormone, follicle stimulating hormone and prolactin in castrated male rats. Endocrinology. 1979;104:212–217. doi: 10.1210/endo-104-1-212. [DOI] [PubMed] [Google Scholar]
- 7.Salisbury RL, Krieg RJ, Seibel HR. Effects of arginine vasotocin, oxytocin and arginine vasopressin on steroid-induced surges of luteinizing hormone and prolactin in ovariectomized rats. Acta Endocrinol. 1980;94:166–173. doi: 10.1530/acta.0.0940166. [DOI] [PubMed] [Google Scholar]
- 8.Lumpkin MD, Samson WK, Mccann SM. Hypothalamic and pituitary sites of action of oxytocin to alter prolactin secretion in the rat. Endocrinology. 1983;112:1711–1717. doi: 10.1210/endo-112-5-1711. [DOI] [PubMed] [Google Scholar]
- 9.Samson WK, Lumpkin MD, Mccann SM. Evidence for a physiological role for oxytocin in the control of prolactin secretion. Endocrinology. 1986;119:554–560. doi: 10.1210/endo-119-2-554. [DOI] [PubMed] [Google Scholar]
- 10.Burlet A, Chateau M, Czernichow P. Infundibular localization of vasopressin, oxytocin and nuerophysins in the rat: Its relationship with corticotrope function. Brain Res. 1979;168:275–286. doi: 10.1016/0006-8993(79)90169-0. [DOI] [PubMed] [Google Scholar]
- 11.Beny JL, Baertschi AJ. Oxytocin: Major corticotropin-releasing factor secreted from diabetes insipidus rat posterior pituitary in vitro. Neuroendocrinology. 1980;31:261–264. doi: 10.1159/000123085. [DOI] [PubMed] [Google Scholar]
- 12.Gibbs D. Inhibition of corticotropin release during hypothermia: The role of corticotropin releasing factor, vasopressin, and oxytocin. Endocrinology. 1985;116:723–727. doi: 10.1210/endo-116-2-723. [DOI] [PubMed] [Google Scholar]
- 13.Antoni FA. Oxytocin receptors in rat adenohypophysis: Evidence from radioligand binding studies. Endocrinology. 1986;119:2393–2395. doi: 10.1210/endo-119-5-2393. [DOI] [PubMed] [Google Scholar]
- 14.Antoni FA. Receptors mediating CRH effects of vasopressin and oxytocin. Ann. N.Y. Acad. Sci. 1988;512:195–204. doi: 10.1111/j.1749-6632.1987.tb24961.x. [DOI] [PubMed] [Google Scholar]
- 15.Maurer RA. Estradiol regulates the transcription of prolactin gene. J. Biol. Chem. 1982;257:2133–2142. [PubMed] [Google Scholar]
- 16.Seo H. The Pituitary Gland. New York: Raven Press; 1982. Growth hormone and prolactin: Chemistry, gene organization, biosynthesis and regulation of gene expression; pp. 57–82. [Google Scholar]
- 17.Gorski J, Shull J, Durrin J. Estrogen regulation of prolactin gene transcription and chromatin structure. In: MacLeod RM, Scapagnini U, Thorner MO, editors. Prolactin Basic and Clinical Correlates. New York: Springer-Verlag; 1985. pp. 259–269. [Google Scholar]
- 18.Ajika K, Arai K, Okinaga S. Electronmicroscopic localization of vasopressin and oxytocin in the median eminence of normal and hypophysectomized rats. Neuroscience. 1987;22(suppl):S138. [Google Scholar]
- 19.Verbalis JG, Baldwin EF, Ronnekliev OK, Robinson AG. In vitro release of vasopressin and oxytocin from rat median eminence tissue. Neuroendocrinology. 42:481–488. doi: 10.1159/000124491. 19886. [DOI] [PubMed] [Google Scholar]
- 20.Antoni FA, Kovacks KJ, Dohanits J, Makara GB, Holmes MC, Mazurek MF. Hypophysiotrophic function of vasopressin and oxytocin. Brain Res. Bull. 1988;20:729–736. doi: 10.1016/0361-9230(88)90084-6. [DOI] [PubMed] [Google Scholar]
- 21.Buma P, Nieuwenhuys R. Ultrastructural demonstration of oxytocin and vasopressin release sites in the neural lobe and median eminence of the rat by tannic acid and immunogold methods. Neurosci. Lett. 1987;74:151–157. doi: 10.1016/0304-3940(87)90141-8. [DOI] [PubMed] [Google Scholar]
- 22.Antoni FA, Fink G, Sheward WJ. Corticotropin-releasing peptides in rat hypophysial portal blood after paraventricular lesions: A marked reduction in the concentration of corticotropin-releasing factor-41, but no change in vasopressin. J. Endocrinol. 1989;125:175–183. doi: 10.1677/joe.0.1250175. [DOI] [PubMed] [Google Scholar]
- 23.Kato Y, Iwasaki Y, Iwasaki J, Abe H, Yanaihara N, Imura H. Regulation of prolactin secretion. In: Imura H, editor. The Pituitary Gland. New York: Raven Press; 1985. pp. 261–278. [Google Scholar]
- 24.Samson WK, Mogg RJ. Peptidergic control of prolactin secretion. Biomed. Res. 1990;10(S3):107–116. [Google Scholar]
- 25.Clark IJ, Cummins JT. The temporal relationship between GnRH and LH secretion in ovariectomized ewe. Endocrinology. 1982;111:1737–1739. doi: 10.1210/endo-111-5-1737. [DOI] [PubMed] [Google Scholar]
- 26.Sarkar DK. Does LHRH meet the criteria for a hypothalamic releasing factor? Psychoneuroendocrinology. 1983;8:259–275. doi: 10.1016/0306-4530(83)90002-1. [DOI] [PubMed] [Google Scholar]
- 27.Fink G, Aiyer M, Chiappa S, Handerson S, Jamieson M, Levy-Perez V, Pickering A, Sarkar D, Sherwood N, Speight A, Watts A. Gonadotropin-releasing hormone release into the hypophyseal portal blood and mechanism of action. In: McKerns K, Pantic V, editors. Hormonally Active Brain Peptides, Structure and Function. New York: Plenum Press; 1983. pp. 397–426. [Google Scholar]
- 28.Caraty A, Grino M, Locatelli A, Oliver C. Secretion of corticotropin releasing factor (CRF) and vasopressin (AVP) into the hypophysial portal blood of conscious, unstrained rams. Biochem. Biophys. Res. Commun. 1988;155:841–849. doi: 10.1016/s0006-291x(88)80572-2. [DOI] [PubMed] [Google Scholar]
- 29.Caraty A, Locatelli A, Martin GB. Biphasic response in the secretion of gonadotropin-releasing hormone in ovariectomized ewes injected with estradiol. J. Endocrinol. 1989;123:375–381. doi: 10.1677/joe.0.1230375. [DOI] [PubMed] [Google Scholar]
- 30.Moenter SM, Caraty A, Karsch FJ. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology. 1990;127:1375–1384. doi: 10.1210/endo-127-3-1375. [DOI] [PubMed] [Google Scholar]
- 31.Sarkar DK, Minami S. Pituitary portal blood collection in rats: A powerful technique for studying hypothalamic hormone secretion. In: Greenstein B, editor. Neuroendocrine Research Methods. London: Harwood Academic Publishers GMBH; 1991. pp. 235–248. [Google Scholar]
- 32.Plotsky PM. Pathways to the secretion of adrenocorticotropin: A view from the portal. J. Neuroendocrinol. 1991;3:1–9. doi: 10.1111/j.1365-2826.1991.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 33.Sarkar DK, Chiappa SA, Fink G, Sherwood NM. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature. 1976;264:461–463. doi: 10.1038/264461a0. [DOI] [PubMed] [Google Scholar]
- 34.Sherwood NM, Chiappa SA, Sarkar DK, Fink G. Gonadotropin-releasing hormone (GnRH) in pituitary stalk blood from proestrous rats: Effects of anesthetics and relationship between stored and released GnRH and luteinizing hormone. Endocrinology. 1980;107:1410–1417. doi: 10.1210/endo-107-5-1410. [DOI] [PubMed] [Google Scholar]
- 35.Clarke WP, Gala RR. The influence of anesthetics on the estrogen-induced afternoon prolactin surge. Althesin does not block the surge. Life Sci. 1981;29:277–284. doi: 10.1016/0024-3205(81)90244-7. [DOI] [PubMed] [Google Scholar]
- 36.Sarkar DK, Gottschall PE, Meites J. Damage to hypothalamic dopaminergic neurons is associated with development of prolactin-secreting pituitary tumors. Science. 1982;218:684–686. doi: 10.1126/science.7134966. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar DK, Yen SSC. Changes in β-endorphin-like immunoreactivity in pituitary portal blood during the estrous cycle and after ovariectomy in rats. Endocrinology. 1985;116:2075–2079. doi: 10.1210/endo-116-5-2075. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar DK, Mrrsugi N, Frautschy S. Effects of long-term estradiol treatment on hypothalamic secretion of oxytocin and vasoactive intestinal peptide into pituitary portal blood of ovariectomized rats. Society for Gynecologic Investigation, 35th Annual Meeting, Abstr. 1988:173. [Google Scholar]
- 39.Fink G, Koch Y, Ben Aroya N. Release of thyrotropin releasing hormone into hypophysial portal blood is high relative to other neuropeptides and may be related to prolactin secretion. Brain Res. 1982;243:186–189. doi: 10.1016/0006-8993(82)91137-4. [DOI] [PubMed] [Google Scholar]
- 40.Fink G, Robinson ICAF, Tannahill LA. Effects of adrenalectomy and glucocorticoids on the peptides CRF-41, AVP and oxytocin in rat hypophysial portal blood. J. Physiol. 1988;401:329–345. doi: 10.1113/jphysiol.1988.sp017165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chihara K, Arimura A, Schally AV. Immunoreactive somatostatin in rat hypophyseal portal blood: Effects of anesthetics. Endocrinology. 1979;104:1434–1441. doi: 10.1210/endo-104-5-1434. [DOI] [PubMed] [Google Scholar]
- 42.Worthington WC. Blood samples from the pituitary stalk of the rat: Method of collection and factors determining volume. Nature. 1966;210:710–712. doi: 10.1038/210710a0. [DOI] [PubMed] [Google Scholar]
- 43.Gibbs D. High concentration of oxytocin in hypophysial portal plasma. Endocrinology. 1984;114:1216–1218. doi: 10.1210/endo-114-4-1216. [DOI] [PubMed] [Google Scholar]
- 44.Horn AM, Robinson ICAF, Fink G. Oxytocin and vasopressin in rat hypophysial portal blood: Experimental studies in normal and Brattleboro rats. J. Endocrinol. 1985;104:211–224. doi: 10.1677/joe.0.1040211. [DOI] [PubMed] [Google Scholar]
- 45.Gibbs DM. Immunoneutralization of oxytocin attenuates stress-induced corticotropin secretion in the rat. Reg. Peptides. 1985;12:273–277. doi: 10.1016/0167-0115(85)90170-3. [DOI] [PubMed] [Google Scholar]
- 46.Robinson AG, Zimmerman EA. Cerebrospinal fluid and ependymal neurophysin. J. Clin. Invest. 1973;52:1260–1267. doi: 10.1172/JCI107293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mens WB, Van Dam AF, Van Egmond MAH, Bakker EAD, Lergros JJ, Van Wimersma Greidanus TB. Frontiers in Hormone Research. Vol. 9. Basel: Karger; 1982. Neurohypophyseal hormones in cerebrospinal fluid; pp. 119–130. [Google Scholar]
- 48.Robinson FICA. Neurohypophysial peptides in cerebro-spinal fluid. Prog. Brain Res. 1983;60:129–145. doi: 10.1016/S0079-6123(08)64381-2. [DOI] [PubMed] [Google Scholar]
- 49.Rhodes CH, Morrell JI, Pfaff DW. Changes in oxytocin content in the magnocellular neurons of the rat hypothalamus following water deprivation or estrogen treatment. Quantitative immunohistological studies. Cell Tissue Res. 216:47–55. doi: 10.1007/BF00234544. [DOI] [PubMed] [Google Scholar]
- 50.Rhodes CH, Morrell JI, Pfaff DW. Estrogen-concentrating neurophysin-containing hypothalamic magnocellular neurons in the vasopressin-deficient (Brattleboro) rat: A study combining steroid autoradiography and immunocytochemistry. J. Neurosci. 1982;2:1718–1724. doi: 10.1523/JNEUROSCI.02-12-01718.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akaishi T, Sakuma Y. Estrogen excites oxytocinergic, but not vasopressinergic cells in the paraventricular nucleus of female rat hypothalamus. Brain Res. 1985;335:302–305. doi: 10.1016/0006-8993(85)90481-0. [DOI] [PubMed] [Google Scholar]
- 52.Caldwell JD, Greer ER, Johnson MF, Prange AJ, Jr, Pedersen CA. Oxytocin and vasopressin immunoreactivity in hypothalamic and extrahypothalamic sites in late pregnant and postpartum rats. Neuroendocrinology. 1986;46:39–57. doi: 10.1159/000124794. [DOI] [PubMed] [Google Scholar]
- 53.Falconer J, Mitchell MD, Mountford LA, Robinson JS. Plasma oxytocin concentrations during the menstrual cycle in the Rhesus monkey. Macaca mulatta. J. Reprod. Fertil. 1980;59:69–72. doi: 10.1530/jrf.0.0590069. [DOI] [PubMed] [Google Scholar]
- 54.Jirikowski GF, Caldwell JD, Pedersen CA, Stumpf WE. Estradiol influences oxytocin-immunoreactive brain systems. Neuroscience. 25:237–248. doi: 10.1016/0306-4522(88)90022-x. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell MD, Haynes PJ, Anderson ABM, Turbull AC. Oxytocin in human ovulation. Lancet. 1980;2:704. doi: 10.1016/s0140-6736(80)92751-8. [DOI] [PubMed] [Google Scholar]
- 56.Robinson AG, Ferin M, Zimmerman EA. Plasma neurophysin levels in monkeys: Emphasis on the hypothalamic response to estrogen and ovarian events. Endocrinology. 1976;98:468–475. doi: 10.1210/endo-98-2-468. [DOI] [PubMed] [Google Scholar]
- 57.Forsling ML. Regulation of oxytocin release. In: Ganten D, Pfaff D, editors. Neurobiology of Oxytocin. Current Topics in Neuroendocrinology. Vol. 6. Berlin: Springer-Verlag; 1981. pp. 19–54. [Google Scholar]
- 58.Sarkar DK, Giass DM. Cyclic variation of oxytocin in the blood of pituitary portal vessels of rats. Neuroendocrinology. 1984;39:481–483. doi: 10.1159/000124024. [DOI] [PubMed] [Google Scholar]
- 59.Sarkar DK. Immunoneutralization of oxytocin attenuates preovulatory prolactin secretion during proestrus in the rat. Neuroendocrinology. 1988;48:214–216. doi: 10.1159/000125012. [DOI] [PubMed] [Google Scholar]
- 60.Johnston CA, Negro-Vilar A. Role of oxytocin on prolactin secretion during proestrus and in different physiological or pharmacological paradigms. Endocrinology. 1988;122:341–350. doi: 10.1210/endo-122-1-341. [DOI] [PubMed] [Google Scholar]
- 61.Ben-Jonathan N, Oliver C, Weiner HJ, Mical RS, Porter JC. Dopamine in hypophysial portal plasma of the rat during the estrous cycle and throughout pregnancy. Endocrinology. 1977;100:452–458. doi: 10.1210/endo-100-2-452. [DOI] [PubMed] [Google Scholar]
- 62.Raymond V, Beaulieu M, Labrie F. Potent antidopaminergic activity of estradiol at the pituitary level on prolactin release. Science. 1978;200:1173–1175. doi: 10.1126/science.418505. [DOI] [PubMed] [Google Scholar]
- 63.Abe H, Engler D, Moltich ME, Bollinger-Gruber J, Reichlin S. Vasoactive intestinal peptide is a physiological mediator of prolactin release in the rat. Endocrinology. 1985;116:1383–1390. doi: 10.1210/endo-116-4-1383. [DOI] [PubMed] [Google Scholar]
- 64.Grosvenor CE, Shyr SW, Goodman GT, Mena F. Comparison of plasma profiles of oxytocin and prolactin following suckling in rat. Neuroendocrinology. 1986;43:679–685. doi: 10.1159/000124604. [DOI] [PubMed] [Google Scholar]
- 65.Hyde JF, Ben-Jonathan N. Characterization of prolactin-releasing factor in the rat posterior pituitary. Endocrinology. 1988;122:2533–2539. doi: 10.1210/endo-122-6-2533. [DOI] [PubMed] [Google Scholar]
- 66.Mural I, Ben-Jonathan N. Posterior pituitary lobectomy abolishes the suckling-induced rise in prolactin (PRL): Evidence for a PRL-releasing factor in the posterior pituitary. Endocrinology. 1987;121:205–211. doi: 10.1210/endo-121-1-205. [DOI] [PubMed] [Google Scholar]
- 67.Murai I, Reichlin S, Ben-Jonathan N. The peak phase of the proestrus prolactin surge is blocked by either posterior pituitary lobectomy or antisera to vasoactive intestinal peptide. Endocrinology. 1989;124:1050–1055. doi: 10.1210/endo-124-2-1050. [DOI] [PubMed] [Google Scholar]
- 68.Johnston CA, Fagin KD, Negro-Vilar RHA. Differential effect of neurointermediate lobectomy on central oxytocin and vasopressin. Neurosci. Lett. 1990;113:101–106. doi: 10.1016/0304-3940(90)90502-z. [DOI] [PubMed] [Google Scholar]
- 69.Sarkar DK. Evidence for prolactin feedback actions on hypothalamic oxytocin, vasoactive intestinal peptide and dopamine secretion. Neuroendocrinology. 1989;49:520–524. doi: 10.1159/000125161. [DOI] [PubMed] [Google Scholar]
- 70.Ganten D, Pfaff D. Current topics in neuroendocrinology. Vol. 6. Berlin: Springer-Verlag; 1986. Neurobiology of oxytocin. [Google Scholar]
- 71.Pedersen CA, Prange AJ. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc. Natl. Acad. Sci. USA. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kovacs GL. Oxytocin and behavior. In: Ganten D, Pfaff D, editors. Neurobiology of Oxytocin. Current Topics in Neuroendocrinology. Vol. 6. Berlin: Springer-Verlag; 1981. pp. 91–128. [Google Scholar]