Abstract
BACKGROUND:
The association between HIV and emphysema remains incompletely understood. We sought to determine whether HIV is an independent risk factor for emphysema severity and whether markers of HIV severity and systemic biomarkers of inflammation (IL-6), altered coagulation (D-dimer), and immune activation (soluble CD14) are associated with emphysema.
METHODS:
We performed a cross-sectional analysis of 114 participants with HIV infection and 89 participants without HIV infection in the Examinations of HIV-Associated Lung Emphysema (EXHALE) study. Participants underwent chest CT imaging with blinded semiquantitative interpretation of emphysema severity, distribution, and type. We generated multivariable logistic regression models to determine the risk of HIV for radiographic emphysema, defined as > 10% lung involvement. Similar analyses examined associations of plasma biomarkers, HIV RNA, and recent and nadir CD4 cell counts with emphysema among participants with HIV infection.
RESULTS:
Participants with HIV infection had greater radiographic emphysema severity with increased lower lung zone and diffuse involvement. HIV was associated with significantly increased risk for > 10% emphysema in analyses adjusted for cigarette smoking pack-years (OR, 2.24; 95% CI, 1.12-4.48). In multivariable analyses restricted to participants with HIV infection, nadir CD4 < 200 cells/μL (OR, 2.98; 95% CI, 1.14-7.81), and high soluble CD14 level (upper 25th percentile) (OR, 2.55; 95% CI, 1.04-6.22) were associated with increased risk of > 10% emphysema. IL-6 and D-dimer were not associated with emphysema in HIV.
CONCLUSIONS:
HIV is an independent risk factor for radiographic emphysema. Emphysema severity was significantly greater among participants with HIV infection. Among those with HIV, nadir CD4 < 200 cells/μL and elevated soluble CD14 level were associated with emphysema, highlighting potential mechanisms linking HIV with emphysema.
A leading global cause of morbidity and mortality, COPD is common among individuals with HIV infection.1,2 In the general population, emphysema-predominant COPD is associated with impaired health status3,4 and increased risk of pulmonary malignancy,5 cardiovascular disease, chronic kidney disease, cerebrovascular disease,6 osteoporosis,7 and mortality.8 Cigarette smoking9 and α1-antitrypsin deficiency10 are well-established risk factors for emphysema, with growing evidence linking inflammation and aging to emphysema.11,12
An increased risk for bullous emphysema in individuals with HIV infection was reported early in the HIV/AIDS epidemic,1 yet the link between HIV and emphysema remains incompletely understood. Sequelae of Pneumocystis pneumonia, other opportunistic infections, and AIDS-related wasting play a role in destructive lung changes in advanced HIV.13 Early in the antiretroviral therapy (ART) era, however, increased emphysema was described among individuals with HIV infection who had no prior opportunistic lung infections.14
HIV infection is associated with chronic inflammation, endothelial dysfunction, altered coagulation, and immune activation, which are tightly linked to comorbidities and early mortality in HIV, even among those receiving effective ART15‐20; whether emphysema is associated with biomarkers reflective of these factors is unknown. Therefore, in the current study, we determined whether HIV infection is a risk factor for radiographic emphysema in the current ART era, characterizing emphysema semiquantitatively on chest CT scans and determining whether differences in the severity, distribution, and type of emphysema by HIV exist. We explored whether radiographic emphysema is associated with markers of HIV severity and systemic biomarkers of inflammation (IL-6), altered coagulation (D-dimer), and immune activation (soluble CD14 [sCD14]).
Materials and Methods
Study Design and Cohort
We performed a cross-sectional analysis of data from 114 participants with HIV infection and 89 participants without HIV infection enrolled from 2009 to 2012 in the Examinations of HIV-Associated Lung Emphysema (EXHALE) study, a substudy of the Veterans Aging Cohort Study.21 Enrollment was stratified by HIV and smoking status. All participants signed written informed consent. This study was approved by all appropriate institutional review boards. Methodologic details regarding the cohort, data collection, statistical analyses, and institutional review board approval are provided in e-Appendix 1 (142.1KB, pdf) .
Chest CT Scan Examination and Interpretation
Noncontrast CT images were acquired using a standard protocol at enrollment. Supine scans from the lung apices to bases were obtained at end inspiration with multidetector CT scanners calibrated across centers on a standardized lung phantom as part of the research protocol.
Emphysema severity, distribution, and type were determined by a board-certified radiologist trained in thoracic imaging and blinded to clinical history. Emphysema severity was characterized semiquantitatively by visual inspection (Table 1).5,7 Global severity scores of 0 (no emphysema) through 5 (> 75% emphysema) were assigned to indicate emphysema severity throughout the entirety of the lungs.
TABLE 1 ] .
Emphysema Severity Scores
| Score | Emphysematous Involvement, % | Severity of Emphysema |
| 0 | 0 | None/negligible |
| 1 | 1-10 | Trace |
| 2 | 11-25 | Mild |
| 3 | 26-50 | Moderate |
| 4 | 51-75 | Severe |
| 5 | > 75 | Very severe |
Craniocaudal distribution was assessed by presence of emphysema in upper, middle, and lower lung zones, comprising the upper lobes, middle lobe and lingula, and lower lobes, respectively. Diffuse emphysema was defined as the presence of emphysema in each zone. Emphysema type was categorized as centrilobular, paraseptal, panlobular, and bullous.
Other Data Collection
Plasma biomarker levels, including IL-6 (pg/mL), D-dimer (μg/mL), and sCD14 (ng/mL), were measured at enrollment (Laboratory for Clinical Biochemistry Research, University of Vermont).22 HIV RNA levels (copies/mL) and CD4 cell counts (cells/μL) within 12 months were obtained from US Veterans Affairs Healthcare System (VA) laboratory data and ART use from VA pharmacy data21; nadir CD4 cell counts reflected lowest available VA laboratory values. Standardized questionnaires assessed exposures, including cigarette, marijuana, injection drug, and unhealthy alcohol use.23 Smoking status was classified as never (< 100 lifetime cigarettes), former (quit > 1 year ago), and current (smoked within the past year). Pack-years were based on average number of cigarettes per day and years smoked. COPD was defined by a postbronchodilator FEV1/FVC ratio less than the lower limit of normal; reference populations for spirometry and diffusing capacity of the lung for carbon monoxide (Dlco) were based on National Health and Nutrition Examination Survey III data.24,25 Pneumonia history was based on International Classification of Diseases, Ninth Revision codes from VA administrative databases.
Statistical Analysis
Baseline characteristics were compared by HIV status and emphysema using Wilcoxon rank sum tests for continuous and χ2 or Fisher exact tests for categorical variables. P < .05 was considered statistically significant. Because the median global severity score was 1 (corresponding to 1%-10% emphysema), we used a threshold of > 1 (ie, > 10% emphysema) in logistic regression models as the outcome. Elevated biomarker levels were defined as the upper 25th percentile.
To determine the independent risk of emphysema associated with HIV infection and biomarkers, we generated multivariable logistic regression models that included all participants. Pack-years of cigarette smoking are strongly associated with emphysema and demonstrate a dose-response relationship.14,26,27 In addition, pack-years is a continuous variable, providing more information on smoking history than smoking status alone. As such, pack-years was selected a priori as an important variable to include in all adjusted models. Age was not included due to covariance with pack-years. Biomarkers were retained in multivariable models if they remained statistically significant. Because we could not determine the temporal relationship between emphysema and pneumonia in the data and because pneumonia may be in the causal pathway by which HIV increases emphysema risk, we did not include pneumonia history in the main multivariable model. Nonetheless, to explore this potential association, a model including HIV, pack-years, and pneumonia history was generated. Similar analyses restricted to participants with HIV infection examined the association of HIV severity and biomarkers with emphysema.
Results
Baseline Characteristics
Most participants were black men, and those with HIV infection were slightly older (Table 2). Although there was no significant difference in cigarette smoking, marijuana and injection drug use, prior bacterial pneumonia and TB were significantly more common in participants with HIV infection. Most with HIV infection were receiving ART, had undetectable HIV RNA, and had recent CD4 ≥ 200 cells/μL, although more than one-half had nadir CD4 < 200 cells/μL. sCD14 and IL-6 levels were significantly higher among participants with HIV infection (P < .001). Participants with HIV infection had a significantly lower BMI, although few were underweight.
TABLE 2 ] .
Baseline Characteristics of Participants by HIV Status
| Characteristic | HIV Infection (n = 114) | No HIV Infection (n = 89) | P Value |
| Age, y | 55 (50-58) | 52 (48-57) | .07 |
| Male sex | 97 | 85 | .002 |
| Race/ethnicity | .1 | ||
| Black | 67 | 69 | |
| White | 18 | 24 | |
| Hispanic/other | 15 | 7 | |
| BMI, kg/m2 | 25.8 (23.4-29.3) | 28.5 (25.9-33.8) | < .001 |
| Cigarette smoking history | .7 | ||
| Never smoker | 16 | 20 | |
| Former smoker | 22 | 24 | |
| Pack-y | 18 (13-43) | 18 (1-39) | .5 |
| Current smoker | 62 | 56 | |
| Pack-y | 27 (12-41) | 22 (11-34) | .3 |
| Other substance abuse, ever | |||
| Marijuana (smoked) | 85 | 72 | .04 |
| Alcohol | 24 | 21 | .6 |
| Injection drug use | 32 | 10 | < .001 |
| COPD (FEV1/FVC < LLN) | 20 | 12 | .1 |
| Dlco, % predicted | 62.5 (53.6-74.4) | 67.0 (59.1-76.9) | .07 |
| FEV1, % predicted | 91.4 (81.9-103.2) | 91.6 (79.2-102.6) | 1.0 |
| Prior pneumonia | |||
| Bacterial, community acquired | 20 | 3 | < .001 |
| Pneumocystis jirovecii | 1 | 0 | 1.0 |
| Mycobacterium tuberculosis | 8 | 1 | .03 |
| Emphysema severity | .01 | ||
| None/negligible (0%) | 40 | 46 | |
| Trace (1%-10%) | 27 | 37 | |
| Mild (11%-25%) | 19 | 8 | |
| Moderate (26%-50%) | 5 | 8 | |
| Severe (51%-75%) | 9 | 1 | |
| Very severe (> 75%) | 0 | 0 | |
| Emphysema typea | |||
| Centrilobular | 57 | 48 | .2 |
| Paraseptal | 33 | 30 | .7 |
| Panlobular | 3 | 0 | .3 |
| Bullous | 19 | 15 | .4 |
| Serum biomarkers | |||
| IL-6, pg/mL | 1.81 (1.28-3.43) | 1.23 (0.94-2.07) | < .001 |
| sCD14, ng/mL | 1,671 (1,472-2,128) | 1,386 (1,171-1,569) | < .001 |
| D-dimer, μg/mL | 0.26 (0.17-0.47) | 0.28 (0.17-0.42) | 1.0 |
| HIV-related variablesb | |||
| CD4 count, cells/μL | 438 (310-605) | ... | ... |
| CD4 < 200 cells/μL | 14 | ... | ... |
| Nadir CD4 < 200 cells/μL | 61 | ... | ... |
| Detectable HIV RNA (≥ 400 copies/mL) | 20 | ... | ... |
| ART use | 93 | ... | ... |
Data are presented as median (interquartile range) or %. ART = antiretroviral therapy; Dlco = diffusing capacity of the lung for carbon monoxide; LLN = lower limit of normal; sCD14 = soluble CD14.
Percents do not add to 100 because participants may have had emphysema in more than one lung zone and more than one type of emphysema.
Analyses for HIV-related variables are restricted to participants with HIV infection.
Emphysema Severity, Distribution, and Type
Participants with HIV infection were more likely to have a greater percentage of lung involved with emphysema based on global severity scores (Table 2). Thirty-three percent of those with HIV infection had > 10% emphysema compared with only 17% of those without HIV infection (P = .01). Among participants with HIV infection, 41% with nadir CD4 < 200 cells/μL had > 10% emphysema compared with 22% with higher nadir CD4 counts (P = .04) (e-Table 1 (142.1KB, pdf) ).
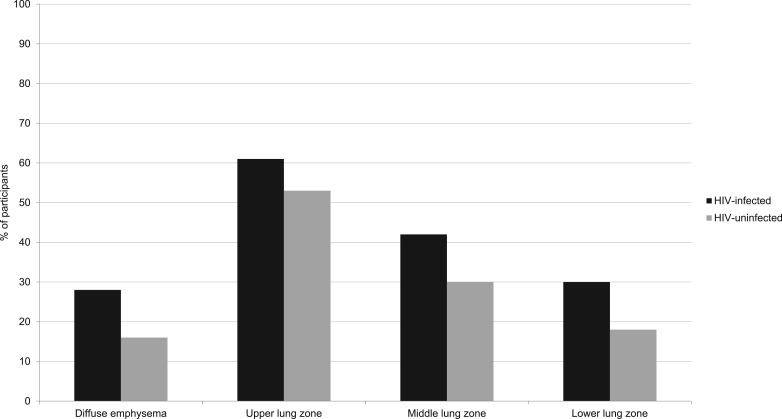
Emphysema distribution also differed by HIV (Fig 1). Nearly all participants with emphysema had upper lung zone involvement, but because those with HIV infection also had more lower lung involvement (P = .05), they were significantly more likely to have diffuse emphysema compared with those without HIV infection (28% vs 16%, P = .04).
Figure 1 –
Emphysema distribution by HIV status.
However, emphysema type, including bullous emphysema, was similar in participants with and without HIV infection (Table 2). Many had more than one emphysema type. Centrilobular emphysema was the predominant type followed by paraseptal emphysema.
Risk Factors for > 10% Emphysema
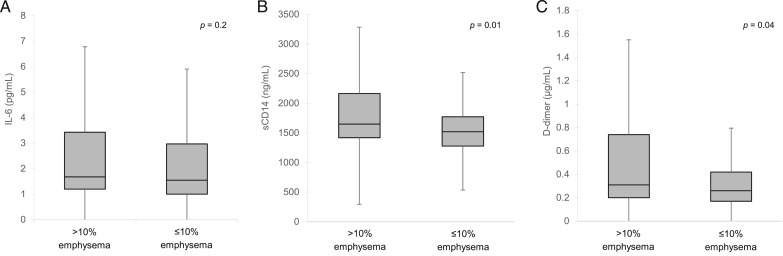
When comparing all participants, significantly more individuals with > 10% emphysema had HIV (Table 3). Those with > 10% emphysema were also significantly more likely to have spirometrically diagnosed COPD, lower Dlco, lower BMI, heavier smoking, marijuana use, and prior bacterial pneumonia. Median sCD14 and D-dimer levels were higher in this group (Fig 2).
TABLE 3 ] .
Baseline Characteristics of Participants by > 10% Emphysema
| Characteristic | > 10% Emphysema (n = 53) | ≤ 10% Emphysema (n = 150) | P Value |
| Age, y | 57 (52-61) | 52 (47-57) | < .001 |
| Male sex | 96 | 91 | .2 |
| Race/ethnicity | .3 | ||
| Black | 70 | 68 | |
| White | 25 | 19 | |
| Hispanic/other | 6 | 13 | |
| BMI, kg/m2 | 24.2 (21.2-27.8) | 28.1 (25.2-32.3) | < .001 |
| Cigarette smoking history | .001 | ||
| Never smoker | 2 | 23 | |
| Former smoker | 22 | 23 | |
| Pack-y | 41 (15-50) | 17 (3-33) | .07 |
| Current smoker | 76 | 54 | |
| Pack-y | 36 (18-48) | 21 (10 -34) | .002 |
| Other substance abuse, ever | |||
| Marijuana (smoked) | 92 | 75 | .007 |
| Alcohol | 22 | 23 | .8 |
| Injection drug use | 24 | 22 | .7 |
| COPD (FEV1/FVC < LLN) | 36 | 9 | < .001 |
| Dlco, % predicted | 56.9 (47.4-64.2) | 68.4 (58.6-78.3) | < .001 |
| FEV1, % predicted | 88.4 (75.6-98.3) | 92.1 (82-104.7) | .06 |
| Prior pneumonia | |||
| Bacterial, community acquired | 22 | 9 | .02 |
| Pneumocystis jirovecii | 0 | 1 | 1.0 |
| Mycobacterium tuberculosis | 9 | 3 | .1 |
| HIV infection | 72 | 51 | .008 |
| HIV-related variables (n = 114)a | |||
| CD4 count, cells/μL | 384 (304-591) | 447 (323-633) | .3 |
| CD4 < 200 cells/μL | 21 | 11 | .2 |
| Nadir CD4 < 200 cells/μL | 74 | 54 | .04 |
| Detectable HIV RNA (≥ 400 copies/mL) | 24 | 18 | .6 |
| ART use | 92 | 93 | 1.0 |
Data are presented as median (interquartile range) or %. See Table 1 legend for expansion of abbreviations.
Analyses for HIV-related variables are restricted to participants with HIV infection.
Figure 2 –
Serum biomarkers by radiographic emphysema. A, IL-6 (pg/mL). B, sCD14 (ng/mL). C, D-dimer (μg/mL). sCD14 = soluble CD14.
In bivariate analyses, HIV infection was associated with a significantly increased risk for > 10% radiographic emphysema (unadjusted OR, 2.47; 95% CI, 1.25-4.87) (Table 4). Age, pack-years, ever smoking, prior pneumonia, marijuana use, and elevated sCD14 and D-dimer levels were also important risk factors for > 10% emphysema. Injection drug use was not significantly associated with > 10% emphysema in the sample.
TABLE 4 ] .
ORs for Associations of Baseline Characteristics and > 10% Emphysema on CT Scan Among Participants With and Without HIV Infection
| OR (95% CI) for > 10% Emphysema | ||||
| Characteristic | Unadjusted | Adjusted for Pack-y | Adjusted for Pack-y and History of Pneumonia | Adjusted for Pack-y and High sCD14a |
| HIV infection | 2.47 (1.25-4.87)b | 2.24 (1.12-4.48)b | 1.84 (0.90-3.76) | 1.55 (0.71-3.41) |
| sCD14, upper 25th percentile | 2.63 (1.32-5.21)b | … | … | 2.30 (1.02-5.19)b |
| IL-6, upper 25th percentile | 1.41 (0.70-2.84) | … | … | … |
| D-dimer, upper 25th percentile | 2.14 (1.07-4.25)b | … | … | … |
| History of pneumonia | 2.85 (1.34-6.07)b | … | 2.26 (0.96-5.34) | … |
| Agec | 2.54 (1.59-4.06)d | … | … | … |
| Male sex | 2.63 (0.57-12.0) | … | … | … |
| Race | ||||
| Black | Reference | … | … | … |
| White | 1.28 (0.60-2.74) | … | … | … |
| Hispanic/other | 0.41 (0.12-1.48) | … | … | … |
| Cigarette smoking, pack-ye | 1.34 (1.13-1.59)b | 1.36 (1.13-1.63)b | 1.35 (1.13-1.62)b | 1.39 (1.15-1.68)b |
| Smoking status | ||||
| Never smoker | Reference | … | … | … |
| Former smoker | 11.3 (1.38-93.0)b | … | … | … |
| Current smoker | 16.4 (2.16-125)b | … | … | … |
| Smoking marijuana | 3.75 (1.26-11.2)b | … | … | … |
| Injection drug use | 1.13 (0.53-2.43) | … | … | … |
See Table 1 legend for expansion of abbreviation.
The final multivariable model includes 197 participants (97% of the analytic cohort).
P < .05.
10-y increments.
P < .001.
Increments of 10 pack-y.
In a logistic regression model adjusting for pack-years, the association with HIV infection remained significant (OR, 2.24; 95% CI, 1.12-4.48) (Table 4). A nested multivariable model additionally adjusting for high sCD14 level markedly attenuated the association between HIV infection and > 10% emphysema (OR, 1.55; 95% CI, 0.71-3.41). In a separate model adjusting for pneumonia history, the association of HIV with emphysema was also attenuated (OR, 1.84; 95% CI, 0.90-3.76).
HIV Severity, Biomarkers, and Emphysema
Among participants with HIV infection, those with > 10% emphysema were significantly more likely to have nadir CD4 < 200 cells/μL than those with ≤ 10% emphysema (74% vs 54%, P = .04) (e-Table 2 (142.1KB, pdf) ). Significantly more participants with HIV infection and > 10% emphysema had spirometrically diagnosed COPD and were current smokers. When restricted to participants with HIV infection, sCD14 levels were higher among participants with > 10% emphysema than in those with ≤ 10% emphysema (1,883 vs 1,648, P = .05); there were no significant differences in IL-6 or D-dimer values by emphysema.
In bivariate analyses restricted to participants with HIV infection, low nadir CD4 cell count was associated with increased odds of > 10% emphysema (OR, 2.39; 95% CI, 1.02-5.62) (Table 5). Age, pack-years, and prior pneumonia were also associated with > 10% emphysema. High sCD14 level was a significant risk factor for radiographic emphysema among participants with HIV but not among those without HIV (data not shown). We did not detect an association of ART use and HIV RNA with emphysema. In a multivariable model restricted to participants with HIV infection adjusting for pack-years, both nadir CD4 < 200 cells/μL (OR, 2.98; 95% CI, 1.14-7.81) and high sCD14 (OR, 2.55; 95% CI, 1.04-6.22) retained a significant association with risk of > 10% emphysema.
TABLE 5 ] .
ORs for Associations of Baseline Characteristics and > 10% Emphysema on CT Among Participants With HIV Infection
| OR (95% CI) for > 10% Emphysema | ||
| Characteristic | Unadjusted | Adjusteda |
| Nadir CD4 < 200 cells/μL | 2.39 (1.02-5.62)b | 2.98 (1.14-7.81)b |
| sCD14, upper 25th percentile | 3.95 (1.20-12.9)b | 2.55 (1.04-6.22)b |
| IL-6, upper 25th percentile | 0.77 (0.32-1.85) | … |
| D-dimer, upper 25th percentile | 2.02 (0.86-4.76) | … |
| ART use | 0.82 (0.18-3.66) | … |
| Recent CD4 < 200 cells/μL | 2.27 (0.77-6.64) | … |
| Detectable HIV RNA | 1.37 (0.53-3.56) | … |
| History of pneumonia | 2.45 (1.04-5.74)b | … |
| Agec | 2.19 (1.23-3.92)b | … |
| Male sex | 1.00 (0.09-11.5) | … |
| Race | ||
| Black | Reference | … |
| White | 1.52 (0.56-4.13) | … |
| Hispanic/other | 0.25 (0.05-1.17) | … |
| Cigarette smoking (pack-y)d | 1.24 (1.02-1.51)b | 1.29 (1.05-1.59)b |
| Smoking status | ||
| Never smoker | Reference | … |
| Former smoker | 7.00 (0.77-63.8) | … |
| Current smoker | 10.9 (1.36-87.8)b | … |
| Smoking marijuana | 4.13 (0.88-19.3) | … |
| Injection drug use | 1.11 (0.47-2.60) | … |
See Table 1 legend for expansion of abbreviations.
This final multivariable model includes 111 participants (97% of the analytic cohort with HIV infection).
P < .05.
10-y increments.
Increments of 10 pack-y.
Discussion
We found that radiographic emphysema severity was overall significantly greater in individuals with HIV infection than in those without HIV infection and that HIV infection was an independent risk factor for emphysema defined by > 10% emphysema on chest CT scan using semiquantitative methods. In the present cohort, the upper lung zone was involved in nearly all participants with emphysema. Interestingly, we found that individuals with HIV infection may be more likely to have diffuse emphysema involving the lower lung in addition to the upper and middle lung zones, with results approaching statistical significance.
We also explored the association between systemic biomarkers and emphysema, focusing on markers known to be elevated in HIV infection, reflecting general inflammation (IL-6), immune activation (sCD14), and altered coagulation (D-dimer). Consistent with prior studies, participants with HIV infection compared with those without had significantly higher median IL-6 and sCD14 values despite the majority receiving ART.15,22 We found that elevated sCD14 level was strongly associated with radiographic emphysema in participants with HIV infection. Nested models adjusting for HIV infection and sCD14 level demonstrated substantial attenuation of the association between HIV and emphysema.
The findings suggest that elevated sCD14 is a marker of radiographic emphysema in HIV infection and support the hypothesis that increased risk of emphysema in HIV may be mediated by immune activation. Mucosal epithelial leakiness may be an underlying cause of persistent immune activation during suppressive ART, allowing microbial products, including lipopolysaccharide, to leak into the systemic circulation and activate the immune system.28‐31 Elevated sCD14 levels may reflect greater immune activation as a result of mucosal bacterial translocation despite ART.16,20,32 High sCD14 is associated with increased mortality20 and neurocognitive impairment33 among individuals with HIV infection. Elevated sCD14 levels have also been identified in BAL fluid of smokers in the general population34 and in patients with ARDS,35 supporting a potential link with lung disease. When stratified by HIV, we found that sCD14 was strongly associated with radiographic emphysema in participants with HIV infection but not in those without HIV infection, further suggesting that monocyte activation related to microbial translocation may play a unique role in emphysema in HIV. Given the present sample size, however, we cannot definitively rule out a similar association among individuals without HIV infection.
In addition, we demonstrated that among individuals with HIV infection, nadir CD4 < 200 cells/μL is independently associated with > 10% emphysema. Nadir CD4 cell count reflects past HIV-related immunosuppression and predicts immune recovery.36 Low nadir CD4 counts may induce incompletely reversible defects in immune function37 and correlates with cardiovascular38 and chronic kidney39 diseases. Low nadir CD4 is also a marker for increased likelihood of prior opportunistic infections. In the present population, 23% of participants with HIV infection who had nadir CD4 < 200 cells/μL continued to have CD4 < 200 cells/μL at EXHALE enrollment despite most receiving ART with undetectable HIV RNA. Whether low nadir CD4 cell count is a risk factor for emphysema because of greater immune dysfunction, prior pulmonary infections, or other unmeasured confounders is unclear from this cross-sectional study.
Taken together, the present findings point to intriguing differences that may contribute to emphysema in individuals with HIV infection. First, these data suggest that sCD14 is an important marker of radiographic emphysema in individuals with HIV infection and support the hypothesis that microbial translocation and monocyte activation may be involved in emphysema pathogenesis in HIV. Whether this is mediated by HIV severity remains unknown.32
Second, the increased lower lung and, therefore, diffuse emphysema in individuals with HIV infection points to potential vascular-related pathways consistent with the finding that systemic levels of sCD14 are associated with emphysema severity. Because blood flow tends to be greater in basal and dependent lung segments,40 endothelial dysfunction in the setting of chronic HIV-related immune activation may lead to greater lower lung involvement and diffuse emphysema. Interestingly, this distribution shares features of α1-antitrypsin deficiency. Low α1-antitrypsin levels have been associated with HIV infection and elevated plasma sCD14 levels.41,42
To assess emphysema severity, we used a validated, semiquantitative approach.5,7,43 Consistent with other studies, CT scans were scored by a single radiologist.5,7,43 Studies have reported low interobserver variability for emphysema assessment.44‐46 Although we did not include purely quantitative assessments, other studies suggested a strong correlation between density mask and visual assessment of emphysema.43,47 Compared with purely quantitative analysis, visual assessment provides important structural information.48
We used the median emphysema severity value (corresponding to > 10% involvement) in logistic regression models because the sample size limited utility of statistical methods such as ordinal and multinomial regression. Semiquantitative cut points (> 10% and < 10% emphysema) have been used to differentiate normal and mildly diseased lungs from those with clinically important emphysema.3,14,49‐51 Other studies suggested that any degree of emphysema is associated with worse patient outcomes, including increased incidence of lung cancer,7 low bone mineral density,9 and decreased glomerular filtration rate43; risk for these outcomes further increases with greater emphysema severity. The clinical significance of varying emphysema thresholds requires further investigation.
Emphysema progression may be linked to latent or subclinical pulmonary infections.52 We found that prior pneumonia was associated with radiographic emphysema in bivariate analyses. In a multivariable model, pneumonia history attenuated the association of HIV with emphysema, although to a lesser degree than elevated sCD14 level. However, evaluation of the impact of pneumonia on emphysema development and progression is limited in this cross-sectional analysis because we do not know whether emphysema was already established at the time of prior pneumonia or whether pneumonia occurred before and contributed to emphysema development. Further evaluation requires longitudinal studies.
This study has several other limitations. The sample size is relatively small and limits conclusions regarding when significant associations were detected and when they were not; validation within other cohorts is required. Participants were predominantly male veterans, although the cohort was racially diverse and from multiple US sites.21 Although participants with and without HIV infection were similar in terms of demographics and smoking history and we adjusted for pack-years, residual confounding remains possible. Nonetheless, the results are consistent with work in a larger cohort demonstrating an independent decrease in Dlco among men with HIV infection.53 The present findings suggest that decreased Dlco in HIV may be due to greater emphysema severity. Finally, abstracted nadir CD4 cell count may not reflect a participant’s true nadir. In the present cohort, however, 61% of participants with HIV infection had nadir CD4 < 200 cells/μL, consistent with a prior observation that 51% of patients establishing HIV care at VA medical centers had CD4 < 200 cells/μL.54
In conclusion, we found that HIV infection is an independent risk factor for emphysema in the current ART era. Radiographic emphysema severity was overall significantly greater among individuals with HIV infection. The association of HIV with emphysema appears mediated by elevated sCD14 levels, suggesting an important role for immune activation in emphysema pathogenesis in HIV. Additionally, among those with HIV, nadir CD4 < 200 cells/μL and elevated sCD14 level were associated with radiographic emphysema. The trend toward increased lower lung zone and diffuse emphysema in individuals with HIV infection further suggests that vascular-related mechanisms may be important. Additional studies are needed to investigate mechanisms leading to emphysema and the impact of varying degrees of radiographic emphysema on patient outcomes.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: E. F. A. and K. C. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. E. F. A. and K. C. contributed to the study concept and design, data analysis and interpretation, and drafting and review of the manuscript for important intellectual content; K. M. A., C. W., M. B. G., M. C. R.-B., S. T. B., G. W. S. H., J. K., and A. C. J. contributed to the data analysis and interpretation and drafting and review of the manuscript for important intellectual content; and D. R., P. J. L., L. M. S., and A. S. contributed to the drafting and review of the manuscript for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Wongtrakool reports receiving grants from the Department of Veterans Affairs Biomedical Laboratory Research and Development Program. Dr Goetz reports receiving grants from the National Institutes of Health during conduct of this study. Dr Brown holds shares of common stock in Pfizer, Inc, and Cepheid. Drs Attia, Akgün, Rodriguez-Barradas, Rimland, Soo Hoo, Kim, Lee, Schnapp, Sharafkhaneh, Justice, and Crothers have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design and conduct of the study, the collection, analysis and interpretation of the data, or the preparation and approval of the manuscript. The material presented is the result of work supported with resources and the use of facilities at participating VA medical centers. The contents do not represent the views of the Department of Veterans Affairs or the United States government.
Other contributions: The authors thank the veterans who participated in EXHALE and the coordinators who made the study possible.
Additional information: The e-Appendix and e-Tables can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- ART
antiretroviral therapy
- Dlco
diffusing capacity of the lung for carbon monoxide
- sCD14
soluble CD14
- VA
US Veterans Affairs Healthcare System
Footnotes
Dr Schnapp is currently at the Medical University of South Carolina (Charleston, SC).
Part of this article has been presented in abstract form at Gairdner, UBC, and St. Paul’s Hospital 2013 Symposium on COPD, December 3-4, 2013, Vancouver, BC, Canada, and the Conference on Retroviruses and Opportunistic Infections, March 3-6, 2014, Boston, MA.
FUNDING/SUPPORT: This work was supported by the National Heart, Lung, and Blood Institute (NHLBI) at the National Institutes of Health (NIH) [R21 HL120391 to Dr Schnapp and R01 HL090342 to Dr Crothers]. This research was funded in part by a 2012 developmental grant from the University of Washington Center for AIDS Research, an NIH-funded program under award number P30-AI-027757, which is supported by the following NIH institutes and centers: National Institute of Allergy and Infectious Diseases, National Cancer Institute, National Institute of Mental Health, National Institute on Drug Abuse, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NHLBI, and National Institute on Aging.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Petrache I, Diab K, Knox KS, et al. HIV associated pulmonary emphysema: a review of the literature and inquiry into its mechanism. Thorax. 2008;63(5):463-469. [DOI] [PubMed] [Google Scholar]
- 2.Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183(3):388-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boschetto P, Quintavalle S, Zeni E, et al. Association between markers of emphysema and more severe chronic obstructive pulmonary disease. Thorax. 2006;61(12):1037-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han MK, Bartholmai B, Liu LX, et al. Clinical significance of radiologic characterizations in COPD. COPD. 2009;6(6):459-467. [DOI] [PubMed] [Google Scholar]
- 5.Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178(7):738-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Divo M, Cote C, de Torres JP, et al. ; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155-161. [DOI] [PubMed] [Google Scholar]
- 7.Bon J, Fuhrman CR, Weissfeld JL, et al. Radiographic emphysema predicts low bone mineral density in a tobacco-exposed cohort. Am J Respir Crit Care Med. 2011;183(7):885-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johannessen A, Skorge TD, Bottai M, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187(6):602-608. [DOI] [PubMed] [Google Scholar]
- 9.Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest. 2002;121(5_suppl):121S-126S. [DOI] [PubMed] [Google Scholar]
- 10.Sandford AJ, Silverman EK. Chronic obstructive pulmonary disease. 1: susceptibility factors for COPD the genotype-environment interaction. Thorax. 2002;57(8):736-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karrasch S, Holz O, Jörres RA. Aging and induced senescence as factors in the pathogenesis of lung emphysema. Respir Med. 2008;102(9):1215-1230. [DOI] [PubMed] [Google Scholar]
- 12.MacNee W. Aging, inflammation, and emphysema. Am J Respir Crit Care Med. 2011;184(12):1327-1329. [DOI] [PubMed] [Google Scholar]
- 13.Guillemi SA, Staples CA, Hogg JC, et al. Unexpected lung lesions in high resolution computed tomography (HRTC) among patients with advanced HIV disease. Eur Respir J. 1996;9(1):33-36. [DOI] [PubMed] [Google Scholar]
- 14.Diaz PT, King MA, Pacht ER, et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med. 2000;132(5):369-372. [DOI] [PubMed] [Google Scholar]
- 15.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuller LH, Tracy R, Belloso W, et al. ; INSIGHT SMART Study Group. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker J, Ayenew W, Quick H, et al. High-density lipoprotein particles and markers of inflammation and thrombotic activity in patients with untreated HIV infection. J Infect Dis. 2010;201(2):285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker JV, Neuhaus J, Duprez D, et al. ; INSIGHT SMART Study Group. Inflammation predicts changes in high-density lipoprotein particles and apolipoprotein A1 following initiation of antiretroviral therapy. AIDS. 2011;25(17):2133-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubé MP, Sattler FR. Inflammation and complications of HIV disease. J Infect Dis. 2010;201(12):1783-1785. [DOI] [PubMed] [Google Scholar]
- 20.Sandler NG, Wand H, Roque A, et al. ; INSIGHT SMART Study Group. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203(6):780-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): overview and description. Med Care. 2006;44(suppl 2):S13-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armah KA, McGinnis K, Baker J, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis. 2012;55(1):126-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA; Ambulatory Care Quality Improvement Project (ACQUIP). The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789-1795. [DOI] [PubMed] [Google Scholar]
- 24.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179-187. [DOI] [PubMed] [Google Scholar]
- 25.Neas LM, Schwartz J. The determinants of pulmonary diffusing capacity in a national sample of US adults. Am J Respir Crit Care Med. 1996;153(2):656-664. [DOI] [PubMed] [Google Scholar]
- 26.Patel BD, Coxson HO, Pillai SG, et al. ; International COPD Genetics Network. Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178(5):500-505. [DOI] [PubMed] [Google Scholar]
- 27.Grydeland TB, Dirksen A, Coxson HO, et al. Quantitative computed tomography: emphysema and airway wall thickness by sex, age and smoking. Eur Respir J. 2009;34(4):858-865. [DOI] [PubMed] [Google Scholar]
- 28.Nazli A, Chan O, Dobson-Belaire WN, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6(4):e1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chege D, Sheth PM, Kain T, et al. ; Toronto Mucosal Immunology Group. Sigmoid Th17 populations, the HIV latent reservoir, and microbial translocation in men on long-term antiretroviral therapy. AIDS. 2011;25(6):741-749. [DOI] [PubMed] [Google Scholar]
- 30.Mehandru S, Poles MA, Tenner-Racz K, et al. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006;3(12):e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365-1371. [DOI] [PubMed] [Google Scholar]
- 32.Romero-Sánchez M, González-Serna A, Pacheco YM, et al. Different biological significance of sCD14 and LPS in HIV-infection: importance of the immunovirology stage and association with HIV-disease progression markers. J Infect. 2012;65(5):431-438. [DOI] [PubMed] [Google Scholar]
- 33.Lyons JL, Uno H, Ancuta P, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr. 2011;57(5):371-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Regueiro V, Campos MA, Morey P, et al. Lipopolysaccharide-binding protein and CD14 are increased in the bronchoalveolar lavage fluid of smokers. Eur Respir J. 2009;33(2):273-281. [DOI] [PubMed] [Google Scholar]
- 35.Martin TR, Rubenfeld GD, Ruzinski JT, et al. Relationship between soluble CD14, lipopolysaccharide binding protein, and the alveolar inflammatory response in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;155(3):937-944. [DOI] [PubMed] [Google Scholar]
- 36.Negredo E, Massanella M, Puig J, et al. Nadir CD4 T cell count as predictor and high CD4 T cell intrinsic apoptosis as final mechanism of poor CD4 T cell recovery in virologically suppressed HIV-infected patients: clinical implications. Clin Infect Dis. 2010;50(9):1300-1308. [DOI] [PubMed] [Google Scholar]
- 37.Siddique MA, Hartman KE, Dragileva E, et al. Low CD4+ T cell nadir is an independent predictor of lower HIV-specific immune responses in chronically HIV-1-infected subjects receiving highly active antiretroviral therapy. J Infect Dis. 2006;194(5):661-665. [DOI] [PubMed] [Google Scholar]
- 38.Lang S, Mary-Krause M, Simon A, et al. ; French Hospital Database on HIV (FHDH)–ANRS CO4. HIV replication and immune status are independent predictors of the risk of myocardial infarction in HIV-infected individuals. Clin Infect Dis. 2012;55(4):600-607. [DOI] [PubMed] [Google Scholar]
- 39.Ganesan A, Krantz EM, Huppler Hullsiek K, et al. ; Infectious Disease Clinical Research Program HIV/STI Working Group. Determinants of incident chronic kidney disease and progression in a cohort of HIV-infected persons with unrestricted access to health care. HIV Med. 2013;14(2):65-76. [DOI] [PubMed] [Google Scholar]
- 40.Murray JF, Nadel JA. Textbook of Respiratory Medicine. Philadelphia, PA: WB Saunders Company; 1994. [Google Scholar]
- 41.Bryan CL, Beard KS, Pott GB, et al. HIV infection is associated with reduced serum alpha-1-antitrypsin concentrations. Clin Invest Med. 2010;33(6):E384-E389. [DOI] [PubMed] [Google Scholar]
- 42.Sandström CS, Novoradovskaya N, Cilio CM, Piitulainen E, Sveger T, Janciauskiene S. Endotoxin receptor CD14 in PiZ α-1-antitrypsin deficiency individuals. Respir Res. 2008;9:34-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chandra D, Stamm JA, Palevsky PM, et al. The relationship between pulmonary emphysema and kidney function in smokers. Chest. 2012;142(3):655-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Remy-Jardin M, Remy J, Boulenguez C, Sobaszek A, Edme JL, Furon D. Morphologic effects of cigarette smoking on airways and pulmonary parenchyma in healthy adult volunteers: CT evaluation and correlation with pulmonary function tests. Radiology. 1993;186(1):107-115. [DOI] [PubMed] [Google Scholar]
- 45.Tylén U, Boijsen M, Ekberg-Jansson A, Bake B, Löfdahl CG. Emphysematous lesions and lung function in healthy smokers 60 years of age. Respir Med. 2000;94(1):38-43. [DOI] [PubMed] [Google Scholar]
- 46.Vehmas T, Kivisaari L, Huuskonen MS, Jaakkola MS. Effects of tobacco smoking on findings in chest computed tomography among asbestos-exposed workers. Eur Respir J. 2003;21(5):866-871. [DOI] [PubMed] [Google Scholar]
- 47.Park KJ, Bergin CJ, Clausen JL. Quantitation of emphysema with three-dimensional CT densitometry: comparison with two-dimensional analysis, visual emphysema scores, and pulmonary function test results. Radiology. 1999;211(2):541-547. [DOI] [PubMed] [Google Scholar]
- 48.Barr RG, Berkowitz EA, Bigazzi F, et al. ; COPDGene CT Workshop Group. A combined pulmonary-radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. COPD. 2012;9(2):151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitaguchi Y, Fujimoto K, Kubo K, Honda T. Characteristics of COPD phenotypes classified according to the findings of HRCT. Respir Med. 2006;100(10):1742-1752. [DOI] [PubMed] [Google Scholar]
- 50.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162(3):1102-1108. [DOI] [PubMed] [Google Scholar]
- 51.Fujimoto K, Kitaguchi Y, Kubo K, Honda T. Clinical analysis of chronic obstructive pulmonary disease phenotypes classified using high-resolution computed tomography. Respirology. 2006;11(6):731-740. [DOI] [PubMed] [Google Scholar]
- 52.Morris A, Sciurba FC, Norris KA. Pneumocystis: a novel pathogen in chronic obstructive pulmonary disease? COPD. 2008;5(1):43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crothers K, McGinnis K, Kleerup E, et al. HIV infection is associated with reduced pulmonary diffusing capacity. J Acquir Immune Defic Syndr. 2013;64(3):271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gandhi NR, Skanderson M, Gordon KS, Concato J, Justice AC. Delayed presentation for human immunodeficiency virus (HIV) care among veterans: a problem of access or screening? Med Care. 2007;45(11):1105-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement