Abstract
Bronchopulmonary C-fibers and a subset of mechanically sensitive, acid-sensitive myelinated sensory nerves play essential roles in regulating cough. These vagal sensory nerves terminate primarily in the larynx, trachea, carina, and large intrapulmonary bronchi. Other bronchopulmonary sensory nerves, sensory nerves innervating other viscera, as well as somatosensory nerves innervating the chest wall, diaphragm, and abdominal musculature regulate cough patterning and cough sensitivity. The responsiveness and morphology of the airway vagal sensory nerve subtypes and the extrapulmonary sensory nerves that regulate coughing are described. The brainstem and higher brain control systems that process this sensory information are complex, but our current understanding of them is considerable and increasing. The relevance of these neural systems to clinical phenomena, such as urge to cough and psychologic methods for treatment of dystussia, is high, and modern imaging methods have revealed potential neural substrates for some features of cough in the human.
Cough preserves the gas-exchanging functions of the lung by facilitating clearance of aspirate, inhaled particulate matter, accumulated secretions, and irritants that are either inhaled or formed at sites of mucosal inflammation. An impaired cough reflex can have acute, dire consequences.1-4 Under normal conditions, therefore, cough serves an important protective role in the airways and lungs. However, cough is also a mechanism for the spread of life-threatening respiratory tract infections, including many forms of influenza, TB, severe acute respiratory syndrome, and Bordetella pertussis, the gram-negative bacteria responsible for whooping cough. Furthermore, in diseases such as asthma, COPD, gastroesophageal reflux disease (GERD), and rhinosinusitis, cough may become excessive and harmful to the airway mucosa and adversely impact patient quality of life.5,6 These contrasting functions and consequences of cough highlight the importance of balancing therapy-targeting cough, such that the defensive functions of the reflex are preserved, while limiting the role of cough in spreading harmful illnesses and adversely impacting patient sense of well-being.
Most of our understanding about the afferent (sensory) neuronal pathways regulating the cough reflex comes from studies performed in animals. These studies implicate vagal afferent nerves innervating the larynx, trachea, carina, and large intrapulmonary bronchi with terminations largely confined to the airway mucosa,7-10 which we describe, as well as their role in regulating cough. In this article we provide an update to the 2006 Diagnosis and Management of Cough: ACCP Evidence-Based Clinical Practice Guidelines by detailing the recent research, including an increasing number of studies conducted in human subjects designed to elucidate the anatomic and neurophysiologic components of cough. In addition, we have attempted to provide a clinical perspective with particular emphasis on what is known about cough in patients as well as mechanisms accounting for the excessive coughing and cough hypersensitivity associated with acute, subacute, and chronic conditions associated with cough.
Properties of Airway Afferent Nerve Subtypes and Evidence for Their Potential Role in Regulating Human Cough
Bronchopulmonary vagal afferent nerves arise bilaterally from the inferior (nodose) and superior (jugular) vagal ganglia and differ in their chemical and mechanical responsiveness, in addition to their anatomic, embryological, and physiologic attributes.11,12 When evaluated in isolation, these attributes are often not sufficiently specific to differentiate afferent nerves. When combined, clear patterns of anatomy and physiology emerge that help distinguish specific nerve subtypes (Fig 1). Understanding the differences in vagal afferent nerve subtypes is critical to understanding the physiology and pathophysiology of cough, and thus the functional, anatomic, and neurochemical attributes of vagal afferent nerve subtypes are described below.
Figure 1 –
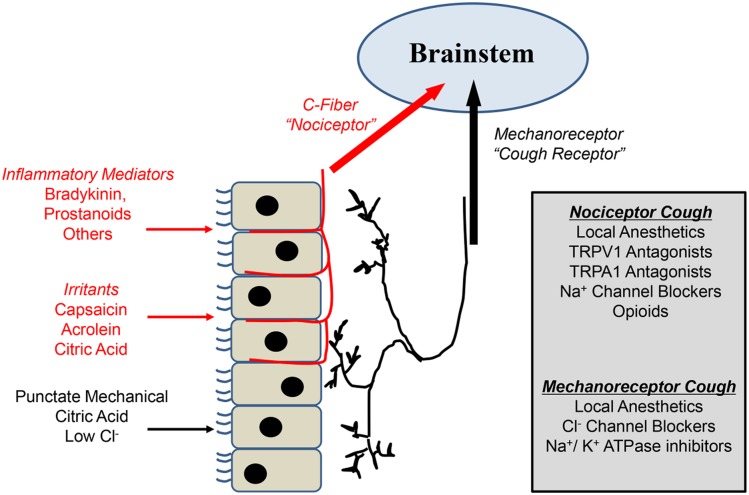
Peripheral mechanisms of cough. A dual-sensory neuron model subserving cough. Extensive studies in animals and humans support the concept that at least two subtypes of primary sensory neurons can induce coughing when stimulated. C-fiber nociceptors, which have their terminals in and around the mucosa surface of the airways, are sensitive to a wide variety of inhaled or locally produced chemical mediators, which may either activate or sensitize nociceptor nerve endings. Mechanically sensitive “cough receptors” are positioned beneath the epithelium in the large airways and are relatively insensitive to most chemical mediators (with the exception of low pH) but are exquisitely sensitive to punctate stimuli delivered to the mucosal surface (for example, inhaled particulate matter). A number of peripherally acting compounds that block nociceptor and or mechanoreceptor activation to reduce coughing are listed in the gray box. TRPA1 = transient receptor potential A1; TRPV1 = transient receptor potential vanilloid 1.
C-Fibers
The majority of bronchopulmonary vagal afferent nerves are unmyelinated C-fibers.13-16 In addition to their conduction velocity (< 2 m/s), airway vagal afferent C-fibers are distinguished from lung stretch receptors by their relative insensitivity to mechanical stimulation and lung inflation (Fig 2). C-fibers are further differentiated from lung stretch receptors by their sensitivity to bradykinin and activators of the ion channels, transient receptor potential vanilloid 1 (TRPV1) (eg, capsaicin, protons), and transient receptor potential ankyrin 1 (TRPA1) (eg, ozone, allyl isothiocyanate). Other inflammatory mediators and environmental irritants that somewhat selectively activate bronchopulmonary C-fibers include prostaglandin E2, ozone, nicotine, adenosine, and serotonin.13-20
Figure 2 –
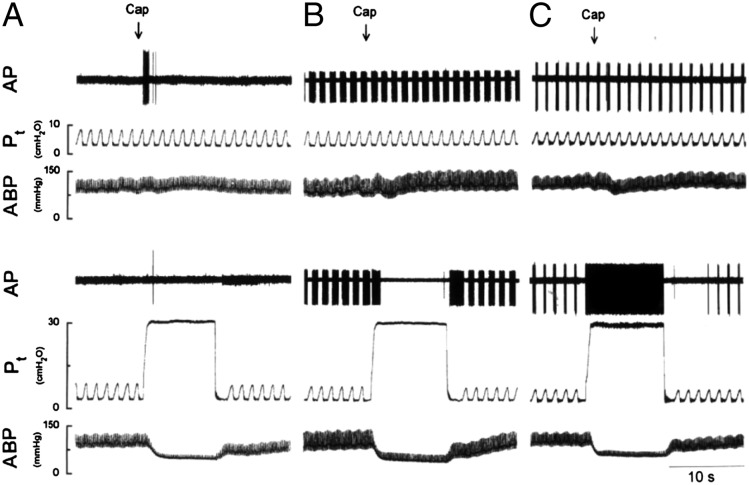
Representative traces of single-unit recordings from airway vagal afferent nerve subtypes in anesthetized rats. A, Airway C-fibers are quiescent during tidal breathing and relatively unresponsive to lung inflation. However, C-fibers respond vigorously to IV injected capsaicin. B and C, Rapidly adapting receptors (RARs) (B) and slowly adapting receptors (SARs) (C) are sporadically active during the respiratory cycle. Neither subtype of mechanoreceptor responds to capsaicin, but both respond intensely when the lungs are inflated. Note that RARs are easily differentiated from SARs by their rapid adaptation during sustained lung inflation. ABP = arterial BP; AP = action potential; Cap = capsaicin; Pt = tracheal pressure. (Reprinted with permission from Ho et al.11)
Morphologic studies in rats and in guinea pigs have identified afferent C-fiber terminations in the airway epithelium as well as other effector structures within the airway wall.21-24 Such studies are aided by the unique expression of TRPV1 and substance P in rat and guinea pig C-fibers. Comparable structures have been identified in human airway mucosa and the airway mucosa of other species, including terminals labeled immunohistochemically for TRPV1, but their identity as C-fiber terminations has not been proven.25-28 Considerably less substance P-containing innervation has been localized to human airways, in comparison with that seen in rats and guinea pigs.26 This makes it unlikely that neurokinin-dependent axonal reflexes play a prominent role in humans (see later).
Bronchopulmonary afferent C-fiber subtypes have been described in several species.13,15,16 These subtypes are differentiated by their ganglionic origin (nodose vs jugular), sites of termination in the airways and lungs, chemical sensitivity, neurochemistry, and the reflexes initiated upon their activation. Whether similar physiologic distinctions between bronchial and pulmonary afferent C-fibers can be defined in humans is unknown.
C-fibers are activated somewhat selectively by capsaicin, bradykinin, TRPA1 activators, and protons, and each of these stimuli initiate coughing in patients and awake animals. The coughing evoked by these stimuli in animals is prevented by prior capsaicin desensitization (which selectively renders C-fibers unresponsive to subsequent challenges). In animals, these cough responses are inhibited by neurokinin receptor antagonists. As described above, neurokinins, such as substance P, are uniquely expressed by airway C-fibers in animals. All of these observations provide compelling evidence for an essential role of C-fibers in cough.9,10,29
Widdicombe Cough Receptors
More than one-half a century ago, John Widdicombe described a subtype of myelinated vagal afferent nerves innervating the trachea, mainstem, and segmental bronchi that played an essential role in cough reflexes evoked from the airways mucosa of anesthetized cats.30-32 He called these afferent nerves “cough receptors,” a flawed term, but a term that has persisted in the literature in the nearly 6 decades since. This seminal work by Widdicombe has been substantiated in multiple studies since, and it is now well established that, in addition to C-fibers, a subset of myelinated, mechanically sensitive, capsaicin-insensitive vagal afferent nerves plays an essential role in cough.10,33
Cough receptors can be differentiated from C-fibers and lung stretch receptors by their axon conduction velocity (5 m/s), which is considerably faster than C-fibers (< 1 m/s) but slower than lung stretch receptors (≥ 15 m/s). These mechanically sensitive vagal afferents do not normally express the ion channels TRPV1 or TRPA1 and are, thus, insensitive to the chemical irritants capsaicin and allyl isothiocyanate. These afferents are, however, activated by protons, perhaps through expression of acid-sensing ion channels. In guinea pigs, the cell bodies of the cough receptors are located in the nodose ganglia, whereas the cell bodies of the C-fibers regulating cough are located in the jugular ganglia. Cough receptors do not synthesize neuropeptides under normal conditions but use glutamate as their primary transmitter at their central terminals in the brainstem.33-35
At various times since his original discoveries, Widdicombe referred to the cough receptors by other terms, including rapidly adapting receptors (RARs) (see later) and irritant receptors.7,12 Because they are myelinated and adapt rapidly to a punctate (touch-like, as opposed to stretch-like) mechanical stimulation, it is tempting to conclude that the cough receptors are merely RARs that innervate the extrapulmonary airways. But unlike RARs, which primarily innervate the intrapulmonary airways, the cough receptor terminations are found exclusively in the extrapulmonary airways (larynx, trachea, mainstem bronchi). Also, unlike RARs, the cough receptors are utterly unresponsive to a wide variety of irritants, spasmogens, and autacoids that induce airway smooth muscle contraction and decrease lung compliance, including methacholine, histamine, leukotriene C4, substance P, neurokinin A, 5-hydroxytryptamine, adenosine triphosphate (ATP), and adenosine.10 All of these stimuli have been shown to activate RARs, and yet none of them reliably evokes coughing in animals or in patients.36-39
The peripheral terminals of cough receptors have been described in guinea pigs (Fig 3). These terminals are found in abundance in the airway mucosa, assuming a stereotypical position in the airway wall, with complex dendritic arbors that are invariably arranged along the circumferential axis of the airways.23 Terminations are confined to the space between the smooth muscle and the epithelial cell layers. This location for cough receptor terminations may explain the relative insensitivity of cough receptors to epithelium removal or airway smooth muscle contraction. It is of interest that this location in the extracellular matrix is a site of extensive remodeling in several chronic diseases in patients.40 Comparable structures have been identified in the airway mucosa of other species, including humans.12,41
Figure 3 –
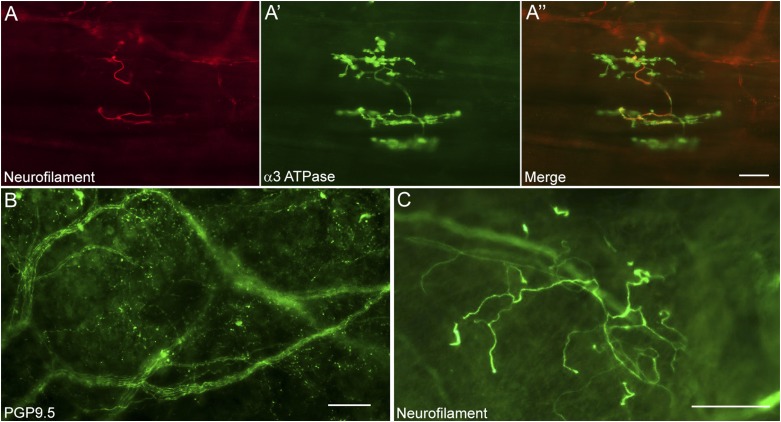
Structural organization of airway nerve terminals in guinea pigs and humans. A-A′′, The arrangement of a cough receptor terminal in the guinea pig airways. Heavy chain neurofilaments (A) are expressed in the cough receptor axon and major branch points, indicative of the myelinated nature of these sensory nerve subtypes. These major branch points give rise to a more complex terminal structure (A′) that is defined by the expression of an isozyme of the Na+/K+ ATPase containing the α3 subunit (α3 ATPase). B, Staining of nerve fibers innervating the human airways revealed using the pan-neuronal marker protein gene product 9.5 (PGP9.5) (original magnification × 20). C, Some of these fibers express heavy chain neurofilament proteins and are comparable to the structures in guinea pigs that give rise to cough receptor terminals (original magnification × 10). Scale bars represent 50 μm.
Pulmonary Stretch Receptors
Lung stretch receptors are characterized by their responses to sustained lung inflation. Both RARs and slowly adapting receptors (SARs) are activated by sustained lung inflation, but RARs are active primarily during the dynamic phase of lung inflation, whereas SARs continue firing throughout the distension. RARs are also differentiated from SARs by their responses to lung deflation/lung collapse.11,42-46
The anatomy of RAR terminations in the airway wall is unknown. Functional studies of RARs suggest that they terminate within or beneath the epithelium, primarily in the intrapulmonary airways.31,44 Such terminal sites might explain RAR sensitivity to lung collapse and/or lung deflation, although their responsiveness to alterations in dynamic lung compliance (and thus their sensitivity to bronchospasm) also suggests an association with the airway smooth muscle. The sustained activation of RARs, produced by dynamic lung inflation, bronchospasm, or lung collapse, indicates that adaptation of RARs is not attributable to an electrophysiological adaptation. RARs may, thus, be better defined as dynamic airway mechanoreceptors.
SARs are highly sensitive to the mechanical forces imposed upon the lung during breathing. SAR activity increases sharply during inspiration and peaks just prior to the initiation of expiration.11,43 SARs are, thus, believed to be the primary afferent fibers involved in the Hering-Breuer inflation reflex, which terminates inspiration and initiates expiration when the lungs are adequately inflated.43 SAR terminal structures have been identified in the intrapulmonary airways and lungs of rabbits.47 These terminals assume a complex and varying position within the airway wall but are found primarily in the peripheral airways (associated with alveoli or bronchioles). Occasionally, but not uniformly, SAR dendritic arbors are associated with the bronchiolar smooth muscle, in which case they have been termed smooth muscle-associated receptors. Some species variations in SAR termination patterns (eg, extrapulmonary vs intrapulmonary) have been described.10,43
Despite some imprecise semantics, whereby researchers group cough receptors with the intrapulmonary RARs into a single, heterogenous class of vagal afferent nerves that are called either “irritant receptors” or RARs, there is little evidence to suggest that either RARs or SARs, defined here as those afferents responding to sustained lung inflation, directly initiate coughing upon activation. Both RARs and SARs are spontaneously active during eupnea, and it would be hard to envision a scenario whereby their action potential patterning could change, such that cough and not eupnea is encoded. Electrophysiologic recordings from the vagus nerve in rabbits suggest that SAR activity does not increase prior to or during evoked cough responses.48 Conversely, SARs are activated by sustained lung inflations and RARs are activated by negative intraluminal pressures (such as those produced by inspiratory efforts against a closed glottis), and neither stimulus reliably initiates coughing in animals or in humans.49,50 Bronchospasm is also an effective stimulus for RARs but largely ineffective at initiating cough.9,10,36-39 Both subtypes of stretch receptors play a critical role in regulating respiratory rate and tidal volumes at eupnea, and their activity likely modulates sensitivity to tussive stimuli. RARs may regulate the duration and magnitude of the inspiratory and expiratory phases of coughing. A primary role in cough initiation for either RARs or SARs seems unlikely. Unlike their role in regulating the duration of inspiratory and expiratory phases during breathing, volume-related feedback from SARs has no effect on the duration of breathing phases of tracheobronchial cough.51
Interactions Between Afferent Nerve Subtypes Evoking Cough
Peripheral Interactions
Although C-fibers and the cough receptors subserve primary roles in cough initiation, other bronchopulmonary afferent nerve subtypes likely modulate cough pattern and sensitivity to tussive stimuli. Some of these sensory nerve interactions depend upon the peripheral consequences of the reflexes initiated upon their activation.9,10,13 These reflexes include changes in respiratory rate and tidal volume and also alterations in autonomic nerve activity in the airways and lungs and throughout the cardiovascular system. For example, SARs and RARs regulate tidal volumes and the timing of inspiration and expiration.43,44 The peak expiratory flows achieved during cough are directly related to inspiratory volumes,52 and in this way lung stretch receptors will modulate cough. Lung stretch receptors also indirectly regulate airway caliber and, thus, resistance to airflow through airway autonomic pathways.53,54 Although there are conflicting results in the literature, anticholinergics have been shown to modulate cough responsiveness and at least partially reduce cough severity in some patients.55 Airway sympathetic nerves may also regulate cough by modulating mucosal blood flow, which may clear irritants contained in airway surface liquid and thus reduce their concentrations in the biophase, thereby reducing excitation of underlying sensory nerves.56
Axonal reflexes, which result in the release of neuropeptides from the peripheral terminals of afferent C-fibers, independent of any involvement of the CNS, are unlikely to regulate human lung physiology and pathophysiology, given the paucity of neuropeptide-containing sensory nerves in the human airway mucosa.9,57 This contrasts sharply with previous studies performed in rats and guinea pigs, wherein the majority of C-fiber terminals in the airways mucosa contain the neuropeptides substance P, neurokinin A, and calcitonin gene-related peptide. In these species, neuropeptide-dependent axonal reflexes play a prominent role in lung responses to inflammation and other noxious insults.58 Comparable nerve terminals lacking neuropeptides have been localized to human airways.25-27 However, these nerve terminals have the subcellular machinery for peripheral neurotransmitter release. Perhaps nonpeptide transmitters, including the purine transmitter ATP, may be released peripherally from human airway C-fibers by axonal reflexes. Administered exogenously, ATP evokes coughing and sensation of dyspnea in patients, and a clinical study described a profound reduction in chronic cough rates upon administration of a potent purinergic/P2X3 receptor antagonist in patients with chronic cough.59-61
Central Interactions
Vagal afferent nerves may also interact in the CNS to regulate cough. Anatomic and functional studies have demonstrated convergence of vagal afferents at sites of brainstem integration, particularly in the nucleus of the solitary tract (nTS), a region in the caudal brainstem where many vagal sensory nerves project.62,63 Such convergent projections lead to synergistic regulation of reflex bronchospasm.64 Evidence for synergistic interactions between cough receptors and C-fibers has also been documented in studies of cough. Thus, in anesthetized guinea pigs, C-fiber activation does not evoke cough but greatly sensitizes the cough reflex evoked by activating the cough receptors.65 This sensitizing effect of bronchopulmonary C-fiber activation through CNS-dependent mechanisms is comparable to the processes of central sensitization described in studies of pain in somatic tissues.66,67 Comparable central interactions may contribute to the enhanced cough responsiveness evoked by allergic inflammation and associated with gastroesophageal reflux and upper airway diseases, presumably reflecting facilitatory central interactions of esophageal or trigeminal afferent pathways with cough circuits in the brain.9,10,68
Not all central interactions among afferent pathways promote enhanced cough responsiveness. Activation of a subset of pulmonary C-fibers has been shown to inhibit evoked coughing in dogs, cats, and guinea pigs, and circumstantial evidence suggests a comparable inhibition of cough evoked by pulmonary C-fiber activation in patients.10,69,70 These inhibitory effects occur coincident with pulmonary chemoreflexes, which results in profound changes in respiration and dyspnea and almost certainly depends upon inhibitory interactions between respiratory pattern generators and central pattern regulators of cough. Cough may also be inhibited by activation of cold-sensitive/menthol-sensitive trigeminal afferent nerves innervating the upper airways and nose (see later). The central pathways accounting for these upper and lower airway nerve interactions have not been determined.
Complex and poorly defined central (and perhaps peripheral) interactions between cough neural pathways and changes in arterial oxygen and CO2 can also regulate coughing. Hypoxia depresses cough in animal models and increases capsaicin thresholds in humans.71,72 Repetitive coughing in animal models induces profound hyperventilation and metabolic alkalemia, such that central arterial levels of CO2 fall well below apneic threshold.73 These low levels of CO2 do not depress cough efforts as they would respiratory muscle activity during breathing.74
Extrapulmonary Modulation of Cough
Cough Evoked From the Ear (Arnold Reflex)
Afferent nerves carried by the auricular branch of the vagus nerve (Arnold nerve) innervate the external auditory meatus.75 In a small subset of individuals (< 5%), several visceral reflexes, including cough, may be evoked by mechanical stimulation of the ear. Tracing studies in cats show that the cell bodies of the afferent neurons carried by the Arnold nerve are located in the jugular ganglia and terminate in several areas throughout the brainstem, including the nTS.76 It is interesting that, in guinea pigs, the nociceptors that modulate cough also have their cell bodies in the jugular ganglia and terminate centrally in the same region of the nTS.9,10 It seems likely that the cough initiated by stimulation of the ear involves integration of both ongoing airway vagal afferent nerve input and the additional afferent input arising from the ear.
Nasal Afferent Nerve Regulation of the Cough Reflex
Rhinosinusitis and other conditions involving the upper airways are common comorbidities in patients with chronic cough. Precisely why upper airways disease is associated with chronic cough is unclear. Direct stimulation of the nasal mucosa does not initiate cough. There has been speculation that postnasal drip may be responsible for the coughing resulting from upper airways disorders, but there seems to be considerable variance in the prevalence of postnasal drip among patients with chronic cough.5,6
An alternative explanation for cough associated with rhinosinusitis would be a sensitizing effect of upper airway afferent nerves, resulting in enhanced cough responsiveness and thus coughing in a subset of individuals. This hypothetical mechanism assumes that trigeminal afferent pathways can promote the likelihood of cough by lowering the threshold of the central cough pattern generator to subsequent vagal afferent input, perhaps to the point where even innocuous or physiologic stimuli arising from the lower airways could promote coughing. Thus, cough reflex sensitivity is enhanced by upper airways disease, and an additional lower airway trigger for cough results in a clinical presentation that is responsive to aggressive medical therapy targeting upper airway disease. There is precedent in animals and in patients for enhanced cough sensitivity evoked by upper airway irritation. Acute allergic inflammation, challenges with mediators associated with allergic inflammation, or stimulation of TRPA1- or TRPV1-responsive upper airway sensory nerves increases responsiveness to tussive challenge.77-80
A subset of upper airway afferent nerves may also reduce cough frequency and responsiveness to tussive challenge. Menthol has been in use clinically for centuries for its soothing effects on mucosal surfaces and is commonly formulated in cough remedies. Menthol and components of eucalyptus and camphor may potentially work, in part, to produce their cooling and soothing effects by activating the ion channel transient receptor potential M8 (TRPM8). Unlike TRPV1 and TRPA1, which evoke painful and noxious sensations and reflexes, TRPM8 activation is soothing, a counterirritant to effects evoked by nociceptors. These opposing effects of TRPA1/TRPV1 and TRPM8 suggest differential expression by sensory nerves. Molecular and functional studies find little, if any, expression of TRPM8 in nerves innervating the lower airways (where C-fibers comprise the majority of vagal afferent innervation) but find a prominent expression of TRPM8 by trigeminal nerves innervating the upper airways. Molecular analyses confirm that a subset of TRPM8+ nasal afferent nerves do not coexpress TRPV1 or TRPA1. Activation of these upper airway afferents reduces cough responsiveness in animals and humans and may reduce cough frequency in patients with acute cough.81,82
Cough Initiated From the Pharynx
Infusion of fluid into the pharynx or pharyngeal stimulation with air puffs in awake or anesthetized human subjects can initiate coughing or the urge to cough under certain circumstances.83-86 The pharyngeal afferent nerves regulating cough are either vagal in origin or arise from the glossopharyngeal nerves or from nerve branches emanating from the trigeminal ganglion.87 The physiologic properties of the pharyngeal afferent nerves regulating cough are poorly defined, but they may be similar to the cough receptors innervating the larynx, trachea, and bronchi. Thus, mechanical stimulation, postnasal drip, and a water bolus placed into the pharynx evoke vigorous coughing in human subjects and in animals, whereas capsaicin may be unable to initiate coughing when applied selectively to the pharynx.83-86
Cough Initiated From the Esophagus
There are conflicting reports regarding the ability of esophageal afferent nerve activation to initiate coughing.87 In animals, neither distension nor luminal challenge of the esophagus with acid or capsaicin evokes coughing.9,10 Comparably negative studies have been reported in humans. However, there are also reports of cough evoked from the esophagus in humans. Moreover, the association between chronic cough and GERD is quite clear, and the ability to markedly reduce coughing in at least a subset of patients with GERD, by effective medical or surgical treatment of the reflux disease, argues strongly for the hypothesis that reflux initiates coughing in susceptible patients.5,6,88 Studies combining cough monitoring with impedance measurements also implicate reflux as a driver of cough in a subset of patients (Fig 4).89 The easiest explanation for these results is that refluxate is aspirated into the airways and directly activates the airway vagal afferent nerves regulating cough.87,90 But pH/impedance monitoring in patients with GERD provides little evidence that refluxate needs to reach the proximal esophagus, much less the airways, to initiate pulmonary symptoms or coughing.87,89 The available evidence suggests that locally or centrally mediated airway reflexes (promoting mucous secretion, bronchospasm, cough) can be initiated directly from the stomach or the esophagus.91-93 For example, many species have neuronal projections between esophageal and airway autonomic ganglia that, when activated, produce physiologic effects in the airways (including mucous secretion or bronchoconstriction) that could promote coughing.93 Alternatively, the interactions between the GI tract (GIT) and airway afferent pathways may occur in the central nervous system. Although untested directly, and thus unproven, esophageal and airway afferent nerves are known to project to similar brainstem regions and could converge centrally to regulate and initiate parallel and overlapping reflex effects in the airways and GIT.94,95 Alternatively, portions of the GIT and airways share a common blood supply and, as such, it is conceivable that inflammatory mediators or cells arising from the esophagus as a consequence of reflux could impinge upon airway sensory pathways to produce cough.
Figure 4 –
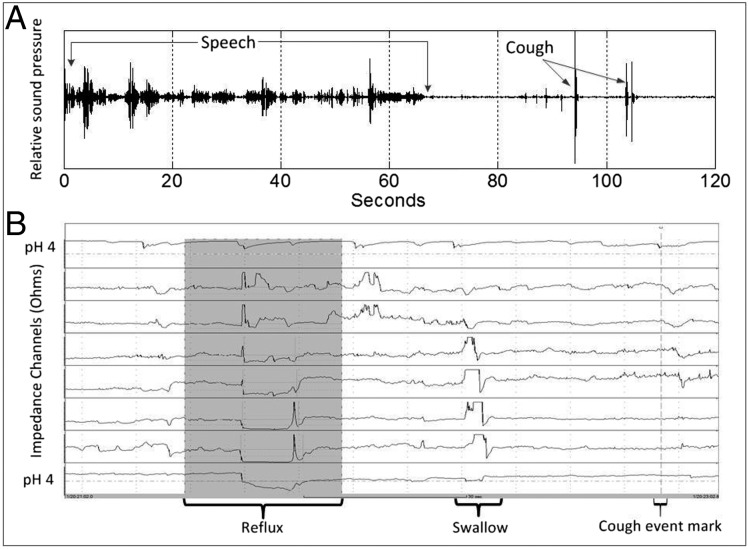
A, Time-synchronized acoustic recording; B, esophageal pH impedance. Acoustic recording shows a period of speech followed by two cough sound waveforms. The gray area in the impedance trace shows a retrograde fall in esophageal impedance associated with a fall in pH below 4 in the distal pH channel, indicative of an acid reflux event. As the cough sounds commence within 2 min of the start of the reflux event, they are considered associated. The cough event marker is created by the patient pressing a button on the impedance device to document the coughing. (Data from Smith et al.89)
Studies in patients with GERD provide evidence that refluxate may also sensitize the cough reflex.94-96 Acid challenges to the esophagus markedly reduce the concentration of inhaled capsaicin required to evoke cough or directly result in coughing. Ing and colleagues95 reported that this sensitizing effect of the esophageal challenge could be prevented by inhalation of atropine, suggesting that a CNS-dependent reflex, initiated from the esophagus, resulted in bronchospasm and/or mucous secretion in the airways that initiated coughing. Medical treatment of GERD has also been reported to greatly reduce sensitivity to tussive stimuli.88 This sensitizing effect of refluxate or experimental acid challenges likely depends on CNS integration and amplification of parallel vagal sensory inputs controlling cough.68
Central Regulation of Cough
Our current understanding of the central regulation of cough can be separated into three main areas: (1) brainstem processing of afferent information, (2) organization of the brainstem control network, and (3) higher brain (encompassing subcortical and cortical) circuits that support the role of consciousness, perception, and emotion in the expression of cough.
Brainstem Processing of Afferent Information
Vagal afferent fibers enter the brainstem via the nTS, where they synapse on second-order interneurons.97 The nTS cough-related interneuronal circuit has been proposed to have a “relay” function for transmission of information to populations of neurons in the brainstem that regulate breathing.98-101 The core of the cough network is located in the ventrolateral region of the medulla and is hypothesized to participate in breathing and rhythm regulation for cough.98,100-102 However, there are no studies of the response patterns and functional interactions of nTS neurons during cough. Studies have used transneuronal tracing with herpes simplex virus to map central circuits from the extrathoracic trachea.103 These studies showed that a broad network of neurons in the nTS participated in central processing of tracheal afferent information. This work supports the idea that the role of the nTS in generating cough has been significantly underestimated. What is clear is that this area of the brainstem does not simply relay vagal sensory afferent information through to the rest of the nervous system.
Cough and breathing can be differentially regulated by primary afferent inputs to the brainstem.51,104,105 This supports the concept that the regulation of cough is not an extension of our understanding of that for breathing but rather it is customized to the function of the behavior. In particular, the lack of an important role for hyperventilation in the control of cough is consistent with the fact that ventilation increases significantly during this behavior. This alteration in the regulatory system is necessary to prevent a loss of respiratory muscle excitability as CO2 falls during coughing. The depression of cough induced by hypoxia is less well understood. This effect may be related to a transition in priority of the respiratory muscle control system from maintaining blood gasses under normal conditions while allowing episodes of coughing to clear the airways to a focus on maintaining ventilation during hypoxia, which is a significant threat to normal gas exchange. The specific importance of the permissive/facilitatory effect of SARs on cough is also not well understood but may help optimize respiratory muscle performance106 and facilitate expulsive airflows that occur when cough is elicited at higher lung volumes.52
Organization of the Brainstem Control System
A network of neurons that includes the ventrolateral medulla, raphe nuclei, and pons is responsible for controlling the duration of the inspiratory, compressive, and expulsive phases of coughing as well as the magnitude of motor activation of the respiratory muscles during this behavior.98,99,101,102,107-109 This network also regulates respiratory muscles to produce breathing.102 Evidence supporting the concept of an anatomically distributed, but shared, network for both cough and breathing comes from a variety of preclinical studies showing that brainstem neurons in these locations alter their activities during both behaviors.98,99,101,102,108-110 These neurons are synaptically connected in ways that can explain motor drive to respiratory muscles during both behaviors.98,99,101 The process by which selected populations of this distributed brainstem network alter their activities to account for both behaviors is known as reconfiguration.98,101,102
Although complex, the reconfigured brainstem network hypothesis for cough does not yet account for the differential regulation of this behavior relative to breathing. Furthermore, this network cannot account for the phenotype of response to cough-suppressive drugs, first shown by May and Widdicombe111 > 50 years ago. Centrally active antitussive drugs suppress cough in a dose-dependent manner at doses that have no effect on breathing.111,112 By definition, there must be some “neural module” in this brainstem network that is not required for breathing but is important for the full expression of cough. Some investigators have approached this problem using microinjection methods to apply antitussive drugs and/or neurotransmitter agonists and antagonists to various brainstem regions.113-117 It is clear that familiar brainstem areas, such as the nTS, could participate in the actions of antitussive drugs. It is also clear that other areas not currently believed to have a command function for cough could be substrates for cough suppressants.113,115 These studies support the concept that there may be no single anatomic brainstem area or “center” that is susceptible to antitussive drugs but that these compounds may act at multiple sites when administered systemically. The extent to which these sites may contain undescribed elements of the brainstem network for cough is unknown, but these findings do support the idea that, even though our current conceptualization of the brainstem cough network is complex, it probably represents an underestimate of its true complexity.
Higher Brain Processing Systems in Cough
Coughing, like swallowing, belching, urinating, and defecating, is unique in that there is higher brain (cortical and subcortical) control of this visceral reflex (Fig 5).118-120 Higher brain control of coughing can manifest both as inhibition (eg, coughing that is suppressed until a break in a live performance or activity) and also as voluntary cough. Patients with persistent cough typically experience a recurrent need to cough or clear their airways (ie, an urge to cough)118 and are acutely aware of the ongoing sense of irritation in the airways (which can present, for example, as an itch or scratch in the throat or chest). Perceptual awareness requires cortical processing of the sensory information originating in the airways, and this promotes behavioral coughing in an attempt to clear the airways and satiate the urge. The implications of this conscious control of cough are severalfold. First, behavioral cough, rather than reflex cough, may contribute significantly to chronic cough in disease, and this could have implications for antitussive therapies. For example, Vertigan and Gibson121,122 reported that 49% of a patient cohort diagnosed with chronic refractory cough reported coughing deliberately in response to perceived irritations in the throat. Perhaps related to this, placebos can have a profound effect on coughing, the urge to cough having been studied experimentally.123,124 As such, any study of a novel or existing cough treatment should include a blinded (preferably double-blinded) placebo arm. Furthermore, speech therapy is proving beneficial in a subset of chronic coughers,122 which may reflect regulatory processes in this higher-order cough circuitry. It is also clear, however, that cough on occasion may be unrelated to a physiologic disease (eg, GERD, asthma), and thus a psychologic issue needs to be considered (“psychogenic cough”).125 Addressing the psychologic impact of coughing on a patient’s sense of well-being should therefore be a component of any treatment regimen for chronic cough, whether an underlying cause is identified, because many patients with cough present with symptoms of anxiety, sleep disturbance, and/or depressive illness.126 Because of this, it may be difficult to resolve whether the behavioral symptoms are due to the impact of intractable and unexplained cough or somehow the cause of the cough itself.
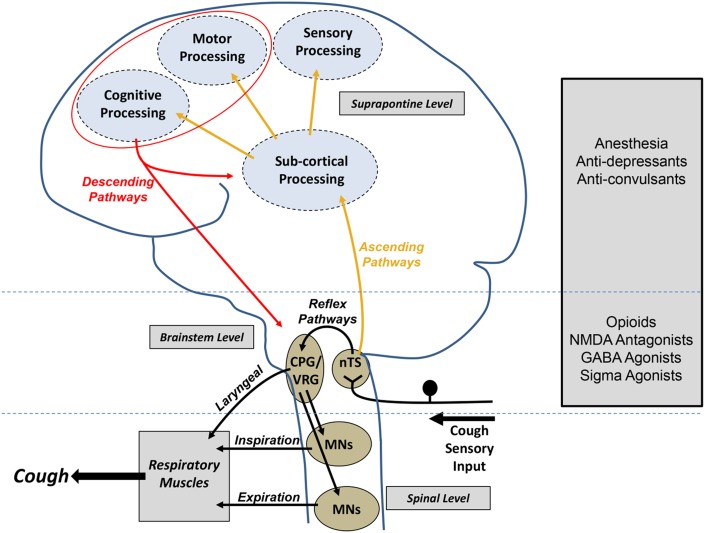
Figure 5 –
Central mechanisms regulating cough. Airway sensory neurons project to the brainstem, where they terminate predominately in the nTS. Projections from the nTS can reflexively induce coughing by modifying activity of the respiratory CPG, a collection of neurons that generates respiratory rhythm in the VRG of the brainstem. Output from the CPG via MNs provides the drive to respiratory muscles needed to elicit the cough motor pattern. Superimposed on this reflex pathway is a complex higher brain network that also receives inputs from the nTS in the brainstem. Higher brain processing gives rise to respiratory sensations and emotions associated with airway irritation as well as enabling a higher level of motor control over the basic reflexive cough pathways. Centrally acting antitussive agents likely affect both brainstem and higher brain processes to modify coughing. CPG = central pattern generator; GABA = gamma-aminobutyric acid; MN = motor neuron; NMDA = N-methyl-D-aspartate; nTS = nucleus tractus solitarius; VRG = ventral respiratory group.
The sensory and motor pathways composing the higher brain regulation of coughing in humans have received considerable attention.118-120 Uniquely, more is known about the higher brain control of cough in humans than in animals, which is not the case for other aspects of cough physiology. These processes are complex, but nevertheless it is of value to provide a summary of the major points. The urge to cough evoked by capsaicin inhalation in healthy volunteers is encoded in a brain network that includes the primary sensory, insula, prefrontal, and posterior parietal cerebral cortices.118,120 The activity patterns in these regions suggest that the primary sensory cortex is principally responsible for encoding urge-to-cough intensity, whereas the insula cortex encodes the magnitude of the incoming sensory input from the airways, and the prefrontal and posterior parietal cortices likely contribute to attention and localizing the site of irritation.120 Voluntary cough suppression requires brain regions similar to those needed for inhibiting other types of motor patterns, including activity in the inferior frontal gyrus, anterior mid-cingulate cortex, insula cortex, and supplementary motor area,119,127,128 although it is presently unclear where in the cough reflex pathway these suppressive circuits act. Not surprisingly, voluntary cough is associated with activity in premotor and motor cortices and in the cerebellum. Of particular interest, voluntary cough may proceed without input to the brainstem cough circuits described above, presumably relying on direct higher-brain control of respiratory motor neurons in the spinal cord.119,128 This is obviously in stark contrast to cough evoked by airways irritation. Placebo suppression of cough is correlated with activity in the dorsolateral prefrontal cortex,97 a region known to be necessary for placebo analgesia.129 The limbic brain and, in particular, the orbitofrontal cortex contribute to the affective components (such as the sense of unpleasantness) associated with airways irritation and cough.118,119,130
The Clinical Perspective
The anatomic and neurophysiologic processes responsible for the initiation and regulation of cough are complex131 and may not be considered immediately relevant by the clinician managing his/her patient with troublesome cough. However, the body of literature devoted to this area should be viewed as a valuable resource to help the clinician understand how and why his/her patient coughs and provide rationale for a targeted and systematic approach to treatment. In its simplest form, clinical cough is a reflex event beginning with activation of vagal afferent sensory nerves located in pulmonary and extrapulmonary sites that project the “signal” centrally, where it undergoes modulation, resulting in the generation of the appropriate efferent motor response. This concept of distinct afferent sites has provided the rationale for the use of the “anatomic diagnostic protocol” to evaluate and manage patients with cough.132,133 This approach of directing investigations and trials of therapy at various anatomic sites has now become embedded in clinical practice, leading to a broad recognition that asthma, GERD, and upper airways disease are, at least in adult patients, the most common causes of chronic cough.5,134-136 However, why the vast majority of patients with these common conditions do not develop chronic cough remains unclear. Adequate treatment of one or more of these causes is effective in the majority of patients, although a significant minority remains troubled with an unexplained, refractory chronic cough, despite extensive investigation and trials of therapy.136,137
Much of what is known about the regulation of cough by neural structures within the lung and at extrapulmonary sites, such as the nose, pharynx, and esophagus, has been obtained from experiments involving the mechanical probing and chemical stimulation of laboratory animals.138,139 Although these models, particularly those involving anesthetized animals, may not faithfully reproduce the human condition, they have provided clinically important information. The most sensitive regions of the airways for mechanically eliciting cough are the larynx and the tracheal and bronchial bifurcations.9,10,138 The idea that “mechanosensitive” receptors are located proximally within the airways seems entirely logical and consistent with the principal role of cough in protecting the lungs. When these processes are impaired, important clinical problems arise. The extension of the original animal experiments on mechanical activation of the airway to human studies has provided some important information of how cough and airway-protective reflexes may be modified in various clinical scenarios. These include studies designed to determine the impact of premature birth,139 aging,140 and disease141,142 on the integrity of the cough reflex. Cough can also be initiated with chemical stimuli. In experiments conducted in cats, Widdicombe30-32 reported a more vigorous response to chemical stimulation when delivered to distal bronchi, compared with the trachea and more proximal larger airways. The regional sensitivity of human airways to chemical activation was studied > 30 years later by Hansson and colleagues.143 Using single-breath aerosols of capsaicin in healthy human subjects, they noted that coughing was more readily induced by small droplet particles (deposited more distally in the airway) than by large droplet particles (deposited more proximally).143 The notion that “chemosensitive” receptors are present in the distal airways, a region of the lung considered to be sparsely innervated,144 provides some rationale for cough as a prominent symptom in clinical conditions such as idiopathic pulmonary fibrosis and bronchiolitis, both characterized by disease distributed more peripherally in the airways.
Coughing in response to experimental airway challenge with a range of physical and chemical irritants, including cold air and aerosols of capsaicin, citric acid, histamine, and charcoal dust, has been reported in both healthy subjects and patients with disease.145-149 A number of clinically relevant observations have emerged from these studies. First, subjects undergoing tussive challenge often report a “tickle” sensation at the top center of their chest, accompanied by the “urge” or desire to cough. These sensory symptoms are also commonly described by patients with chronic cough.150 Second, it has consistently been observed that female subjects exhibit heightened cough responses to chemical tussive stimuli.151,152 Whether this explains the predominance of female patients among those seeking help for chronic cough is not known. Third, patients typically report triggering of their cough by low-level physical (mechanical and thermal) and chemical stimuli (eg, scents, odors). This feature can often be the most troublesome cough-associated adverse occurrence to patients and contributes to their impaired health status.153,154 The notion that the cough reflex may be sensitized is central to current understanding of clinical cough.150 This hypersensitive state may exist transiently, as typically seen with a respiratory viral infection, where the accompanying cough is termed “acute” if it diminishes in < 3 weeks. For the majority of patients with cough, however, their cough and associated sensitivity last > 8 weeks, termed “chronic” by definition. It is not yet clear whether this “hypersensitive state” occurs directly from the effects of inflammatory mediators arising from disease in relevant anatomic sites “sensitizing” receptors expressed on afferent nerves. Alternative possibilities include the central amplification of otherwise normal afferent signals or the failure of descending inhibitory pathways in the higher center to exert their suppressing effect. Irrespective of the exact mechanism, a resolution of this sensitization, by whatever means (eg, the natural resolution of the viral infection, the withdrawal of the angiotensin-converting enzyme inhibitor, or the effective treatment of the underlying cause), is critical to the improvement of the cough. On the other hand, it is also unclear why a hypersensitive state is transient in some patients, whereas it becomes permanent in others.
The maturational and developmental aspects of the cough reflex have important clinical implications.139 Chemical and mechanical activation is effective at initiating protective reflexes, including apneas and swallowing in the newborn. As the infant matures, these responses become less pronounced. The age at which the cough reflex in children becomes fully matured is not known, but aging per se can impact cough to varying degrees. The efficacy of coughing may be reduced, as respiratory muscle strength decreases with age.155 However, the threshold for initiating coughing does not change.156-158 By contrast, the elderly’s capacity to suppress cough is diminished,158 as is the sensation of the urge to cough following airways irritation.157 Whether these characteristics have a bearing on the likelihood of coughing in the elderly is unclear, although aspiration pneumonia is more common in old age.
Conclusions
Coughing is directly evoked by stimulation of a subset of bronchopulmonary C-fibers and a mechanically sensitive, acid-sensitive subtype of myelinated airway mechanoreceptors. Their activation may result directly in coughing, whereas their coincident activation greatly increases responsiveness to tussive stimuli. Cough can also be modulated by other bronchopulmonary, vagal afferent nerve subtypes and afferent nerves terminating in extrapulmonary locations, including the upper airways, pharynx, and esophagus.
Evidence for extensive remodeling of airway afferent innervation in diseases associated with cough has been conflicting.159-163 Perhaps chronic coughing occurs, not because of a hyperinnervation of the airways, but because there are more triggers for coughing in the airways or in other organs associated with coughing. Consistent with this notion, inflammatory mediators are elevated in the BAL fluid of patients with asthma and chronic coughers without asthma, and therapeutics that target identified triggers of coughing or inflammation have proven effective in treating cough in several patient populations.164-173 The precise terminal locations of the afferent nerves driving chronic cough are not yet known.
Although effects in animals have been reported,174 it is unknown whether any meaningful changes in afferent nerve excitability or ion channel expression occur in chronic cough in patients. Therapeutic strategies targeting the interactions between the afferent nerves regulating cough at the level of the CNS, the mechanisms underlying action potential generation, and the encoding mechanisms of cough are all viable therapeutic strategies for cough suppression (Fig 5). Emerging therapeutic research strategies based on these rationales are priorities to advancing the field at this time and include blockers of TRPV1 and/or TRPA1, voltage-gated sodium-channel blockers (selectively targeting the sodium channels [eg, voltage-gated sodium channel 1.7] expressed by airways sensory nerves), N-methyl-D-aspartate-receptor/channel blockers, and other therapeutic strategies aimed at reducing afferent nerve excitability within the airways.175-177
Acknowledgments
Author contributions: L. M. served as guarantor of the article and as corresponding author. B. J. C., S. B. M., and L. M. wrote the first draft of the manuscript; J. A. S. and S. B. M. prepared the figures and figure legends; A. B. C., D. C. B., and J. A. S. refined the subsequent drafts of the manuscript; and all contributors approved the final version.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Canning chairs a scientific advisory board for Cerecor Inc, a company that has sought development of new drugs for treating cough, including therapies protected by patents held jointly by Cerecor Inc and by Dr Canning. Dr Canning is also a member of the scientific advisory board for Holaira, Inc and has recently served as a consultant for and/or received research funding from Afferent Pharmaceuticals; Almirall S.A.; Circuit Therapeutics, Inc; Eli Lilly and Company; GlaxoSmithKline; Merck & Co, Inc; and Palatin Technologies, Inc. Dr Bolser receives no financial compensation for participating in the Expert Cough Panel. He has acted as a paid consultant for Merck & Co, Inc in the past year on matters unrelated to the content of this manuscript. He also is a cofounder of two startup companies, Sensory Integrated Solutions, LLC and Airway Assistance, LLC. He holds an equity stake in each but receives no financial compensation from these companies. Dr Smith is a named inventor on a patent owned by the University Hospital of South Manchester, which describes novel techniques for detecting cough from sound recordings. This patent is licensed to a medical device company, but Dr Smith has not received any financial rewards as a result. Dr Smith's institution has received grant funding from Afferent Pharmaceuticals. Dr McGarvey receives university grant monies and pharmaceutical company grant monies as follows: Bionorica SE: Global Research Initiative Award (2014); Laboratory Animal Science Association /NC3Rs (2011-2014); British Heart Foundation: infrastructure grant (2010-2011); RBHSC Clinical Fellowship (2010-2012); Laboratory Animal Science Association /NC3Rs (2009-2010); Asthma UK (2009-2010); NI Chest Heart & Stroke Association (2009-2011). Dr McGarvey reports that his institution has received grant income for studies undertaken by him related to basic science and clinical aspects of cough. Drs Chang and Mazzone have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Collaborators: Todd M. Adams, MD; Kenneth W. Altman, MD, PhD; Alan F. Barker, MD; Surinder S. Birring, MBChB, MD; Fiona Blackhall, MD, PhD; Donald, C. Bolser, PhD; Louis-Philippe Boulet, MD, FCCP; Sidney S. Braman, MD, FCCP; Christopher Brightling, MBBS, PhD, FCCP; Priscilla Callahan-Lyon, MD; Brendan Canning, PhD; Anne Bernadette Chang, MBBS, PhD, MPH; Remy Coeytaux, MD, PhD; Terrie Cowley; Paul Davenport, PhD; Rebecca L. Diekemper, MPH; Satoru Ebihara, MD, PhD; Ali A. El Solh, MD, MPH; Patricio Escalante, MD, FCCP; Anthony Feinstein, MPhil, PhD; Stephen K. Field, MD; Dina Fisher, MD; Cynthia T. French, PhD, ANP-BC; Peter Gibson, MBBS; Philip Gold, MD, FCCP; Cameron Grant, MBChB, PhD; Susan M. Harding, MD, FCCP; Anthony Harnden, MBChB; Adam T. Hill, MBChB, MD; Richard S. Irwin, MD, Master FCCP; Peter J. Kahrilas, MD; Karina A. Keogh, MD; Andrew P. Lane, MD; Sandra Zelman Lewis, PhD; Kaiser Lim, MD; Mark A. Malesker, PharmD, FCCP; Peter Mazzone, MD, MPH, FCCP; Stuart Mazzone, PhD, FCCP; Lorcan McGarvey, MD; Alex Molasiotis, PhD, RN; M. Hassan Murad, MD, MPH; Peter Newcombe, PhD; Huong Q. Nguyen, PhD, RN; John Oppenheimer, MD; David Prezant, MD; Tamara Pringsheim, MD; Marcos I. Restrepo, MD, MSc, FCCP; Mark Rosen, MD, Master FCCP; Bruce Rubin, MEngr, MD, MBA; Jay H. Ryu, MD, FCCP; Jaclyn Smith, MBChB, PhD; Susan M. Tarlo, MBBS, FCCP; Ronald B. Turner, MD; Anne Vertigan, PhD, MBA; Gang Wang, MD, PhD; Kelly Weir, MsPath.
Role of sponsors: CHEST was the sole supporter of these guidelines, this article, and the innovations addressed within.
Other contributions: We thank other panelists participating in the guidance development process for their review of this article. We thank Peter West MBiolSci, PhD, University of Manchester, England, for production of the human images in Figure 3.
ABBREVIATIONS
- ATP
adenosine triphosphate
- GERD
gastroesophageal reflux disease
- GIT
gastrointestinal tract
- nTS
nucleus tractus solitarius
- RAR
rapidly adapting receptor
- SAR
slowly adapting receptor
- TRPA1
transient receptor potential A1
- TRPM8
transient receptor potential M8
- TRPV1
transient receptor potential vanilloid 1
Footnotes
FUNDING/SUPPORT: The American College of Chest Physicians was the sole supporter of these guidelines, this article, and the innovations addressed within. Dr Bolser received National Institutes of Health funding [Grant R01 HL104315].
DISCLAIMER: American College of Chest Physician guidelines are intended for general information only, are not medical advice, and do not replace professional medical care and physician advice, which always should be sought for any medical condition. The complete disclaimer for this guideline can be accessed at http://dx.doi.org/10.1378/chest.1464S1.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
Contributor Information
on behalf of the CHEST Expert Cough Panel:
Todd M. Adams, Kenneth W. Altman, Alan F. Barker, Surinder S. Birring, Fiona Blackhall, Donald, C. Bolser, Louis-Philippe Boulet, Sidney S. Braman, Christopher Brightling, Priscilla Callahan-Lyon, Brendan Canning, Anne Bernadette Chang, Remy Coeytaux, Terrie Cowley, Paul Davenport, Rebecca L. Diekemper, Satoru Ebihara, Ali A. El Solh, Patricio Escalante, Anthony Feinstein, Stephen K. Field, Dina Fisher, Cynthia T. French, Peter Gibson, Philip Gold, Cameron Grant, Susan M. Harding, Anthony Harnden, Adam T. Hill, Richard S. Irwin, Peter J. Kahrilas, Karina A. Keogh, Andrew P. Lane, Sandra Zelman Lewis, Kaiser Lim, Mark A. Malesker, Peter Mazzone, Stuart Mazzone, Lorcan McGarvey, Alex Molasiotis, M. Hassan Murad, Peter Newcombe, Huong Q. Nguyen, John Oppenheimer, David Prezant, Tamara Pringsheim, Marcos I. Restrepo, Mark Rosen, Bruce Rubin, Jay H. Ryu, Jaclyn Smith, Susan M. Tarlo, Ronald B. Turner, Anne Vertigan, Gang Wang, and Kelly Weir
References
- 1.Fleming PJ, Bryan AC, Bryan MH. Functional immaturity of pulmonary irritant receptors and apnea in newborn preterm infants. Pediatrics. 1978;61(4):515-518. [PubMed] [Google Scholar]
- 2.Schramm CM. Current concepts of respiratory complications of neuromuscular disease in children. Curr Opin Pediatr. 2000;12(3):203-207. [DOI] [PubMed] [Google Scholar]
- 3.Mosconi P, Langer M, Cigada M, Mandelli M; Intensive Care Unit Group for Infection Control. Epidemiology and risk factors of pneumonia in critically ill patients. Eur J Epidemiol. 1991;7(4):320-327. [DOI] [PubMed] [Google Scholar]
- 4.Niimi A, Matsumoto H, Ueda T, et al. Impaired cough reflex in patients with recurrent pneumonia. Thorax. 2003;58(2):152-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1_suppl):1S-23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet. 2008;371(9621):1364-1374. [DOI] [PubMed] [Google Scholar]
- 7.Widdicombe JG. Afferent receptors in the airways and cough. Respir Physiol. 1998;114(1):5-15. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson JA. The role of capsaicin-sensitive C-fibre afferent nerves in the cough reflex. Pulm Pharmacol. 1996;9(5-6):315-321. [DOI] [PubMed] [Google Scholar]
- 9.Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol. 2006;152(3):223-242. [DOI] [PubMed] [Google Scholar]
- 10.Canning BJ, Chou YL. Cough sensors. I. Physiological and pharmacological properties of the afferent nerves regulating cough. Handb Exp Pharmacol. 2009;(187):23-47. [DOI] [PubMed] [Google Scholar]
- 11.Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol. 2001;127(2-3):113-124. [DOI] [PubMed] [Google Scholar]
- 12.Widdicombe J. Airway receptors. Respir Physiol. 2001;125(1-2):3-15. [DOI] [PubMed] [Google Scholar]
- 13.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1-110. [DOI] [PubMed] [Google Scholar]
- 14.Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol. 2001;125(1-2):47-65. [DOI] [PubMed] [Google Scholar]
- 15.Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556(pt 3):905-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nassenstein C, Taylor-Clark TE, Myers AC, et al. Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J Physiol. 2010;588(pt 23):4769-4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor-Clark TE, Undem BJ. Sensing pulmonary oxidative stress by lung vagal afferents. Respir Physiol Neurobiol. 2011;178(3):406-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nassenstein C, Kwong K, Taylor-Clark T, et al. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol. 2008;586(6):1595-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birrell MA, Belvisi MG, Grace M, et al. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med. 2009;180(11):1042-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grace M, Birrell MA, Dubuis E, Maher SA, Belvisi MG. Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax. 2012;67(10):891-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baluk P, Nadel JA, McDonald DM. Substance P-immunoreactive sensory axons in the rat respiratory tract: a quantitative study of their distribution and role in neurogenic inflammation. J Comp Neurol. 1992;319(4):586-598. [DOI] [PubMed] [Google Scholar]
- 22.Hunter DD, Undem BJ. Identification and substance P content of vagal afferent neurons innervating the epithelium of the guinea pig trachea. Am J Respir Crit Care Med. 1999;159(6):1943-1948. [DOI] [PubMed] [Google Scholar]
- 23.Mazzone SB, Reynolds SM, Mori N, et al. Selective expression of a sodium pump isozyme by cough receptors and evidence for its essential role in regulating cough. J Neurosci. 2009;29(43):13662-13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricco MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol. 1996;496(pt 2):521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundberg JM, Hökfelt T, Martling CR, Saria A, Cuello C. Substance P-immunoreactive sensory nerves in the lower respiratory tract of various mammals including man. Cell Tissue Res. 1984;235(2):251-261. [DOI] [PubMed] [Google Scholar]
- 26.Lamb JP, Sparrow MP. Three-dimensional mapping of sensory innervation with substance p in porcine bronchial mucosa: comparison with human airways. Am J Respir Crit Care Med. 2002;166(9):1269-1281. [DOI] [PubMed] [Google Scholar]
- 27.Groneberg DA, Niimi A, Dinh QT, et al. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med. 2004;170(12):1276-1280. [DOI] [PubMed] [Google Scholar]
- 28.Dey RD, Altemus JB, Zervos I, Hoffpauir J. Origin and colocalization of CGRP- and SP-reactive nerves in cat airway epithelium. J Appl Physiol (1985). 1990;68(2):770-778. [DOI] [PubMed] [Google Scholar]
- 29.Forsberg K, Karlsson JA. Cough induced by stimulation of capsaicin-sensitive sensory neurons in conscious guinea-pigs. Acta Physiol Scand. 1986;128(2):319-320. [DOI] [PubMed] [Google Scholar]
- 30.Widdicombe JG. Respiratory reflexes from the trachea and bronchi of the cat. J Physiol. 1954;123(1):55-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widdicombe JG. Receptors in the trachea and bronchi of the cat. J Physiol. 1954;123(1):71-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Widdicombe JG. Respiratory reflexes excited by inflation of the lungs. J Physiol. 1954;123(1):105-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004;557(pt 2):543-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canning BJ, Mori N. An essential component to brainstem cough gating identified in anesthetized guinea pigs. FASEB J. 2010;24(10):3916-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canning BJ, Mori N. Encoding of the cough reflex in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R369-R377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnes NC, Piper PJ, Costello JF. Comparative effects of inhaled leukotriene C4, leukotriene D4, and histamine in normal human subjects. Thorax. 1984;39(7):500-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joos G, Pauwels R, van der Straeten M. Effect of inhaled substance P and neurokinin A on the airways of normal and asthmatic subjects. Thorax. 1987;42(10):779-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujimura M, Sakamoto S, Kamio Y, Matsuda T. Effects of methacholine induced bronchoconstriction and procaterol induced bronchodilation on cough receptor sensitivity to inhaled capsaicin and tartaric acid. Thorax. 1992;47(6):441-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shinagawa K, Kojima M, Ichikawa K, Hiratochi M, Aoyagi S, Akahane M. Participation of thromboxane A(2) in the cough response in guinea-pigs: antitussive effect of ozagrel. Br J Pharmacol. 2000;131(2):266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164(10 pt 2):S28-S38. [DOI] [PubMed] [Google Scholar]
- 41.Baluk P, Gabella G. Afferent nerve endings in the tracheal muscle of guinea-pigs and rats. Anat Embryol (Berl). 1991;183(1):81-87. [DOI] [PubMed] [Google Scholar]
- 42.Yu J. Spectrum of myelinated pulmonary afferents. Am J Physiol Regul Integr Comp Physiol. 2000;279(6):R2142-R2148. [DOI] [PubMed] [Google Scholar]
- 43.Schelegle ES, Green JF. An overview of the anatomy and physiology of slowly adapting pulmonary stretch receptors. Respir Physiol. 2001;125(1-2):17-31. [DOI] [PubMed] [Google Scholar]
- 44.Widdicombe J. Functional morphology and physiology of pulmonary rapidly adapting receptors (RARs). Anat Rec A Discov Mol Cell Evol Biol. 2003;270(1):2-10. [DOI] [PubMed] [Google Scholar]
- 45.Jonzon A, Pisarri TE, Coleridge JC, Coleridge HM. Rapidly adapting receptor activity in dogs is inversely related to lung compliance. J Appl Physiol (1985). 1986;61(5):1980-1987. [DOI] [PubMed] [Google Scholar]
- 46.Pack AI, DeLaney RG. Response of pulmonary rapidly adapting receptors during lung inflation. J Appl Physiol. 1983;55(3):955-963. [DOI] [PubMed] [Google Scholar]
- 47.Yu J, Wang YF, Zhang JW. Structure of slowly adapting pulmonary stretch receptors in the lung periphery. J Appl Physiol (1985). 2003;95(1):385-393. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto S. The activities of lung stretch and irritant receptors during cough. Neurosci Lett. 1988;90(1-2):125-129. [DOI] [PubMed] [Google Scholar]
- 49.Nishino T, Sugimori K, Hiraga K, Hond Y. Influence of CPAP on reflex responses to tracheal irritation in anesthetized humans. J Appl Physiol (1985). 1989;67(3):954-958. [DOI] [PubMed] [Google Scholar]
- 50.Lavorini F, Fontana G, Chellini E, Magni C, Pistolesi M, Widdicombe J. The Fontana paradoxical reflex? Chest. 2011;140(3):586-588. [DOI] [PubMed] [Google Scholar]
- 51.Bolser DC, Davenport PW. Volume-timing relationships during cough and resistive loading in the cat. J Appl Physiol (1985). 2000;89(2):785-790. [DOI] [PubMed] [Google Scholar]
- 52.Smith JA, Aliverti A, Quaranta M, et al. Chest wall dynamics during voluntary and induced cough in healthy volunteers. J Physiol. 2012;590(pt 3):563-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Canning BJ, Reynolds SM, Mazzone SB. Multiple mechanisms of reflex bronchospasm in guinea pigs. J Appl Physiol (1985). 2001;91(6):2642-2653. [DOI] [PubMed] [Google Scholar]
- 54.Richardson CA, Herbert DA, Mitchell RA. Modulation of pulmonary stretch receptors and airway resistance by parasympathetic efferents. J Appl Physiol. 1984;57(6):1842-1849. [DOI] [PubMed] [Google Scholar]
- 55.Lowry R, Wood A, Johnson T, Higenbottam T. Antitussive properties of inhaled bronchodilators on induced cough. Chest. 1988;93(6):1186-1189. [DOI] [PubMed] [Google Scholar]
- 56.Mazzone SB, Lim LH, Wagner EM, Mori N, Canning BJ. Sympathetic nerve-dependent regulation of mucosal vascular tone modifies airway smooth muscle reactivity. J Appl Physiol (1985). 2010;109(5):1292-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barnes PJ. Neurogenic inflammation in the airways. Respir Physiol. 2001;125(1-2):145-154. [DOI] [PubMed] [Google Scholar]
- 58.Lundberg JM, Saria A, Brodin E, Rosell S, Folkers K. A substance P antagonist inhibits vagally induced increase in vascular permeability and bronchial smooth muscle contraction in the guinea pig. Proc Natl Acad Sci U S A. 1983;80(4):1120-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Basoglu OK, Pelleg A, Essilfie-Quaye S, Brindicci C, Barnes PJ, Kharitonov SA. Effects of aerosolized adenosine 5′-triphosphate vs adenosine 5′-monophosphate on dyspnea and airway caliber in healthy nonsmokers and patients with asthma. Chest. 2005;128(4):1905-1909. [DOI] [PubMed] [Google Scholar]
- 60.Beijer S, Gielisse EA, Hupperets PS, et al. Intravenous ATP infusions can be safely administered in the home setting: a study in pre-terminal cancer patients. Invest New Drugs. 2007;25(6):571-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abdulqwai R, McCarthy B, Layton G, Woodcock A, Ford A, Smith JA. Inhibition of ATP-gated P2X3 channels by AF-219: an effective anti-tussive mechanism in chronic cough. Abstract 1965. Presented at: European Respiratory Society Annual Congress; September 7-11, 2013; Barcelona, Spain.
- 62.Mazzone SB, Canning BJ. Central nervous system control of the airways: pharmacological implications. Curr Opin Pharmacol. 2002;2(3):220-228. [DOI] [PubMed] [Google Scholar]
- 63.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol (1985). 2006;101(2):618-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mazzone SB, Canning BJ. Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R86-R98. [DOI] [PubMed] [Google Scholar]
- 65.Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol. 2005;569(pt 2):559-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma QP, Woolf CJ. Involvement of neurokinin receptors in the induction but not the maintenance of mechanical allodynia in rat flexor motoneurones. J Physiol. 1995;486(pt 3):769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765-1769. [DOI] [PubMed] [Google Scholar]
- 68.Canning BJ, Mazzone SB. Reflex mechanisms in gastroesophageal reflux disease and asthma. Am J Med. 2003;115(suppl 3A):45S-48S. [DOI] [PubMed] [Google Scholar]
- 69.Tatar M, Webber SE, Widdicombe JG. Lung C-fibre receptor activation and defensive reflexes in anaesthetized cats. J Physiol. 1988;402:411-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tatar M, Sant’Ambrogio G, Sant’Ambrogio FB. Laryngeal and tracheobronchial cough in anesthetized dogs. J Appl Physiol (1985). 1994;76(6):2672-2679. [DOI] [PubMed] [Google Scholar]
- 71.Tatár M, Korpás J, Polácek H, Záhradný V. Changes induced by severe hypoxia in respiratory defence reflexes in anaesthetized cats. Respiration. 1986;49(2):114-121. [DOI] [PubMed] [Google Scholar]
- 72.Eckert DJ, Catcheside PG, Stadler DL, McDonald R, Hlavac MC, McEvoy RD. Acute sustained hypoxia suppresses the cough reflex in healthy subjects. Am J Respir Crit Care Med. 2006;173(5):506-511. [DOI] [PubMed] [Google Scholar]
- 73.Tomori Z, Sedíková V, Javorka K. Changes in blood gas tensions and acid-base balance during respiratory reflexes. Physiol Bohemoslov. 1973;22(3):305-314. [PubMed] [Google Scholar]
- 74.Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol. 1999;59(4):299-331. [DOI] [PubMed] [Google Scholar]
- 75.Tekdemir I, Aslan A, Elhan A. A clinico-anatomic study of the auricular branch of the vagus nerve and Arnold’s ear-cough reflex. Surg Radiol Anat. 1998;20(4):253-257. [PubMed] [Google Scholar]
- 76.Nomura S, Mizuno N. Central distribution of primary afferent fibers in the Arnold’s nerve (the auricular branch of the vagus nerve): a transganglionic HRP study in the cat. Brain Res. 1984;292(2):199-205. [DOI] [PubMed] [Google Scholar]
- 77.Plevkova J, Kollarik M, Brozmanova M, Revallo M, Varechova S, Tatar M. Modulation of experimentally-induced cough by stimulation of nasal mucosa in cats and guinea pigs. Respir Physiol Neurobiol. 2004;142(2-3):225-235. [DOI] [PubMed] [Google Scholar]
- 78.Plevkova J, Brozmanova M, Pecova R, Tatar M. Effects of intranasal capsaicin challenge on cough reflex in healthy human volunteers. J Physiol Pharmacol. 2004;55(suppl 3):101-106. [PubMed] [Google Scholar]
- 79.Buday T, Brozmanova M, Biringerova Z, et al. Modulation of cough response by sensory inputs from the nose - role of trigeminal TRPA1 versus TRPM8 channels. Cough. 2012;8(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tatar M, Plevkova J, Brozmanova M, Pecova R, Kollarik M. Mechanisms of the cough associated with rhinosinusitis. Pulm Pharmacol Ther. 2009;22(2):121-126. [DOI] [PubMed] [Google Scholar]
- 81.Plevkova J, Kollarik M, Poliacek I, Brozmanova M, Surdenikova L, Tatar M, Mori N, Canning BJ. The role of trigeminal nasal TRPM8-expressing afferent neurons in the antitussive effects of menthol. J Appl Physiol (1985). 2013;115(2):268-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morice AH, Marshall AE, Higgins KS, Grattan TJ. Effect of inhaled menthol on citric acid induced cough in normal subjects. Thorax. 1994;49(10):1024-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nishino T, Hasegawa R, Ide T, Isono S. Hypercapnia enhances the development of coughing during continuous infusion of water into the pharynx. Am J Respir Crit Care Med. 1998;157(3 pt 1):815-821. [DOI] [PubMed] [Google Scholar]
- 84.Nishino T. Physiological and pathophysiological implications of upper airway reflexes in humans. Jpn J Physiol. 2000;50(1):3-14. [DOI] [PubMed] [Google Scholar]
- 85.Hegland KW, Pitts T, Bolser DC, Davenport PW. Urge to cough with voluntary suppression following mechanical pharyngeal stimulation. Bratisl Lek Listy (Tlacene Vyd). 2011;112(3):109-114. [PMC free article] [PubMed] [Google Scholar]
- 86.Yoshida Y, Tanaka Y, Hirano M, Nakashima T. Sensory innervation of the pharynx and larynx. Am J Med. 2000;108(suppl 4a):51S-61S. [DOI] [PubMed] [Google Scholar]
- 87.Smith JA, Houghton LA. The oesophagus and cough: laryngo-pharyngeal reflux, microaspiration and vagal reflexes. Cough. 2013;9(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benini L, Ferrari M, Sembenini C, et al. Cough threshold in reflux oesophagitis: influence of acid and of laryngeal and oesophageal damage. Gut. 2000;46(6):762-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith JA, Decalmer S, Kelsall A, et al. Acoustic cough-reflux associations in chronic cough: potential triggers and mechanisms. Gastroenterology. 2010;139(3):754-762. [DOI] [PubMed] [Google Scholar]
- 90.Harding SM. Recent clinical investigations examining the association of asthma and gastroesophageal reflux. Am J Med. 2003;115(suppl 3A):39S-44S. [DOI] [PubMed] [Google Scholar]
- 91.Lang IM, Medda BK, Shaker R. Mechanisms of reflexes induced by esophageal distension. Am J Physiol Gastrointest Liver Physiol. 2001;281(5):G1246-G1263. [DOI] [PubMed] [Google Scholar]
- 92.German VF, Corrales R, Ueki IF, Nadel JA. Reflex stimulation of tracheal mucus gland secretion by gastric irritation in cats. J Appl Physiol. 1982;52(5):1153-1155. [DOI] [PubMed] [Google Scholar]
- 93.Mazzone SB, McGovern AE. Innervation of tracheal parasympathetic ganglia by esophageal cholinergic neurons: evidence from anatomic and functional studies in guinea pigs. Am J Physiol Lung Cell Mol Physiol. 2010;298(3):L404-L416. [DOI] [PubMed] [Google Scholar]
- 94.Wu DN, Yamauchi K, Kobayashi H, et al. Effects of esophageal acid perfusion on cough responsiveness in patients with bronchial asthma. Chest. 2002;122(2):505-509. [DOI] [PubMed] [Google Scholar]
- 95.Ing AJ, Ngu MC, Breslin AB. Pathogenesis of chronic persistent cough associated with gastroesophageal reflux. Am J Respir Crit Care Med. 1994;149(1):160-167. [DOI] [PubMed] [Google Scholar]
- 96.Javorkova N, Varechova S, Pecova R, et al. Acidification of the oesophagus acutely increases the cough sensitivity in patients with gastro-oesophageal reflux and chronic cough. Neurogastroenterol Motil. 2008;20(2):119-124. [DOI] [PubMed] [Google Scholar]
- 97.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol (1985). 2006;101(2):618-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol (1985). 1998;84(6):2020-2035. [DOI] [PubMed] [Google Scholar]
- 99.Baekey DM, Morris KF, Gestreau C, Li Z, Lindsey BG, Shannon R. Medullary respiratory neurones and control of laryngeal motoneurones during fictive eupnoea and cough in the cat. J Physiol. 2001;534(pt 2):565-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shannon R, Baekey DM, Morris KF, Lindsey BG. Brainstem respiratory networks and cough. Pulm Pharmacol. 1996;9(5-6):343-347. [DOI] [PubMed] [Google Scholar]
- 101.Shannon R, Baekey DM, Morris KF, Li Z, Lindsey BG. Functional connectivity among ventrolateral medullary respiratory neurones and responses during fictive cough in the cat. J Physiol. 2000;525(pt 1):207-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shannon R, Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG. Production of reflex cough by brainstem respiratory networks. Pulm Pharmacol Ther. 2004;17(6):369-376. [DOI] [PubMed] [Google Scholar]
- 103.McGovern AE, Davis-Poynter N, Farrell MJ, Mazzone SB. Transneuronal tracing of airways-related sensory circuitry using herpes simplex virus 1, strain H129. Neuroscience. 2012;207:148-166. [DOI] [PubMed] [Google Scholar]
- 104.Hanácek J, Davies A, Widdicombe JG. Influence of lung stretch receptors on the cough reflex in rabbits. Respiration. 1984;45(3):161-168. [DOI] [PubMed] [Google Scholar]
- 105.Sant’Ambrogio G, Sant’Ambrogio FB, Davies A. Airway receptors in cough. Bull Eur Physiopathol Respir. 1984;20(1):43-47. [PubMed] [Google Scholar]
- 106.Iscoe S. Control of abdominal muscles. Prog Neurobiol. 1998;56(4):433-506. [DOI] [PubMed] [Google Scholar]
- 107.O’Connor R, Segers LS, Morris KF, et al. A joint computational respiratory neural network-biomechanical model for breathing and airway defensive behaviors. Front Physiol. 2012;3:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shannon R, Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG. Pontine respiratory group neuron discharge is altered during fictive cough in the decerebrate cat. Respir Physiol Neurobiol. 2004;142(1):43-54. [DOI] [PubMed] [Google Scholar]
- 109.Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG, Shannon R. Medullary raphe neuron activity is altered during fictive cough in the decerebrate cat. J Appl Physiol (1985). 2003;94(1):93-100. [DOI] [PubMed] [Google Scholar]
- 110.Jakus J, Tomori Z, Stránsky A. Activity of bulbar respiratory neurones during cough and other respiratory tract reflexes in cats. Physiol Bohemoslov. 1985;34(2):127-136. [PubMed] [Google Scholar]
- 111.May AJ, Widdicombe JG. Depression of the cough reflex by pentobarbitone and some opium derivatives. Br Pharmacol Chemother. 1954;9(3):335-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bolser DC, Hey JA, Chapman RW. Influence of central antitussive drugs on the cough motor pattern. J Appl Physiol (1985). 1999;86(3):1017-1024. [DOI] [PubMed] [Google Scholar]
- 113.Poliacek I, Wang C, Corrie LW, Rose MJ, Bolser DC. Microinjection of codeine into the region of the caudal ventral respiratory column suppresses cough in anesthetized cats. J Appl Physiol (1985). 2010;108(4):858-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Poliacek I, Corrie LW, Rose MJ, Wang C, Bolser DC. Influence of microinjections of D,L-homocysteic acid into the Botzinger complex area on the cough reflex in the cat. J Physiol Pharmacol. 2008;59(suppl 6):585-596. [PMC free article] [PubMed] [Google Scholar]
- 115.Poliacek I, Corrie LW, Wang C, Rose MJ, Bolser DC. Microinjection of DLH into the region of the caudal ventral respiratory column in the cat: evidence for an endogenous cough-suppressant mechanism. J Appl Physiol (1985). 2007;102(3):1014-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mutolo D, Bongianni F, Cinelli E, Fontana GA, Pantaleo T. Modulation of the cough reflex by antitussive agents within the caudal aspect of the nucleus tractus solitarii in the rabbit. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R243-R251. [DOI] [PubMed] [Google Scholar]
- 117.Mutolo D, Bongianni F, Fontana GA, Pantaleo T. The role of excitatory amino acids and substance P in the mediation of the cough reflex within the nucleus tractus solitarii of the rabbit. Brain Res Bull. 2007;74(4):284-293. [DOI] [PubMed] [Google Scholar]
- 118.Mazzone SB, McLennan L, McGovern AE, Egan GF, Farrell MJ. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Respir Crit Care Med. 2007;176(4):327-332. [DOI] [PubMed] [Google Scholar]
- 119.Mazzone SB, McGovern AE, Koo K, Farrell MJ. Mapping supramedullary pathways involved in cough using functional brain imaging: comparison with pain. Pulm Pharmacol Ther. 2009;22(2):90-96. [DOI] [PubMed] [Google Scholar]
- 120.Farrell MJ, Cole LJ, Chiapoco D, Egan GF, Mazzone SB. Neural correlates coding stimulus level and perception of capsaicin-evoked urge-to-cough in humans. Neuroimage. 2012;61(4):1324-1335. [DOI] [PubMed] [Google Scholar]
- 121.Vertigan AE, Gibson PG. Chronic refractory cough as a sensory neuropathy: evidence from a reinterpretation of cough triggers. J Voice. 2011;25(5):596-601. [DOI] [PubMed] [Google Scholar]
- 122.Vertigan AE, Gibson PG. The role of speech pathology in the management of patients with chronic refractory cough. Lung. 2012;190(1):35-40. [DOI] [PubMed] [Google Scholar]
- 123.Leech J, Mazzone SB, Farrell MJ. Brain activity associated with placebo suppression of the urge-to-cough in humans. Am J Respir Crit Care Med. 2013;188(9):1069-1075. [DOI] [PubMed] [Google Scholar]
- 124.Leech J, Mazzone SB, Farrell MJ. The effect of placebo conditioning on capsaicin-evoked urge to cough. Chest. 2012;142(4):951-957. [DOI] [PubMed] [Google Scholar]
- 125.Irwin RS, Glomb WB, Chang AB. Habit cough, tic cough, and psychogenic cough in adult and pediatric populations: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(suppl 1):174S-179S. [DOI] [PubMed] [Google Scholar]
- 126.Dicpinigaitis PV, Tso R, Banauch G. Prevalence of depressive symptoms among patients with chronic cough. Chest. 2006;130(6):1839-1843. [DOI] [PubMed] [Google Scholar]
- 127.Jahfari S, Waldorp L, van den Wildenberg WP, Scholte HS, Ridderinkhof KR, Forstmann BU. Effective connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. J Neurosci. 2011;31(18):6891-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Simonyan K, Saad ZS, Loucks TM, Poletto CJ, Ludlow CL. Functional neuroanatomy of human voluntary cough and sniff production. Neuroimage. 2007;37(2):401-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Amanzio M, Benedetti F, Porro CA, Palermo S, Cauda F. Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp. 2013;34(3):738-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mazzone SB, Cole LJ, Ando A, Egan GF, Farrell MJ. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J Neurosci. 2011;31(8):2948-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Canning BJ. Anatomy and neurophysiology of the cough reflex: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(suppl 1):33S-47S. [DOI] [PubMed] [Google Scholar]
- 132.Irwin RS, Corrao WM, Pratter MR. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am Rev Respir Dis. 1981;123(4 pt 1):413-417. [DOI] [PubMed] [Google Scholar]
- 133.Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis. 1990;141(3):640-647. [DOI] [PubMed] [Google Scholar]
- 134.Morice AH, McGarvey L, Pavord I; British Thoracic Society Cough Guideline Group. Recommendations for the management of cough in adults. Thorax. 2006;61(suppl 1):i1-i24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McGarvey LP, Heaney LG, Lawson JT, et al. Evaluation and outcome of patients with chronic non-productive cough using a comprehensive diagnostic protocol. Thorax. 1998;53(9):738-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Haque RA, Usmani OS, Barnes PJ. Chronic idiopathic cough: a discrete clinical entity? Chest. 2005;127(5):1710-1713. [DOI] [PubMed] [Google Scholar]
- 137.Pratter MR. Unexplained (idiopathic) cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1_suppl):220S-221S. [DOI] [PubMed] [Google Scholar]
- 138.Canning BJ, Farmer DG, Mori N. Mechanistic studies of acid-evoked coughing in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R454-R463. [DOI] [PubMed] [Google Scholar]
- 139.Thach BT. Maturation of cough and other reflexes that protect the fetal and neonatal airway. Pulm Pharmacol Ther. 2007;20(4):365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ebihara S, Ebihara T, Kohzuki M. Effect of aging on cough and swallowing reflexes: implications for preventing aspiration pneumonia. Lung. 2012;190(1):29-33. [DOI] [PubMed] [Google Scholar]
- 141.Aviv JE, Sacco RL, Mohr JP, et al. Laryngopharyngeal sensory testing with modified barium swallow as predictors of aspiration pneumonia after stroke. Laryngoscope. 1997;107(9):1254-1260. [DOI] [PubMed] [Google Scholar]
- 142.Phua SY, McGarvey L, Ngu M, Ing A. The differential effect of gastroesophageal reflux disease on mechanostimulation and chemostimulation of the laryngopharynx. Chest. 2010;138(5):1180-1185. [DOI] [PubMed] [Google Scholar]
- 143.Hansson L, Wollmer P, Dahlbäck M, Karlsson JA. Regional sensitivity of human airways to capsaicin-induced cough. Am Rev Respir Dis. 1992;145(5):1191-1195. [DOI] [PubMed] [Google Scholar]
- 144.Fox B, Bull TB, Guz A. Innervation of alveolar walls in the human lung: an electron microscopic study. J Anat. 1980;131(pt 4):683-692. [PMC free article] [PubMed] [Google Scholar]
- 145.Simonsson BG, Jacobs FM, Nadel JA. Role of autonomic nervous system and the cough reflex in the increased responsiveness of airways in patients with obstructive airway disease. J Clin Invest. 1967;46(11):1812-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Choudry NB, Fuller RW, Pride NB. Sensitivity of the human cough reflex: effect of inflammatory mediators prostaglandin E2, bradykinin, and histamine. Am Rev Respir Dis. 1989;140(1):137-141. [DOI] [PubMed] [Google Scholar]
- 147.Doherty MJ, Mister R, Pearson MG, Calverley PM. Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax. 2000;55(8):643-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ferrari M, Olivieri M, Sembenini C, et al. Tussive effect of capsaicin in patients with gastroesophageal reflux without cough. Am J Respir Crit Care Med. 1995;151(2 pt 1):557-561. [DOI] [PubMed] [Google Scholar]
- 149.O’Connell F, Thomas VE, Pride NB, Fuller RW. Capsaicin cough sensitivity decreases with successful treatment of chronic cough. Am J Respir Crit Care Med. 1994;150(2):374-380. [DOI] [PubMed] [Google Scholar]
- 150.McGarvey L, McKeagney P, Polley L, MacMahon J, Costello RW. Are there clinical features of a sensitized cough reflex? Pulm Pharmacol Ther. 2009;22(2):59-64. [DOI] [PubMed] [Google Scholar]
- 151.Fujimura M, Kasahara K, Kamio Y, Naruse M, Hashimoto T, Matsuda T. Female gender as a determinant of cough threshold to inhaled capsaicin. Eur Respir J. 1996;9(8):1624-1626. [DOI] [PubMed] [Google Scholar]
- 152.Kastelik JA, Thompson RH, Aziz I, Ojoo JC, Redington AE, Morice AH. Sex-related differences in cough reflex sensitivity in patients with chronic cough. Am J Respir Crit Care Med. 2002;166(7):961-964. [DOI] [PubMed] [Google Scholar]
- 153.French CL, Irwin RS, Curley FJ, Krikorian CJ. Impact of chronic cough on quality of life. Arch Intern Med. 1998;158(15):1657-1661. [DOI] [PubMed] [Google Scholar]
- 154.Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MD, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax. 2003;58(4):339-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lalley PM. The aging respiratory system—pulmonary structure, function and neural control. Respir Physiol Neurobiol. 2013;187(3):199-210. [DOI] [PubMed] [Google Scholar]
- 156.Katsumata U, Sekizawa K, Ebihara T, Sasaki H. Aging effects on cough reflex. Chest. 1995;107(1):290-291. [DOI] [PubMed] [Google Scholar]
- 157.Ebihara S, Ebihara T, Kanezaki M, et al. Aging deteriorated perception of urge-to-cough without changing cough reflex threshold to citric acid in female never-smokers. Cough. 2011;7(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Leow LP, Beckert L, Anderson T, Huckabee ML. Changes in chemosensitivity and mechanosensitivity in aging and Parkinson’s disease. Dysphagia. 2012;27(1):106-114. [DOI] [PubMed] [Google Scholar]
- 159.O’Connell F, Springall DR, Moradoghli-Haftvani A, et al. Abnormal intraepithelial airway nerves in persistent unexplained cough? Am J Respir Crit Care Med. 1995;152(6 pt 1):2068-2075. [DOI] [PubMed] [Google Scholar]
- 160.Howarth PH, Springall DR, Redington AE, Djukanovic R, Holgate ST, Polak JM. Neuropeptide-containing nerves in endobronchial biopsies from asthmatic and nonasthmatic subjects. Am J Respir Cell Mol Biol. 1995;13(3):288-296. [DOI] [PubMed] [Google Scholar]
- 161.Lucchini RE, Facchini F, Turato G, et al. Increased VIP-positive nerve fibers in the mucous glands of subjects with chronic bronchitis. Am J Respir Crit Care Med. 1997;156(6):1963-1968. [DOI] [PubMed] [Google Scholar]
- 162.Chanez P, Springall D, Vignola AM, et al. Bronchial mucosal immunoreactivity of sensory neuropeptides in severe airway diseases. Am J Respir Crit Care Med. 1998;158(3):985-990. [DOI] [PubMed] [Google Scholar]
- 163.Lee SY, Kim MK, Shin C, et al. Substance P-immunoreactive nerves in endobronchial biopsies in cough-variant asthma and classic asthma. Respiration. 2003;70(1):49-53. [DOI] [PubMed] [Google Scholar]
- 164.Jatakanon A, Lalloo UG, Lim S, Chung KF, Barnes PJ. Increased neutrophils and cytokines, TNF-alpha and IL-8, in induced sputum of non-asthmatic patients with chronic dry cough. Thorax. 1999;54(3):234-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brightling CE, Ward R, Wardlaw AJ, Pavord ID. Airway inflammation, airway responsiveness and cough before and after inhaled budesonide in patients with eosinophilic bronchitis. Eur Respir J. 2000;15(4):682-686. [DOI] [PubMed] [Google Scholar]
- 166.Dicpinigaitis PV, Dobkin JB, Reichel J. Antitussive effect of the leukotriene receptor antagonist zafirlukast in subjects with cough-variant asthma. J Asthma. 2002;39(4):291-297. [DOI] [PubMed] [Google Scholar]
- 167.Birring SS, Parker D, Brightling CE, Bradding P, Wardlaw AJ, Pavord ID. Induced sputum inflammatory mediator concentrations in chronic cough. Am J Respir Crit Care Med. 2004;169(1):15-19. [DOI] [PubMed] [Google Scholar]
- 168.Chaudhuri R, McMahon AD, Thomson LJ, et al. Effect of inhaled corticosteroids on symptom severity and sputum mediator levels in chronic persistent cough. J Allergy Clin Immunol. 2004;113(6):1063-1070. [DOI] [PubMed] [Google Scholar]
- 169.Hartl D, Griese M, Nicolai T, et al. Pulmonary chemokines and their receptors differentiate children with asthma and chronic cough. J Allergy Clin Immunol. 2005;115(4):728-736. [DOI] [PubMed] [Google Scholar]
- 170.Xie S, Macedo P, Hew M, Nassenstein C, Lee KY, Chung KF. Expression of transforming growth factor-beta (TGF-beta) in chronic idiopathic cough. Respir Res. 2009;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ferreira FdeA, Filho LV, Rodrigues JC, Bush A, Haslam PL. Comparison of atopic and nonatopic children with chronic cough: bronchoalveolar lavage cell profile. Pediatr Pulmonol. 2007;42(10):857-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chang AB, Gibson PG, Ardill J, McGarvey LP. Calcitonin gene-related peptide relates to cough sensitivity in children with chronic cough. Eur Respir J. 2007;30(1):66-72. [DOI] [PubMed] [Google Scholar]
- 173.Mund E, Christensson B, Grönneberg R, Larsson K. Noneosinophilic CD4 lymphocytic airway inflammation in menopausal women with chronic dry cough. Chest. 2005;127(5):1714-1721. [DOI] [PubMed] [Google Scholar]
- 174.Fischer A, McGregor GP, Saria A, Philippin B, Kummer W. Induction of tachykinin gene and peptide expression in guinea pig nodose primary afferent neurons by allergic airway inflammation. J Clin Invest. 1996;98(10):2284-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Muroi Y, Ru F, Kollarik M, et al. Selective silencing of Na(V)1.7 decreases excitability and conduction in vagal sensory neurons. J Physiol. 2011;589(pt 23):5663-5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Muroi Y, Ru F, Chou YL, Carr MJ, Undem BJ, Canning BJ. Selective inhibition of vagal afferent nerve pathways regulating cough using Nav 1.7 shRNA silencing in guinea pig nodose ganglia. Am J Physiol Regul Integr Comp Physiol. 2013;304(11):R1017-R1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dicpinigaitis PV, Morice AH, Birring SS, et al. Antitussive drugs—past, present, and future. Pharmacol Rev. 2014;66(2):468-512. [DOI] [PMC free article] [PubMed] [Google Scholar]