Abstract
Falls and fall-related injuries cause extremely costly and potentially fatal health problems in people post-stroke. However, there is no global indicator of walking instability for detecting which individuals will have increased risk of falls. The purposes of this study were to directly quantify walking stability in stroke survivors and neurologically intact controls and to determine which stability measures would reveal the changes in walking stability following stroke. This study thus provided an initial step to establish objective measures for identifying potential fallers. Nine post-stroke individuals and nine controls walked on a treadmill at four different speeds. We computed short-term local divergence exponent (LDE) and maximum Floquet multiplier (maxFM) of the trunk motion, average and variability of dynamic margins of stability (MOS) and step spatiotemporal measures. Post-stroke individuals demonstrated larger short-term LDE (p = 0.002) and maxFM (p = 0.041) in the mediolateral (ML) direction compared to the controls but remained orbitally stable (maxFM < 1). In addition, post-stroke individuals walked with greater average step width (p = 0.003) but similar average ML MOS (p = 0.154) compared to the controls. Post-stroke individuals also exhibited greater variability in all MOS and step measures (all p < 0.005). Our findings indicate that post-stroke individuals walked with greater local and orbital instability and gait variability than neurologically intact controls. The results suggest that short-term LDE of ML trunk motion and the variability of MOS and step spatiotemporal measures detect the changes in walking stability associated with stroke. These stability measures may have the potential for identifying those post-stroke individuals at increased risk of falls.
Keywords: Stroke, Gait, Dynamic stability, Non-linear dynamics, Margins of stability
1. Introduction
Falls and fall-related injuries cause extremely costly health problems in people following stroke [1–3]. Walking is the most common activity at the time of a fall [2–5]. Falls cause physical injuries (e.g., hip fractures), psychologically deleterious consequences (e.g., depression), and hospitalization, which lead to more disability and possible death [3]. Researchers have examined many potential risk factors for falls [1–4]. Due to the diversity of stroke-specific impairments and the multifactorial nature of falls, there is no agreement on what measurements best predict which post-stroke individuals will have an increased risk of falls. Identification of those individuals is needed so that interventions can be implemented sooner to prevent falls, injuries and the associated costs.
Walking stability reflects the ability to respond to external (e.g., irregular surface) or internal (e.g., noise in muscle force generation) perturbations without falling [6]. Understanding the differences in walking stability between post-stroke and neurologically intact individuals would be an initial step to establish objective measures for identifying potential fallers in people post-stroke. Stroke survivors often have reduced lower-limb joint excursions, inadequate forward propulsion, and hyperactive stretch reflexes that lead to an unstable and inefficient gait pattern [7]. Physical therapy assessments are used to provide valid and reliable scales in evaluating particular impairments. However, some of these tests may not reflect dynamic balance levels during community walking [1,8]. In addition, the discrete activities as performed in these assessments may be insufficient to capture complex walking dynamics [9]. Examining overall walking stability, which reflects the resultant effects of individual deficits, may better explain falls in the post-stroke population, especially since most falls occur during walking [2–5].
Another important issue related to falls post-stroke is how gait speed affects walking stability. There was a U-shaped relationship between preferred gait speed and falls in elders, that is individuals with faster and slower gaits were at higher risk of falls than those with normal gait speeds [10,11]. Slower gait might reflect multiple health issues that could be responsible for falls whereas faster gait might reflect high physical expectation of those individuals, thus exposing to more and/or greater environmental hazards [10,12]. Although it is not possible to determine the precise cause of how gait speed increases falls incidence, examining the effects of walking speeds on the stability of post-stroke gait would provide insights into how gait speed might have affected the risk of falls.
Techniques employing short-term local divergence exponent (LDE) and maximum Floquet multiplier (maxFM) are used to quantify local and orbital stability of human walking, respectively [6,13,14]. These two stability measures quantify how sensitive the human locomotor system is to naturally occurring perturbations during walking. It is unknown if the sensitivity of locomotor system to small perturbations will predict its capacity to resist all perturbations without falling [14]. However, these measures that quantify responses to small perturbations can directly reflect the increased instability of human walking resulting from larger perturbations [13,15,16]. The amplitude of short-term LDE and maxFM increased substantially and responded specifically to different types of external perturbations [13,16]. In addition, short-term LDE was associated with fall history in elders in a retrospective study [17]. Moreover, recent modeling studies also provide support for using short-term LDE to predict fall risk [18,19] although those models with the features of general aging or altered morphology may not be applicable to the post-stroke gait. Thus, assessing local and/or orbital stability of post-stroke gait may have the potential for detecting the increased instability associated with stroke.
The dynamic margins of stability (MOS) approach proposed by Hof et al. (2005) [20] has been used to quantify instantaneous walking stability. MOS is defined as the distance between a “velocity-adjusted” or “extrapolated” position of the center of mass (COM) and the boundaries of an individual’s base of support (BOS) [20]. To prevent falling during walking, the horizontal velocity of the COM must be considered because maintaining walking stability may be impossible if the COM velocity is directed outward at a large enough magnitude even if the projection of a person’s COM still lies within the BOS [20]. Foot placement may be used to control the amplitudes of MOS so the projection of the extrapolated COM remains within the BOS [21]. Humans appear to walk with similar amplitude of average lateral MOS [22] and significantly greater variability of lateral MOS when exposed to experimental perturbations [23], or when voluntarily changing step length or width [24]. This suggests that quantifying MOS variability may be more useful than the average MOS in assessing individual walking stability.
The purpose of this study was to quantify walking stability in post-stroke and neurologically intact individuals. We examined local and orbital stability, MOS, and gait variability. Stride-to-stride variability in the control of human walking was suggested as an independent predictor of falling in the elderly [25]. For people post-stroke, changes in gait variability are also related to poor motor performance outcomes [26]. We examined which measures would reveal the changes in walking stability following stroke and how walking speed would affect the stability of post-stroke gait. We hypothesized that these measures could detect differences in walking stability between post-stroke and healthy individuals. We also hypothesized that slower walking speed would lead to improved stability in people post-stroke. We based these hypotheses on previous findings in healthy young and elderly individuals and individuals with neuropathy, showing that slower walking is more stable than fast walking [27–30].
2. Method
2.1. Subjects
Nine post-stroke subjects (four female, five male, age: 60.8 ± 9.0 (45–71) years, post-stroke duration: 3.4 ± 3.3 (0.7–9.3) years; lower-extremity Fugl-Meyer score: 27 ±4 (20–34), mean ± STD (range)) and nine gender- and age-matched (±5 years) neurologically intact controls (age: 61.7 ± 10.0 (43–75) years) gave written informed consent and participated in the study. The University’s Institutional Review Board approved the protocol. None of the subjects reported a fall during the three months prior to the experiment. The exclusion criteria for the post-stoke subjects included moderate/severe chronic white matter disease or cerebellar stroke on MRI, neglect/hemianopia, history of lower-extremity joint replacement, or any medical condition, other than stroke, that affects walking ability.
2.2. Experimental protocol
All subjects walked on a treadmill at four different speeds: 60%, 80%, 100% of their preferred walking speed (PWS) and fastest attainable speed (FAS). PWS was determined from five trials of overground walking over 10 m (PWS: stroke: 1.0 ± 0.3 (0.55–1.35) m/s; control: 1.4 ± 0.1 (1.3–1.5) m/s). After ~5 min of familiarization to the treadmill walking, FAS was determined as the fastest speed subjects confirmed that they could sustain for 1 min (FAS: stroke: 1.1 ± 0.2 (0.66–1.44) m/s; control: 1.7 ± 0.2 (1.5–2.0) m/s). Each speed was tested three times in a pseudo-randomized order for 1 min. We chose this short testing duration to prevent excluding subjects with low endurance.
2.3. Data acquisition and analysis
We collected 3-dimensional (3D) kinematics and ground reaction forces while subjects walked on an instrumented treadmill (1200 Hz, Bertec Corp., Columbus, OH, USA). The 3D kinematic data were recorded using an 8-camera video system (120 Hz, Motion Analysis Corporation, Santa Rosa, CA, USA) with 46 reflective markers attached on the lower body, trunk and over the C7 vertebra. We used commercial software (Visual3D, C-Motion Inc., Germantown, MD, USA) for initial data processing.
2.3.1. Local and orbital stability analyses
Techniques to quantify local and orbital stability are well established [6,13,14,28,31,32]. Briefly, data of 30 continuous strides were first extracted for each trial [30]. Typically, recording at least 150 continuous strides is recommended for these stability assessments [33,34]. This was not practically feasible in this patient population. However, by analyzing the same number of strides across all subjects and conditions, comparisons of the different dependent measures made across groups and conditions should remain valid [14,30,33,34].
For local stability analyses, the data were re-sampled to 3000 total data points, approximately 100 data points per stride. Delay embedded state spaces were reconstructed independently from the anterior–posterior (AP), mediolateral (ML), and vertical (VT) velocities of non-filtered C7 vertebral marker data [27]. The 3-dimensional motions of this C7 vertebral marker were used to represent trunk movements during walking [27]. The reconstructed state spaces included the original data and its time delayed copies (Eq. (1)):
| (1) |
where S(t) was the 5-dimensional state vector, x(t) was the original 1-dimensional C7 vertebral marker velocity data, and τ was the time delay. Fixed time delays were set equal to 25, 30, and 15 data samples (approximately 25%, 30%, and 15% of a gait cycle) for the AP, ML, and VT directions, respectively, for each subject and condition [32].
We quantified local stability by computing the short-term local divergence exponent (LDE) on the reconstructed state spaces of C7 vertebral marker movement [13]. LDE quantified how quickly neighboring movement trajectories in a state space diverge over time. We identified nearest neighbors and calculated Euclidean distances (i.e., divergence) between neighboring trajectories in the state space as a function of time. We averaged the logarithmic divergence over all original pairs of initially nearest neighbors.
| (2) |
where dj(i) represents the Euclidean distance between the jth pair of initially nearest neighbors after i discrete time steps (i.e., iΔt)and 〈〉 represents the average over all j pairs. Short-term LDE was estimated from the slope of a linear least-square fit to the mean log divergence curve across the span of 0–100 data samples [27,30]. Positive LDE indicates local instability. A larger value of LDE indicates more unstable and sensitive to local perturbations.
Floquet multipliers (FM) were used to estimate orbital stability, reflecting how a system responds to small perturbations discretely from one cycle to the next [31]. Since Floquet analysis assumes purely periodic dynamics, the state spaces were first divided for individual strides and then each stride was time normalized to 101 samples [28]. Poincaré sections were then defined for each percent of the stride cycle as:
| (3) |
where Sk is the state of the system at stride k at each given Poincaré section (i.e., at each % of the stride cycle) and Sk + 1 is the state of the system at the next stride. Fixed points at each Poincaré section were defined from the average trajectory across all strides in a given trial:
| (4) |
Orbital stability at each Poincaré section was examined by evaluating the effects of small perturbations away from the fixed points, using a linearized approximation of Eq. (4):
| (5) |
where J(S*) is the Jacobian matrix for the system at each Poincaré section. The FM are the eigenvalues of J(S*), which quantify how small perturbations grow or diminish for a given Poincaré section between one cycle and the next cycle. We calculated the maximum FM (maxFM) for each percent of the gait cycle and then averaged these maxFMs across the cycle for each trial. If maxFM is smaller than one, deviations away from the fixed point on average shrink by the subsequent strides and this indicates orbitally stable.
2.3.2. Dynamic margins of stability (MOS)
MOS were computed as the distances between the extrapolated center of mass (XCOM) positions and the boundaries of the BOS [20]. The XCOM position was calculated as:
| (6) |
where x is the COM position, ẋ is the COM velocity, and
| (7) |
where g is 9.81 m/s2 and l is the equivalent pendulum length taken as the height of the COM during quiet standing. We computed anterior–posterior MOS (MOSAP) as the anterior–posterior distance between the XCOM and the front toe marker of the leading foot. Mediolateral MOS (MOSML) was computed as the lateral distance between the XCOM and the lateral toe marker of the leading foot. We calculated MOSAP and MOSML at heel strikes for each foot for each trial [23].
2.3.3. Gait variability
We computed the standard deviations of step width, length and time from each trial. We also calculated mean standard deviations (meanSD) of the C7 marker positions and velocities to quantify overall variability of subjects’ displacements (i.e., drift) on the treadmill and stride-to-stride trunk movement variability, respectively [27]. The meanSD of the C7 marker positions and velocities were calculated across strides at each normalized time point of the gait cycle and then averaged over the whole gait cycle to produce a single measure of the mean variability for each trial.
2.4. Statistics
We used mixed-design ANOVAs to test for differences in short-term LDE and maxFM of the trunk motion in all three directions, margins of stability, step measures and variability of mediolateral trunk motion and position between groups and speeds (2 groups × 4 speeds). We also used another mixed-design ANOVA to test for differences in the MOS and step measures among legs (control, stroke unaffected, and stroke affected) and speeds (3 legs × 4 speeds). Due to the substantial difference in walking speed among subjects, we used actual walking speeds as a covariate for all statistical analyses. We set the significance level at p < 0.05 and used Tukey honestly significant difference (THSD) post hoc tests for pair-wise comparisons if a main effect (e.g., leg) was detected. All statistical analyses were performed in JMP version 10.0.2 (SAS institute Inc., Cary, NC, USA).
3. Results
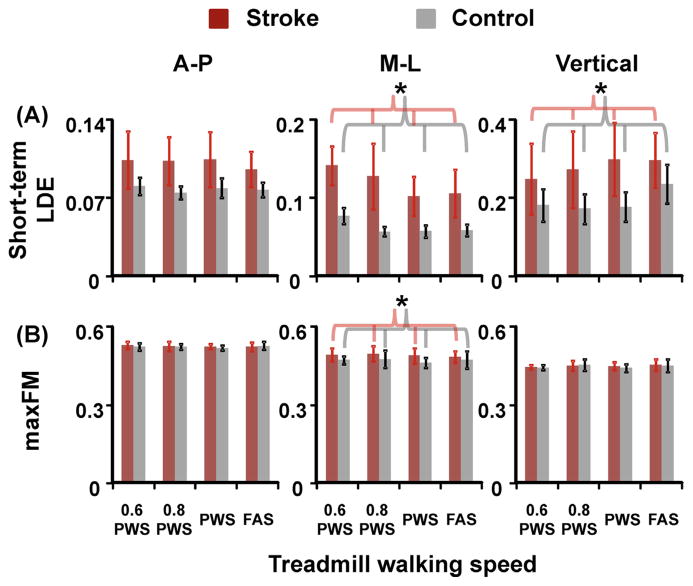
Post-stroke individuals had significantly larger, positive values of short-term LDE, indicating more local instability, for C7 movements in the mediolateral (p = 0.002) and vertical directions (p = 0.001) than the controls (Fig. 1). In addition, post-stroke individuals had significantly larger short-term LDE of mediolateral C7 movements at 0.6 PWS and 0.8 PWS compared to PWS and FAS (THSD, p < 0.05). For orbital stability, post-stroke individuals had significantly larger maxFM, indicating more orbital instability, only for C7 movements in the mediolateral direction (p = 0.041) compared to the controls. In addition, the amplitudes of maxFM were all smaller than one for all participants, indicating orbitally stable (i.e., perturbations decayed after successive strides).
Fig. 1.
(A) Short-term LDE and (B) maxFM, indicating local stability and orbital stability of the C7 vertebral marker movement, in the anterior–posterior, mediolateral, and vertical directions for stroke (red bars) and control (grey bars) subjects. Error bars are ±1 STD. * Indicates significant difference between stroke and control groups. In addition, there was a speed effect for short-term LDE of mediolateral C7 movements. Note that a larger value of short-term LDE or maxFM indicates greater instability of the trunk motion represented by the C7 vertebral marker velocity profile. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
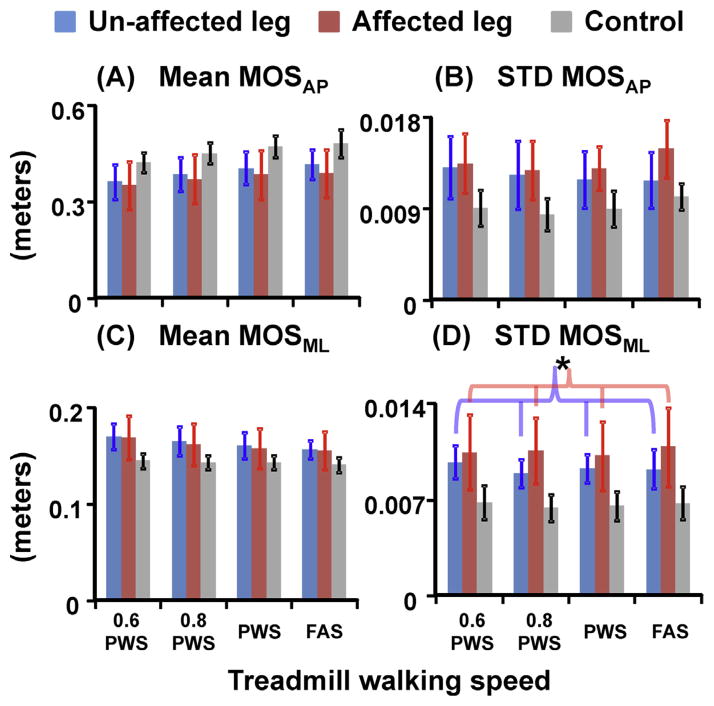
Post-stroke individuals had significantly smaller average MOSAP (p = 0.042) but greater variability of MOS both in anterioposterior and mediolateral directions (both p < 0.001) compared to the controls (Fig. 2). In addition, the variability of MOSML was significantly greater at the affected leg compared to the unaffected leg (THSD, p < 0.05) in post-stroke individuals. For the average MOSML, there was no group effect (p = 0.154). Moreover, post-stroke individuals walked with significantly greater MOSAP but significantly smaller MOSML at PWS and FAS compared to 0.6 PWS (THSD, p < 0.05).
Fig. 2.
(A) Mean and (B) variability of MOSAP, and (C) mean and (D) variability of MOSML for unimpaired control leg (grey bars), stroke unaffected leg (blue bars), and stroke affected leg (red bars). Error bars are ±1 STD. * Indicates significant difference between the unaffected and affected legs in post-stroke individuals (THSD, p < 0.05). There was a significant group effect for average MOSAP, MOSAP variability, and MOSML variability. In addition, there was a speed effect for average MOSAP and average MOSML. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
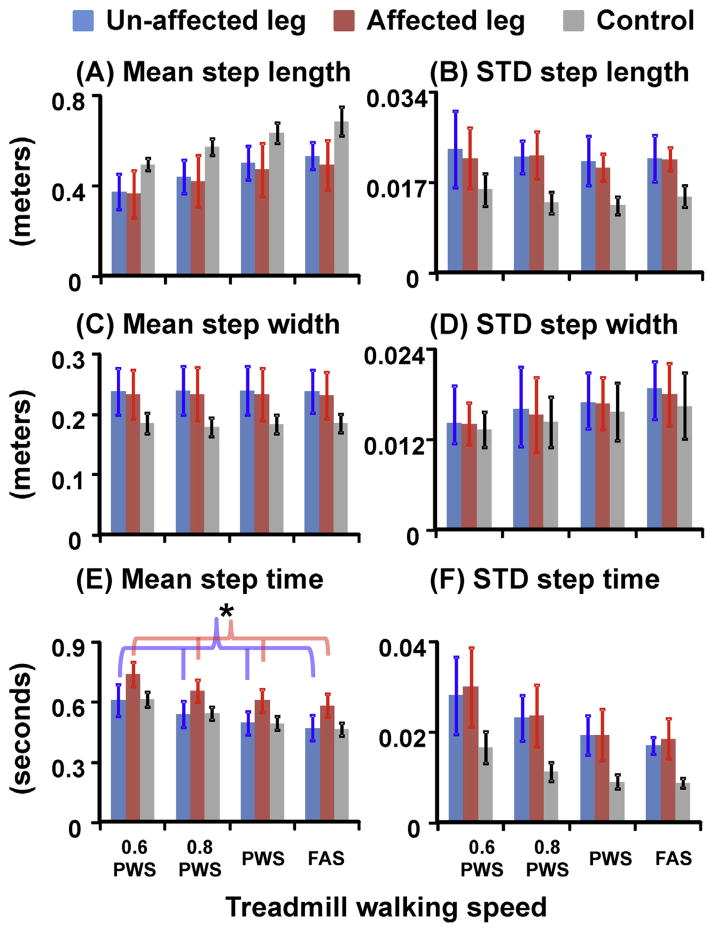
Post-stroke individuals showed a trend to walk with slightly shorter average step lengths (p = 0.092) but significantly greater average step widths (p = 0.003) than the controls (Fig. 3). In addition, post-stroke individuals showed greater variability in all step spatiotemporal measures (all p < 0.005) compared to the controls. Although there was no difference in the average step time between groups, post-stroke individuals had longer average step time at the affected leg compared to the unaffected leg (THSD, p < 0.05). Moreover, post-stroke individuals walked with significantly greater average step length and step width variability but significantly less average and variability of step time at PWS and FAS compared to 0.6 PWS and 0.8 PWS (THSD, p < 0.05).
Fig. 3.
(A) Mean and (B) variability of step length, (C) mean and (D) variability of step width, and (E) mean and (F) variability of step time for unimpaired control leg (grey bars), stroke unaffected leg (blue bars), and stroke affected leg (red bars). Step time was defined as the time interval from the contralateral heel strikes to the following ipsilateral heel strikes. Error bars are ±1 STD. * Indicates significant difference between the unaffected and affected legs in post-stroke individuals (THSD, p < 0.05). There was a significant group effect for average step width and the variability in step length, width, and time. In addition, there was a speed effect for average step length, step width variability, average and variability of step time. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Post-stroke individuals had significantly greater mediolateral trunk position variability (p < 0.001) (i.e., more lateral drift on the treadmill) and a trend to exhibit slightly greater mediolateral trunk velocity variability (p = 0.098) compared to the controls (Fig. 4). In addition, post-stroke individuals had significantly smaller trunk position variability but significantly greater trunk velocity variability at 0.6 PWS compared to PWS and FAS (THSD, p < 0.05). There were no group or speed effects for the trunk position or velocity variability in the anterioposterior or vertical directions.
Fig. 4.
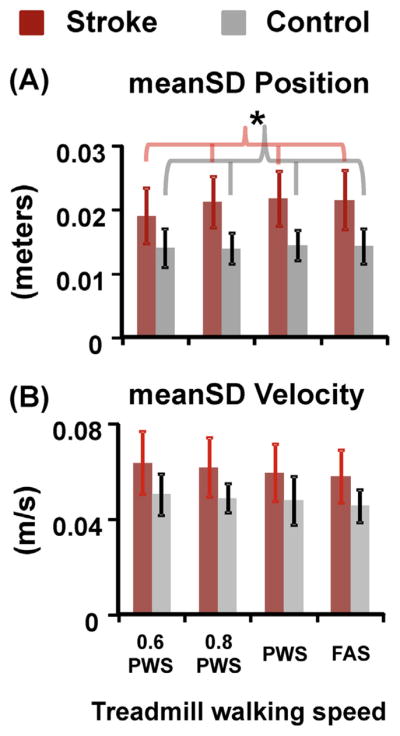
(A) meanSD of C7 marker position and (B) meanSD of C7 marker velocity in the mediolateral direction for stroke (red bars) and control (grey bars) subjects. Error bars are ±1 STD. * Indicates significant difference between stroke and control groups. In addition, there was a speed effect for mediolateral C7 marker position variability and velocity variability in stroke subjects. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Our findings support our hypothesis that post-stroke individuals would show greater walking instability compared to neurologically intact controls. Post-stroke individuals walked with greater local and orbital instability than the controls but remained orbitally stable. In addition, the differences in short-term LDE between the stroke and control groups were much larger than the differences in maxFM between the groups. These results are consistent with the previous findings in elders, people with neuropathy, or unimpaired, young adults under destabilizing conditions, demonstrating larger differences in local stability than the differences in orbital stability between groups or conditions [13,28]. Our results also indicate that short-term LDE detects biological (e.g., stroke) alterations relevant to walking stability.
Our findings do not support our hypothesis that slower walking would lead to improved stability in people post-stroke as suggested by previous findings in healthy individuals and individuals with neuropathy [27–30]. The post-stroke individuals tested here were slightly more locally stable in the vertical direction but significantly more unstable in the mediolateral direction at slower speeds. Preferred walking speeds (PWS) of the stroke subjects in this study ranged from 0.55 to 1.35 m/s. It is possible that stroke subjects with low PWS already chose a slow speed that would allow them to walk stably and additional slowing down (e.g., 0.6 PWS) lead not to additional improvement, but rather to deterioration. In addition, previous studies did not test the speeds slower than 0.7 m/s. It is not known if any further slowing down out of the comfortable zone would still lead to improved stability.
The choice of time delays for constructing state spaces affects the actual values of short-term LDE and maxFM. We set fixed time delays of 25, 30, and 15 data samples for the AP, ML, and VT trunk motion [32] in this study. We also re-analyzed our data with different time delays used in previous studies such as a fixed time lag of 10 data samples for all subjects/conditions [30], different time lags for each subject/condition determined from the first minimum of the average mutual information (AMI) function [27], or a fixed time lag of the averaged, first minimum of the AMI from all subjects/conditions [13]. We found that the differences between the groups in short-term LDE were consistent for all time delays. However, the group effects for maxFM were not consistent when using other time delays. Regardless of different time delays used, the differences in maxFM between the groups were small.
Post-stroke individuals walked with increased step width but similar average ML MOS compared to the controls. In addition, post-stroke individuals exhibited greater variability in MOS, step measures and mediolateral trunk position. Although post-stroke individuals had slightly greater average ML MOS, their variability of MOS measures was much greater compared to the controls. The likelihood of experiencing a very small or negative MOS during walking may have been greater in post-stroke individuals, which would increase the chance of falls [20]. Moreover, it is commonly assumed that walking with a wider stride improves lateral stability. McAndrew and Dingwell [24] showed that increasing step width voluntarily increased ML MOS but reduced AP MOS and caused greater variability of the MOS measures for both directions in unimpaired, young adults. On the contrary, increased average step width was associated with falls in the elderly [25]. It is possible that the velocity or acceleration profile of the trunk in the ML direction becomes more variable when walking with wider steps [25,32], which may worsen the lateral stability. Our findings also indicate that taking wider steps alone in people post-stroke may not be sufficient to regain the same level of stability as neurologically intact individuals.
The current study compared walking stability of post-stroke and neurologically intact individuals, as an initial step of establishing objective measures to detect those post-stroke individuals at increased risk of falls. Short-term LDE of trunk motion and the variability of MOS and step measures detect the effects of stroke on walking stability. Although these stability measures can directly reflect the increased instability resulting from experimentally induced perturbations [13,15,16], it is unclear to what extent these measures are related to actual falls. Thus, it is necessary to validate these stability measures [14] before applying them to detect potential fallers clinically.
Acknowledgments
The authors thank the UD stroke research core and research PTs for recruiting and screening subjects. This work was partially supported by NIH grants HD038582 and GM103333.
Footnotes
The study was partially published as an abstract in the Proceedings of the 42nd Annual Meeting of Society for Neuroscience (New Orleans, Louisiana, 2012) and the 18th Annual Meeting of Gait & Clinical Movement Analysis Society (Cincinnati, Ohio, 2013).
5. Conflict of interest
There are no conflicts of interest in this work.
References
- 1.Harris JE, Eng JJ, Marigold DS, Tokuno CD, Louis CL. Relationship of balance and mobility to fall incidence in people with chronic stroke. Phys Ther. 2005;85:150–8. [PubMed] [Google Scholar]
- 2.Jorgensen L, Engstad T, Jacobsen BK. Higher incidence of falls in long-term stroke survivors than in population controls: depressive symptoms predict falls after stroke. Stroke. 2002;33:542–7. doi: 10.1161/hs0202.102375. [DOI] [PubMed] [Google Scholar]
- 3.Batchelor FA, Mackintosh SF, Said CM, Hill KD. Falls after stroke. Int J Stroke. 2012;7:482–90. doi: 10.1111/j.1747-4949.2012.00796.x. [DOI] [PubMed] [Google Scholar]
- 4.Weerdesteyn V, de Niet M, van Duijnhoven HJ, Geurts AC. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45:1195–213. [PubMed] [Google Scholar]
- 5.Belgen B, Beninato M, Sullivan PE, Narielwalla K. The association of balance capacity and falls self-efficacy with history of falling in community-dwelling people with chronic stroke. Arch Phys Med Rehabil. 2006;87:554–61. doi: 10.1016/j.apmr.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Dingwell JB, Kang HG. Differences between local and orbital dynamic stability during human walking. J Biomech Eng. 2007;129:586–93. doi: 10.1115/1.2746383. [DOI] [PubMed] [Google Scholar]
- 7.Lamontagne A, Stephenson JL, Fung J. Physiological evaluation of gait disturbances post stroke. Clin Neurophysiol. 2007;118:717–29. doi: 10.1016/j.clinph.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther. 2008;88:559–66. doi: 10.2522/ptj.20070205. [DOI] [PubMed] [Google Scholar]
- 9.Bowden MG, Clark DJ, Kautz SA. Evaluation of abnormal synergy patterns poststroke: relationship of the Fugl-Meyer Assessment to hemiparetic locomotion. Neurorehabil Neural Repair. 2010;24:328–37. doi: 10.1177/1545968309343215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quach L, Galica AM, Jones RN, Procter-Gray E, Manor B, Hannan MT, et al. The nonlinear relationship between gait speed and falls: the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston Study. J Am Geriatr Soc. 2011;59:1069–73. doi: 10.1111/j.1532-5415.2011.03408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelsey JL, Procter-Gray E, Hannan MT, Li W. Heterogeneity of falls among older adults: implications for public health prevention. Am J Public Health. 2012;102:2149–56. doi: 10.2105/AJPH.2012.300677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelsey JL, Procter-Gray E, Berry SD, Hannan MT, Kiel DP, Lipsitz LA, et al. Reevaluating the implications of recurrent falls in older adults: location changes the inference. J Am Geriatr Soc. 2012;60:517–24. doi: 10.1111/j.1532-5415.2011.03834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McAndrew PM, Wilken JM, Dingwell JB. Dynamic stability of human walking in visually and mechanically destabilizing environments. J Biomech. 2011;44:644–9. doi: 10.1016/j.jbiomech.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruijn SM, Meijer OG, Beek PJ, van Dieen JH. Assessing the stability of human locomotion: a review of current measures. J R Soc Interface. 2013;10:20120999. doi: 10.1098/rsif.2012.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Schooten KS, Sloot LH, Bruijn SM, Kingma H, Meijer OG, Pijnappels M, et al. Sensitivity of trunk variability and stability measures to balance impairments induced by galvanic vestibular stimulation during gait. Gait Posture. 2011;33:656–60. doi: 10.1016/j.gaitpost.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Sinitksi EH, Terry K, Wilken JM, Dingwell JB. Effects of perturbation magnitude on dynamic stability when walking in destabilizing environments. J Biomech. 2012;45:2084–91. doi: 10.1016/j.jbiomech.2012.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toebes MJ, Hoozemans MJ, Furrer R, Dekker J, van Dieen JH. Local dynamic stability and variability of gait are associated with fall history in elderly subjects. Gait Posture. 2012;36:527–31. doi: 10.1016/j.gaitpost.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Roos PE, Dingwell JB. Influence of simulated neuromuscular noise on the dynamic stability and fall risk of a 3D dynamic walking model. J Biomech. 2011;44:1514–20. doi: 10.1016/j.jbiomech.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruijn SM, Bregman DJ, Meijer OG, Beek PJ, van Dieen JH. Maximum Lyapunov exponents as predictors of global gait stability: a modelling approach. Med Eng Phys. 2012;34:428–36. doi: 10.1016/j.medengphy.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Hof AL, Gazendam MG, Sinke WE. The condition for dynamic stability. J Biomech. 2005;38:1–8. doi: 10.1016/j.jbiomech.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Hof AL, Vermerris SM, Gjaltema WA. Balance responses to lateral perturbations in human treadmill walking. J Exp Biol. 2010;213:2655–64. doi: 10.1242/jeb.042572. [DOI] [PubMed] [Google Scholar]
- 22.Rosenblatt NJ, Grabiner MD. Measures of frontal plane stability during treadmill and overground walking. Gait Posture. 2010;31:380–4. doi: 10.1016/j.gaitpost.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 23.McAndrew Young PM, Wilken JM, Dingwell JB. Dynamic margins of stability during human walking in destabilizing environments. J Biomech. 2012;45:1053–9. doi: 10.1016/j.jbiomech.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAndrew Young PM, Dingwell JB. Voluntary changes in step width and step length during human walking affect dynamic margins of stability. Gait Posture. 2012;36:219–24. doi: 10.1016/j.gaitpost.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45:313–20. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 26.Balasubramanian CK, Neptune RR, Kautz SA. Variability in spatiotemporal step characteristics and its relationship to walking performance post-stroke. Gait Posture. 2009;29:408–14. doi: 10.1016/j.gaitpost.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dingwell JB, Marin LC. Kinematic variability and local dynamic stability of upper body motions when walking at different speeds. J Biomech. 2006;39:444–52. doi: 10.1016/j.jbiomech.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Dingwell JB, Kang HG, Marin LC. The effects of sensory loss and walking speed on the orbital dynamic stability of human walking. J Biomech. 2007;40:1723–30. doi: 10.1016/j.jbiomech.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Kang HG, Dingwell JB. Effects of walking speed, strength and range of motion on gait stability in healthy older adults. J Biomech. 2008;41:2899–905. doi: 10.1016/j.jbiomech.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.England SA, Granata KP. The influence of gait speed on local dynamic stability of walking. Gait Posture. 2007;25:172–8. doi: 10.1016/j.gaitpost.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurmuzlu Y, Basdogan C. On the measurement of dynamic stability of human locomotion. J Biomech Eng. 1994;116:30–6. doi: 10.1115/1.2895701. [DOI] [PubMed] [Google Scholar]
- 32.McAndrew Young PM, Dingwell JB. Voluntarily changing step length or step width affects dynamic stability of human walking. Gait Posture. 2012;35:472–7. doi: 10.1016/j.gaitpost.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruijn SM, van Dieen JH, Meijer OG, Beek PJ. Statistical precision and sensitivity of measures of dynamic gait stability. J Neurosci Methods. 2009;178:327–33. doi: 10.1016/j.jneumeth.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 34.Kang HG, Dingwell JB. Intra-session reliability of local dynamic stability of walking. Gait Posture. 2006;24:386–90. doi: 10.1016/j.gaitpost.2005.11.004. [DOI] [PubMed] [Google Scholar]