Abstract
The various neurological complications associated with HIV-1 infection, specifically HIV-associated neurocognitive disorders (HAND) persist as a major public health burden worldwide. Despite the widespread use of anti-retroviral therapy, the prevalence of HAND is significantly high. HAND results from the direct effects of an HIV-1 infection as well as secondary effects of HIV-1-induced immune reaction and inflammatory response. Complement, a critical mediator of innate and acquired immunity, plays important roles in defeating many viral infections by the formation of a lytic pore or indirectly by opsonization and recruitment of phagocytes. While the role of complement in the pathogenesis of HIV-1 infection and HAND has been previously recognized for over fifteen years, it has been largely underestimated thus far. Complement can be activated through HIV-1 envelope proteins, mannose binding lectins (MBL) and anti-HIV-1 antibodies. Complement not only fights against HIV-1 infection but also enhances HIV-1 infection. Also, HIV-1 can hijack complement regulators such as CD59 and CD55 and can utilize these regulators and factor H to escape from complement attack. Normally, complement levels in brain are much lower than plasma levels and there is no or little complement deposition in brain cells. Interestingly, local production and deposition of complement are dramatically increased in HIV-1-infected brain, indicating that complement may contribute to the pathogenesis of HAND. Here, we review the current understanding of the role of complement in HIV-1 infection and HAND as well as potential therapeutic approaches targeting to the complement system for the treatment and eradications of HIV-1 infection.
Keywords: Complement, complement regulation, CD59, ILYd4, HIV-1, HAND
Introduction
The various neurological complications associated with HIV-1 infection, specifically HIV-associated neurocognitive disorders (HAND), remain to be a severe clinical problem for individuals infected with HIV-1, which persists as a major public health burden worldwide (Spudich and Gonzalez-Scarano, 2012). Despite the widespread use of anti-retroviral therapy, the prevalence of HAND is significantly high. It is widely accepted that HAND results from the direct effect of HIV-1 infection as well as secondary effects of HIV-1-induced immune reaction and inflammatory response (Letendre, 2011). However, our current understanding of the pathogenesis of HAND remains unclear and requires extensive investigation. Due to current limitations in treatment and the inability to eradicate HIV-1 infection, latency and persistence of viral infection are likely to represent big challenges for the foreseeable future.
Complement, a critical mediator of innate and acquired immunity, plays important roles in defeating many viral infections by the formation of a lytic pore or indirectly by opsonization and recruitment of phagocytes (Datta and Rappaport, 2006) and also contributes to the pathogenesis of many chronic immune and inflammatory diseases including autoimmune diseases, atherosclerosis (Wu et al., 2009), aneurysm (Wu et al., 2010), and non-viral-related neurological disorders such as Alzheimer’s disease (Eikelenboom et al., 1989), multiple sclerosis (Rus et al., 2005), and Huntington’s disease (Dalrymple et al., 2007), etc. While the role of complement in the pathogenesis of HIV-1 infection and HAND development has been previously recognized for over fifteen years, it has been largely underestimated thus far. Recent clinical and experimental evidence further indicates that complement may participate in the development of HIV-1 infections and HAND development. In addition, the urgent need for understanding the role of innate immunity including complement in HIV-1 infection is specifically highlighted by the facts that 1) results from large-scale clinical trials of both antibody- and T cell-targeted immunogens have given largely disappointing outcomes and 2) although some short-lived protection was observed in the most recent phase III HIV vaccine trial (Rerks-Ngarm et al., 2009) and the rapid killing effect was found in rhesus macaques chronically infected with the R5 tropic simian-human immunodeficiency virus (SHIV) with antibody-mediated immunotherapy (Shingai et al., 2013), the mechanism(s) of these protections are poorly understood (Borrow et al., 2010). Therefore, it is imperative to further understand the role of innate immunity such as the complement system in the pathogenesis of HIV-1 infection and HIV-related complications including HAND for the development of novel approaches of HIV-1 prevention and treatment. Here we will discuss 1) the complement system, 2) the role of complement in HIV-1 infection, 3) the potential role of complement in HAND, and 4) novel therapeutic approaches for eradication HIV-1 infection for the treatment of HIV-1 related complications including HAND.
Complement activation and complement regulation
Complement system
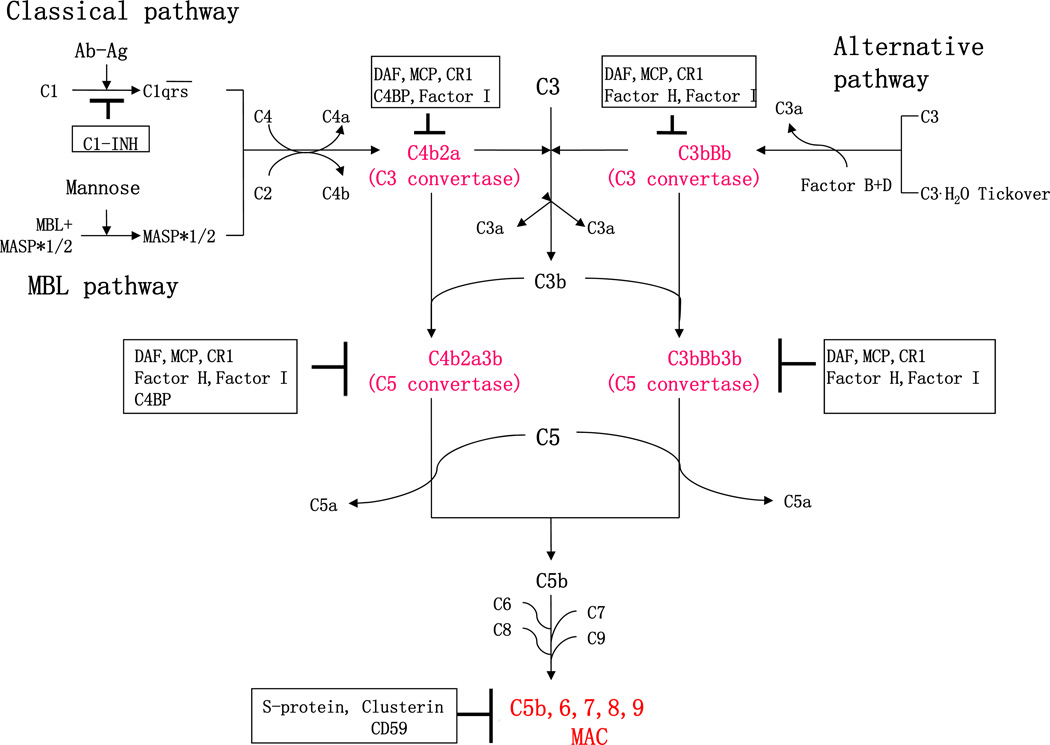
The complement system, an important effector of both innate and acquired immunity, consists of more than 30 components and complement regulators and functions as the first line of defense against invading pathogens. Depending on different activators, complement activation occurs through three distinct pathways: classical, alternative and MBL pathways in plasma or on the pathogen surface (Figure 1). All three of these pathways merge to induce the activation and cleavage of C3, a critical component which triggers the formation of the terminal membrane attack complex (MAC) (Yu et al., 2010; Zhou et al., 2008).
Figure 1. Complement activation and regulation.
The complement system is activated through three different activation pathways: classical, alternative and MBL cascades. The three complement activation pathways converge at the C3 level. Formation of C3 or C5 convertases as well as the membrane attack complement (MAC), a final activation complex (as highlighted in red text) are regulated by a number of complement regulators (highlighted within black squares). Ab, Antibody; Ag, Antigen; C1-INH, C1-inhibitor; MBL, Mannose-binding lectin; MASP, MBL-associated serine proteases; DAF, decay-accelerating factor; MCP, membrane co-factor protein; CR, complement receptor; C4BP, C4b-binding protein; and MAC, membrane attack complex.
The classical pathway is activated by binding of the Fc portion of antigen-bound antibody to C1, which results in the formation of an active C1-complex. Then the C1-complex can recruit and cleave C4 and C2, generating inactive fragments (C4a and C2b) and C3 convertase C4bC2a, which cleaves C3 into C3a and C3b (Figure 1). The formation of C3 convertase C4bC2a is also involved in the MBL pathway (Morgan and Gasque, 1997). Different from the classical pathway, the MBL pathway targets pathogenic molecules by the MBL protein family and ficolins, which recognize and bind to carbohydrate ligands located on the surface of pathogens, such as viruses, bacteria, and fungi. The MBL-associated serine proteases (MASPs) are then activated and cleave C4 and C2 to generate C4bC2a (Figure 1). Unlike the classical and MBL pathways which are activated via recognition of pathogens, activation of the alternative pathway relies on C3 (H2O), the spontaneous hydrolysis of C3, which constitutively exists at low levels and behaves similar to C3b. C3(H2O) binds to factor B (FB), and then serves as a substrate for the serine protease factor D (FD), resulting in cleavage of FB by FD and formation of C3(H2O)Bb, which acts as C4bC2a (Figure 1). Either C4bC2a or C3bBb can cleave C3 into anaphylatoxin C3a and active C3b. Further, active C3b forms C5 convertase such as the C4bC2bC3b from the classical and MBL pathways and (C3b)2Fbb from alternative pathway. The C5 convertase then cleaves C5 to form C5b, and C5a, another anaphylatoxin. The terminal complement activation pathway is induced initially by C5b, followed by the sequential condensation of the C6 form to C5b6, and then C7, C8, and C9. Polymerization of C9 bound to the C5b-8 complex forms the MAC, an end-product of the complement activation pathways (Figure 1). The MAC forms a lytic pore in the lipid bilayer membrane that allows the free passage of solutes and water across the membrane and destroys membrane integrity, followed by killing of foreign pathogens and cells (Figure 1) (Mayer, 1984).
Furthermore, the byproducts produced in complement activation, such as C1q, C3b, iC3b, and C4b, are critical opsonins for host defense against pathogens and for the disposal of immune complexes and dead cellular debris by the phagocytosis/lysis effect of the immune cells (macrophages, neutrophils, natural killer [NK] cells, etc.) through their surface receptors binding to these byproducts. For instance, the small fragment byproducts such as C5a, an anaphylatoxins, also play an important role in inflammation and especially in host defense against parasites (Rees-Roberts et al., 2010) (Mosser and Brittingham, 1997). These anaphylatoxins can cause mast cell and basophil degranulation, with the release of histamine and other substances that increase vascular permeability and stimulate smooth muscle constriction. C3a and C5a are potent leukocyte chemoattractants, and can also activate these immune effector cells by binding to cell surface receptors (Haas and van Strijp, 2007). Among these anaphylatoxins, C5a has the most potent biological activity (Guo and Ward, 2005).
Complement proteins are mainly synthesized by hepatocytes in the liver and the liver accounts for 80%~90% of plasma complement components and their soluble regulators (Qin and Gao, 2006). Many other non-hepatic cells including macrophages, endothelial cells, neutrophils and lymphocytes also produce complement proteins. Local synthesis of complement also occurs in the brain, heart, lung, joints, intestines, skeletal muscle and the bone marrow (Morgan and Gasque, 1997). Specifically in the brain, under normal condition, complement levels are much lower than in the plasma. However, as discussed below, complement production may be significantly increased in the brain in inflammatory conditions such as HIV-1 infection of the brain or during abnormal immune responses such as in individuals with autoimmune diseases. The local production of complement in tissues may also play a critical role in disease pathogenesis. Indeed, the absence of locally synthesized complement component C3 is capable of modulating the rejection of renal allografts in vivo and regulating T-cell responses in vivo and in vitro (Pratt et al., 2002).
Complement regulation
To protect the host from complement attack, a variety of plasma and membrane-bound proteins participate in the regulation of complement activation (Figure 1) (Morgan, 1999). In plasma, factor I (fI) plays an important role in the control of active C3b by the cleavage of C3b into inactive iC3b and C3d. This function of fI requires co-factor activity such as factor H (fH), a soluble regulatory protein which also decreases the stability of C3bBb and competes with FB in binding to C3b. C4 binding protein is another co-factor of fI, which promotes the cleavage of C4b. Moreover, a C1 inhibitor, also termed SERPING1, inactivates C1r, C1s and MBL-associated proteins (MASPs) by irreversible binding at the very beginning of the classical and MBL pathways (Yu et al., 2010; Zhou et al., 2008). Membrane-bound regulators include membrane cofactor protein (MCP/CD46), decay-accelerating factor (DAF/CD55) and complement receptor 1 (CR 1). MCP protects those cells by functioning as co-factor of fI (Brodbeck et al., 2000). DAF dissociates C4bC2a generated in the classical and MBL pathways (Davitz et al., 1986; Medof et al., 1987; Nicholson-Weller et al., 1985), while CR1 interacts with C3b as a co-factor of fI, and decays the activity of C3 convertase. CD59, a membrane-bound regulator, is a key regulator of MAC formation. It inhibits the association of C9 and C5b-8, which suppresses the polymerization of C9, namely MAC formation (Sugita et al., 1989).
Of note, extensive evidence obtained from knockout mice deficient in these complement regulators and humans with genetic mutations of the complement regulators indicates that CD59 is much more relevant than DAF, MCP and other complement regulators in protecting cells from MAC formation and MAC-induced phenomena (Fisicaro, 2000). Of note, the CD59 knockout mouse exhibits paroxysmal nocturnal hemoglobinuria (PNH), a complement-mediated hemolytic disease due to the deficiency of complement regulators such as CD59 and DAF. Not only does the CD59 knockout mouse exhibit a full PNH-like anemia, but it also exhibits progressive loss of male fertility (Holt et al., 2001; Qin et al., 2009; Qin et al., 2003). Nevo et al (2013) published 5 patients of North-African Jewish origin from 4 unrelated families and reported that a Cys89Tyr genetic mutation in CD59 is associated with a failure of proper localization of the CD59 protein on the cell surface (Nevo et al., 2013). This mutation is manifested clinically in infancy by chronic hemolysis and relapsing peripheral demyelinating disease. Importantly, 4 patients remain alive and the oldest one is 5 years of age at the time of publication (Nevo et al., 2013), which indicates that this complete loss of function mutation does not cause early death. The findings in this recent paper are comparable to those from an index case report from Japan describing a man who had a global deficiency of hCD59 due to single nucleotide deletions in the CD59 gene, which placed the gene product out of frame and introduced a premature stop codon (Yamashina et al., 1990). This subject expressed a severe PNH phenotype from the unusually young age of thirteen and also had a stroke that left him with permanent neurological damage (Yamashina et al., 1990). In contrast, an isolated deficiency of DAF has been described in four families that had an unusual blood group phenotype termed Inab (Lin et al., 1988; Telen and Green, 1989). Although DAF was completely absent from all circulating cells (Reid et al., 1991), none of the propositi had symptoms suggestive of PNH. In addition, an mDAF knockout mouse did not show any evidence of intravascular hemolysis (Sun et al., 1999). Also, although the role of S-protein as an inhibitor of MAC formation in tissues has been suggested, the S-protein knockout mouse did not show any detectable phenotype and instead developed normally and was fully fertile (Zheng et al., 1995). This indicates that, at least in mice, the S-protein is not essential for survival nor does it play a major role in restricting complement activation or MAC formation.
The delicate balance between complement activation and regulation
There is a delicate balance between complement activation and regulation on autologous cells, which is subject to perturbation by either increased complement activation or decreased regulation. The perturbation may cause a variety of immune diseases and chronic diseases (Yu et al., 2010). It is very likely that this delicate balance between complement activity and regulation varies in tissues because of differential expression of complement regulatory proteins and focal activation of complement proteins (Acosta et al., 2004). Indeed, extensive study has shown an offset balance between complement activation and complement regulation, which contributes to the pathogenesis of inflammatory or immune diseases, such as systemic lupus erythematosus (Abe et al., 1998), rheumatoid arthritis (Breitner et al., 1995), Alzheimer’s disease (Gasque et al., 1995), atherosclerosis (Wu et al., 2009), aneurysm (Martinez-Pinna et al., 2013; Wu et al., 2010; Zhou et al., 2012), and acute renal transplant rejection (Pratt et al., 2002). In the case of the progression of HIV-1 infection, there may be a delicate balance between complement activation and regulation in HIV-1 particles and HIV-1 infected cells, which may contribute to the development of latency and persistence of HIV-1 infection in acute and chronic stages of the infection (Dierich et al., 1996; Humbert and Dietrich, 2006; Saifuddin et al., 1995; Schmitz et al., 1995). However, in the case of the development of HAND, there may be an offset balance between complement activation and regulation, which may contribute to the neuronal damage in the response to HIV-1 infection as discussed below.
Complement activation in HIV-infection
Complement activation initiated by HIV-1 envelope proteins and MBL
As a central effector of the innate immune system, the complement system plays an important role in the protection against viral infection. As early as the 1970s, human serum was found to have the ability to inactivate and lyse a number of RNA tumor viruses in a complement-dependent manner, but no antibody activity was found to play a role in this event (Cooper et al., 1976; Welsh et al., 1975; Welsh et al., 1976). In the case of HIV-1, studies have shown that HIV-1 infection also can directly activate the complement system but that HIV-1 virions are themselves resistant to complement-mediated lysis. After crossing the mucosal surface, the direct binding of the HIV-1 surface protein, gp41, to C1q was reported to be responsible for activation of the classical complement pathway (Ebenbichler et al., 1991) (Spear et al., 1991). Meanwhile, gp120, another HIV-1 envelope protein, can also activate complement in an antibody depend manner via classical pathway (Susal et al., 1994). In addition, activation of the MBL-pathway can be triggered by the binding of gp120 and MBL (Saifuddin et al., 2000). As a result, complement activation initiated by gp41 and gp120 or MBL occurs at a very early stage of HIV-1 infection even without HIV-1 specific antibodies. Plasma mannose-binding lectin (MBL) in the circulation plays an important role in innate immunity by binding to carbohydrates on micro-organisms including HIV-1. MBL binds to HIV-1 envelope proteins, i.e. gp120 and gp41, which activates complement activation (Ezekowitz et al., 1989; Haurum et al., 1993; Saifuddin et al., 2000). This complement activation initiated by the lectin pathway affects the clearance of HIV-1 from the blood, mediates uptake by tissue macrophage (Ying et al., 2004), directly neutralizes HIV-1 (Ezekowitz et al., 1989), and enhances antibody-mediated neutralization (Hart et al., 2003; Ying et al., 2004).
In support of this notion, recent genetic evidence indicates that MBL deficiency is associated with an increased risk of vertical HIV-1 transmission (Mangano et al., 2008) and the association between HIV-1 disease progression and MBL deficiency is most pronounced in children less than 2 years of age, probably due to immaturity of their adaptive immunity (Israels et al., 2012). Three genetic studies of Chinese populations have demonstrated that MBL deficiencies are associated with increased susceptibility to HIV-1 infection and disease progression (Sheng et al., 2010) (Tan et al., 2009) (Li et al., 2013) and serum circulating levels of normal MBL in HIV-1-infected patients could be an important auxiliary biological marker in association with CD4(+) T cell counts in the evaluation of HIV-1 disease progression (Tan et al., 2009). These findings are consistent with studies previously conducted by Dzwonek et al (2006) in the UK (Dzwonek et al., 2006). Further, the results from a study among a North Indian population also suggest that homozygosity for the codon 54 allele within exon 1 of the MBL-2 gene, which is associated with low MBL production correlates with increased susceptibility to HIV-1 infection in the studied population (Chatterjee et al., 2011). Taken together, emerging evidence strongly supports a role for MBL in HIV-1 infection and even in disease progression. However, how MBL participates in disease progression and whether MBL can be utilized as a therapeutic target for the treatment of HIV-1 infection and prevention of disease progression still require further investigation.
Complement activation initiated by anti-HIV-1 antibodies
After seroconversion, HIV-1 specific antibodies further facilitate activation of complement by binding to HIV-1 particles (Prohaszka et al., 1995; Stoiber et al., 2001). Antibody responses to HIV-1 are generally vigorous at all stages of viral infection (Huber and Trkola, 2007; Morris, 2002). Within a few weeks of infection, antibodies against the viral envelope (gp120 and gp41), core (Gag), and matrix (p17) become detectable in the plasma of HIV-1-positive individuals (Aasa-Chapman et al., 2004; Belec et al., 1995; Binley et al., 1997; Pellegrin et al., 1996; Pincus et al., 1994; Richman et al., 2003). Antibody levels mount in response to the gradual increase in viral load and appear to be maintained at high levels throughout the disease (Humbert and Dietrich, 2006). Why does such a vigorous and sustained antibody response fail to play its role in the containment of HIV-1 replication and cytotoxicity? It is currently believed that only a fraction of the elicited antibodies bear neutralizing activity, which is not sufficient to prevent the initiation of HIV-1 infection by blocking viral attachment to its CD4 receptor (Huber and Trkola, 2007).
Furthermore, recent comparative studies on the immune responsiveness of HIV-1- and HIV-2-infected hosts have revealed that antibodies binding to HIV-2 structures facilitate the more efficient use of complement through activating the classical pathway than antibodies binding to HIV-1 and therefore may be one factor contributing to the strong antiviral activity elicited by HIV-2, but not HIV-1 (Ozkaya Sahin et al., 2013)
Nevertheless, several HIV-specific antibodies which can activate complement system have been described, including both neutralizing antibodies and non-neutralizing (Spear et al., 1993) (Gregersen et al., 1990; Huber and Trkola, 2007; Pantophlet and Burton, 2006). Huber et al. found that antibody-mediated complement virolysis develops rapidly and effectively in the early course of HIV-1 infection (Huber et al., 2006). Several reports have demonstrated complement-mediated virolysis occurs in vivo and complement can also augment the effect of neutralizing antibodies both in vivo and in vitro (Gauduin et al., 1997; Posner et al., 1992). Serum from uninfected C1q- or C3-deficient individuals used as source of complement do not exhibit any anti-HIV-1 activity, suggesting that the classical pathway dominates the complement-mediated virolysis (Prohaszka et al., 1995) (Aasa-Chapman et al., 2005). Although the complement system is strongly activated by HIV-1 infection, free virus is surprisingly resistant to complement-mediated virolysis (Stoiber et al., 1997). This may depend on the expression of complement regulatory proteins such as CD46, CD59 and CD55 on infected cells (Montefiori et al., 1994; Saifuddin et al., 1995; Schmitz et al., 1995). Indeed, we have demonstrated that the inhibition of human CD59 activation in the HIV-1 envelope unleashed the capacity of anti-HIV-1 antibodies from HIV-1 infection patients at different stages of infections to induce complement-mediated virolysis of HIV-1 particles (Hu et al., 2010).
Complement-mediated enhancement of HIV-1 infection
On the other hand, complement activation itself also presents opportunities for enhancement of HIV-1 infection. Szabo et al found a strong association between the extent of complement-mediated antibody-dependent enhancement of HIV-1 infection and plasma viral load in HIV-1 patients during a 17 month observation period (Szabo et al., 1999). These findings provide clinical evidence supporting the notion that complement-mediated antibody-dependent enhancement correlates with HIV-1 replication in vivo, and contributes to the progression of HIV-1 disease. The first event involved is the opsonization of virus with fragments produced by complement activation, such as C3 fragments and C5a (Pruenster et al., 2005; Stoiber et al., 2008b). Deposition of these fragments on the surface of the viron can result in enhanced interaction between HIV-1 and CR-positive cells. For CR-positive but CD4 and chemokine receptor-negative cells like B cells and erythrocytes, HIV-1 can only attach to their surface via CRs but cannot productively infect these cells, which can lead to further trafficking of the virus to other tissues and permissive cells. But cells positive for both CRs and CD4 such as CD4+ T cells, will be infected directly (Speth et al., 2003). Several lines of independent evidence indicate that CD4-gp120, C3d-CR2, iC3b-CR3 and gp41-CD3 interactions may facilitate viral transmission and replication (Lund et al., 1995) (June et al., 1991; Reisinger et al., 1990). Stimulating CR1 and CR3 with monoclonal antibodies or C3 fragments results in the augmentation of viral replication in infected monocytes, accompanied by NF-kB translocation (Thieblemont et al., 1995) (Griffin et al., 1991). In addition to the interaction between virus-bound C3 fragments and CR2 present on the target cells which contributes to the complement-mediated enhancing effect for HIV-1 infection, fixation of C1q to intact virions also resulted in enhanced productive HIV-1 infection in an MT-4 cell culture model (Prohaszka et al., 1997). Complement-enhanced loading and infection was also observed in monocyte-derived DCs, which is believed to play a major role in the transmission of HIV-1 to CD4+ T cells (Bajtay et al., 2004; Pruenster et al., 2005). Besides opsonization, anaphylatoxins C3a and C5a generated by cleavage of C3 and C5 can induce inflammation, recruit DCs and macrophages for HIV-1 infection at an early stage and facilitate cellular adhesion. An increase of TNF-alpha and IL-6 has been reported in the presence of C5a and its metabolite, C5adesArg. Both TNF-alpha and IL-6 are responsible for promoting HIV-1 infection and replication. Inhibition of C5aR dramatically reversed the enhancement of HIV-1 infection induced by C5a and C5adesArg (Kacani et al., 2001).
Complement regulators present in HIV-1 and SIV envelopes escapes complement attack
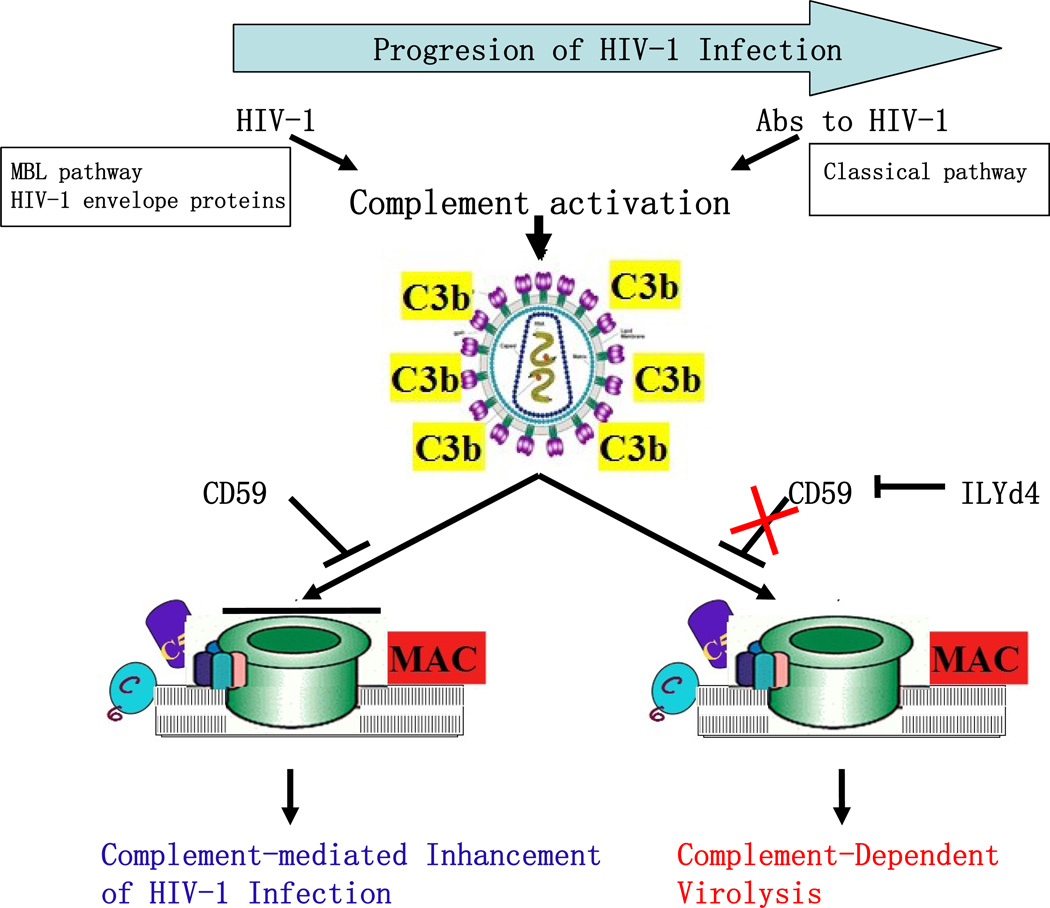
HIV-1 virions escape complement-mediated attack and remain highly infective, even though both the HIV-1 envelope proteins, through binding of gp120/gp41 and MBL, and anti-HIV-1 antibodies in the blood of HIV-1 infected individuals are capable of activating the complement cascades as discussed above (Figure 2) (Ebenbichler et al., 1991; Stoiber et al., 2003). Indeed, HIV-1 virions from infected individuals accumulate C3 on their surface, an indication of complement activation, but HIV-1 virions have long been recognized to be very resistant to lysis by complement attack (Figure 2) (Stoiber et al., 2008a). Interestingly, all other mammalian RNA viruses tested thus far can be inactivated by complement-mediated lysis using human serum (Speth et al., 2003). Furthermore, opsonization of HIV-1 with C3 enhances its infectivity by facilitating the interaction of HIV-1 viral particles with complement-receptor-positive cells such as monocytes/macrophage and dendritic cells (Stoiber et al., 2001). This experimental and clinical evidence indicates that MAC pores are not completely formed in the case of HIV-1 infection, leading to viral escape (Figure 2).
Figure 2. Roles of complement in the pathogenesis of HIV-1 infection and novel approach for inhibition of complement regulators including CD59 with its inhibitor, ILYd4 (domain 4 of intermedilysin).
MBL, Mannose-binding lectin; MAC, membrane attack complex; and Abs, antibodies.
The underlying mechanism of viral escape is due at least in part to the presence of regulators of complement activation such as CD59 and CD55 in the viral envelope, which the virus recruits from the host cell in the budding process (Figure 2) (Dierich et al., 1996; Saifuddin et al., 1995; Schmitz et al., 1995). Additionally, binding of the fluid phase complement regulator factor H to HIV-1 provides the virus further protection from complement attack (Stoiber et al., 1996). The presence of complement regulators such as CD59 on the external surface of the viral envelope provides resistance to MAC-mediated lytic effects, which may explain why certain human pathogenic viruses are not neutralized by complement in human fluids even when they induce a strong antibody response. Indeed, the deficiency or inhibition of hCD59 on the surface of either the viral envelope or the infected cell membrane sensitizes them to the lytic effect of complement (Figure 2)(Saifuddin et al., 1997; Saifuddin et al., 1995; Schmitz et al., 1995). These results were further confirmed by our recent finding that inhibition of human CD59 activity by a novel anti-hCD59 inhibitor sensitizes the anti-HIV-1 antibodies from different HIV-1 infected patients to lyse either experimental HIV-1 particles or primary HIV-1 particles isolated from patients at different stages of HIV-1 infection through MAC-mediated lytic effect (Hu et al., 2010).
Consistent with this observation, SIV can also hijack CD46, CD55 and CD59 from infected cells and incorporate these proteins into its envelope. Montefiori et al in 1994 demonstrated that CD46, CD55, and CD59 are common surface constituents of both HIV-1 and SIV (Montefiori et al., 1994). The presence of these proteins within the SIV envelope may also provide one of the major mechanisms for SIV to escape immune attack from complement-mediated virolysis activated by the classical pathway such as anti-SIV antibodies and the lectin pathway such as MBL (Montefiori et al., 1994).
In summary, although HIV-1 envelope proteins, MBL and anti-HIV-1 antibodies are able to initiate complement activation of HIV-1 particles and HIV-1 infected cells during the course of disease progression, this complement activation fails to clear and eradicate HIV-1 from infected cells and from the circulation due to the presence of complement regulators including CD59 within the HIV-1 envelope and on HIV-1 infected cells. Instead, HIV-1 takes advantages of complement activation to utilize complement byproducts and interact with complement receptors on circulating cells for spreading and enhancing HIV-1 infection. These findings clearly indicate that there may be a delicate balance between complement activation and regulation within HIV-1 particles and HIV-1 infected cells, which may contribute to the pathogenesis of HIV-1 infection. However, the exact role of the complement system in HIV-1 infection remains unclear. Therefore, it is imperative to further investigate how exactly this balance contributes to the development of HIV-1 infection including viral latency and persistence in order to develop novel and effective approaches to maximize the utilization of innate and adaptive immunities for the eradication of HIV-1 infection and the treatment of AIDS-related neurodegenerative diseases such as HAND.
Complement in AIDS-related neurodegenerative diseases such as HAND
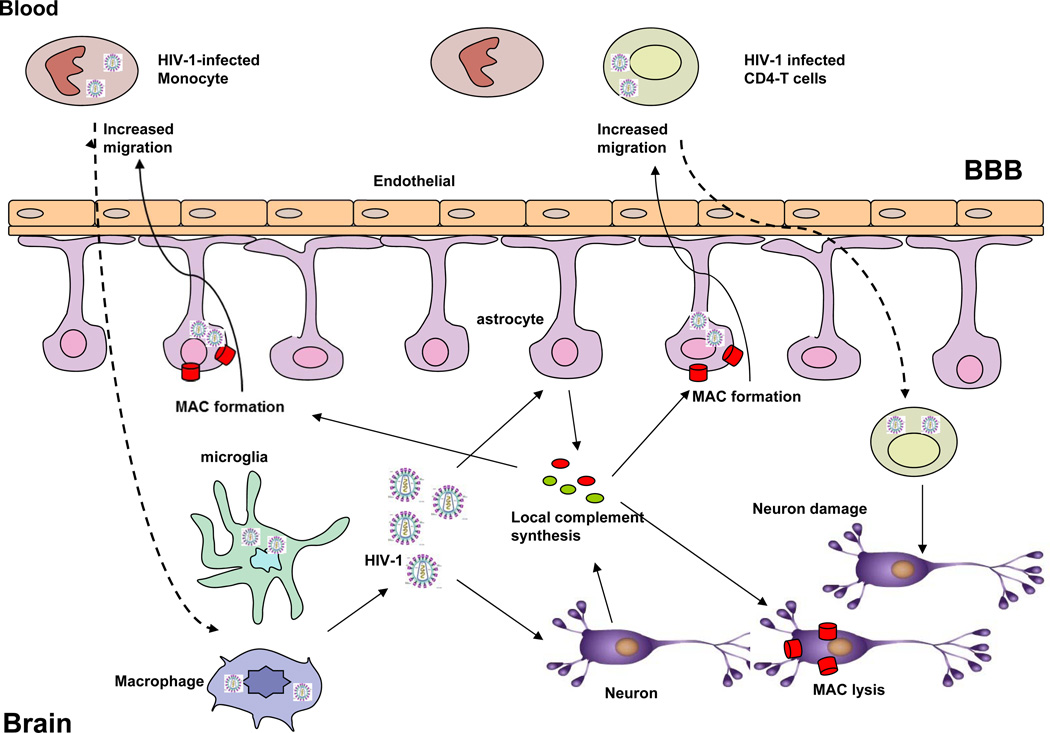
HAND is the result of neural damage caused by HIV-1 replication and immune activation (Letendre, 2011). In the course of HAND, HIV-1 may cross the blood brain barrier (BBB) primarily through monocytes or lymphocytes at the early stage of infection and can persist in the central nervous system (CNS) without symptom for many years (Figure 3). After crossing the BBB, HIV-1-infected monocytes become macrophages. Activated macrophages and resident microglia can replicate HIV-1 and express neurotoxic molecules that can activate astrocytes and other cells (Letendre, 2011). Astrocytes are an important component of the BBB by surrounding brain microvascular endothelial cells. After infection or activation, astrocytes increase BBB permeability, which could promote the migration of monocytes and lymphocytes, thereby mediating neuronal injury, the proximal biological event underpinning clinical neurological and cognitive disease (HAND) (Figure 3) (Letendre, 2011). In turn, HIV-1 infected cells have gradually come to be recognized as a major source of the latent virus and persistence of HIV-1 infection. However, the exact pathogenesis of HAND due to neuronal loss and injury remains unclear. There is increasing clinical and experimental evidence indicating the critical role of complement, a critical mediator of innate and acquired immunity in the pathogenesis of HAND.
Figure 3. Potential roles of complement in the pathogenesis of HIV-1-related neurodegenerative disorders.
Complement enhances HIV-1 infection through interaction of complement receptors with macrophages and lymphocytes, MAC stimulates the migration of HIV-1-infected macrophages and lymphocytes, which may contributes to HIV-1 trafficking to the brain, thereby infecting brain cells such as microglia and astrocytes. These HIV-1 infected brain cells increase the production of complement, leading to the formation of MAC. Increased MAC may induce neuronal damage, which results in HIV-1-related neurodegenerative disorders. BBB, brain blood barrier.
Induction of complement component synthesis in brain cells by HIV-1
The liver is the primary organ to synthesize plasma complement proteins (Qin and Gao, 2006). In normal conditions, it is difficult for plasma complement proteins to reach CNS tissues in the presence of an intact BBB. Therefore, local complement production in response to abnormal insults such as foreign pathogens in the brain may be even more important for the pathogenesis of neurodegenerative diseases than it is in other tissues. Nearly all complement components and regulators can be locally produced in the brain although levels of complement in the normal brain are typically low (Speth et al., 2002a; Speth et al., 2005). Different cells of the brains have varying sensitivity to the effects of complement attack, which may be due to the level of complement regulators in the respective cell type (Speth et al., 2002a). For example, neurons and neuroblastoma cell lines are rapidly lysed due to the formation of the MAC on their membranes since they express low levels of most complement inhibitors (CD59, MCP, C1-INH, fH) and lack DAF (Speth et al., 2002a). However, other brain cells such as astrocytes and microglia are relatively resistant to MAC lytic effects since they express higher levels of complement regulators such as CD59, MCP and DAF than neuronal cells (Speth et al., 2002a).
In contrast, when HIV-1 infects brain cells, it induces the production of complement components in infected cells and increases complement deposition in the CNS. Incubation of different astrocytic cell lines and primary astrocytes with HIV-1 induced a marked upregulation (about 10 time fold increase) of the expression of the complement factors C2 and C3 both at the mRNA level and as secreted proteins in cell culture supernatants (Speth et al., 2001). The synthesis of other secreted factors such as C1q, C4, C5, C7 and C9 or membrane-bound complement regulators such as CD55, CD59 and CD46 were not found to be altered (Speth et al., 2001). Importantly, the C3 protein secreted after incubation of the cells with HIV-1 was shown to be biologically active as it could participate in the complement cascade (Speth et al., 2001). The underlying mechanism by which HIV-1 induces the up-regulation of C3 expression in brain astrocytes is mainly related to adenylate cyclase activation with upregulation of cyclic AMP. Besides whole HIV-1 virions, the purified viral proteins Nef and gp41 are biologically active in upregulating C3, whereas Tat, gp120, and gp160 were not able to modulate C3 synthesis (Speth et al., 2001). Consistently, neurons were also able to respond upon incubation with HIV-1 with increased C3 synthesis (Speth et al., 2002b). Further promoter mapping analysis of C3 defined the IL-6/IL-1beta responsive element in the promoter of C3 as a central element in the response to HIV-1 exposure (Bruder et al., 2004).
Down-regulation of the complement regulator, CD59, in human neuronal and glial cell lines treated with HIV-1 gp41 peptides
In addition to increased complement component synthesis in response to HIV-1 infection, down-regulation of the complement regulator, CD59, has been demonstrated previously. Chong and Lee (2000) demonstrated that an immunodominant gp41 peptide (ID, aa 598–613) as well as a recombinant gp41 protein encompassing this domain markedly reduced CD59 expression on the surface of both human neuronal and glial cell lines at either the mRNA or the protein level in a dose dependent manner whereas p24 and control peptide had little effect (Chong and Lee, 2000). It would be very interesting to further investigate whether increased complement activation and down-regulation of complement regulators in these cells also occurs in vivo and whether these events would break the delicate balance between complement activation and regulation, thereby participating in the pathogenesis of neuroAIDS.
Complement components in infected CNS tissues
Consistently, clinical and experimental results obtained from HIV-1-infected patients and SIV-infected monkeys have shown increased complement deposition and complement activation products in HIV-1 or SIV-infected CNS tissues and CSF. The National NeuroAIDS Tissue Consortium (NNTC) performed a brain gene expression array to elucidate pathophysiology of HIV-1-associated neurocognitive disorders (Gelman et al., 2012). Gelman et al analyzed the global gene profiles of neocortex, white matter, and neostriatum among HIV-1 infected subjects with no substantial neurocognitive impairment (NCI), with substantial NCI without HIV-1 encephalitis (HIVE); and those with substantial NCI and HIVE in comparison with uninfected controls. They demonstrated that HIV-1 RNA loads in brain tissue were three log (10) units higher in patients with HIVE than other groups and over 1,900 gene probes were regulated. Complement components were strongly up-regulated in the neostriatum of the HIVE group. These results provide direct clinical evidence supporting the role of complement in the pathogenesis of HIV-1-related CNS disorders. In support of this observation, using immunohistochemical staining, Speth et al. analyzed the synthesis of complement in the brains of SIV-infected rhesus macaques and documented that the cerebral synthesis of complement factors C1q and C3 was strongly up-regulated in SIV-infected monkeys compared to the spontaneous synthesis in uninfected control monkeys (Speth et al., 2004). Astrocytes, neurons, microglia, infiltrating macrophages and multinuclear giant cells all contributed to the high amounts of C1q and C3 observed in the brain. C1q and C3 were not only present intracellularly but also secreted as enhanced extracellular tissue staining was visible in the brains of the SIV-infected monkeys. The secreted C1q and C3 deposited on the membrane of neurons could contribute to neuronal damage through increasing the formation of membrane-driven lytic MAC attack (Speth et al., 2004).
Complement components in cerebrospinal fluid (CSF)
The results regarding increased complement deposition in the CNS tissues of HIV-infected patients and SIV-infected monkeys are consistent with a previous report that the level of C3 is increased in the CSF of patients with HIV-1-infection and signs of CNS dysfunction compared to the CSF of uninfected persons (Jongen et al., 2000). Jongen et al found that CSF from patients with CNS dysfunction associated with HIV-1 infections had a higher level of mean C3 and C4 index values (index values: CSF/serum ratio for C3 and C4), and C3 and C4 concentrations than the patients without any evident CNS diseases and HIV infection (Jongen et al., 2000). This finding suggests that intrathecal C3 and C4 production is up-regulated in the CNS of HIV-1 infected patients. Consistent with this notion, Reboul, et al demonstrated that intrathecal production of C4 was found frequently and was highly correlated with increased serum C4 though intrathecal C3 levels were decreased in most of the HIV-1 patients with CNS dysfunction. This finding further indicates that complement activation is a frequent accompaniment of the intrathecal immune response in HIV-1-positive patients. They further demonstrated that intrathecal anti-HIV-1 antibody synthesis was not correlated with clinical severity or neurological involvement (Reboul et al., 1989). Furthermore, CSF from the patients with multiple sclerosis and CNS dysfunction associated with systemic lupus erythematosus, in which complement plays a predominant role, had abnormal level of complement components in the CSF. For instance, there was a significant increase in the levels of C3, C4, or soluble MAC in CSF from patients with CNS involvement in systemic lupus erythematosus and with multiple sclerosis compared with controls (Jongen et al., 2000; Sanders et al., 1987; Sanders et al., 1986).
However, Rozek et al found that C3 was down-regulated in CSF from HIV-1-infected people with HAND compared to HIV-1-infected people without HAND (Rozek et al., 2007). Decreased levels of C3 in the CSF may result from complement activation in the CNS associated with HIV-1 infections. The level of C3 changes may reflect the different stages of CNS disease progression in infected individuals.
Mannose binding lectin (MBL) and neurosAIDS
As discussed above, activation of complement through MBL leads to pathogen opsonization and phagocytosis and MBL deficiency is linked to HIV transmission and disease progression. The role of complement in the pathogenesis of neurosAIDS has also been supported by HIV-1 patients with a genetic deficiency in MBL. MBL2-O/O genotypes, which result in lower expression of MBL, have been demonstrated to be associated with more rapid HIV-1-related disease progression, including CNS impairment, predominantly in children younger than 2 years (Singh et al., 2008). In a retrospective study utilizing archived post-mortem brain tissues, Singh et al further demonstrated that 1) MBL was expressed in neurons, astrocytes, microglia, and oligodendrocytes of the frontal cortex of the HIV-1-infected brain, 2) there were 30% to 40% more MBL-positive brain cells in HIVE vs non-HIVE cases (P = 0.01, paired t-test), 3) there was increased MBL expression in the neuronal axons of HIVE cases, and 4) there were 3- to 4-fold higher levels of 78 kD MBL trimers in HIVE vs non-HIVE cases (Singh et al., 2011). These results suggest that MBL could cause neuroinflammation and neuronal injury through the MBL complement activation pathway.
Taken together, emerging evidence indicates that abnormal complement activation takes places, while the complement regulation system is down-regulated in HIV-1 infected brains (Figure 3). The offset balance between complement activation and regulation may contribute to neuronal damage in response to HIV-1 infection (Figure 3). However, the role of complement in HIV-1 associated neuropathogenesis remains elusive and further understanding will require in vivo investigation with animal models. However, such studies will be challenging due to the lack of suitable small size animal models that completely recapitulate human HIV-1-related HAND. However, the availability of a number of complement deficient mice may be useful for assessing the role of complement in the pathogenesis of human diseases.
Complement-related therapeutic approaches for the treatment of HIV-1 infections
HIV-1 infection leading to AIDS and AIDS-related CNS disease is still a major public health challenge (Beck et al., 2008). Current active anti-retroviral treatment (HAART), can successfully control plasma levels of HIV-1 RNA below the limits of detection, but cannot eliminate HIV-1-infected cells or trace levels of free virions. If HAART is discontinued or becomes ineffective due to drug side effects or development of drug resistance, the HIV-1 contained in stable reservoirs rapidly rebounds and disease progression resumes (Cohen et al., 2008). Today, we have been successfully conquered and eradicated many viral infection and have cured the complications resulting from their infection. Indeed, immunotherapies including therapeutic antibodies for cancer therapy are very promising for the treatment of many cancers such as lymphomas and leukemias. However, we still face unprecedented challenge in developing an effective vaccine to eliminate and eradicate HIV-1 infection. Nevertheless, the development of a preventative vaccine remains the best strategy to control the HIV-1 epidemic and is a public health priority. None of the HIV-1 vaccine candidates have shown significant efficacy in patients thus far (De Rosa and McElrath, 2008; Girard et al., 2006; Kutzler and Weiner, 2008; Lu, 2008; Robinson, 2007). These formidable scientific challenges may be related to the high genetic variability of the virus, the lack of immune correlates of protection, limitations of the existing animal models and logistical problems associated with the conduction of multiple clinical trials (Letvin, 2005; Richman et al., 2009; Walker and Burton, 2008). For these reason, there are clinically unmet needs for novel approaches for the treatment and eradication of HIV-1 infection. Extensive evidence obtained from clinical and experimental studies supports the critical role of complement for therapeutic antibodies or vaccine-based cancer therapy (Zhou et al., 2008) (Ge et al., 2011; Hu et al., 2011; Kim et al., 2013; Pawluczkowycz et al., 2009). Why can this be done with HIV-1 infection and HIV-1-related complications such as HAND?
As extensively reviewed above, HIV-1 has also evolved many escape mechanisms to avoid innate and adaptive immunity to fight against HIV-1 infections. Hijacking complement regulators including CD59 from host cells is a major mechanism for HIV-1 to escape complement-mediated lytic attack (Figure 2). Therefore, we and others have actively been searching for complement-targeted therapeutics that would sensitize HIV-1 virions or HIV-1-infected cells to the lytic effect of activated complement (Dierich et al., 1996).
To enhance the efficacy of complement-mediated HIV-1 lysis, Xu et al have proposed a new approach that a novel activator of complement, consisting of a target domain (C3-binding region of complement receptor type 2) linked to a complement-activating human IgG1 Fc domain (CR2-Fc), would target and amplify complement deposition on HIV-1 virions (Xu et al., 2009).
We have previously reported the development of a recombinant form of the fourth domain of the bacterial toxin intermedilysin (ILY) (the recombinant domain 4 of intermedilysin [ILYd4]), a 114 aa protein that inhibits human CD59 function with high affinity and specificity (Figure 2). ILY is a pore forming toxin that exclusively lyses human cells because it binds with high affinity and specificity to hCD59 but not to CD59 from other species, as reported by Giddings, et al. (Giddings et al., 2004) and confirmed using our hCD59 transgenic mice (Hu et al., 2008). Binding of ILY to hCD59 occurs through domain 4 (D4) while the three other domains (D1, D2 and D3) of ILY form the lytic transmembrane pore (Giddings et al., 2004). Because the D4 of ILY binds to a region of hCD59 encompassing its active site (amino acids 42–58) (Giddings et al., 2004; Tweten, 2005), we reasoned that ILYd4 may also inhibit human CD59 activity (Zhou et al., 2008). After we demonstrated that ILYd4 indeed inhibits hCD59 function and thereby enhances antibody-activated complement-mediated virolysis of HIV-1, as reported in the 2008 annual meeting of The American Society of Immunologists (http://www.fasebj.org/cgi/content/meeting_abstract/22/2_MeetingAbstracts/522), we initiated a collaboration with Dr. Yu’s group at Indiana University to further investigate the potential application of ILYd4 for HIV-1 treatment (Hu et al., 2010).
In collaboration with Dr. Yu and colleagues (2010), we further documented that in the presence of rILYd4, HIV-1 virions derived experimentally or primary HIV-1 isolates collected from HIV-1–infected patients became highly sensitive to complement-mediated lysis activated by anti–HIV-1 antibodies present in the plasma of HIV-1–infected individuals (Hu et al., 2010). We also showed that ILYd4 together with serum or plasma from HIV-1–infected patients as a source of anti–HIV-1 antibodies and complement did not mediate complement-mediated lysis of either erythrocytes or peripheral blood mononuclear cells (Figure 2)(Hu et al., 2010). Furthermore, recent studies have also shown that CD59 is incorporated into both cell line-derived and plasma primary HCV virions (a major virus frequently co-infected in HIV-1 infected drug abusers) at levels that protect against complement-mediated lysis (Amet et al., 2011). The direct addition of CD59 inhibitor, ILYd4, into plasma from HCV-infected patients rendered endogenous plasma virions sensitive to complement-mediated lysis (Amet et al., 2011). These results indicate that inhibition of CD59 activity through its inhibitor such as ILYd4 may serve as a novel agent to abrogate hCD59 function, thereby unleashing the ability of vaccine- or viral infection-induced antibodies to specifically eliminate HIV-1 or HCV virions and infected cells through enhancing complement-mediated virolysis (Figure 2)(Amet et al., 2011; Hu et al., 2010). ILYd4 has significant potential as an anti-HIV-1 and HCV therapeutic agent that warrants further testing in animal studies and in human clinical trials.
Conclusion
Complement, a critical mediator of innate and adaptive immunity, plays several diverse roles in the neuropathogenesis of HIV-1 infection. The complement system can be activated in response to HIV-1 infection in the circulation and the CNS through HIV-1 envelope proteins, MBL and anti-HIV antibodies. Paradoxically, complement not only fights against HIV-1 infection but also enhances HIV-1 infection. Complement may also contribute to the pathogenesis of HIV-1-related CNS diseases. However, HIV-1 hijacks complement regulators such as CD59 and CD55 and utilizes these regulators and Fh to escape from complement attack. On the one hand, there may be a delicate balance between complement activation and complement regulations in HIV-1 infection, which may contribute to development of HIV-1 latency and persistence. On the other hand, there may be an offset balance in HIV-1-infected brains, which may contribute to the development of HIV-1 related HAND. It is imperative for us to clearly understand the role of complement in HIV-1 infection and HIV-1-related HAND although in vivo studies in animal models will be a significant challenge due to the lack of appropriate mouse models. Although several approaches have been proposed for enhancing complement activation in HIV-1 infection for the treatment and prevention of HIV-1 infection and HIV-1-related HAND, they still requires for further evaluation and extensive in vivo investigation.
Acknowledgments
The authors gratefully acknowledge support from the National Institutes of Health grants NIHR21CA141324 and 1R01CA166144 (to X.B.Q.).
Footnotes
Conflicts of interest:
The authors declare that they have no conflict of interest.
Reference
- Aasa-Chapman MM, Hayman A, Newton P, Cornforth D, Williams I, Borrow P, Balfe P, McKnight A. Development of the antibody response in acute HIV-1 infection. AIDS (London, England) 2004;18:371–381. doi: 10.1097/00002030-200402200-00002. [DOI] [PubMed] [Google Scholar]
- Aasa-Chapman MM, Holuigue S, Aubin K, Wong M, Jones NA, Cornforth D, Pellegrino P, Newton P, Williams I, Borrow P, et al. Detection of antibody-dependent complement-mediated inactivation of both autologous and heterologous virus in primary human immunodeficiency virus type 1 infection. Journal of virology. 2005;79:2823–2830. doi: 10.1128/JVI.79.5.2823-2830.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe K, Miyazaki M, Koji T, Furusu A, Ozono Y, Harada T, Sakai H, Nakane PK, Kohno S. Expression of decay accelerating factor mRNA and complement C3 mRNA in human diseased kidney. Kidney international. 1998;54:120–130. doi: 10.1046/j.1523-1755.1998.00961.x. [DOI] [PubMed] [Google Scholar]
- Acosta J, Qin X, Halperin J. Complement and complement regulatory proteins as potential molecular targets for vascular diseases. Curr Pharm Des. 2004;10:203–211. doi: 10.2174/1381612043453441. [DOI] [PubMed] [Google Scholar]
- Amet T, Ghabril M, Chalasani N, Byrd D, Hu N, Grantham A, Liu Z, Qin X, He JJ, Yu Q. CD59 incorporation protects hepatitis C virus against complement-mediated destruction. Hepatology (Baltimore, Md. 2011 doi: 10.1002/hep.24686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajtay Z, Speth C, Erdei A, Dierich MP. Cutting edge: productive HIV-1 infection of dendritic cells via complement receptor type 3 (CR3, CD11b/CD18) J Immunol. 2004;173:4775–4778. doi: 10.4049/jimmunol.173.8.4775. [DOI] [PubMed] [Google Scholar]
- Beck EJ, Santas XM, Delay PR. Why and how to monitor the cost and evaluate the cost-effectiveness of HIV services in countries. AIDS (London, England) 2008;22(Suppl 1):S75–S85. doi: 10.1097/01.aids.0000327626.77597.fa. [DOI] [PubMed] [Google Scholar]
- Belec L, Dupre T, Prazuck T, Tevi-Benissan C, Kanga JM, Pathey O, Lu XS, Pillot J. Cervicovaginal overproduction of specific IgG to human immunodeficiency virus (HIV) contrasts with normal or impaired IgA local response in HIV infection. The Journal of infectious diseases. 1995;172:691–697. doi: 10.1093/infdis/172.3.691. [DOI] [PubMed] [Google Scholar]
- Binley JM, Klasse PJ, Cao Y, Jones I, Markowitz M, Ho DD, Moore JP. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. Journal of virology. 1997;71:2799–2809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P, Shattock RJ, Vyakarnam A, Group EW. Innate immunity against HIV: a priority target for HIV prevention research. Retrovirology. 2010;7:84. doi: 10.1186/1742-4690-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitner S, Storkel S, Reichel W, Loos M. Complement components C1q, C1r/C1s, and C1INH in rheumatoid arthritis. Correlation of in situ hybridization and northern blot results with function and protein concentration in synovium and primary cell cultures. Arthritis and rheumatism. 1995;38:492–498. doi: 10.1002/art.1780380406. [DOI] [PubMed] [Google Scholar]
- Brodbeck WG, Mold C, Atkinson JP, Medof ME. Cooperation between decay-accelerating factor and membrane cofactor protein in protecting cells from autologous complement attack. J Immunol. 2000;165:3999–4006. doi: 10.4049/jimmunol.165.7.3999. [DOI] [PubMed] [Google Scholar]
- Bruder C, Hagleitner M, Darlington G, Mohsenipour I, Wurzner R, Hollmuller I, Stoiber H, Lass-Florl C, Dierich MP, Speth C. HIV-1 induces complement factor C3 synthesis in astrocytes and neurons by modulation of promoter activity. Molecular immunology. 2004;40:949–961. doi: 10.1016/j.molimm.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Rathore A, Yamamoto N, Dhole TN. Mannose-binding lectin (+54) exon-1 gene polymorphism influence human immunodeficiency virus-1 susceptibility in North Indians. Tissue antigens. 2011;77:18–22. doi: 10.1111/j.1399-0039.2010.01563.x. [DOI] [PubMed] [Google Scholar]
- Chong YH, Lee MJ. Expression of complement inhibitor protein CD59 in human neuronal and glial cell lines treated with HIV-1 gp41 peptides. Journal of neurovirology. 2000;6:51–60. doi: 10.3109/13550280009006382. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Hellmann N, Levy JA, DeCock K, Lange J. The spread, treatment, and prevention of HIV-1: evolution of a global pandemic. The Journal of clinical investigation. 2008;118:1244–1254. doi: 10.1172/JCI34706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper NR, Jensen FC, Welsh RM, Jr, Oldstone MB. Lysis of RNA tumor viruses by human serum: direct antibody-independent triggering of the classical complement pathway. The Journal of experimental medicine. 1976;144:970–984. doi: 10.1084/jem.144.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple A, Wild EJ, Joubert R, Sathasivam K, Bjorkqvist M, Petersen A, Jackson GS, Isaacs JD, Kristiansen M, Bates GP, et al. Proteomic profiling of plasma in Huntington's disease reveals neuroinflammatory activation and biomarker candidates. Journal of proteome research. 2007;6:2833–2840. doi: 10.1021/pr0700753. [DOI] [PubMed] [Google Scholar]
- Datta PK, Rappaport J. HIV and complement: hijacking an immune defense. Biomed Pharmacother. 2006;60:561–568. doi: 10.1016/j.biopha.2006.07.087. [DOI] [PubMed] [Google Scholar]
- Davitz MA, Low MG, Nussenzweig V. Release of decay-accelerating factor (DAF) from the cell membrane by phosphatidylinositol-specific phospholipase C (PIPLC) J Exp Med. 1986;163:1150–1161. doi: 10.1084/jem.163.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa SD, McElrath MJ. T cell responses generated by HIV vaccines in clinical trials. Curr Opin HIV AIDS. 2008;3:375–379. doi: 10.1097/COH.0b013e3282fbaaa7. [DOI] [PubMed] [Google Scholar]
- Dierich MP, Stoiber H, Clivio A. A "complement-ary" AIDS vaccine. Nat Med. 1996;2:153–155. doi: 10.1038/nm0296-153. [DOI] [PubMed] [Google Scholar]
- Dzwonek A, Novelli V, Bajaj-Elliott M, Turner M, Clapson M, Klein N. Mannose-binding lectin in susceptibility and progression of HIV-1 infection in children. Antiviral therapy. 2006;11:499–505. [PubMed] [Google Scholar]
- Ebenbichler CF, Thielens NM, Vornhagen R, Marschang P, Arlaud GJ, Dierich MP. Human immunodeficiency virus type 1 activates the classical pathway of complement by direct C1 binding through specific sites in the transmembrane glycoprotein gp41. The Journal of experimental medicine. 1991;174:1417–1424. doi: 10.1084/jem.174.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P, Hack CE, Rozemuller JM, Stam FC. Complement activation in amyloid plaques in Alzheimer's dementia. Virchows Archiv B, Cell pathology including molecular pathology. 1989;56:259–262. doi: 10.1007/BF02890024. [DOI] [PubMed] [Google Scholar]
- Ezekowitz RA, Kuhlman M, Groopman JE, Byrn RA. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. The Journal of experimental medicine. 1989;169:185–196. doi: 10.1084/jem.169.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasque P, Fontaine M, Morgan BP. Complement expression in human brain. Biosynthesis of terminal pathway components and regulators in human glial cells and cell lines. J Immunol. 1995;154:4726–4733. [PubMed] [Google Scholar]
- Gauduin MC, Parren PW, Weir R, Barbas CF, Burton DR, Koup RA. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- Ge X, Wu L, Hu W, Fernandes S, Wang C, Li X, Brown JR, Qin X. rILYd4, a human CD59 inhibitor, enhances complement-dependent cytotoxicity of ofatumumab against rituximab-resistant B-cell lymphoma cells and chronic lymphocytic leukemia. Clin Cancer Res. 2011;17:6702–6711. doi: 10.1158/1078-0432.CCR-11-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Chen T, Lisinicchia JG, Soukup VM, Carmical JR, Starkey JM, Masliah E, Commins DL, Brandt D, Grant I, et al. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PloS one. 2012;7:e46178. doi: 10.1371/journal.pone.0046178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings KS, Zhao J, Sims PJ, Tweten RK. Human CD59 is a receptor for the cholesterol-dependent cytolysin intermedilysin. Nat Struct Mol Biol. 2004;11:1173–1178. doi: 10.1038/nsmb862. [DOI] [PubMed] [Google Scholar]
- Girard MP, Osmanov SK, Kieny MP. A review of vaccine research and development: the human immunodeficiency virus (HIV) Vaccine. 2006;24:4062–4081. doi: 10.1016/j.vaccine.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Gregersen JP, Mehdi S, Baur A, Hilfenhaus J. Antibody- and complement-mediated lysis of HIV-infected cells and inhibition of viral replication. Journal of medical virology. 1990;30:287–293. doi: 10.1002/jmv.1890300411. [DOI] [PubMed] [Google Scholar]
- Griffin GE, Leung K, Folks TM, Kunkel S, Nabel GJ. Induction of NF-kappa B during monocyte differentiation is associated with activation of HIV-gene expression. Research in virology. 1991;142:233–238. doi: 10.1016/0923-2516(91)90062-8. [DOI] [PubMed] [Google Scholar]
- Guo RF, Ward PA. Role of C5a in inflammatory responses. Annual review of immunology. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- Haas PJ, van Strijp J. Anaphylatoxins: their role in bacterial infection and inflammation. Immunol Res. 2007;37:161–175. doi: 10.1007/BF02697367. [DOI] [PubMed] [Google Scholar]
- Hart ML, Saifuddin M, Spear GT. Glycosylation inhibitors and neuraminidase enhance human immunodeficiency virus type 1 binding and neutralization by mannose-binding lectin. The Journal of general virology. 2003;84:353–360. doi: 10.1099/vir.0.18734-0. [DOI] [PubMed] [Google Scholar]
- Haurum JS, Thiel S, Jones IM, Fischer PB, Laursen SB, Jensenius JC. Complement activation upon binding of mannan-binding protein to HIV envelope glycoproteins. AIDS (London, England) 1993;7:1307–1313. doi: 10.1097/00002030-199310000-00002. [DOI] [PubMed] [Google Scholar]
- Holt DS, Botto M, Bygrave AE, Hanna SM, Walport MJ, Morgan BP. Targeted deletion of the CD59 gene causes spontaneous intravascular hemolysis and hemoglobinuria. Blood. 2001;98:442–449. doi: 10.1182/blood.v98.2.442. [DOI] [PubMed] [Google Scholar]
- Hu W, Ferris SP, Tweten RK, Wu G, Radaeva S, Gao B, Bronson RT, Halperin JA, Qin X. Rapid conditional targeted ablation of cells expressing human CD59 in transgenic mice by intermedilysin. Nat Med. 2008;14:98–103. doi: 10.1038/nm1674. [DOI] [PubMed] [Google Scholar]
- Hu W, Ge X, You T, Xu T, Zhang J, Wu G, Peng Z, Chorev M, Aktas BH, Halperin JA, et al. Human CD59 inhibitor sensitizes rituximab-resistant lymphoma cells to complement-mediated cytolysis. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Yu Q, Hu N, Byrd D, Amet T, Shikuma C, Shiramizu B, Halperin JA, Qin X. A High-Affinity Inhibitor of Human CD59 Enhances Complement-Mediated Virolysis of HIV-1: Implications for Treatment of HIV-1/AIDS. J Immunol. 2010;184:359–368. doi: 10.4049/jimmunol.0902278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Fischer M, Misselwitz B, Manrique A, Kuster H, Niederost B, Weber R, von Wyl V, Gunthard HF, Trkola A. Complement lysis activity in autologous plasma is associated with lower viral loads during the acute phase of HIV-1 infection. PLoS medicine. 2006;3:e441. doi: 10.1371/journal.pmed.0030441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Trkola A. Humoral immunity to HIV-1: neutralization and beyond. J Intern Med. 2007;262:5–25. doi: 10.1111/j.1365-2796.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- Humbert M, Dietrich U. The role of neutralizing antibodies in HIV infection. AIDS Rev. 2006;8:51–59. [PubMed] [Google Scholar]
- Israels J, Scherpbier HJ, Frakking FN, van de Wetering MD, Kremer LC, Kuijpers TW. Mannose-binding lectin and the risk of HIV transmission and disease progression in children: a systematic review. The Pediatric infectious disease journal. 2012;31:1272–1278. doi: 10.1097/INF.0b013e3182678bc4. [DOI] [PubMed] [Google Scholar]
- Jongen PJ, Doesburg WH, Ibrahim-Stappers JL, Lemmens WA, Hommes OR, Lamers KJ. Cerebrospinal fluid C3 and C4 indexes in immunological disorders of the central nervous system. Acta neurologica Scandinavica. 2000;101:116–121. doi: 10.1034/j.1600-0404.2000.101002116.x. [DOI] [PubMed] [Google Scholar]
- June RA, Schade SZ, Bankowski MJ, Kuhns M, McNamara A, Lint TF, Landay AL, Spear GT. Complement and antibody mediate enhancement of HIV infection by increasing virus binding and provirus formation. AIDS (London, England) 1991;5:269–274. doi: 10.1097/00002030-199103000-00004. [DOI] [PubMed] [Google Scholar]
- Kacani L, Banki Z, Zwirner J, Schennach H, Bajtay Z, Erdei A, Stoiber H, Dierich MP. C5a and C5a(desArg) enhance the susceptibility of monocyte-derived macrophages to HIV infection. J Immunol. 2001;166:3410–3415. doi: 10.4049/jimmunol.166.5.3410. [DOI] [PubMed] [Google Scholar]
- Kim MK, Breitbach CJ, Moon A, Heo J, Lee YK, Cho M, Lee JW, Kim SG, Kang DH, Bell JC, et al. Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans. Science translational medicine. 2013;5:185ra163. doi: 10.1126/scitranslmed.3005361. [DOI] [PubMed] [Google Scholar]
- Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9:776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S. Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Topics in antiviral medicine. 2011;19:137–142. [PMC free article] [PubMed] [Google Scholar]
- Letvin NL. Progress toward an HIV vaccine. Annu Rev Med. 2005;56:213–223. doi: 10.1146/annurev.med.54.101601.152349. [DOI] [PubMed] [Google Scholar]
- Li H, Fu WP, Hong ZH. Replication study in Chinese Han population and meta-analysis supports association between the MBL2 gene polymorphism and HIV-1 infection. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2013;20:163–170. doi: 10.1016/j.meegid.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Lin RC, Herman J, Henry L, Daniels GL. A family showing inheritance of the Inab phenotype. Transfusion. 1988;28:427–429. doi: 10.1046/j.1537-2995.1988.28588337329.x. [DOI] [PubMed] [Google Scholar]
- Lu S. Immunogenicity of DNA vaccines in humans: it takes two to tango. Hum Vaccin. 2008;4:449–452. doi: 10.4161/hv.4.6.6179. [DOI] [PubMed] [Google Scholar]
- Lund O, Hansen J, Soorensen AM, Mosekilde E, Nielsen JO, Hansen JE. Increased adhesion as a mechanism of antibody-dependent and antibody-independent complement-mediated enhancement of human immunodeficiency virus infection. Journal of virology. 1995;69:2393–2400. doi: 10.1128/jvi.69.4.2393-2400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano A, Rocco C, Marino SM, Mecikovsky D, Genre F, Aulicino P, Bologna R, Sen L. Detrimental effects of mannose-binding lectin (MBL2) promoter genotype XA/XA on HIV-1 vertical transmission and AIDS progression. The Journal of infectious diseases. 2008;198:694–700. doi: 10.1086/590498. [DOI] [PubMed] [Google Scholar]
- Martinez-Pinna R, Madrigal-Matute J, Tarin C, Burillo E, Esteban-Salan M, Pastor-Vargas C, Lindholt JS, Lopez JA, Calvo E, de Ceniga MV, et al. Proteomic analysis of intraluminal thrombus highlights complement activation in human abdominal aortic aneurysms. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:2013–2020. doi: 10.1161/ATVBAHA.112.301191. [DOI] [PubMed] [Google Scholar]
- Mayer MM. Complement. Historical perspectives and some current issues. Complement (Basel, Switzerland) 1984;1:2–26. [PubMed] [Google Scholar]
- Medof ME, Lublin DM, Holers VM, Ayers DJ, Getty RR, Leykam JF, Atkinson JP, Tykocinski ML. Cloning and characterization of cDNAs encoding the complete sequence of decay-accelerating factor of human complement. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:2007–2011. doi: 10.1073/pnas.84.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori DC, Cornell RJ, Zhou JY, Zhou JT, Hirsch VM, Johnson PR. Complement control proteins, CD46, CD55, and CD59, as common surface constituents of human and simian immunodeficiency viruses and possible targets for vaccine protection. Virology. 1994;205:82–92. doi: 10.1006/viro.1994.1622. [DOI] [PubMed] [Google Scholar]
- Morgan BP. Regulation of the complement membrane attack pathway. Crit Rev Immunol. 1999;19:173–198. [PubMed] [Google Scholar]
- Morgan BP, Gasque P. Extrahepatic complement biosynthesis: where, when and why? Clin Exp Immunol. 1997;107:1–7. doi: 10.1046/j.1365-2249.1997.d01-890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L. Neutralizing antibody responses to HIV-1 infection. IUBMB Life. 2002;53:197–199. doi: 10.1080/15216540212656. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Brittingham A. Leishmania, macrophages and complement: a tale of subversion and exploitation. Parasitology. 1997;115(Suppl):S9–S23. doi: 10.1017/s0031182097001789. [DOI] [PubMed] [Google Scholar]
- Nevo Y, Ben-Zeev B, Tabib A, Straussberg R, Anikster Y, Shorer Z, Fattal-Valevski A, Ta-Shma A, Aharoni S, Rabie M, et al. CD59 deficiency is associated with chronic hemolysis and childhood relapsing immune-mediated polyneuropathy. Blood. 2013;121:129–135. doi: 10.1182/blood-2012-07-441857. [DOI] [PubMed] [Google Scholar]
- Nicholson-Weller A, Spicer DB, Austen KF. Deficiency of the complement regulatory protein, "decay-accelerating factor," on membranes of granulocytes, monocytes, and platelets in paroxysmal nocturnal hemoglobinuria. The New England journal of medicine. 1985;312:1091–1097. doi: 10.1056/NEJM198504253121704. [DOI] [PubMed] [Google Scholar]
- Ozkaya Sahin G, Holmgren B, Sheik-Khalil E, da Silva Z, Nielsen J, Nowroozalizadeh S, Mansson F, Norrgren H, Aaby P, Fenyo EM, et al. Effect of complement on HIV-2 plasma antiviral activity is intratype specific and potent. Journal of virology. 2013;87:273–281. doi: 10.1128/JVI.01640-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantophlet R, Burton DR. GP120: target for neutralizing HIV-1 antibodies. Annu Rev Immunol. 2006;24:739–769. doi: 10.1146/annurev.immunol.24.021605.090557. [DOI] [PubMed] [Google Scholar]
- Pawluczkowycz AW, Beurskens FJ, Beum PV, Lindorfer MA, van de Winkel JG, Parren PW, Taylor RP. Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than by RTX. J Immunol. 2009;183:749–758. doi: 10.4049/jimmunol.0900632. [DOI] [PubMed] [Google Scholar]
- Pellegrin I, Legrand E, Neau D, Bonot P, Masquelier B, Pellegrin JL, Ragnaud JM, Bernard N, Fleury HJ. Kinetics of appearance of neutralizing antibodies in 12 patients with primary or recent HIV-1 infection and relationship with plasma and cellular viral loads. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:438–447. doi: 10.1097/00042560-199604150-00003. [DOI] [PubMed] [Google Scholar]
- Pincus SH, Messer KG, Nara PL, Blattner WA, Colclough G, Reitz M. Temporal analysis of the antibody response to HIV envelope protein in HIV-infected laboratory workers. The Journal of clinical investigation. 1994;93:2505–2513. doi: 10.1172/JCI117260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MR, Elboim HS, Cannon T, Cavacini L, Hideshima T. Functional activity of an HIV-1 neutralizing IgG human monoclonal antibody: ADCC and complement-mediated lysis. AIDS research and human retroviruses. 1992;8:553–558. doi: 10.1089/aid.1992.8.553. [DOI] [PubMed] [Google Scholar]
- Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8:582–587. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- Prohaszka Z, Hidvegi T, Ujhelyi E, Stoiber H, Dierich MP, Susal C, Fust G. Interaction of complement and specific antibodies with the external glycoprotein 120 of HIV-1. Immunology. 1995;85:184–189. [PMC free article] [PubMed] [Google Scholar]
- Prohaszka Z, Nemes J, Hidvegi T, Toth FD, Kerekes K, Erdei A, Szabo J, Ujhelyi E, Thielens N, Dierich MP, et al. Two parallel routes of the complement-mediated antibody-dependent enhancement of HIV-1 infection. AIDS (London, England) 1997;11:949–958. doi: 10.1097/00002030-199708000-00002. [DOI] [PubMed] [Google Scholar]
- Pruenster M, Wilflingseder D, Banki Z, Ammann CG, Muellauer B, Meyer M, Speth C, Dierich MP, Stoiber H. C-type lectin-independent interaction of complement opsonized HIV with monocyte-derived dendritic cells. European journal of immunology. 2005;35:2691–2698. doi: 10.1002/eji.200425940. [DOI] [PubMed] [Google Scholar]
- Qin X, Gao B. The complement system in liver diseases. Cell Mol Immunol. 2006;3:333–340. [PubMed] [Google Scholar]
- Qin X, Hu W, Song W, Grubissich L, Hu X, Wu G, Ferris S, Dobarro M, Halperin JA. Generation and phenotyping of mCd59a and mCd59b double-knockout mice. Am J Hematol. 2009;84:65–70. doi: 10.1002/ajh.21319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Krumrei N, Grubissich L, Dobarro M, Aktas H, Perez G, Halperin JA. Deficiency of the mouse complement regulatory protein mCd59b results in spontaneous hemolytic anemia with platelet activation and progressive male infertility. Immunity. 2003;18:217–227. doi: 10.1016/s1074-7613(03)00022-0. [DOI] [PubMed] [Google Scholar]
- Reboul J, Schuller E, Pialoux G, Rey MA, Lebon P, Allinquant B, Brun-Vezinet F. Immunoglobulins and complement components in 37 patients infected by HIV-1 virus: comparison of general (systemic) and intrathecal immunity. Journal of the neurological sciences. 1989;89:243–252. doi: 10.1016/0022-510x(89)90026-9. [DOI] [PubMed] [Google Scholar]
- Rees-Roberts D, Mullen LM, Gounaris K, Selkirk ME. Inactivation of the complement anaphylatoxin C5a by secreted products of parasitic nematodes. International journal for parasitology. 2010;40:527–532. doi: 10.1016/j.ijpara.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid ME, Mallinson G, Sim RB, Poole J, Pausch V, Merry AH, Liew YW, Tanner MJ. Biochemical studies on red blood cells from a patient with the Inab phenotype (decay-accelerating factor deficiency) Blood. 1991;78:3291–3297. [PubMed] [Google Scholar]
- Reisinger EC, Vogetseder W, Berzow D, Kofler D, Bitterlich G, Lehr HA, Wachter H, Dierich MP. Complement-mediated enhancement of HIV-1 infection of the monoblastoid cell line U937. AIDS (London, England) 1990;4:961–965. doi: 10.1097/00002030-199010000-00003. [DOI] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England journal of medicine. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson HL. HIV/AIDS vaccines: 2007. Clin Pharmacol Ther. 2007;82:686–693. doi: 10.1038/sj.clpt.6100408. [DOI] [PubMed] [Google Scholar]
- Rozek W, Ricardo-Dukelow M, Holloway S, Gendelman HE, Wojna V, Melendez LM, Ciborowski P. Cerebrospinal fluid proteomic profiling of HIV-1-infected patients with cognitive impairment. Journal of proteome research. 2007;6:4189–4199. doi: 10.1021/pr070220c. [DOI] [PubMed] [Google Scholar]
- Rus H, Cudrici C, Niculescu F. C5b-9 complement complex in autoimmune demyelination and multiple sclerosis: dual role in neuroinflammation and neuroprotection. Ann Med. 2005;37:97–104. doi: 10.1080/07853890510007278. [DOI] [PubMed] [Google Scholar]
- Saifuddin M, Hart ML, Gewurz H, Zhang Y, Spear GT. Interaction of mannose-binding lectin with primary isolates of human immunodeficiency virus type 1. The Journal of general virology. 2000;81:949–955. doi: 10.1099/0022-1317-81-4-949. [DOI] [PubMed] [Google Scholar]
- Saifuddin M, Hedayati T, Atkinson JP, Holguin MH, Parker CJ, Spear GT. Human immunodeficiency virus type 1 incorporates both glycosyl phosphatidylinositol-anchored CD55 and CD59 and integral membrane CD46 at levels that protect from complement-mediated destruction. J Gen Virol. 1997;78:1907–1911. doi: 10.1099/0022-1317-78-8-1907. [DOI] [PubMed] [Google Scholar]
- Saifuddin M, Parker CJ, Peeples ME, Gorny MK, Zolla-Pazner S, Ghassemi M, Rooney IA, Atkinson JP, Spear GT. Role of virion-associated glycosylphosphatidylinositol-linked proteins CD55 and CE59 in complement resistance of cell derived and primary isolates of HIV-1. J Exp Med. 1995;182:501–509. doi: 10.1084/jem.182.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders ME, Alexander EL, Koski CL, Frank MM, Joiner KA. Detection of activated terminal complement (C5b-9) in cerebrospinal fluid from patients with central nervous system involvement of primary Sjogren's syndrome or systemic lupus erythematosus. J Immunol. 1987;138:2095–2099. [PubMed] [Google Scholar]
- Sanders ME, Koski CL, Robbins D, Shin ML, Frank MM, Joiner KA. Activated terminal complement in cerebrospinal fluid in Guillain-Barre syndrome and multiple sclerosis. J Immunol. 1986;136:4456–4459. [PubMed] [Google Scholar]
- Schmitz J, Zimmer JP, Kluxen B, Aries S, Bogel M, Gigli I, Schmitz H. Antibody-dependent complement-mediated cytotoxicity in sera from patients with HIV-1 infection is controlled by CD55 and CD59. The Journal of clinical investigation. 1995;96:1520–1526. doi: 10.1172/JCI118190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng A, Lan J, Wu H, Lu J, Wang Y, Chu Q, Jia Z, Song M, Liu L, Wang W. A clinical case-control study on the association between mannose-binding lectin and susceptibility to HIV-1 infection among northern Han Chinese population. International journal of immunogenetics. 2010;37:445–454. doi: 10.1111/j.1744-313X.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M, Jr, Lifson JD, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Lieser A, Ruan PK, Fenton T, Spector SA. An age-dependent association of mannose-binding lectin-2 genetic variants on HIV-1-related disease in children. The Journal of allergy and clinical immunology. 2008;122:173–180. doi: 10.1016/j.jaci.2008.05.025. 180 e171–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Nathamu S, Adame A, Alire TU, Dumaop W, Gouaux B, Moore DJ, Masliah E H.I.V.N.R.C.G. Expression of mannose binding lectin in HIV-1-infected brain: implications for HIV-related neuronal damage and neuroAIDS. Neurobehavioral HIV medicine. 2011;3:41–52. doi: 10.2147/NBHIV.S19969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear GT, Sullivan BL, Takefman DM, Landay AL, Lint TF. Human immunodeficiency virus (HIV)-infected cells and free virus directly activate the classical complement pathway in rabbit, mouse and guinea-pig sera; activation results in virus neutralization by virolysis. Immunology. 1991;73:377–382. [PMC free article] [PubMed] [Google Scholar]
- Spear GT, Takefman DM, Sullivan BL, Landay AL, Zolla-Pazner S. Complement activation by human monoclonal antibodies to human immunodeficiency virus. Journal of virology. 1993;67:53–59. doi: 10.1128/jvi.67.1.53-59.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth C, Dierich MP, Gasque P. Neuroinvasion by pathogens: a key role of the complement system. Molecular immunology. 2002a;38:669–679. doi: 10.1016/s0161-5890(01)00104-3. [DOI] [PubMed] [Google Scholar]
- Speth C, Dierich MP, Sopper S. HIV-infection of the central nervous system: the tightrope walk of innate immunity. Molecular immunology. 2005;42:213–228. doi: 10.1016/j.molimm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Speth C, Schabetsberger T, Mohsenipour I, Stockl G, Wurzner R, Stoiber H, Lass-Florl C, Dierich MP. Mechanism of human immunodeficiency virus-induced complement expression in astrocytes and neurons. Journal of virology. 2002b;76:3179–3188. doi: 10.1128/JVI.76.7.3179-3188.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth C, Stockl G, Mohsenipour I, Wurzner R, Stoiber H, Lass-Florl C, Dierich MP. Human immunodeficiency virus type 1 induces expression of complement factors in human astrocytes. Journal of virology. 2001;75:2604–2615. doi: 10.1128/JVI.75.6.2604-2615.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth C, Stoiber H, Dierich MP. Complement in different stages of HIV infection and pathogenesis. Int Arch Allergy Immunol. 2003;130:247–257. doi: 10.1159/000070211. [DOI] [PubMed] [Google Scholar]
- Speth C, Williams K, Hagleitner M, Westmoreland S, Rambach G, Mohsenipour I, Schmitz J, Wurzner R, Lass-Florl C, Stoiber H, et al. Complement synthesis and activation in the brain of SIV-infected monkeys. Journal of neuroimmunology. 2004;151:45–54. doi: 10.1016/j.jneuroim.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Spudich S, Gonzalez-Scarano F. HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harbor perspectives in medicine. 2012;2:a007120. doi: 10.1101/cshperspect.a007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoiber H, Banki Z, Wilflingseder D, Dierich MP. Complement-HIV interactions during all steps of viral pathogenesis. Vaccine. 2008a;26:3046–3054. doi: 10.1016/j.vaccine.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Stoiber H, Clivio A, Dierich MP. Role of complement in HIV infection. Annu Rev Immunol. 1997;15:649–674. doi: 10.1146/annurev.immunol.15.1.649. [DOI] [PubMed] [Google Scholar]
- Stoiber H, Kacani L, Speth C, Wurzner R, Dierich MP. The supportive role of complement in HIV pathogenesis. Immunological reviews. 2001;180:168–176. doi: 10.1034/j.1600-065x.2001.1800115.x. [DOI] [PubMed] [Google Scholar]
- Stoiber H, Pinter C, Siccardi AG, Clivio A, Dierich MP. Efficient destruction of human immunodeficiency virus in human serum by inhibiting the protective action of complement factor H and decay accelerating factor (DAF, CD55) The Journal of experimental medicine. 1996;183:307–310. doi: 10.1084/jem.183.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoiber H, Soederholm A, Wilflingseder D, Gusenbauer S, Hildgartner A, Dierich MP. Complement and antibodies: a dangerous liaison in HIV infection? Vaccine. 2008b;26(Suppl 8):I79–I85. doi: 10.1016/j.vaccine.2008.11.050. [DOI] [PubMed] [Google Scholar]
- Stoiber H, Speth C, Dierich MP. Role of complement in the control of HIV dynamics and pathogenesis. Vaccine. 2003;21(Suppl 2):S77–S82. doi: 10.1016/s0264-410x(03)00203-2. [DOI] [PubMed] [Google Scholar]
- Sugita Y, Tobe T, Oda E, Tomita M, Yasukawa K, Yamaji N, Takemoto T, Furuichi K, Takayama M, Yano S. Molecular cloning and characterization of MACIF, an inhibitor of membrane channel formation of complement. J Biochem (Tokyo) 1989;106:555–557. doi: 10.1093/oxfordjournals.jbchem.a122893. [DOI] [PubMed] [Google Scholar]
- Sun X, Funk CD, Deng C, Sahu A, Lambris JD, Song WC. Role of decay-accelerating factor in regulating complement activation on the erythrocyte surface as revealed by gene targeting. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:628–633. doi: 10.1073/pnas.96.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susal C, Kirschfink M, Kropelin M, Daniel V, Opelz G. Complement activation by recombinant HIV-1 glycoprotein gp120. J Immunol. 1994;152:6028–6034. [PubMed] [Google Scholar]
- Szabo J, Prohaszka Z, Toth FD, Gyuris A, Segesdi J, Banhegyi D, Ujhelyi E, Minarovits J, Fust G. Strong correlation between the complement-mediated antibody-dependent enhancement of HIV-1 infection and plasma viral load. AIDS (London, England) 1999;13:1841–1849. doi: 10.1097/00002030-199910010-00005. [DOI] [PubMed] [Google Scholar]
- Tan Y, Liu L, Luo P, Wang A, Jia T, Shen X, Wang M, Zhang S. Association between mannose-binding lectin and HIV infection and progression in a Chinese population. Molecular immunology. 2009;47:632–638. doi: 10.1016/j.molimm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Telen MJ, Green AM. The Inab phenotype: characterization of the membrane protein and complement regulatory defect. Blood. 1989;74:437–441. [PubMed] [Google Scholar]
- Thieblemont N, Haeffner-Cavaillon N, Haeffner A, Cholley B, Weiss L, Kazatchkine MD. Triggering of complement receptors CR1 (CD35) and CR3 (CD11b/CD18) induces nuclear translocation of NF-kappa B (p50/p65) in human monocytes and enhances viral replication in HIV-infected monocytic cells. J Immunol. 1995;155:4861–4867. [PubMed] [Google Scholar]
- Tweten RK. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun. 2005;73:6199–6209. doi: 10.1128/IAI.73.10.6199-6209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320:760–764. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- Welsh RM, Jr, Cooper NR, Jensen FC, Oldstone MB. Human serum lyses RNA tumour viruses. Nature. 1975;257:612–614. doi: 10.1038/257612a0. [DOI] [PubMed] [Google Scholar]
- Welsh RM, Jr, Jensen FC, Cooper NR, Oldstone MB. Inactivation of lysis of oncornaviruses by human serum. Virology. 1976;74:432–440. doi: 10.1016/0042-6822(76)90349-4. [DOI] [PubMed] [Google Scholar]
- Wu G, Chen T, Shahsafaei A, Hu W, Bronson RT, Shi GP, Halperin JA, Aktas H, Qin X. Complement regulator CD59 protects against angiotensin II-induced abdominal aortic aneurysms in mice. Circulation. 2010;121:1338–1346. doi: 10.1161/CIRCULATIONAHA.108.844589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Hu W, Shahsafaei A, Song W, Dobarro M, Sukhova GK, Bronson RR, Shi GP, Rother RP, Halperin JA, et al. Complement regulator CD59 protects against atherosclerosis by restricting the formation of complement membrane attack complex. Circulation research. 2009;104:550–558. doi: 10.1161/CIRCRESAHA.108.191361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhang C, Jia L, Wen C, Liu H, Wang Y, Sun Y, Huang L, Zhou Y, Song H. A novel approach to inhibit HIV-1 infection and enhance lysis of HIV by a targeted activator of complement. Virology journal. 2009;6:123. doi: 10.1186/1743-422X-6-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashina M, Ueda E, Kinoshita T, Takami T, Ojima A, Ono H, Tanaka H, Kondo N, Orii T, Okada N, et al. Inherited complete deficiency of 20-kilodalton homologous restriction factor (CD59) as a cause of paroxysmal nocturnal hemoglobinuria. The New England journal of medicine. 1990;323:1184–1189. doi: 10.1056/NEJM199010253231707. [DOI] [PubMed] [Google Scholar]
- Ying H, Ji X, Hart ML, Gupta K, Saifuddin M, Zariffard MR, Spear GT. Interaction of mannose-binding lectin with HIV type 1 is sufficient for virus opsonization but not neutralization. AIDS research and human retroviruses. 2004;20:327–335. doi: 10.1089/088922204322996563. [DOI] [PubMed] [Google Scholar]
- Yu Q, Yu R, Qin X. The good and evil of complement activation in HIV-1 infection. Cellular & molecular immunology. 2010 doi: 10.1038/cmi.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Saunders TL, Camper SA, Samuelson LC, Ginsburg D. Vitronectin is not essential for normal mammalian development and fertility. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:12426–12430. doi: 10.1073/pnas.92.26.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HF, Yan H, Stover CM, Fernandez TM, Rodriguez de Cordoba S, Song WC, Wu X, Thompson RW, Schwaeble WJ, Atkinson JP, et al. Antibody directs properdin-dependent activation of the complement alternative pathway in a mouse model of abdominal aortic aneurysm. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E415–E422. doi: 10.1073/pnas.1119000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Hu W, Qin X. The role of complement in the mechanism of action of rituximab for B-cell lymphoma: implications for therapy. Oncologist. 2008;13:954–966. doi: 10.1634/theoncologist.2008-0089. [DOI] [PubMed] [Google Scholar]