Abstract
BACKGROUND:
Cell-free hemoglobin (CFH) is a potent nitric oxide scavenger associated with poor outcomes in several diseases. Pulmonary arterial hypertension (PAH) is characterized by reduced nitric oxide availability. We hypothesized that CFH would be elevated in PAH and would associate with hemodynamics and clinical outcomes.
METHODS:
We measured CFH in 200 consecutively evaluated patients with PAH, 16 unaffected bone morphogenetic receptor protein type 2 (BMPR2) mutation carriers, 19 healthy subjects, and 29 patients with pulmonary venous hypertension (PVH). CFH values were tested for association with hemodynamics, time to hospitalization, and death.
RESULTS:
CFH was elevated in patients with PAH and BMPR2 carriers compared with healthy subjects and patients with PVH (P ≤ .01 all comparisons). There were no differences in CFH across PAH subtypes. CFH modestly correlated with mean pulmonary artery pressure (ρ = 0.16, P = .03) and pulmonary vascular resistance (ρ = 0.21, P = .01) and inversely with cardiac index (ρ = −0.18, P = .02) in patients with PAH. CFH was not associated with hemodynamic response to nitric oxide or death. Patients with the highest CFH levels had increased risk of PAH-related hospitalization when adjusted for age, sex, and PAH cause (hazard ratio, 1.69; 95% CI ,1.08-2.66; P = .02).
CONCLUSIONS:
CFH is elevated in patients with PAH and BMPR2 carriers compared with healthy subjects and patients with PVH. Elevated CFH levels are independently associated with an increased risk of hospitalization. Further study is required to understand the mechanism of CFH elevation and the potential pathologic contribution of CFH in PAH.
Hemoglobin, when released from the RBC, is a potent oxidant1,2 and vasoconstrictor3‐7 associated with poor clinical outcomes.8 Cell-free hemoglobin (CFH) levels are elevated in the plasma of patients with sickle cell anemia,3,9 sepsis,8 and after RBC transfusion.5 In all of these patient populations, CFH has been associated with poor outcomes, including the risk of acute kidney injury,1 myocardial infarction,10 and death.8 Potential mechanisms underlying this association include the ability of CFH to injure the vascular endothelium,6,11 cause oxidative injury,1 and scavenge nitric oxide,3 all of which lead to vasoconstriction.
Pulmonary arterial hypertension (PAH) is characterized, in part, by vasoconstriction of the pulmonary vascular bed.12 Activation of the nitric oxide signaling pathway is a major therapeutic avenue in PAH.13‐15 In an animal model of hypoxia-induced PAH, infusion of cell-free hemoglobin in mice was associated with increased pulmonary artery pressure (PAP) and right ventricular size.16 In humans with PAH, abnormalities in proteins responsible for hemoglobin processing have been reported.17,18 There are no reports of CFH measurement in the general PAH population or any association with hemodynamics or clinical outcomes. Therapies directed toward preventing the negative effects of CFH are currently being developed19; these therapies could also be studied in patients with PAH if CFH is found to be associated with poor clinical outcomes.
We used a prospective institutional registry and biorepository to test the hypothesis that CFH would be elevated in PAH compared with healthy subjects and patients with pulmonary venous hypertension (PVH), in whom elevated pulmonary pressures are not related to nitric oxide imbalance. We also compared CFH levels in carriers of a mutation associated with heritable PAH, bone morphogenetic protein receptor type 2 (BMPR2), who did not have PAH at the time of enrollment. We further hypothesized that CFH levels would be associated with severity of pulmonary vascular disease and clinical outcomes. The purpose of this study was to determine whether a link exists between elevation in CFH, a potent nitric oxide scavenger, and PAH, a disease characterized by decreased nitric oxide availability.
Materials and Methods
Study Populations
The Vanderbilt University Institutional Review Board approved this study, and all patients gave written informed consent (Vanderbilt University IRB numbers 9401 and 111530). Subjects with PAH for this study were consecutively enrolled in the Vanderbilt Pulmonary Hypertension Research Cohort, a prospective institutional registry containing detailed clinical information and biologic specimens collected over 30 years.20 We identified 200 consecutive patients with PAH presenting for their initial evaluation in the Vanderbilt Pulmonary Vascular Clinic between 2007 and 2012. The Vanderbilt Pulmonary Hypertension Research Cohort also includes unaffected mutation carriers (UMCs) of a BMPR2 mutation. Patients with PAH were diagnosed by experienced clinicians according to consensus guidelines.21 PAH was defined as an invasively measured mean pulmonary artery pressure (mPAP) ≥ 25 mm Hg as well as a pulmonary wedge pressure (PWP) or left ventricular end-diastolic pressure ≤ 15 mm Hg. PAH patient inclusion was restricted to patients with idiopathic PAH (IPAH), heritable PAH (HPAH), or portopulmonary PAH and PAH associated with congenital heart disease and connective tissue disease.
Additionally, we studied three control populations: healthy subjects without known cardiopulmonary disease, patients with PVH, and UMCs. UMCs were studied to determine whether genetic predisposition to PAH is associated with elevated CFH as a potential marker of subclinical pulmonary vascular disease or a mediator of pulmonary vascular disease. We selected consecutive patients with PVH due to ischemic or nonischemic cardiomyopathy enrolled between 2010 and 2012 in the Vanderbilt Main Heart Registry, which includes clinical information and plasma samples from consenting patients evaluated at the Vanderbilt Heart and Vascular Institute. Inclusion in this group required mPAP > 25 mm Hg and PWP > 15 mm Hg measured on right-sided heart catheterization (RHC) closest to the date of blood draw. All patients with PVH had a transpulmonary gradient (mPAP minus PWP) ≤ 18 mm Hg, indicating the absence of a significant vasoreactive contribution to pulmonary hypertension.22
RHC was performed with a balloon-tipped catheter using hemodynamic and fluoroscopic guidance. Heart rate, right atrial pressure, PAP, PWP, and cardiac output were recorded from the RHC closest to the date of blood draw. Cardiac index, pulmonary vascular resistance, and stroke volume were calculated from standard formulas. In patients with PAH, acute vasodilator testing was performed using inhaled nitric oxide, as described previously by our group.23
Blood Collection and Analysis
Samples were obtained at the time of clinic visits or hospitalization for patients, and via the Vanderbilt General Clinical Research Center for healthy control subjects and UMCs. Patient samples were not specifically obtained prior to the initiation of therapy or RHC. Plasma samples were collected using standard blood sampling techniques into ethylenediaminetetraacetic acid plasma tubes. Plasma was collected either from central venous access catheters or via venipuncture. Ethylenediaminetetraacetic tubes were centrifuged within 45 min at 4,000 rpm and the plasma fraction immediately aliquoted as 200-μL aliquots and stored at −80°C. CFH was measured in duplicate in human plasma using spectrophotometric methods with the QuantiChrom Hemoglobin Assay Kit (BioAssay Systems).
Statistical Analysis
All values are reported as mean ± SD or median (interquartile range [IQR]) and categorical variables as absolute value and percent. Between-group differences were calculated using Mann-Whitney U test or χ2 test. Correlation coefficients were calculated using the Spearman method. The impact of CFH on hemodynamics was assessed using linear regression with adjustment for age, sex, and PAH cause. Time to event analysis was performed using the Kaplan-Meier log-rank test and Cox regression to assess the impact of CFH on time to first hospitalization or death. We prespecified a Cox regression analysis controlling for PAH cause, age, and sex, as these variables are known to influence survival and clinical course. Statistical analyses were performed using SPSS 20 software (IBM).
Results
We measured CFH in 200 patients with PAH, 29 patients with PVH, 19 healthy subjects, and 16 UMCs. The following subtypes of PAH were represented: IPAH (n = 85), HPAH (n = 25), connective tissue disease-associated (CTD-PAH) (n = 63), congenital heart disease (n = 21), and portopulmonary PAH (n = 6). Demographic, laboratory, clinical, and hemodynamic data for the tested cohorts are shown in Table 1. Invasive hemodynamics were not obtained in the control or UMC cohorts.
TABLE 1 ] .
Demographics and Clinical Data
| Variable | PAH (n = 200) | PVH (n = 29) | Control (n = 19) | UMC (n = 16) |
| Female sex, % | 155 (78) | 7 (24) | 14 (74) | 11 (69) |
| Age, y | 50 (18) | 57 (11) | 34 (11) | 47 (16) |
| Race, No. (%) | ||||
| White | 176 (88) | 27 (94) | 17 (89) | 16 (100) |
| Black | 24 (12) | 1 (3) | 2 (11) | … |
| Other | 2 (< 1) | 1 (3) | … | … |
| BMI, kg/m2 | 30 (7) | 27 (4) | N/A | 25 (7) |
| NYHA | 2.4 (0.8) | 2.9 (0.5) | … | … |
| 6MWD, m | 316 (113) | 327 (87) | … | … |
| BNP, pg/mL | 371 (628) (n = 116) | 861 (770) | … | … |
| Creatinine, mg/dL | 1.1 (0.4) (n = 151) | 1.9 (2.3) | … | … |
| Hemoglobin, mg/dL | 13.8 (2.2) (n = 138) | 12.4 (1.8) | … | … |
| Hemodynamics | ||||
| Heart rate, beats per min | 77 (15) | 66 (18) | … | … |
| RAP, mm Hg | 10 (11) | 11 (7) | … | … |
| Systolic PAP, mm Hg | 77 (21) | 57 (14) | … | … |
| mPAP, mm Hg | 49 (13) | 38 (9) | … | … |
| PWP, mm Hg | 10 (6) | 22 (7) | … | … |
| Cardiac index, L/min/m2 | 2.6 (1.1) | 2.4 (0.5) | … | … |
| PVR, Wood Units | 9.0 (4.9) | 3.7 (2.3) | … | … |
| Mvo2, % | 66 (9) | 63 (8) | … | … |
| Δ mPAP | 5 (6) | N/A | … | … |
| Δ PVR | 1.2 (2.5) | N/A | … | … |
Data shown as mean (SD) except where indicated. Δ Values indicate baseline minus post-nitric oxide inhalation. 6MWD = 6-min walk distance; BNP = brain natriuretic peptide; mPAP = mean pulmonary artery pressure; mPVR = mean pulmonary vascular resistance; Mvo2 = mixed venous oxygen saturation; N/A = not available; NYHA = New York Heart Association; PAH = pulmonary arterial hypertension; PAP = pulmonary artery pressure; PVH = pulmonary venous hypertension; PVR = pulmonary vascular resistance; PWP = pulmonary wedge pressure; RAP = right atrial pressure; UMC = unaffected carrier of bone morphogenetic protein receptor type II mutation.
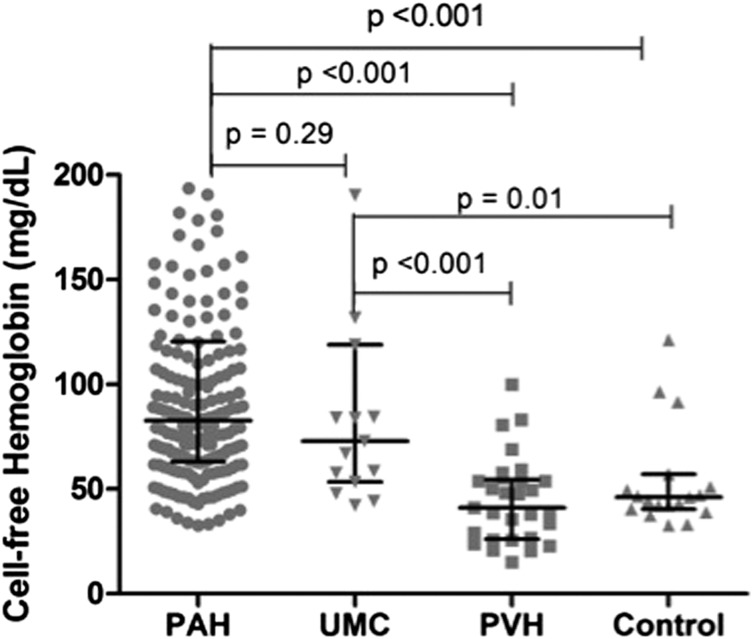
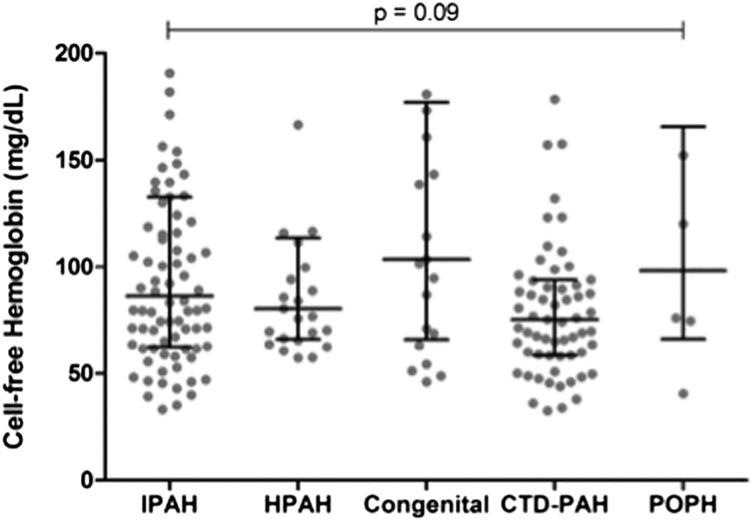
Plasma CFH was significantly elevated in patients with PAH compared with both control subjects and patients with PVH (Fig 1), with median levels that were approximately twofold higher, whereas there was no difference between the PVH and control cohorts. In addition, CFH was elevated in UMCs compared with both the PVH and control cohorts. No difference was found in CFH across PAH subtypes (Fig 2). We found no significant difference in CFH level in patients who were on one or more PAH-specific therapies at the time of blood draw (n = 169, 85%) compared with treatment-naive patients (101 ± 63 mg/dL vs 129 ± 102 mg/dL, respectively; P = .15).
Figure 1 –
Cell-free hemoglobin (CFH) by group. CFH is significantly elevated in patients with PAH compared with healthy subjects and patients with PVH. No difference was found between PAH and UMC. UMCs had higher CFH than healthy control subjects and patients with PVH. Across-group comparison was initially performed using the Kruskal-Wallis test (P < .001). Between-group comparisons were performed using the Mann-Whitney U test. Bars denote median (interquartile range). PAH = pulmonary arterial hypertension; PVH = pulmonary venous hypertension; UMC = unaffected mutation carrier.
Figure 2 –
CFH by PAH subtype. No significant difference in CFH was found across different subtypes of PAH. Across-group comparison performed using Kruskal-Wallis test (P = .09). Bars denote median (interquartile range). CTD-PAH = connective tissue disease-associated PAH; HPAH = heritable PAH; IPAH = idiopathic PAH; POPH = portopulmonary hypertension. See Figure 1 legend for expansion of other abbreviations.
To study whether patients with PAH had increased release of CFH into the circulation, we analyzed markers of RBC fragility (RBC distribution width [RDW], mean corpuscular volume [MCV]), markers of hemolysis (total bilirubin, peripheral smear analysis for schistocytes), and liver function (total bilirubin, aspartate aminotransferase, alanine aminotransferase, albumin) drawn at the time of CFH measurements. All of these indexes were available on 103 (51.5%) of the PAH cohort. There was no association between CFH and RDW, MCV, alanine aminotransferase, or albumin, nor were schistocytes seen in any of the patients. Increased levels of CFH were significantly associated with increased total bilirubin (ρ = 0.26, P < .01) and aspartate aminotransferase (ρ = 0.21, P = .04). Additionally, the association of CFH with total bilirubin was independent of the presence of chronic liver disease (β = 0.03; 95% CI, 0.01-0.05; P < .01; after controlling for chronic liver disease, for every increase of CFH by 10 mg/dL, there was an associated increase in total bilirubin by 0.03 mg/dL). We prospectively reviewed blood smears from 15 patients with PAH, none of which showed evidence of hemolysis.
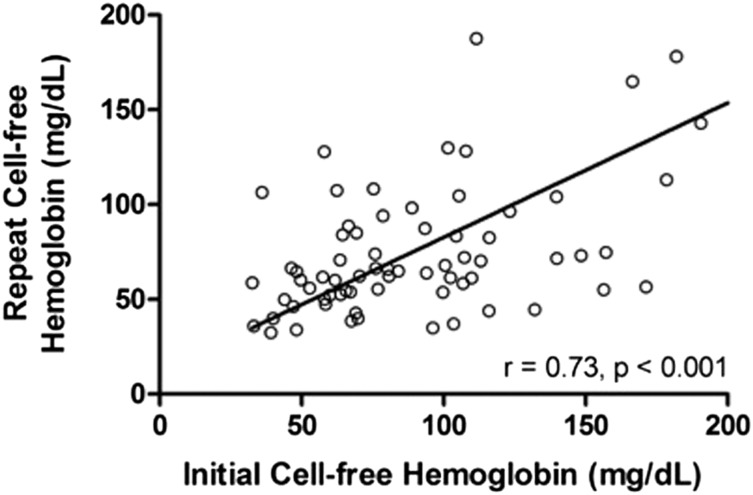
To determine whether CFH in PAH reflects transient physiologic conditions or whether elevations are stable over time, we repeated CFH measurement at the next clinical evaluation in patients with the highest and lowest 10 values among the IPAH, HPAH, and CTD-PAH populations. The correlation between baseline and repeat values was strong (ρ = 0.73, P < .001) (Fig 3), indicating elevated values remain stable over time (median interval, 6 months; IQR, 4-12 months).
Figure 3 –
Correlation of baseline and repeat CFH values in patients with PAH. CFH was measured at the time of first clinical evaluation and repeated at the next clinical visit in patients with the highest and lowest 10 values among the IPAH, HPAH, and CTD-PAH populations. We found a strong correlation between initial and repeat measurements indicting that CFH values are relatively stable over time and do not simply reflect transient physiologic conditions. Line indicates best-fit Spearman correlation. See Figure 1 and 2 legends for expansion of abbreviations.
In linear regression analysis adjusted for age, sex, and PAH cause, we found no impact of CFH level on mPAP (β = 0.07; 95% CI, −0.01 to 0.04; P = .32) or cardiac index (β = −0.11; 95% CI, −0.004 to 0.001; P = .16). We found a modest but statistically significant effect of CFH on pulmonary vascular resistance (PVR) (β = 0.16; 95% CI, 0.001-0.02; P = .037). CFH was modestly correlated with mPAP, PVR, and cardiac index in patients with PAH (Table 2). The median time from blood draw to RHC was 1.8 months (IQR, 0.4-17 months). The majority of patients with PAH (176 of 200, 88%) underwent acute vasodilator testing with inhaled nitric oxide. We found no association between CFH value and the change in mPAP (ρ = −0.06, P = .40) or change in PVR (ρ = 0.05, P = .56). We dichotomized patients with IPAH according to whether they met clinical criteria for an acute vasodilator response, defined as a fall in mPAP > 10 mm Hg to an absolute value < 40 mm Hg.24 Other PAH causes were excluded because of rare vasodilator responsiveness. When comparing patients with IPAH meeting clinical criteria for an acute vasodilator response (n = 5) to those without a significant response (n = 80), there was a large numerical difference in CFH level that did not meet statistical significance (71 ± 30 mg/dL vs 112 ± 73 mg/dL, P = .22).
TABLE 2 ] .
Correlation of Cell-Free Hemoglobin With Hemodynamics
| Variable | Correlation Coefficient | P Value |
| RAP | −0.01 | .910 |
| mPAP | 0.16 | .028 |
| mPWP | 0.024 | .740 |
| Cardiac index | −0.18 | .023 |
| PVR | 0.21 | .005 |
| Mvo2 | −0.07 | .360 |
Correlation coefficients analyzed using Spearman method. mPWP = mean pulmonary wedge pressure. See Table 1 legend for expansion of other abbreviations.
Clinical Outcomes
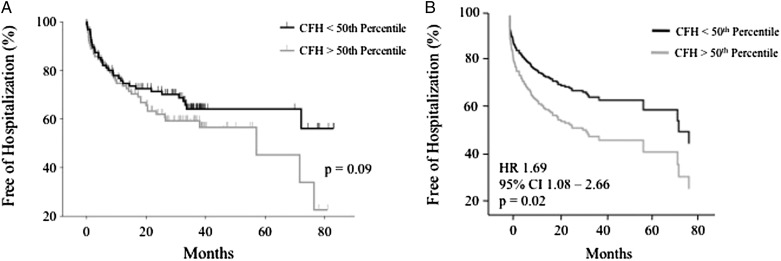
We analyzed the relationship between CFH level, time to first hospitalization, and death in the PAH cohort. There was no difference in CFH between patients who died (75 mg/dL; IQR, 60-107) and those who survived through the most current follow-up (83 mg/dL; IQR, 60-101; P = .3). In time-to-event analysis of patients with CFH above and below the 50th percentile, there was no association between CFH level and time to death (log rank, P = .71). There was a trend toward significance in the time to first hospitalization (log rank, P = .09) (Fig 4A). After controlling for age, sex, and cause of PAH, CFH above the 50th percentile was significantly associated with an increased risk of PAH-related hospitalization compared with patients with CFH below the 50th percentile (hazard ratio, 1.69; 95% CI, 1.08-2.66; P = .02) (Fig 4B). We found no association between death and CFH above and below the 50th percentile when controlling for age, sex, and cause of PAH (hazard ratio, 0.97; 95% CI, 0.45-2.11; P = .94).
Figure 4 –
Impact of CFH level on time to first PAH-related hospitalization. A, Kaplan-Meier curves of PAH-related hospitalization for patients above and below the 50th percentile of CFH level. There was no significant difference between patients with CFH above or below the 50th percentile in time to hospitalization. B, Cox proportional hazards analysis. After controlling for age, sex, and cause of PAH, CFH above the 50th percentile was associated with a 69% increase in the risk of hospitalization. HR = hazard ratio. See Figure 1 legend for expansion of other abbreviation.
Discussion
We have shown that the potent vasoconstrictor CFH is significantly elevated in a large cohort of patients with PAH compared with healthy control subjects and patients with PVH. Increased levels of CFH in patients with PAH were associated with increased mPAP amd PVR and decreased cardiac index. CFH was elevated in all subtypes of PAH tested, with no differences observed among them. In a cohort of unaffected carriers of a BMPR2 mutation, CFH was also significantly elevated compared with healthy control subjects and patients with PVH. Finally, we demonstrated a relationship between CFH and time to PAH-related hospitalization when controlling for variables that are known to influence outcomes in PAH (age, sex, and cause). To our knowledge, this is the first study to identify elevation of the nitric oxide scavenger CFH and a relationship with clinical outcomes in a large cohort of patients with PAH.
In both human studies and animal models of disease, CFH produces a pathobiology that shares common features with PAH.11 In patients with sickle cell disease, CFH is known to induce vasoconstriction and NO consumption.3,25 In an animal model of CFH exposure, a vasoconstrictive effect was confirmed and CFH induced vascular endothelial damage, both of which are features of PAH.11 Given common findings between PAH pathology and diseases characterized by increased CFH, the role of CFH in the pathophysiology of PAH deserves further study. The strong association between CFH and clinical outcomes in other diseases suggests a potentially valuable therapeutic target. Therapies targeting the prevention of CFH accumulation are currently under investigation and may represent an additional avenue for increasing NO availability in PAH.
CFH can accumulate through overproduction in the setting of hemolysis or underprocessing to the ultimate waste product of bilirubin via abnormalities in haptoglobin, hemopexin, CD163, or heme oxygenase-1.26 Our study was not designed to provide a mechanism for CFH accumulation in PAH, but we did not observe significant RBC fragility based on measurement of RDW, MCV, and manual review of blood smears. However, other groups have demonstrated that RDW is increased in patients with pulmonary hypertension and provides incremental prognostic value over established biomarkers, such as NTpro-brain natriuretic peptide and 6-min walk distance.27,28 There was an association between increased CFH and total bilirubin that was independent of chronic liver disease, possibly suggesting increased RBC turnover, but there was no evidence of high-grade, mechanical hemolysis. Kline et al29 and Zagorski et al30 have shown that hemolysis due to shear stress may be responsible for CFH elevation observed in humans with and experimental models of acute pulmonary embolism. Iron deficiency is highly prevalent in PAH and is associated with worse disease severity and clinical outcomes.31‐34 It is possible that the increased RBC turnover observed in our study contributes to iron deficiency by increasing the need for further RBC production, thus, depleting iron stores. This is only speculative, however, as we did not measure markers of iron metabolism in our study. We did not test for hemoglobin processing abnormalities, such as haptoglobin, hemopexin, or CD163, in our cohort. Our finding of elevated CFH in the absence of overt hemolysis warrants further interrogation of the hemoglobin processing pathways and markers of iron metabolism in PAH.
Our control groups were selected to maximize the clinical relevance of our findings. Comparison with healthy subjects confirmed that CFH is elevated in PAH. Comparison with patients with PVH, in whom elevated pulmonary pressure is passively transmitted from the left atrium, implies that primary pulmonary vascular pathology may contribute to elevation in CFH. The finding of elevated CFH in UMCs compared with control subjects suggests that CFH may be a subclinical marker of disease in patients genetically predisposed to develop PAH or that CFH may be a mediator of disease and not solely a biomarker. Polymorphisms in the BMPR2 gene have also been associated with increased risk of developing pulmonary hypertension in patients with sickle cell disease,35 suggesting that BMPR2 may modify an interaction between pulmonary vascular pathology and abnormalities of hemoglobin processing.
Limitations
The assay used in this study uses a spectrophotometric method for detection of hemoglobin. A limitation of this method may be decreased specificity in detecting only plasma CFH; however, others and we have used spectrophotometric assays to report CFH levels in human studies.8,36,37 We addressed potential selection bias by including consecutively enrolled patients with PAH, UMCs, and healthy subjects. Patients with PVH meeting prespecified hemodynamic criteria were a convenience cohort selected from the Vanderbilt Main Heart Registry. The association between CFH and invasive hemodynamics in the cohort was modest, and we did not find an association between CFH and acute response to nitric oxide; however, the interval between blood draw and RHC was variable—simultaneous measurement of CFH and hemodynamics may have demonstrated a stronger relationship. Despite a large numerical difference, our cohort was likely underpowered to detect a difference in CFH between acute vasodilator responders (n = 5) and those without a response (n = 80). The association between CFH and response to nitric oxide warrants further study in larger cohorts enriched with long-term calcium channel blocker responders. Age and sex were not comprehensively explored as potential confounders according to phenotypic category, although to our knowledge no previous studies of CFH in other patient populations have observed differences according to sex or age.
Our analysis of time to first hospitalization may be biased by loss of follow-up for patients who were treated at other hospitals. However, potentially missed hospitalizations in these patients would tend to underestimate rather than overestimate any association. Finally, our study was not designed to elucidate the mechanism for CFH elevation in PAH; potential mechanistic links between CFH and PAH is an area of ongoing investigation in our experimental models and clinical cohorts.
Conclusions
We found that the nitric oxide scavenger CFH is elevated and modestly correlated with hemodynamics in a large cohort of patients with PAH, a disease characterized by reduced nitric oxide availability. Furthermore, CFH was elevated in unaffected BMPR2 mutation carriers compared with healthy subjects and patients with PVH. Finally, we showed in patients with PAH that elevation of CFH is independently associated with increased risk of hospitalization after adjusting for other factors known to influence outcomes in PAH. Further study of the mechanism of CFH elevation in PAH is required to draw conclusions about its potential as a therapeutic target.
Acknowledgments
Author contributions: E. L. B. is the guarantor and takes responsibility for the integrity of the work. E. L. B. contributed to study design, collected and analyzed the data, performed statistical analysis, wrote initial and final drafts of the manuscript, and approved the final, submitted version of the manuscript; D. R. J. contributed to study design, collected and analyzed the data, performed statistical analysis, revised drafts of the manuscript, and approved the final, submitted version of the manuscript; E. D. A., J. A. B., and L. B. W. contributed to study design, revised drafts of the manuscript, and approved the final, submitted version of the manuscript; L. A. W. contributed to study design, collected and analyzed the data, and approved the final, submitted version of the manuscript; and A. R. H. contributed to study design, analyzed the data, revised drafts of the manuscript, and approved the final, submitted version of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Hemnes has received grants from Pfizer Inc and the National Institutes of Health and has been a consultant to Pfizer Inc and United Therapeutics Corporation. Drs Brittain, Janz, Austin, Bastarache, and Ware and Ms Wheeler have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the American Heart Association or the National Institutes of Health.
ABBREVIATIONS
- BMPR2
bone morphogenetic protein receptor type II
- CFH
cell-free hemoglobin
- CTD-PAH
connective tissue disease-associated pulmonary arterial hypertension
- HPAH
heritable pulmonary arterial hypertension
- IPAH
idiopathic pulmonary arterial hypertension
- IQR
interquartile range
- MCV
mean corpuscular volume
- mPAP
mean pulmonary artery pressure
- PAH
pulmonary arterial hypertension
- PAP
pulmonary artery pressure
- PVH
pulmonary venous hypertension
- PVR
pulmonary vascular resistance
- PWP
pulmonary wedge pressure
- RDW
RBC distribution width
- RHC
right-sided heart catheterization
- UMC
unaffected mutation carrier
Footnotes
FUNDING/SUPPORT: This work was supported by the American Heart Association Fellow to Faculty Award and American College of Cardiology Foundation/Merck Fellowship (Dr Brittain), the American Heart Association Established Investigator Award (Dr Ware), the National Institutes of Health [Grants T32 HL087738 (Dr Janz), K08 HL093363 (Dr Hemnes), K23 HL0987431 (Dr Austin), R21 HL117676 (Dr Bastarache), and K24 HL103836 (Dr Ware)], and National Center for Research Resources/National Institutes of Health [Grant 1 UL1 RR024975 (Vanderbilt)].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Billings FT, IV, Ball SK, Roberts LJ, II, Pretorius M. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic Biol Med. 2011;50(11):1480-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reeder BJ, Svistunenko DA, Cooper CE, Wilson MT. The radical and redox chemistry of myoglobin and hemoglobin: from in vitro studies to human pathology. Antioxid Redox Signal. 2004;6(6):954–966. [DOI] [PubMed] [Google Scholar]
- 3.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383-1389. [DOI] [PubMed] [Google Scholar]
- 4.Meyer C, Heiss C, Drexhage C, et al. Hemodialysis-induced release of hemoglobin limits nitric oxide bioavailability and impairs vascular function. J Am Coll Cardiol. 2010;55(5):454-459. [DOI] [PubMed] [Google Scholar]
- 5.Gladwin MT, Kim-Shapiro DB. Storage lesion in banked blood due to hemolysis-dependent disruption of nitric oxide homeostasis. Curr Opin Hematol. 2009;16(6):515-523. [DOI] [PubMed] [Google Scholar]
- 6.Minneci PC, Deans KJ, Zhi H, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115(12):3409-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donadee C, Raat NJH, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124(4):465-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janz DR, Bastarache JA, Peterson JF, et al. Association between cell-free hemoglobin, acetaminophen, and mortality in patients with sepsis: an observational study. Crit Care Med. 2013;41(3):784-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. 2008;359(21):2254-2265. [DOI] [PubMed] [Google Scholar]
- 10.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299(19):2304-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baek JH, D’Agnillo F, Vallelian F, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122(4):1444-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351(16):1655-1665. [DOI] [PubMed] [Google Scholar]
- 13.Archer SL, Michelakis ED. Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. N Engl J Med. 2009;361(19):1864-1871. [DOI] [PubMed] [Google Scholar]
- 14.Ghofrani H-A, Galiè N, Grimminger F, et al. ; PATENT-1 Study Group. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330-340. [DOI] [PubMed] [Google Scholar]
- 15.Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007;131(6):1917-1928. [DOI] [PubMed] [Google Scholar]
- 16.Buehler PW, Baek JH, Lisk C, et al. Free hemoglobin induction of pulmonary vascular disease: evidence for an inflammatory mechanism. Am J Physiol Lung Cell Mol Physiol. 2012;303(4):L312-L326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bielecki M, Kowal K, Lapinska A, Chyczewski L, Kowal-Bielecka O. Increased release of soluble CD163 by the peripheral blood mononuclear cells is associated with worse prognosis in patients with systemic sclerosis. Adv Med Sci. 2013;58(1):126-133. [DOI] [PubMed] [Google Scholar]
- 18.Tantawy AAG, Adly AAM, Ismail EAR. Soluble CD163 in young sickle cell disease patients and their trait siblings: a biomarker for pulmonary hypertension and vaso-occlusive complications. Blood Coagul Fibrinolysis. 2012;23(7):640-648. [DOI] [PubMed] [Google Scholar]
- 19.National Institutes of Health Clinical Center. Vanderbilt University. Acetominophen for the reduction of oxidative injury in severe sepsis (ACROSS). NCT01739361. ClinicalTrials.gov. Bethesda, MD: National Library of Medicine; 2012. http://clinicaltrials.gov/show/NCT01739361. Updated December 21, 2013.
- 20.Larkin EK, Newman JH, Austin ED, et al. Longitudinal analysis casts doubt on the presence of genetic anticipation in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186(9):892-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(suppl 25):D42-D50. [DOI] [PubMed] [Google Scholar]
- 22.Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation. 2012;126(8):975-990. [DOI] [PubMed] [Google Scholar]
- 23.Robbins IM, Hemnes AR, Pugh ME, et al. High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail. 2014;7(1):116-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sitbon O, Humbert M, Jaïs X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111(23):3105-3111. [DOI] [PubMed] [Google Scholar]
- 25.Machado RF, Gladwin MT. Pulmonary hypertension in hemolytic disorders: pulmonary vascular disease: the global perspective. Chest. 2010;137(6_suppl):30S-38S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaer DJ, Buehler PW. Cell-free hemoglobin and its scavenger proteins: new disease models leading the way to targeted therapies. Cold Spring Harb Perspect Med. 2013;3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhodes CJ, Wharton J, Howard LS, Gibbs JSR, Wilkins MR. Red cell distribution width outperforms other potential circulating biomarkers in predicting survival in idiopathic pulmonary arterial hypertension. Heart. 2011;97(13):1054-1060. [DOI] [PubMed] [Google Scholar]
- 28.Hampole CV, Mehrotra AK, Thenappan T, Gomberg-Maitland M, Shah SJ. Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. Am J Cardiol. 2009;104(6):868-872. [DOI] [PubMed] [Google Scholar]
- 29.Kline JA, Marchick MR, Hogg MM. Reduction in plasma haptoglobin in humans with acute pulmonary embolism causing tricuspid regurgitation. J Thromb Haemost. 2009;7(9):1597-1599. [DOI] [PubMed] [Google Scholar]
- 30.Zagorski J, Marchick MR, Kline JA. Rapid clearance of circulating haptoglobin from plasma during acute pulmonary embolism in rats results in HMOX1 up-regulation in peripheral blood leukocytes. J Thromb Haemost. 2010;8(2):389-396. [DOI] [PubMed] [Google Scholar]
- 31.Ruiter G, Lankhorst S, Boonstra A, et al. Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur Respir J. 2011;37(6):1386-1391. [DOI] [PubMed] [Google Scholar]
- 32.Rhodes CJ, Howard LS, Busbridge M, et al. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: clinical prevalence, outcomes, and mechanistic insights. J Am Coll Cardiol. 2011;58(3):300-309. [DOI] [PubMed] [Google Scholar]
- 33.Soon E, Treacy CM, Toshner MR, et al. Unexplained iron deficiency in idiopathic and heritable pulmonary arterial hypertension. Thorax. 2011;66(4):326-332. [DOI] [PubMed] [Google Scholar]
- 34.Decker I, Ghosh S, Comhair SA, et al. High levels of zinc-protoporphyrin identify iron metabolic abnormalities in pulmonary arterial hypertension. Clin Transl Sci. 2011;4(4):253-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashley-Koch AE, Elliott L, Kail ME, et al. Identification of genetic polymorphisms associated with risk for pulmonary hypertension in sickle cell disease. Blood. 2008;111(12):5721-5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janz DR, Bastarache JA, Sills G, et al. Association between haptoglobin, hemopexin and mortality in adults with sepsis. Crit Care. 2013;17(6):R272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adamzik M, Hamburger T, Petrat F, Peters J, de Groot H, Hartmann M. Free hemoglobin concentration in severe sepsis: methods of measurement and prediction of outcome. Crit Care. 2012;16(4):R125. [DOI] [PMC free article] [PubMed] [Google Scholar]