Abstract
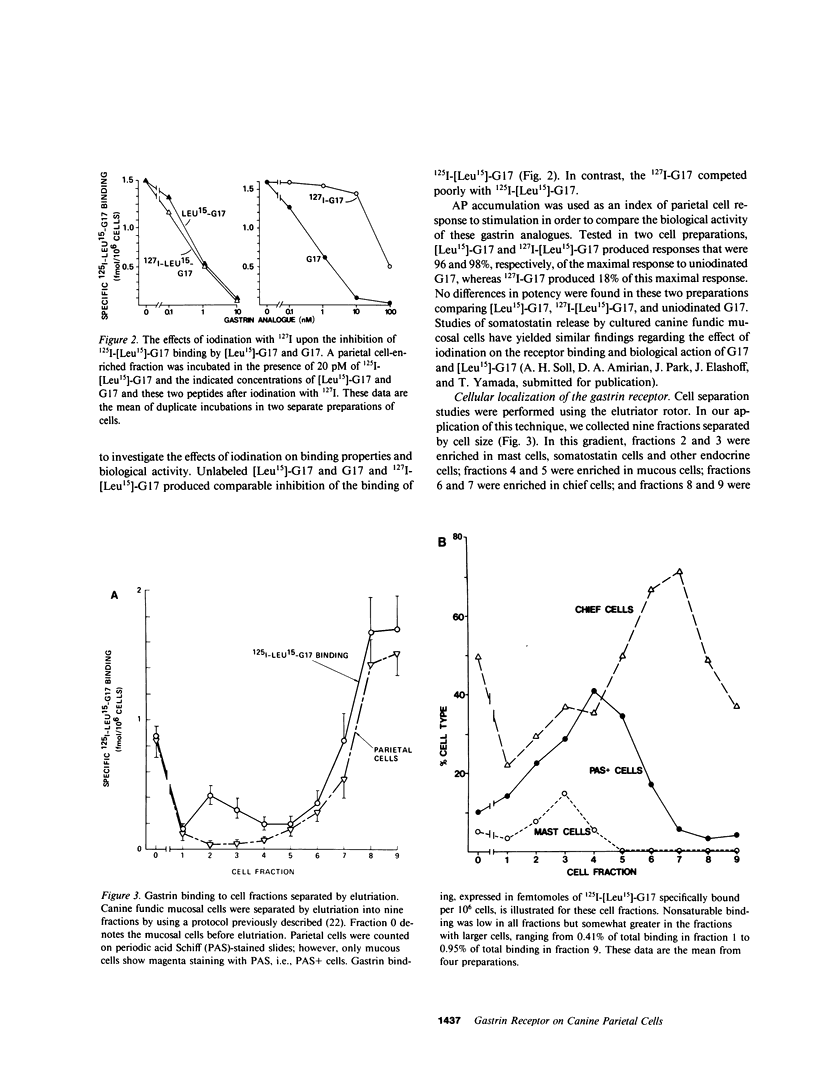
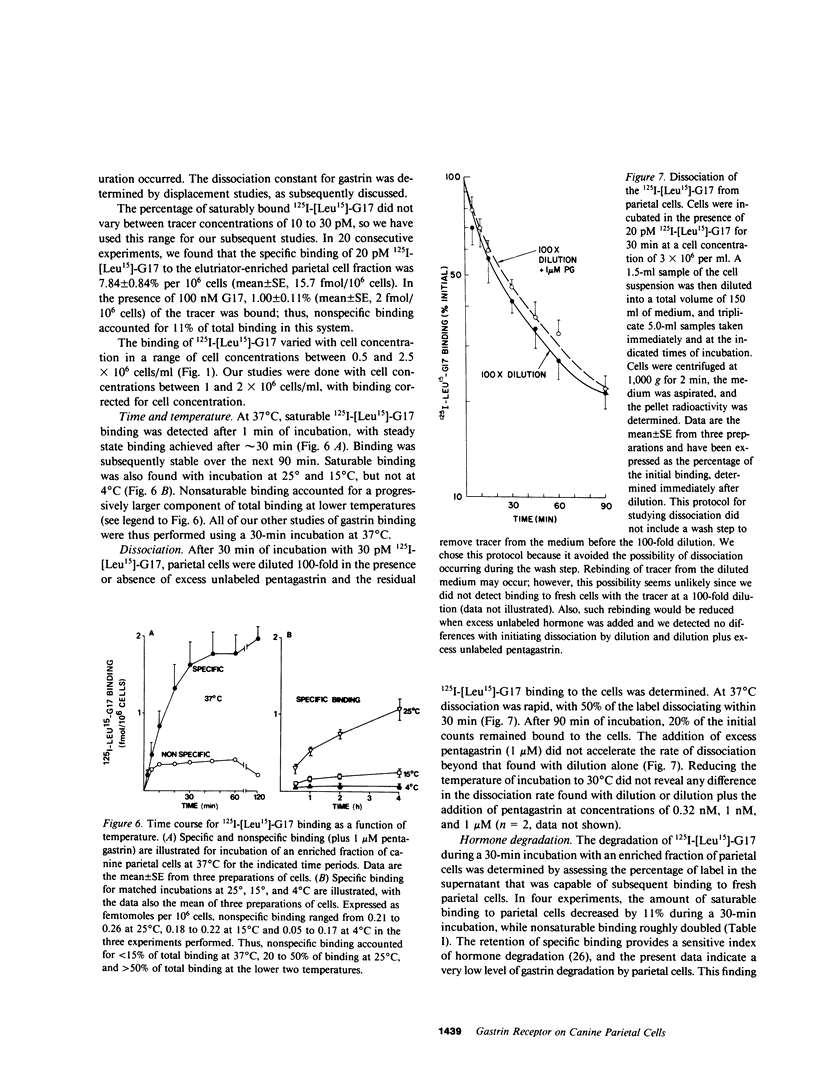
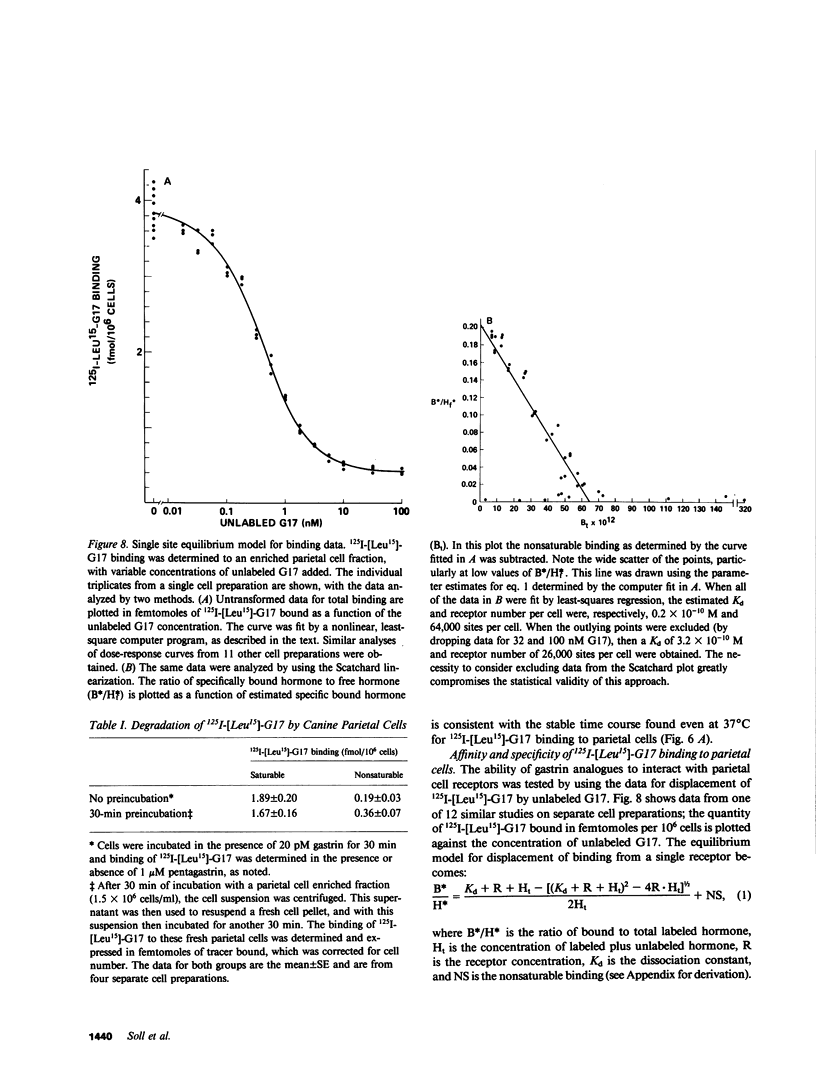
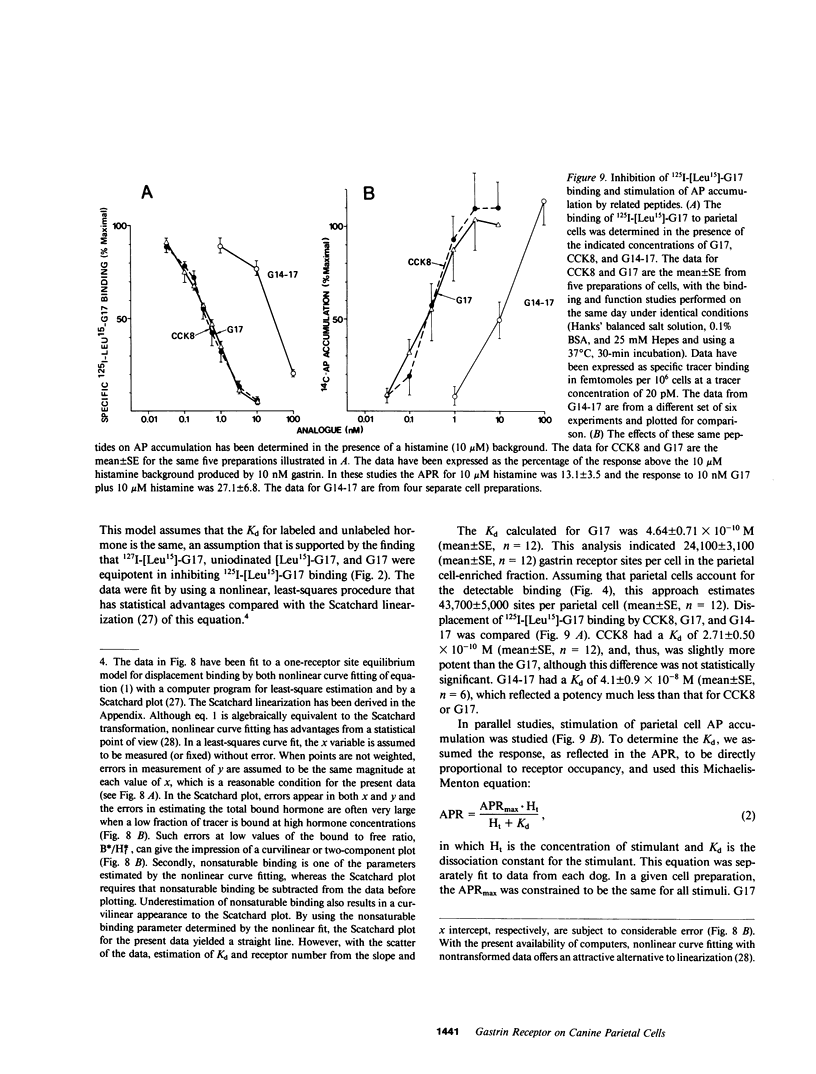
The receptors in the fundic mucosa that mediate gastrin stimulation of acid secretion have been studied. Synthetic human gastrin-17-I (G17) with a leucine substitution in the 15th position ( [Leu15]-G17) was iodinated by chloramine T; high saturable binding was found to enzyme-dispersed canine fundic mucosal cells. 127I-[Leu15]-G17, but not 127I-G17, retained binding potency and biological activity comparable with uniodinated G17. Fundic mucosal cells were separated by size by using an elutriator rotor, and specific 125I-[Leu-15]-G17 binding in the larger cell fractions was highly correlated with the distribution of parietal cells. There was, however, specific gastrin binding in the small cell fractions, not accounted for by parietal cells. Using sequential elutriation and stepwise density gradients, highly enriched parietal and chief cell fractions were prepared; 125I-[Leu15]-G17 binding correlated positively with the parietal cell (r = 0.98) and negatively with chief cell content (r = -0.96). In fractions enriched to 45-65% parietal cells, specific 125I-[Leu15]-G17 binding was rapid, reaching a steady state at 37 degrees C within 30 min. Dissociation was also rapid, with the rate similar after 100-fold dilution or dilution plus excess pentagastrin. At a tracer concentration from 10 to 30 pM, saturable binding was 7.8 +/- 0.8% per 10(6) cells (mean +/- SE) and binding in the presence of excess pentagastrin accounted for 11% of total binding. G17 and carboxyl terminal octapeptide of cholecystokinin (26-33) were equipotent in displacing tracer binding and in stimulating parietal cell function ( [14C]aminopyrine accumulation), whereas the tetrapeptide of gastrin (14-17) had a much lower potency. Proglumide inhibited gastrin binding and selectively inhibited gastrin stimulation of parietal cell function. Canine parietal cells have specific receptors for gastrin that mediate stimulation of parietal cell function. Gastrin receptors were undetectable on chief cells, and yet present on another smaller mucosal cell(s).
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayalon A., Sanders M. J., Thomas L. P., Amirian D. A., Soll A. H. Electrical effects of histamine on monolayers formed in culture from enriched canine gastric chief cells. Proc Natl Acad Sci U S A. 1982 Nov;79(22):7009–7013. doi: 10.1073/pnas.79.22.7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur S., Bacon V. C. A specific gastrin receptor on plasma membranes of antral smooth muscle. Biochem Biophys Res Commun. 1976 Dec 20;73(4):928–933. doi: 10.1016/0006-291x(76)90210-2. [DOI] [PubMed] [Google Scholar]
- Berglindh T., Helander H. F., Obrink K. J. Effects of secretagogues on oxygen consumption, aminopyrine accumulation and morphology in isolated gastric glands. Acta Physiol Scand. 1976 Aug;97(4):401–414. doi: 10.1111/j.1748-1716.1976.tb10281.x. [DOI] [PubMed] [Google Scholar]
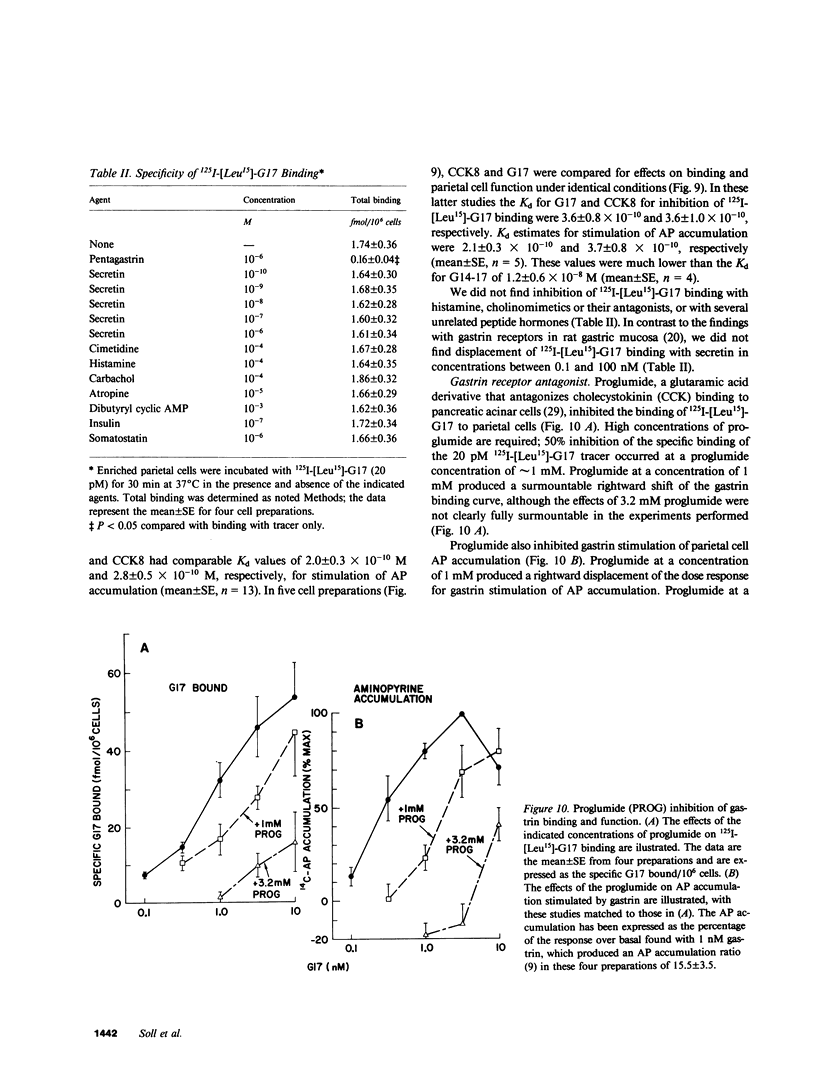
- Berglindh T., Sachs G., Takeguchi N. Ca2+-dependent secretagogue stimulation in isolated rabbit gastric glands. Am J Physiol. 1980 Aug;239(2):G90–G94. doi: 10.1152/ajpgi.1980.239.2.G90. [DOI] [PubMed] [Google Scholar]
- Bergqvist E., Obrink K. J. Gastrin-histamine as a normal sequence in gastric acid stimulation in the rabbit. Ups J Med Sci. 1979;84(2):145–154. doi: 10.3109/03009737909179150. [DOI] [PubMed] [Google Scholar]
- Bitar K. N., Makhlouf G. M. Receptors on smooth muscle cells: characterization by contraction and specific antagonists. Am J Physiol. 1982 Apr;242(4):G400–G407. doi: 10.1152/ajpgi.1982.242.4.G400. [DOI] [PubMed] [Google Scholar]
- Brown J., Gallagher N. D. A specific gastrin receptor site in the rat stomach. Biochim Biophys Acta. 1978 Jan 3;538(1):42–49. doi: 10.1016/0304-4165(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Chew C. S., Hersey S. J. Gastrin stimulation of isolated gastric glands. Am J Physiol. 1982 May;242(5):G504–G512. doi: 10.1152/ajpgi.1982.242.5.G504. [DOI] [PubMed] [Google Scholar]
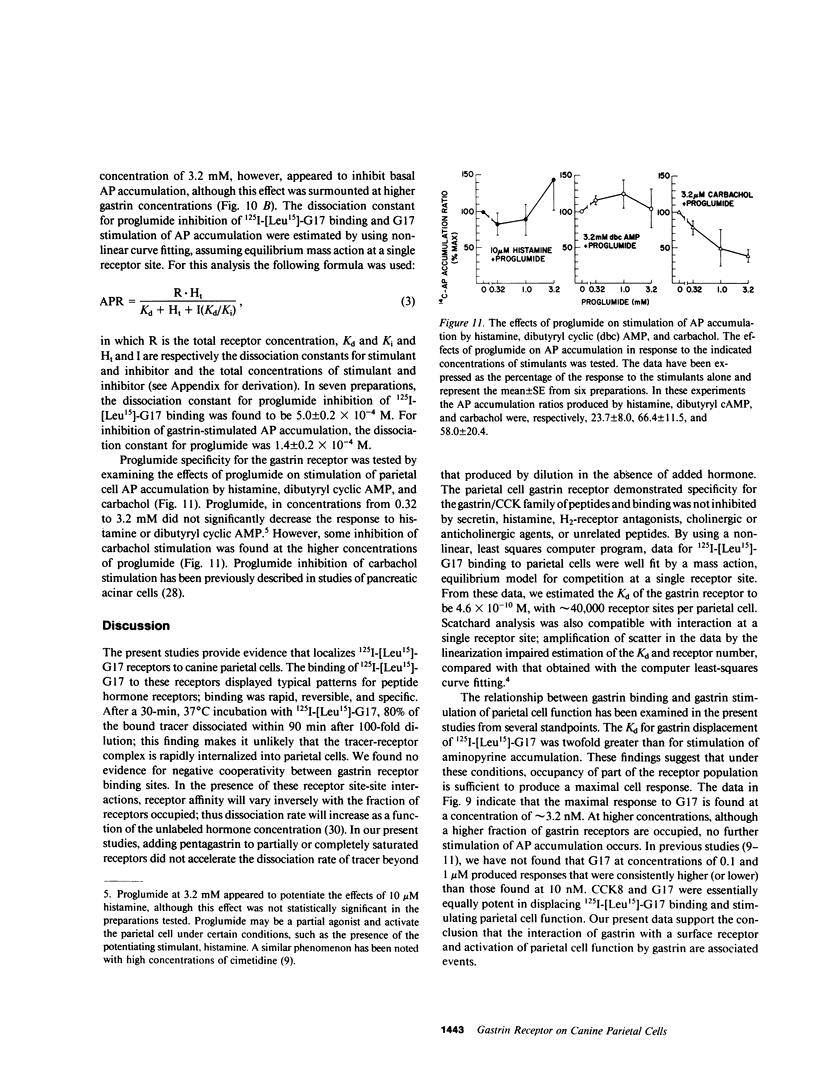
- Dale H. H., Laidlaw P. P. The physiological action of beta-iminazolylethylamine. J Physiol. 1910 Dec 31;41(5):318–344. doi: 10.1113/jphysiol.1910.sp001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMeyts P., Bainco A. R., Roth J. Site-site interactions among insulin receptors. Characterization of the negative cooperativity. J Biol Chem. 1976 Apr 10;251(7):1877–1888. [PubMed] [Google Scholar]
- Dockray G. J., Walsh J. H., Grossman M. I. Biological activity of iodinated gastrins. Biochem Biophys Res Commun. 1976 Mar 22;69(2):339–345. doi: 10.1016/0006-291x(76)90527-1. [DOI] [PubMed] [Google Scholar]
- Feldman M., Walsh J. H., Wong H. C., Richardson C. T. Role of gastrin heptadecapeptide in the acid secretory response to amino acids in man. J Clin Invest. 1978 Feb;61(2):308–313. doi: 10.1172/JCI108940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freychet P., Kahn R., Roth J., Neville D. M., Jr Insulin interactions with liver plasma membranes. Independence of binding of the hormone and its degradation. J Biol Chem. 1972 Jun 25;247(12):3953–3961. [PubMed] [Google Scholar]
- GREGORY R. A., TRACY H. J. THE CONSTITUTION AND PROPERTIES OF TWO GASTRINS EXTRACTED FROM HOG ANTRAL MUCOSA. Gut. 1964 Apr;5:103–114. [PMC free article] [PubMed] [Google Scholar]
- Guldvog I., Gedde-Dahl D., Berstad A. 'Non-active' pepsin secretion compared with stimulated secretion by bethanechol, histamine, pentagastrin, and 2-deoxy-D-glucose. The role of vagal innervation. Scand J Gastroenterol. 1980;15(8):939–948. doi: 10.3109/00365528009181795. [DOI] [PubMed] [Google Scholar]
- Hahne W. F., Jensen R. T., Lemp G. F., Gardner J. D. Proglumide and benzotript: members of a different class of cholecystokinin receptor antagonists. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6304–6308. doi: 10.1073/pnas.78.10.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersey S. J., May D., Schyberg D. Stimulation of pepsinogen release from isolated gastric glands by cholecystokininlike peptides. Am J Physiol. 1983 Feb;244(2):G192–G197. doi: 10.1152/ajpgi.1983.244.2.G192. [DOI] [PubMed] [Google Scholar]
- Hirschowitz B. I., Hutchison G. A. Kinetics of atropine inhibition of pentagastrin-stimulated H+, electrolyte, and pepsin secretion in the dog. Am J Dig Dis. 1977 Feb;22(2):99–107. doi: 10.1007/BF01072950. [DOI] [PubMed] [Google Scholar]
- Håkanson R., Lilja B., Owman C. Cellular localization of histamine and monoamines in the gastric mucosa of man. Histochemie. 1969 Apr 17;18(1):74–86. doi: 10.1007/BF00309904. [DOI] [PubMed] [Google Scholar]
- Jensen R. T., Lemp G. F., Gardner J. D. Interaction of cholecystokinin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2079–2083. doi: 10.1073/pnas.77.4.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlson G., Rosengren E. New approaches to the physiology of histamine. Physiol Rev. 1968 Jan;48(1):155–196. doi: 10.1152/physrev.1968.48.1.155. [DOI] [PubMed] [Google Scholar]
- Lewin M., Soumarmon A., Bali J. P., Bonfils S., Girma J. P., Morgat J. L., Fromageot P. Interaction of 3H-labelled synthetic human gastrin I with rat gastric plasma membranes. Evidence for the existence of biologically reactive gastrin receptor sites. FEBS Lett. 1976 Jul 15;66(2):168–172. doi: 10.1016/0014-5793(76)80495-4. [DOI] [PubMed] [Google Scholar]
- Magous R., Bali J. P., Moroder L., Previero A. Effect of Nin-formylation of the tryptophan residue on gastrin (HG-13) binding and on gastric acid secretion. Eur J Pharmacol. 1982 Jan 8;77(1):11–16. doi: 10.1016/0014-2999(82)90528-3. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Rovati A. L. Inhibition of gastric secretion by anti-gastrinic and H2-blocking agents. Scand J Gastroenterol Suppl. 1976;42:113–118. [PubMed] [Google Scholar]
- Saito A., Sankaran H., Goldfine I. D., Williams J. A. Cholecystokinin receptors in the brain: characterization and distribution. Science. 1980 Jun 6;208(4448):1155–1156. doi: 10.1126/science.6246582. [DOI] [PubMed] [Google Scholar]
- Sanders M. J., Amirian D. A., Ayalon A., Soll A. H. Regulation of pepsinogen release from canine chief cells in primary monolayer culture. Am J Physiol. 1983 Nov;245(5 Pt 1):G641–G646. doi: 10.1152/ajpgi.1983.245.5.G641. [DOI] [PubMed] [Google Scholar]
- Sankaran H., Goldfine I. D., Deveney C. W., Wong K. Y., Williams J. A. Binding of cholecystokinin to high affinity receptors on isolated rat pancreatic acini. J Biol Chem. 1980 Mar 10;255(5):1849–1853. [PubMed] [Google Scholar]
- Soll A. H., Lewin K. J., Beaven M. A. Isolation of histamine-containing cells from rat gastric mucosa: biochemical and morphologic differences from mast cells. Gastroenterology. 1981 Apr;80(4):717–727. [PubMed] [Google Scholar]
- Soll A. H., Lewin K., Beaven M. A. Isolation of histamine-containing cells from canine fundic mucosa. Gastroenterology. 1979 Dec;77(6):1283–1290. [PubMed] [Google Scholar]
- Soll A. H. Potentiating interactions of gastric stimulants on [14 C] aminopyrine accumulation by isolated canine parietal cells. Gastroenterology. 1982 Jul;83(1 Pt 2):216–223. [PubMed] [Google Scholar]
- Soll A. H. Secretagogue stimulation of [14C]aminopyrine accumulation by isolated canine parietal cells. Am J Physiol. 1980 Apr;238(4):G366–G375. doi: 10.1152/ajpgi.1980.238.4.G366. [DOI] [PubMed] [Google Scholar]
- Soll A. H. The actions of secretagogues on oxygen uptake by isolated mammalian parietal cells. J Clin Invest. 1978 Feb;61(2):370–380. doi: 10.1172/JCI108947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H. The interaction of histamine with gastrin and carbamylcholine on oxygen uptake by isolated mammalian parietal cells. J Clin Invest. 1978 Feb;61(2):381–389. doi: 10.1172/JCI108948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stening G. F., Grossman M. I. Gastrin-related peptides as stimulants of pancreatic and gastric secretion. Am J Physiol. 1969 Jul;217(1):262–266. doi: 10.1152/ajplegacy.1969.217.1.262. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Speir G. R., Johnson L. R. Mucosal gastrin receptor. I. Assay standardization and fulfillment of receptor criteria. Am J Physiol. 1979 Sep;237(3):E284–E294. doi: 10.1152/ajpendo.1979.237.3.E284. [DOI] [PubMed] [Google Scholar]
- Vidal y Plana R. R., Cifarelli A., Bizzarri D. Effects of antigastrin drugs on the interaction of 125I-human gastrin with rat gastric mucosa membranes. Hepatogastroenterology. 1980 Feb;27(1):41–47. [PubMed] [Google Scholar]
- Way L. W. Effect of cholecystokinin and caerulein on gastric secretion in cats. Gastroenterology. 1971 Apr;60(4):560–565. [PubMed] [Google Scholar]



