Abstract
BACKGROUND:
Variation in the use of ICUs for low-risk conditions contributes to health system inefficiency. We sought to examine the relationship between ICU use for patients with pulmonary embolism (PE) and cost, mortality, readmission, and procedure use.
METHODS:
We performed a retrospective cohort study including 61,249 adults with PE discharged from 263 hospitals in three states between 2007 and 2010. We generated hospital-specific ICU admission rate quartiles and used a series of multilevel models to evaluate relationships between admission rates and risk-adjusted in-hospital mortality, readmission, and costs and between ICU admission rates and several critical care procedures.
RESULTS:
Hospital quartiles varied in unadjusted ICU admission rates for PE (range, ≤ 15% to > 31%). Among all patients, there was a small trend toward increased use of arterial catheterization (0.6%-1.1%, P < .01) in hospital quartiles with higher levels of ICU admission. However, use of invasive mechanical ventilation (14.4%-7.9%, P < .01), noninvasive ventilation (6.6%-3.0%, P < .01), central venous catheterization (14.6%-11.3%, P < .02), and thrombolytics (11.0%-4.7%, P < .01) in patients in the ICU declined across hospital quartiles. There was no relationship between ICU admission rate and risk-adjusted hospital mortality, costs, or readmission.
CONCLUSIONS:
Hospitals vary widely in ICU admission rates for acute PE without a detectable impact on mortality, cost, or readmission. Patients admitted to ICUs in higher-using hospitals received many critical care procedures less often, suggesting that these patients may have had weaker indications for ICU admission. Hospitals with greater ICU admission may be appropriate targets for improving efficiency in ICU admissions.
As health-care spending continues to rise, policymakers are actively seeking ways to maximize quality while reducing cost and inefficiency.1 ICUs are an important focus for such efforts because of their significant costs and high staffing requirements.2,3 Wide variation in ICU admission practice across hospitals suggests an opportunity to improve the efficiency in the use of this expensive resource.4‐7
Aggressive ICU admission practices, however, can only be deemed inefficient to the extent that they do not improve outcomes.8,9 Studies of patients with heterogeneous diagnoses along with studies limited to specific diseases have revealed significant hospital-level variability in ICU use without differences in mortality.4 Little is known, however, about how ICU use impacts outcomes other than mortality, such as cost or readmission, or how liberal ICU admission practices affect rates of invasive procedures that may contribute to downstream complications.10‐13 Such issues are important for determining whether high ICU-use hospitals are overusing ICU beds or are providing appropriately aggressive care.
To examine the impact of hospital-level variability in ICU use on mortality, readmissions, and costs of care, we used discharge data in three states for patients with discharge diagnoses of pulmonary embolism (PE). PE serves as a representative example of a potentially discretionary ICU admission because it is common, often low risk, and can be cared for in a variety of settings.14‐16 To understand potential mechanisms of how variability in ICU use of PE may impact outcomes, we then examined rates of selected invasive procedures across quartiles of ICU use.
Materials and Methods
Data Source and Study Population
We conducted a retrospective cohort study using hospital discharge records from North Carolina, New York, and Washington between January 1, 2007, and December 31, 2010. Data were obtained from the Healthcare Cost and Use Project’s state inpatient databases (SIDs) from the Agency for Healthcare Research and Quality.17 The SIDs data include nearly 100% of discharges from > 1,000 nonfederal hospitals in 46 states. Data are included for all patients independent of payer. Data on hospital characteristics were obtained from the American Hospital Association’s Annual Survey from 2007 to 2010.18
We identified all adults (age ≥ 18 years) discharged from acute care hospitals with a primary discharge diagnosis of PE as defined by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 415.11, 415.19, and 673.2.19,20 To ensure that included patients had PE as their admission diagnosis, we examined present on admission (POA) rates for the primary diagnosis field.21 Among the states we included that released POA codes to the SIDs (New York and Washington), > 98% of primary discharge diagnoses of PE were marked POA (data not shown).
We excluded hospitals without ICU beds or missing data on ICU beds. We also excluded hospitals with < 25 PE hospitalizations over the study period to improve the reliability of hospital-based estimates.
Study Variables
ICU Admission Rates:
For each hospital, we identified the proportion of hospitalizations with PE admitted to an ICU using the presence of ICU- or coronary care unit-specific room and board charges present among revenue codes. We excluded intermediate care ICUs from this definition.
Outcomes:
Our primary risk-standardized outcomes included hospital-level mortality, readmission, and costs. We defined mortality as death during the index hospitalization, readmission as readmission to any acute care hospital within 30 days of discharge after the index hospitalization, and costs as the total reported hospital charges (adjusted for inflation to 2010 dollars) multiplied by the hospital-specific all-payer cost-to-charge ratio for the year of hospitalization.22 We calculated these outcomes among two populations: (1) all patients with PE and (2) the subset of these patients with ICU stays.
Critical Care Therapies:
For each hospital, we calculated the use of critical care therapies, again among all patients and the subset of patients with ICU stays. We used ICD-9-CM codes to identify selected therapies, including invasive mechanical ventilation (96.70-96.72), noninvasive mechanical ventilation (93.90), pulmonary artery catheterization (89.63, 89.64, 89.66-89.68), central venous catheterization (38.93), arterial catheterization (38.91 and 89.61), and use of thrombolytics (99.10).23
Adjustment Variables:
Adjustment variables included demographic data (age, sex, and payer); admission data (weekday or weekend, emergent or nonemergent, admission source); individual patient comorbidities (as defined by Elixhauser et al24); presence of organ failure using ICD-9-CM codes corresponding to circulatory, renal, neurologic, hematologic, metabolic, and hepatic organ failure (as defined by Angus et al25); use of ICU procedures (central line, arterial line, pulmonary artery catheterization, thrombolytics, invasive mechanical ventilation, and noninvasive positive pressure ventilation); hospital size; total hospital-wide patients with PE across the study period; ICU capacity (as a percentage of total beds); teaching status (defined by the ratio of resident full-time equivalents [FTEs] to beds); medical school affiliation; designation as a critical access hospital; and hospital type (for profit, not for profit, or government). Race and ethnicity were omitted because these data were missing for many of the patients included in the study.
Statistical Analysis
We estimated hospital-specific rates of ICU admission using empirical Bayes posterior estimates from an empty multilevel logistic regression model, where individual hospitalizations were nested within hospitals. This approach accounts for the poor reliability of ICU admission rates calculated from hospitals with few cases.26 We divided hospitals and their patients into quartiles by ICU admission rate and compared patient and hospital characteristics across these quartiles using nonparametric tests for trend. Quartile 1 contained hospitals with the lowest rates of ICU use for PE.
We entered ICU admission rate quartile into a series of multilevel models to estimate its relationship with risk-adjusted in-hospital mortality, readmission, and cost. Finally, we used logistic regression accounting for clustering by hospitals to evaluate the relationship between ICU admission rate quartile and use of each ICU therapy among all patients and the subgroup of patients admitted to the ICU.27 Predictive margins were computed from the multivariable models and reported in the tables and results.
Of particular concern to the validity of our outcome analysis was the possibility of confounding by indication—a type of selection bias that can occur when choice of a treatment is related to an underlying factor (eg, disease severity) that is also related to an outcome of interest.28 Although difficult to address in administrative data, we attempted to limit its impact by adjusting all models for patient and hospital differences that could confound the relationship between ICU admission and each outcome. These included individual organ system failures, need for ICU-level procedures including the administration of thrombolytics, and patient comorbidities.
All data management and analyses were conducted using SAS (V9.2; SAS institute Inc) and Stata (V12.0; StataCorp LP). The University of Michigan institutional review board reviewed the protocol for this study and determined that it was not considered Human Subjects Research (University of Michigan Medical School Institutional Review Board, HUM00075319).
Results
Hospital Characteristics
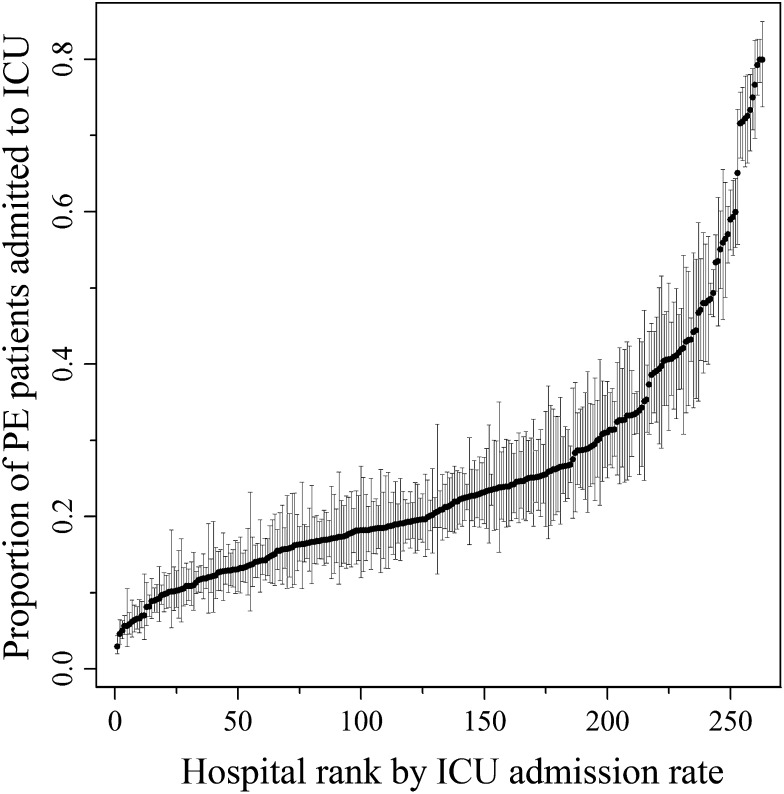
In total, 61,249 patients with a primary discharge diagnosis of PE were included from 263 acute-care hospitals over a 4-year period (Table 1). ICU admission rates varied dramatically across hospitals, ranging from 2.9% to 79.9% (median, 18.4%; interquartile range, 12.0%) (Fig 1). Hospital quartiles of ICU admission for PE were ≤ 15%, > 15% to 21%, > 21% to 31%, and > 31% in the first through fourth quartile, respectively. The median ICU admission rate within each quartile was 11.3%, 18.3%, 23.5%, and 47.1% in quartiles 1 through 4, respectively. The majority of the hospitals in each quartile were designated as not for profit (> 69% in all quartiles), and there was no significant trend across quartiles (P = .15). There was also no significant trend in resident FTEs across quartiles (P = .99). Across all hospitals, 42 hospitals (16.0%) reported having ≤ 100 beds, and 56 (21.3%) reported ≥ 400 beds. There was no significant difference in hospital size or percentage of beds designated as ICU beds across quartiles (P = .09 and P = .82, respectively).
TABLE 1 ] .
Hospital Characteristics
| ICU Admission Rate for PE | |||||
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Characteristic | ≤ 15% | > 15%-21% | > 21%-31% | > 31% | P Valuea |
| Type of hospital (n = 263) | .15 | ||||
| Government | 13 (19.7) | 11 (16.7) | 19 (28.8) | 18 (27.7) | |
| Not for profit | 52 (78.8) | 51 (77.3) | 46 (69.7) | 45 (69.2) | |
| For profit | 1 (1.5) | 4 (6.1) | 1 (1.5) | 2 (3.1) | |
| Critical access hospital (n = 261) | 2 (3.0) | 3 (4.6) | 3 (4.6) | 4 (6.3) | .40 |
| Medical school affiliation (n = 263) | 28 (42.4) | 19 (28.8) | 15 (22.7) | 22 (33.9) | .21 |
| Teaching status (n = 263) | .99 | ||||
| No residents | 32 (48.5) | 43 (65.2) | 35 (53.0) | 34 (52.3) | |
| Minor teaching program (< 0.25 residents/bed) | 23 (34.8) | 11 (16.7) | 20 (30.3) | 20 (30.8) | |
| Major teaching program (> 0.25 residents/bed) | 11 (16.7) | 12 (18.2) | 11 (16.7) | 11 (16.9) | |
| Hospital beds (n = 263) | .09 | ||||
| ≤ 100 | 7 (10.6) | 10 (15.2) | 10 (15.2) | 15 (23.1) | |
| 101-200 | 17 (25.8) | 23 (34.9) | 24 (36.4) | 13 (20.0) | |
| 201-300 | 16 (24.2) | 10 (15.2) | 12 (18.2) | 17 (26.2) | |
| 301-400 | 7 (10.6) | 7 (10.6) | 10 (15.2) | 9 (13.9) | |
| > 400 | 19 (28.8) | 16 (24.2) | 10 (15.2) | 11 (16.9) | |
| Percentage of ICU beds (No. = 263) | .82 | ||||
| ≤ 5 | 13 (19.7) | 8 (12.1) | 12 (18.2) | 13 (20.0) | |
| > 5-7.5 | 20 (30.3) | 20 (30.3) | 18 (27.3) | 15 (23.1) | |
| > 7.5-10 | 13 (19.7) | 19 (28.8) | 13 (19.7) | 18 (27.7) | |
| > 10-12.5 | 11 (19.7) | 8 (12.1) | 14 (21.2) | 8 (12.3) | |
| > 12.5-15 | 2 (3.0) | 2 (3.0) | 3 (4.6) | 5 (7.69) | |
| > 15 | 7 (10.6) | 9 (13.6) | 6 (9.1) | 6 (9.2) | |
| Total patients per hospital with primary discharge diagnosis of PE during study period, median (IQR) | 297 (168-478) | 153 (96-343) | 142.5 (89-219) | 101 (52-190) | < .01 |
| PE ICU admission rate during study period, median (IQR), % | 11.4 (9.1-13.0) | 18.1 (16.8-19.0) | 24.6 (23.1-26.6) | 42.1 (35.1-55.9) | < .01 |
Data are presented as No. (%) unless otherwise noted. IQR = interquartile range; PE = pulmonary embolism.
Using χ2 or nonparametric tests for trend and nonparametric equality of medians tests.
Figure 1 –
ICU admission rates by hospital. Point estimates represent the risk- and reliability-adjusted estimates for ICU admission rate among patients with PE for each hospital, with 84% CIs. When 84% CIs for two points do not overlap, these two points are statistically different (P < .05). PE = pulmonary embolism.
Patient Characteristics
Although there were statistically significant trends across ICU admission quartiles in quartiles of income by zip code, emergent and trauma admissions, and presence of hematologic organ failure, absolute differences were small (P < .01 for all variables). There was no significant difference in the presence of circulatory, renal, neurologic, metabolic, or hepatic organ failures across quartiles (P = .85, P = .34, P = .09, P = .30, and P = 9.95, respectively) (Table 2). There were small trends across quartiles in some individual Elixhauser comorbidities, although again absolute differences were small (Table 3).
TABLE 2 ] .
Patient Characteristics
| ICU Admission Rate for PE | |||||
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Characteristic | ≤ 15% | > 15%-21% | > 21%-31% | > 31% | P Valuea |
| Age, y (n = 60,597) | .08 | ||||
| 18-44 | 4,257 (19.0) | 2,898 (19.0) | 2,198 (18.2) | 1,988 (18.5) | |
| 45-54 | 3,459 (15.4) | 2,335 (15.3) | 1,966 (16.3) | 1,728 (16.1) | |
| 55-64 | 4,358 (19.4) | 2,708 (17.7) | 2,271 (18.8) | 1,955 (18.2) | |
| 65-74 | 4,471 (19.9) | 2,985 (19.5) | 2,324 (19.3) | 2,096 (19.5) | |
| ≥ 75 | 5,923 (26.4) | 4,365 (28.6) | 3,316 (27.5) | 2,996 (27.8) | |
| Female (n = 60,597) | 12,348 (55.0) | 8,576 (56.1) | 6,666 (55.2) | 5,998 (55.7) | .30 |
| Primary insurer (n = 60,450) | .37 | ||||
| Medicare | 9,894 (44.2) | 7,331 (48.1) | 5,823 (48.3) | 4,849 (45.1) | |
| Medicaid | 2,427 (10.8) | 1,593 (10.4) | 1,342 (11.1) | 974 (9.1) | |
| Private pay | 8,662 (38.7) | 5,323 (34.9) | 3,968 (32.9) | 4,008 (37.3) | |
| Self-pay | 805 (3.6) | 646 (4.2) | 599 (5.0) | 709 (6.6) | |
| Other | 604 (2.7) | 359 (2.4) | 331 (2.7) | 203 (1.9) | |
| Quartiles of income by zip code (n = 58,479) | < .01 | ||||
| First quartile (lowest) | 5,510 (25.1) | 4,148 (27.9) | 3,405 (30.0) | 3,066 (29.7) | |
| Second quartile | 5,456 (25.0) | 4,225 (28.4) | 3,737 (32.9) | 2,726 (26.4) | |
| Third quartile | 5,419 (24.7) | 2,584 (17.4) | 2,077 (18.3) | 2,589 (25.0) | |
| Fourth quartile (highest) | 5,538 (25.3) | 3,901 (26.3) | 2,140 (18.8) | 1,958 (18.9) | |
| Elixhauser comorbidities (n = 60,597) | .01 | ||||
| 0 | 2,541 (11.3) | 1,862 (12.2) | 1,338 (11.1) | 1,287 (12.0) | |
| 1 | 4,555 (20.3) | 3,271 (21.4) | 2,535 (21.0) | 2,277 (21.2) | |
| 2 | 5,466 (24.3) | 3,824 (25.0) | 2,907 (24.1) | 2,661 (24.7) | |
| 3 | 4,596 (20.5) | 3,105 (20.3) | 2,458 (20.4) | 2,151 (20.0) | |
| ≥ 4 | 5,310 (23.6) | 3,229 (21.1) | 2,837 (23.5) | 2,387 (22.2) | |
| Admitted via ED (n = 60,532) | 15,698 (70.0) | 11,254 (73.7) | 8,535 (70.7) | 7,532 (70.0) | .90 |
| Admission designated “trauma” or “emergent” (n = 60,584) | 17,722 (78.9) | 12,928 (84.6) | 9,492 (78.6) | 8,773 (81.5) | < .01 |
| Extrapulmonary organ failure | |||||
| Circulatory | 988 (4.4) | 708 (4.6) | 522 (4.3) | 475 (4.4) | .85 |
| Renal | 259 (1.2) | 222 (1.5) | 132 (1.1) | 117 (1.1) | .34 |
| Neurologic | 160 (0.7) | 120 (0.8) | 95 (0.8) | 97 (0.9) | .09 |
| Hematologic | 910 (4.1) | 547 (3.6) | 401 (3.3) | 390 (3.6) | < .01 |
| Metabolic | 383 (1.7) | 258 (1.7) | 186 (1.5) | 172 (1.6) | .30 |
| Hepatic | 107 (0.5) | 74 (0.5) | 57 (0.5) | 51 (0.5) | .95 |
Data are presented as No. (%). See Table 1 legend for expansion of abbreviation.
Using χ2 or nonparametric tests for trend.
TABLE 3 ] .
Elixhauser Comorbidities by Quartile
| ICU Admission Rate for PE | |||||
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Comorbidity | ≤ 15% | > 15%-21% | > 21%-31% | > 31% | P Valuea |
| Congestive heart failure | 2,613 (11.6) | 1,847 (12.1) | 1,445 (12.0) | 1,267 (11.8) | .59 |
| Valvular disease | 1,110 (5.0) | 722 (4.7) | 688 (5.7) | 556 (5.2) | .04 |
| Pulmonary circulation disorders | 1,559 (6.9) | 984 (6.4) | 837 (6.9) | 555 (5.2) | < .01 |
| Peripheral vascular disorders | 833 (3.7) | 543 (3.6) | 387 (3.2) | 402 (3.7) | .44 |
| Hypertension | 11,261 (50.1) | 7,223 (50.5) | 6,046 (50.1) | 5,464 (50.8) | .42 |
| Paralysis | 425 (1.9) | 294 (1.9) | 251 (2.1) | 255 (2.4) | < .01 |
| Other neurologic disorders | 1271 (5.7) | 760 (5.0) | 668 (5.5) | 576 (5.4) | .40 |
| Chronic pulmonary disease | 4,800 (21.4) | 3,287 (21.5) | 2,907 (24.1) | 2,293 (21.3) | .03 |
| Diabetes, uncomplicated | 3,720 (16.6) | 2,508 (16.4) | 2,126 (17.6) | 1,816 (16.9) | .10 |
| Diabetes, complicated | 391 (1.7) | 258 (1.7) | 189 (1.6) | 167 (1.6) | .13 |
| Hypothyroidism | 2,218 (9.9) | 1,460 (9.6) | 1,220 (10.1) | 1,065 (9.9) | .66 |
| Renal failure | 1,949 (8.7) | 1,098 (7.2) | 929 (7.7) | 867 (8.1) | .02 |
| Liver disease | 390 (1.7) | 202 (1.3) | 176 (1.5) | 168 (1.6) | .14 |
| Peptic ulcer disease, excluding bleeding | 9 (< 0.1) | 3 (< 0.1) | 12 (0.1) | 5 (0.1) | .20 |
| AIDS | 30 (0.1) | 19 (0.1) | 18 (0.2) | 24 (0.2) | .07 |
| Lymphoma | 356 (1.6) | 196 (1.3) | 169 (1.4) | 123 (1.1) | .03 |
| Metastatic cancer | 2,213 (9.9) | 1,171 (7.7) | 970 (8.0) | 790 (7.3) | < .01 |
| Solid tumor | 1,404 (6.3) | 883 (5.8) | 756 (6.3) | 595 (5.5) | .05 |
| Rheumatoid arthritis or collagen vascular disease | 747 (3.3) | 433 (2.8) | 394 (3.3) | 349 (3.2) | .86 |
| Coagulopathy | 1,116 (5.0) | 682 (4.5) | 487 (4.0) | 469 (4.4) | < .01 |
| Obesity | 3,078 (13.7) | 1,945 (12.7) | 1,686 (14.0) | 1,432 (13.3) | .80 |
| Weight loss | 778 (3.5) | 370 (2.4) | 342 (2.8) | 275 (2.6) | < .01 |
| Fluid and electrolyte disorders | 3,896 (17.3) | 2,651 (17.3) | 1,950 (16.2) | 1,840 (17.1) | .11 |
| Blood loss anemia | 309 (1.4) | 207 (1.4) | 140 (1.2) | 158 (1.5) | .94 |
| Deficiency anemias | 3,508 (15.6) | 2,213 (14.5) | 1,746 (14.5) | 1,682 (15.6) | .42 |
| Alcohol abuse | 576 (2.6) | 332 (2.2) | 321 (2.7) | 263 (2.4) | .94 |
| Drug abuse | 446 (2.0) | 257 (1.7) | 278 (2.3) | 205 (1.9) | .53 |
| Psychoses | 673 (3.0) | 474 (3.1) | 386 (3.2) | 342 (3.2) | .27 |
| Depression | 2,360 (10.5) | 1,396 (9.1) | 1,286 (10.7) | 1,069 (9.9) | .48 |
Data are presented as No. (%) unless otherwise noted. See Table 1 legend for expansion of abbreviation.
Using nonparametric tests for trend.
ICU Use and Risk-Adjusted Clinical Outcomes
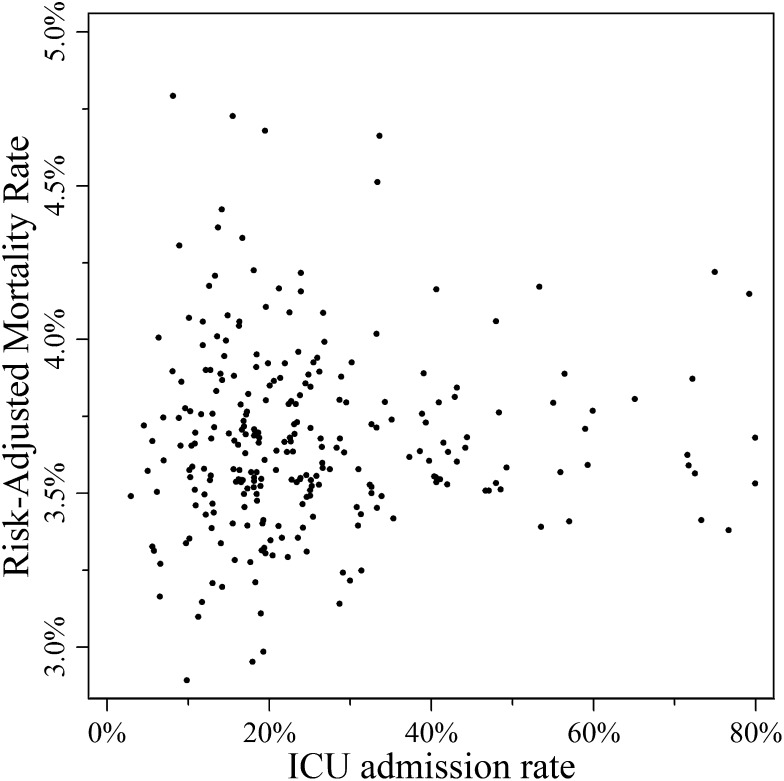
Mortality, readmission, and charge data were available for 60,587 (> 99.9%), 60,597 (100%), and 60,589 (> 99.9%) of participants, respectively. There was no significant relationship between ICU admission rate and risk-adjusted hospital mortality for patients with PEs (Table 4). This finding was consistent when examined at the level of individual hospitals (Fig 2) (r = −0.0001, P = .948) and when grouping hospitals into quartiles of ICU use (Table 5) (P = .88). Similarly, there were no differences in 30-day readmissions (P = .90) and hospital costs (P = .96) across quartiles of ICU use.
TABLE 4 ] .
Hospital Mortality, Cost, and Readmission by ICU Quartile
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Adjusted Outcomea | Admission Rate < 15% | Admission Rate 15%-21% | Admission Rate 21%-31% | Admission Rate 31%-80% | P Value for Trend |
| All hospitalizations | |||||
| Mortality, % | 3.7 (3.6-3.8) | 3.6 (3.6-3.7) | 3.7 (3.6-3.7) | 3.7 (3.6-3.8) | .88 |
| Mean costs | $23,111 ($20,533-$25,689) | $22,982 ($21,052-$24,912) | $22,500 ($20,245-$24,754) | $22,899 ($20,743-$25,056) | .96 |
| 30-d readmission, % | 14.9 (13.5-16.2) | 15.8 (14.3-17.3) | 15.1 (13.6-16.6) | 15.5 (14.0-16.9) | .90 |
| ICU hospitalizations | |||||
| Mortality, % | 9.5 (9.3-9.7) | 9.3 (9.2-9.5) | 9.4 (9.3-9.6) | 9.2 (9.0-9.3) | < .01 |
| Mean costs | $37,876 ($33,682-$42,070) | $36,746 ($33,938-$39,553) | $36,939 ($33,491-$40,388) | $34,968 ($32,093-$37,843) | .86 |
| 30-d readmission, % | 15.1 (14.1-16.1) | 15.9 (14.6-17.2) | 15.2 (14.0-16.3) | 15.2 (14.0-16.5) | .77 |
Numbers in parentheses represent 95% CI.
Adjusted for patient characteristics (admission year; admitted on a weekday or weekend; use of ICU-level therapeutics, such as ventilator, lytics, or invasive access; age; individual comorbidities; sex; payer; admission source; admission type; and individual organ system failures) and hospital characteristics (hospital type and critical access designation, total PE volume, medical school affiliation, number of resident full-time equivalents, total general care and ICU beds, and core-based statistical area).
Figure 2 –
Risk-adjusted in-hospital mortality by ICU admission ranking. Each point represents the risk- and reliability-adjusted estimate for a single hospital’s ICU admission rate and the same hospital’s risk-adjusted mortality.
TABLE 5 ] .
Risk-Adjusted Proportions of Patients Receiving ICU Therapies by ICU Quartile
| Adjusted Proportion of Hospitalizations Receiving Therapy, % | |||||
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Therapy | Admission Rate < 15% | Admission Rate 15%-21% | Admission Rate 21%-31% | Admission Rate 31%-80% | P Value |
| All hospitalizations | |||||
| Invasive mechanical ventilation | 2.6 | 2.7 | 2.7 | 2.8 | .54 |
| Noninvasive mechanical ventilation | 2.1 | 2.0 | 1.8 | 1.7 | .17 |
| Pulmonary artery catheter | 0.1 | 0.1 | 0.2 | 0.2 | .11 |
| Central venous catheter | 4.7 | 5.0 | 5.3 | 5.6 | .19 |
| Arterial catheter | 0.6 | 0.7 | 0.9 | 1.1 | < .01 |
| Thrombolytics | 1.7 | 1.8 | 1.9 | 2.0 | .24 |
| ICU hospitalizations | |||||
| Invasive mechanical ventilation | 14.4 | 11.8 | 9.7 | 7.9 | < .01 |
| Noninvasive mechanical ventilation | 6.6 | 5.1 | 3.9 | 3.0 | < .01 |
| Pulmonary artery catheter | 0.5 | 0.5 | 0.5 | 0.5 | .95 |
| Central venous catheter | 14.6 | 13.4 | 12.3 | 11.3 | .02 |
| Arterial catheter | 3.1 | 3.1 | 3.1 | 3.1 | .99 |
| Thrombolytics | 11.0 | 8.3 | 6.3 | 4.7 | < .01 |
Adjusted for patient characteristics (admission year; admitted on a weekday or weekend; use of ICU-level therapeutics, such as ventilator, lytics, or invasive access; age; individual comorbidities; sex; payer; admission source; admission type; and individual organ system failures) and hospital characteristics (hospital type and critical access designation, total PE volume, medical school affiliation, number of resident full-time equivalents, total general care and ICU beds, and core-based statistical area). See Table 1 legend for expansion of abbreviation.
When limited to the subset of patents hospitalized in an ICU, there was a small but statistically significant inverse relationship between mortality and ICU admission rate quartile (range, 9.5%-9.2%; P < .01). There was no relationship between hospital costs (P = .86) or 30-day readmission (P = .77) and ICU use quartile among patients admitted to an ICU.
ICU Use and Risk-Adjusted Use of ICU Therapies
Among all PE hospitalizations, there was a small increase in arterial catheterization (0.6%-1.1%, P < .01) from the lowest to highest ICU admission quartiles (Table 5). There was no significant difference in the use of mechanical ventilation, noninvasive mechanical ventilation, central venous catheterization, thrombolytics, or pulmonary artery catheterization across quartiles of ICU use.
When limited to patients cared for in an ICU, there was a statistically significant trend toward decreased use of most critical care therapies in hospitals with higher ICU use rates (Table 5). As ICU use quartile increased, there was a decrease in invasive mechanical ventilation (14.4%-7.9%, P < .01), noninvasive mechanical ventilation (6.6%-3.0%, P < .01), central venous catheterization (14.6%-11.3%, P < .02), and thrombolytics (11.0%-4.7%, P < .01) among patients in the ICU. There was no significant difference in pulmonary artery or arterial catheterization for patients in the ICU across quartiles (P = .58 and 0.99, respectively).
Discussion
We found a fourfold variation in ICU use rates across hospital quartiles that was not associated with differences in mortality, readmissions, or hospital costs. Such differences in ICU use in the absence of differences in outcome raise the possibility that hospitals with higher ICU use may be admitting patients to their units with weaker indications for ICU care. This was reflected in a trend toward decreased mortality in higher quartiles of use among the subset of patients admitted to an ICU.
Our study complements past work identifying wide variation in ICU admission decisions and highlights the need for quality improvement in the decision-making process. For many patients, particularly those who require life-sustaining therapies, ICU admission is required to rescue patients from imminent death.29 However, past work demonstrates that ICU admission may be more discretionary for patients at lower risk.4,5,30 Chen et al30 evaluated patients admitted to Veterans Administration hospitals and found significant variation in ICU admission rates between hospitals that persisted after adjustment for both predicted mortality on admission and admission diagnosis. In their mortality analysis, they found a protective effect of ICU admission but only at the highest rates of predicted mortality.30
Additional studies have examined ICU use for lower-risk conditions. Gershengorn et al4 used the New York SID and found significant variation in ICU use for patients with diabetic ketoacidosis that were not associated with differences in hospital length of stay or in-hospital mortality. Safavi et al5 used a national sample of patients with heart failure and found wide variability in ICU admission without a significant mortality difference between hospitals in the top quartile of ICU admission rates and those in the bottom three. Safavi et al5 also found lower rates of use of several ICU therapies, such as mechanical ventilation and vasopressor support, among those patients admitted to an ICU in higher-use hospitals. Our study finds similar results among those patients with PE admitted to an ICU in hospitals with higher ICU admission rates and additionally finds that increased ICU admission rates were not associated with differences in 30-day hospital readmission rates or hospital costs.
There are several potential explanations for the variability in ICU admission rates and the use of invasive procedures among the subset of patients admitted to the ICU. One explanation is that hospitals with higher admission rates have lower thresholds for ICU admission, liberally admitting patients with low severities of illness and diluting the severity of the entire ICU patient population.7 This dilution effect is exemplified by the trend toward decreased mortality and the lower number of patients in the ICU who received mechanical ventilation, central venous catheterization, and thrombolytics in higher ICU-use hospitals. Alternatively, high-admission hospitals may be appropriately using the ICU more liberally to improve patient outcomes. However, this explanation is less likely, as we found only small differences in patient characteristics and extrapulmonary organ failure between high- and low-using hospitals across all patients. These data suggest that hospital variation in the decision to admit a patient to the ICU may be independent of clinical acuity and are perhaps reflective of factors external to the patient, such as bed availability, local practice style, or financial incentives. Alternatively, widespread variation in admitting practices likely reflects a lack of standard triage guidelines for patients presenting with PE. In the absence of accepted triage guidelines, hospitals tailor ICU admission decisions to their population to optimize outcomes of their patients. Yet, in the face of uncertainty about who will benefit most from ICU care, these external factors could play a more significant role in ICU admission decisions.
Notably, we did not find a significant difference in cost across quartiles of ICU use. Costs in this study were estimated using cost-to-charge ratios and, as a result, relied only on hospital charges. The cost data did not account for other typical charges incurred during a hospital stay (eg, physician charges). Professional fees are likely to differ significantly between general care wards and ICUs and may play a role in driving differences in cost in a way not reflected by hospital charges in administrative datasets. Moreover, cost-to-charge ratios are averaged for a particular hospital over a given year and are not provided by diagnosis.
Our study should be interpreted in the context of several limitations. One such limitation is our ability to completely account for severity of illness across hospitals using administrative data. To limit confounding by disease severity, we adjusted for observed factors that reflect disease severity, including organ failure. Furthermore, we collapsed data into quartiles of ICU admission to limit the impact of variation in severity and case mix. Second, we used ICD-9-CM codes at discharge to define our cohort of patients with PE, which have imperfect sensitivity and specificity for true diagnosis of PE. Although discharge diagnoses are not necessarily equivalent to admission diagnoses, our use of POA codes revealed that > 98% of primary discharge diagnoses were present on admission, as discussed earlier. Moreover, Goldman et al21 demonstrated that POA codes were very accurate when compared with direct medical record abstraction, suggesting that the PE cases identified in this study represent PEs diagnosed on admission. Finally, our study is limited to patients with PE, and although it complements previous work among patients with other conditions,4‐6 further work is necessary to assess the validity of these findings in patients without PE.
Conclusions
Intensive care is a costly and limited resource and makes up a significant proportion of health-care spending. Our findings suggest that ICU use for PE varies widely between hospitals without an appreciable relationship with mortality or readmission and leaves open the possibility that ICUs may be overused in some institutions for this condition. Although we observed similar average hospital charges for PEs across all quartiles of ICU use, further work is needed to determine if total charges incurred by patients with PE, including physician fees, vary across hospital quartiles of ICU use. Even if costs are similar, discretionary use of intensive care for patients with PE consumes resources that do not appear to buy improvements in outcome and may be more effectively allocated to those patients with other diagnoses who better stand to benefit. These findings suggest that high-use hospitals may provide an effective target for efforts seeking to increase health-care value through the development of specific ICU admission criteria for PE or the consideration of alternative, less resource-intensive levels of care, such as telemetry or intermediate care.
Acknowledgments
Author contributions: C. R. C. takes full responsibility for the content of the manuscript, including the integrity of the data and accuracy of the analysis. A. J. A. contributed to the study design, analysis, and interpretation of data, writing the initial draft and revising the manuscript, and approval of the final manuscript; C. W. S., H. B. G., and H. W. contributed to the analysis and interpretation of data, writing or revising the manuscript for important intellectual content, and approval of the final manuscript; and C. R. C. contributed to the study design, analysis, and interpretation of data, writing and revising the manuscript, and approval of the final manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
ABBREVIATIONS
- FTE
full-time equivalent
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- PE
pulmonary embolism
- POA
present on admission
- SID
state inpatient database
Footnotes
FUNDING/SUPPORT: This work was supported in part by the Agency for Healthcare Research and Quality [Grant K08HS020672, Dr Cooke], the National Institutes of Health [Grant K23GM104022, Dr Seymour], and the National Institute on Aging [Grant K08AG038477, Dr Wunsch].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Weinberger SE. Providing high-value, cost-conscious care: a critical seventh general competency for physicians. Ann Intern Med. 2011;155(6):386-388. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Kelley MA, Schmitz RJ, White A, Popovich J, Jr; Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS). Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA. 2000;284(21):2762-2770. [DOI] [PubMed] [Google Scholar]
- 3.Halpern NA, Pastores SM. Critical care medicine in the United States 2000-2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38(1):65-71. [DOI] [PubMed] [Google Scholar]
- 4.Gershengorn HB, Iwashyna TJ, Cooke CR, Scales DC, Kahn JM, Wunsch H. Variation in use of intensive care for adults with diabetic ketoacidosis. Crit Care Med. 2012;40(7):2009-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safavi KC, Dharmarajan K, Kim N, et al. Variation exists in rates of admission to intensive care units for heart failure patients across hospitals in the United States. Circulation. 2013;127(8):923-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seymour CW, Iwashyna TJ, Ehlenbach WJ, Wunsch H, Cooke CR. Hospital-level variation in the use of intensive care. Health Serv Res. 2012;47(5):2060-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenthal GE, Sirio CA, Shepardson LB, Harper DL, Rotondi AJ, Cooper GS. Use of intensive care units for patients with low severity of illness. Arch Intern Med. 1998;158(10):1144-1151. [DOI] [PubMed] [Google Scholar]
- 8.Ridley S, Morris S. Cost effectiveness of adult intensive care in the UK. Anaesthesia. 2007;62(6):547-554. [DOI] [PubMed] [Google Scholar]
- 9.Kox M, Pickkers P. “Less is more” in critically ill patients: not too intensive. JAMA Intern Med. 2013;173(14):1369-1372. [DOI] [PubMed] [Google Scholar]
- 10.Ashton CM, Kuykendall DH, Johnson ML, Wray NP, Wu L. The association between the quality of inpatient care and early readmission. Ann Intern Med. 1995;122(6):415-421. [DOI] [PubMed] [Google Scholar]
- 11.Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271(20):1598-1601. [DOI] [PubMed] [Google Scholar]
- 12.Laupland KB, Lee H, Gregson DB, Manns BJ. Cost of intensive care unit-acquired bloodstream infections. J Hosp Infect. 2006;63(2):124-132. [DOI] [PubMed] [Google Scholar]
- 13.Rello J, Ollendorf DA, Oster G, et al. ; VAP Outcomes Scientific Advisory Group. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122(6):2115-2121. [DOI] [PubMed] [Google Scholar]
- 14.Bahloul M, Chaari A, Kallel H, et al. Pulmonary embolism in intensive care unit: predictive factors, clinical manifestations and outcome. Ann Thorac Med. 2010;5(2):97-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353(9162):1386-1389. [DOI] [PubMed] [Google Scholar]
- 16.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(6):585-593. [DOI] [PubMed] [Google Scholar]
- 17.Overview of the Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project website. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed January 10, 2013.
- 18.Healthcare Cost and Utilization Project (HCUP). American Hospital Association (AHA) linkage files. Rockville, MD: Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/db/state/ahalinkage/aha_linkage.jsp. Accessed July 3, 2014. [Google Scholar]
- 19.White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158(14):1525-1531. [DOI] [PubMed] [Google Scholar]
- 20.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171(9):831-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldman LE, Chu PW, Osmond D, Bindman A. The accuracy of present-on-admission reporting in administrative data. Health Serv Res. 2011;46(6 pt 1):1946-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taira DA, Seto TB, Siegrist R, Cosgrove R, Berezin R, Cohen DJ. Comparison of analytic approaches for the economic evaluation of new technologies alongside multicenter clinical trials. Am Heart J. 2003;145(3):452-458. [DOI] [PubMed] [Google Scholar]
- 23.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42(8):801-809. [DOI] [PubMed] [Google Scholar]
- 24.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. [DOI] [PubMed] [Google Scholar]
- 25.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303-1310. [DOI] [PubMed] [Google Scholar]
- 26.Dimick JB, Staiger DO, Birkmeyer JD. Ranking hospitals on surgical mortality: the importance of reliability adjustment. Health Serv Res. 2010;45(6 pt 1):1614-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuhaus JM. Statistical methods for longitudinal and clustered designs with binary responses. Stat Methods Med Res. 1992;1(3):249-273. [DOI] [PubMed] [Google Scholar]
- 28.Bosco JLF, Silliman RA, Thwin SS, et al. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol. 2010;63(1):64-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohn R, Rubenfeld GD, Levy MM, Ubel PA, Halpern SD. Rule of rescue or the good of the many? An analysis of physicians’ and nurses’ preferences for allocating ICU beds. Intensive Care Med. 2011;37(7):1210-1217. [DOI] [PubMed] [Google Scholar]
- 30.Chen LM, Render M, Sales A, Kennedy EH, Wiitala W, Hofer TP. Intensive care unit admitting patterns in the Veterans Affairs health care system. Arch Intern Med. 2012;172(16):1220-1226. [DOI] [PubMed] [Google Scholar]