Abstract
Background
Sonchus arvesis is traditionally reported in various human ailments including hepatotoxicity in Pakistan. Presently we designed to assess the protective effects of methanolic extract of Sonchus arvesis against carbon tetrachloride induced genotoxicity and DNA oxidative damages in hepatic tissues of experimental rats.
Methods
36 male Sprague–Dawley rats were randomly divided into 6 groups to evaluate the hepatoprotective effects of Sonchus arvensis against CCl4 induced genotoxicity, DNA damages and antioxidant depletion. Rats of normal control group were given free access of food and water add labitum. Group II rats received 3 ml/kg of CCl4 (30% in olive oil v/v) via the intraperitoneal route twice a week for four weeks. Group III and IV received 1 ml of 100 mg/kg b.w. and 200 mg/kg b.w. SME via gavage after 48 h of CCl4 treatment whereas group V was given 1 ml of silymarin (100 mg/kg b.w.) after 48 h of CCl4 treatment. Group VI only received 200 mg/kg b.w. SME. Protective effects of SME were checked by measuring serum markers, activities of antioxidant enzymes, genotoxicity and DNA dmages.
Results
Results of the present study showed that treatment of SME reversed the activities of serum marker enzymes and cholesterol profile as depleted with CCl4 treatment. Activities of endogenous antioxidant enzymes of liver tissue homogenate; catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSHpx), glutathione-S-transferase (GST) and glutathione reductase (GSR) were reduced with administration of CCl4, which were returned to the control level with SME treatment. CCl4-induced hepatic cirrhosis decreased hepatic glutathione (GSH) and increased lipid peroxidative products (TBARS), were normalized by treatment with SME. Moreover, administration of CCl4 caused genotoxicity and DNA fragmentation which were significantly restored towards the normal level with SME.
Conclusion
These results reveal that treatment of SME may be useful in the prevention of hepatic stress.
Keywords: Sonchus arvensis, Carbon tetrachloride, Liver cirrhosis, Lipids peroxidation
Background
Carbon tetrachloride (CCl4), a clear, colorless, volatile, heavy and nonflammable industrial liquid, widely used to inducede free radical toxicity in various tissues of experimental animals such as liver, kidneys, heart, lung, testis, brain and blood [1]. CCl4 is converted through hepatic microsomal cytochrome P450 into trichloromethyl-free radical (∙CCl3 or ∙CCl3OO) [2] which in turn, initiate lipid peroxidation process [3, 4]. The most widely accepted mechanism of CCl4 induced hepatotoxicity is the formation of free radicals which is a rate limiting process in tissue peroxidative damage [5, 6]. This free radical and related reactive species may cause oxidative stress, which produces major interconnected changes of cellular metabolism, increases the serum marker enzymes, DNA fragmentation, and destruction of the cells by lipid peroxidation [7]. The accumulation of lipid peroxides introduces hydrophophilic moieties and alters membrane permeability and cell function which causes the loss of hepatic integrity and depressed hepatic function resulting in hepatotoxicity and congestive hepatic failure [8]. To protect the body from such deleterious effects of free radicals, several endogenous enzymatic and non enzymatic systems are provided, but when the formation of free radicals is excessive, additional protective mechanisms of dietary antioxidants may be of a great importance [9]. Maintaining the balance between reactive oxygen species and natural antioxidants is therefore crucial, and could serve as a major mechanism in preventing damage by oxidative stress induced by toxic agents. Cooperative defense systems that protect the body from free radical damage include the antioxidant nutrients and enzymes [10]. Antioxidant and radical scavengers have been used to study the mechanism of CCl4 toxicity as well as to protect tissue cells from CCl4 induced damage by breaking the chain of lipid peroxidation [11]. Numerous studies have shown that horticultural crops and fruits are sources of diverse antioxidant properties, which can protect body against CCl4, induced oxidative stress [12]. Sonchus arvensis is traditionally used in the treatment of kidney stone, gallstone, dysentri, haemorrhoid, gout arthritis, appendicitis, mastitis, hypertension, burn wound, and bruises. The present study was therefore designed to investigate the protective effect of Sonchus arvensis (SME) against CCl4 induced hepatotoxicity in rats.
Methods
Drugs and chemicals
Reduced glutathione (GSH), oxidized glutathione (GSSG), glutathione reductase, gamma-glutamyl p-nitroanilide, glycylglycine, bovine serum albumin (BSA), 1,2-dithio-bis nitro benzoic acid (DTNB), 1-chloro-2,4-dinitrobenzene (CDNB), reduced nicotinamide adenine dinucleotide phosphate (NADPH), CCl4, flavine adenine dinucleotide (FAD), glucose-6-phosphate, Tween-20, 2,6-dichlorophenolindophenol, thiobarbituric acid (TBA), picric acid, sodium tungstate, sodium hydroxide, trichloroacetic acid (TCA) and perchloric acid (PCA) were purchased from Sigma Chemicals Co. USA.
Animals and treatment
Six weeks old, 36 rats (200–210 g) were provided by National Institute of Health Islamabad and were kept in ordinary cages at room temperature of 25 ± 3°C with a 12 h dark/light cycles. They have free access to standard laboratory feed and water, according to the study protocol approved by Ethical Committee of University of Science and Technology Bannu, KPK, Pakistan. To study the hepatoprotective effects of SME, rats were equally divided into 6 groups (six rats). SME was administered after 48 h of CCl4 treatment for four weeks.
Group I: Control; standard diet and water
Group II: CCl4 (3 ml/kg b.w. i.p.)
Group III: CCl4 (3 ml/kg b.w. i.p.) + SME (100 mg/kg b.w. orally)
Group IV: CCl4 (3 ml/kg b.w. i.p.) + SME (200 mg/kg b.w. orally)
Group V: CCl4 (3 ml/kg b.w. i.p.) + Silymarin (100 mg/kg b.w. orally)
Group VI: SME (200 mg/kg b.w. orally) alone
After 24 h of the last treatment, all the animals were weighted, sacrificed, collected the blood while liver were removed, weighted and perfuse in ice-cold saline solution. Liver tissue was treated with liquid nitrogen for further studies.
Assessment of hepatotoxicity
Liver marker enzymes (alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (γ-GT), lipid profile (total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL) and triglyceride were estimated by using standard AMP diagnostic kits (Stattogger Strasse 31b 8045 Graz, Austria).
Assessment of oxidative stress
Hepatic tissue were homogenized in 10 volume of 100 mmol KH2PO4 buffer containing 1 mmol EDTA (pH 7.4) and centrifuged at 12,000 × g for 30 min at 4°C. The supernatant was collected and used for the assessment of antioxidant enzymes. Protein concentration in the supernatant of liver tissue homogenate was determined using crystalline BSA as standard. The entire chemicals used in enzymatic analysis were purchased form sigma.
Catalase assay (CAT)
CAT activities were determined by the method of Chance and Maehly [13] with some modifications. The reaction solution of CAT activities contained: 2.5 ml of 50 mmol phosphate buffer (pH 5.0), 0.4 ml of 5.9 mmol H2O2 and 0.1 ml enzyme extract. Changes in absorbance of the reaction solution at 240 nm were determined after one minute. One unit of CAT activity was defined as an absorbance change of 0.01 as units/min.
Superoxide dismutase assay (SOD)
SOD activity of liver tissue was estimated by the method of Kakkar et al. [14]. Reaction mixture of this method contained: 0.1 ml of phenazine methosulphate (186 μmol), 1.2 ml of sodium pyrophosphate buffer (0.052 mmol; pH 7.0), 0.3 ml of supernatant after centrifugation (1500 × g for 10 min followed by 10000 × g for 15 min) of homogenate was added to the reaction mixture. Enzyme reaction was initiated by adding 0.2 ml of NADH (780 μmol) and stopped after 1 min by adding 1 ml of glacial acetic acid. Amount of chromogen formed was measured by recording color intensity at 560 nm. Results were expressed in units/mg protein.
Glutathione-S-transferase assay (GST)
Glutathione-S-transferase activity was assayed by the method of Habig et al. [15]. The reaction mixture consisted of 1.475 ml phosphate buffer (0.1 mol, pH 6.5), 0.2 ml reduced glutathione (1 mmol), 0.025 ml (CDNB) (1 mmol) and 0.3 ml of homogenate in a total volume of 2.0 ml. The changes in the absorbance were recorded at 340 nm and enzymes activity was calculated as nmol CDNB conjugate formed/min/mg protein using a molar extinction coefficient of 9.6 × 103 M-1 cm-1.
Glutathione reductase assay (GSR)
Glutathione reductase activity was determined by method of Carlberg and Mannervik [16]. The reaction mixture consisted of 1.65 ml phosphate buffer: (0.1 mol; pH 7.6), 0.1 ml EDTA (0.5 mmol), 0.05 ml oxidized glutathione (1 mmol), 0.1 ml NADPH (0.1 mmol) and 0.1 ml of homogenate in a total volume of 2 ml. Enzyme activity was quantitated at 25°C by measuring disappearance of NADPH at 340 nm and was calculated as nmol NADPH oxidized/min/mg protein using molar extinction coefficient of 6.22 × 103 M-1 cm-1.
Glutathione peroxidase assay (GSH-Px)
Glutathione peroxidase activity was assayed by the method of Mohandas et al. [17]. The reaction mixture consisted of 1.49 ml phosphate buffer (0.1 mol; pH 7.4), 0.1 ml EDTA (1 mmol), 0.1 ml sodium azide (1 mmol), 0.05 ml glutathione reductase (1 IU/ml), 0.05 ml GSH (1 mmol), 0.1 ml NADPH (0.2 mmol), 0.01 ml H2O2 (0.25 mmol) and 0.1 ml of homogenate in a total volume of 2 ml. The disappearance of NADPH at 340 nm was recorded at 25°C. Enzyme activity was calculated as nmol NADPH oxidized/min/mg protein using molar extinction coefficient of 6.22 × 103 M-1 cm-1.
Reduced glutathione assay (GSH)
Reduced glutathione was estimated by the method of Jollow et al. [18]. 1.0 ml sample of homogenate was precipitated with 1.0 ml of (4%) sulfosalicylic acid. The samples were kept at 4°C for 1 h and then centrifuged at 1200 × g for 20 min at 4°C. The total volume of 3.0 ml assay mixture contained 0.1 ml filtered aliquot, 2.7 ml phosphate buffer (0.1 mol; pH 7.4) and 0.2 ml DTNB (100 mmol). The yellow color developed was read immediately at 412 nm on a SmartSpecTM plus Spectrophotometer. It was expressed as μmol GSH/g tissue.
Estimation of lipid peroxidation assay (TBARS)
The assay for lipid peroxidation was carried out by the modified method of Iqbal et al. [19]. The reaction mixture in a total volume of 1.0 ml contained 0.58 ml phosphate buffer (0.1 mol; pH 7.4), 0.2 ml homogenate sample, 0.2 ml ascorbic acid (100 mmol), and 0.02 ml ferric chloride (100 mmol). The reaction mixture was incubated at 37°C in a shaking water bath for 1 h. The reaction was stopped by addition of 1.0 ml 10% trichloroacetic acid. Following addition of 1.0 ml 0.67% thiobarbituric acid, all the tubes were placed in boiling water bath for 20 min and then shifted to crushed ice-bath before centrifuging at 2500 × g for 10 min. The amount of TBARS formed in each of the samples was assessed by measuring optical density of the supernatant at 535 nm using spectrophotometer against a reagent blank. The results were expressed as nmol TBARS/min/mg tissue at 37°C using molar extinction coefficient of 1.56 × 105 M-1 cm-1.
DNA fragmentation% assay
DNA fragmentation% assay was conducted using the procedure of Wu et al. [20] with some modifications. The tissue (50 mg) was homogenized in 10 volumes of a TE solution pH 8.0 (5 mmol Tris–HCl, 20 mmol EDTA) and 0.2% triton X-100. 1.0 ml aliquot of each sample was centrifuged at 27,000 × g for 20 min to separate the intact chromatin (pellet, B) from the fragmented DNA (supernatant, T). The pellet and supernatant fractions were assayed for DNA content using a freshly prepared DPA (Diphenylamine) solution for reaction. Optical density was read at 620 nm at (SmartSpecTM Plus Spectrophotometer catalog # 170–2525) spectrophotometer. The results were expressed as amount of % fragmented DNA by the following formula;
DNA ladder assay
DNA was isolated by using the methods of Wu et al. [20] to estimate DNA damages. 5 μg DNA of rats were separately loaded in 1.5% agarose gel containing 1.0 μg/ml ethidium bromide including DNA standards (0.5 μg per well). Electrophoresis was performed for 45 min at 100 Volt. After electrophoresis gel was studied under gel doc system and was photographed through digital camera.
AgNORs count
Silver staining technique was used according to the Trere et al. [21]. The AgNORs technique was performed on dried slides as follows; unstained fixed slides were dewaxed by dipping for 3 minutes in xylene. After complete removal of wax the slides were hydrated in decrease ethanol concentration (90, 70 and 50%) and washed in distilled water for 10 min and dried in an oven. After drying slides were treated with one drop of colloidal solution (2% gelatin and 1% formic acid) and two drops of 50% AgNO3 solution onto the slide and incubated at 35°C for about 8–12 min. The progressive staining was followed under microscope to get golden colored nuclei and brown/black NORs. Then, the slide was washed in distilled water, treated for 1 min with 1% sodium thiosulphate at room temperature to stop the reaction, and washed in tap water. The cells were examined under light microscope at 100 × magnification and number of AgNORs was counted per cell.
Statistical analysis
To determine the treatment effects, one-way analysis of variance was carried by computer software SPSS 13.0. Level of significance among the various treatments was determined by LSD at 0.05% and 0.01% level of probability.
Results
Treatment of CCl4 specifically targets the hepatocytes. CCl4 induced oxidative stress cause lesions in liver along with changes in the liver marker enzymes, biochemical markers and antioxidant defense enzymes and chemicals. The results obtained with CCl4 treatment and changes induced with SME are given below.
Body weight, liver weight
Treatment of CCl4 caused significant reduction (P < 0.01) in body weight while increased the absolute liver and relative liver weight comparatively to control group; were significantly (P < 0.01) restored with treatment of 10 mg/kg b.w., and 200 mg/kg b.w., SME (Table 1).
Table 1.
Effect of SME on body weight, liver weight and relative liver weight
| Treatment | % Increase in body weight | Liver weight (g) | Relative liver weight (% to body weight) |
|---|---|---|---|
| Control | 28.90 ± 2.17++ | 7.0 ± 0.83++ | 0.07 ± 0.002++ |
| 3 ml/kg CCl4 | 19.57 ± 3.02** | 9.6 ± 0.89** | 0.96 ± 0.006** |
| 100 mg/kg SME + CCl4 | 25.28 ± 1.51++ | 7.48 ± 0.70++ | 0.074 ± 0.002++ |
| 200 mg/kg SME + CCl4 | 27.14 ± 2.63++ | 7.14 ± 0.53++ | 0.071 ± 0.003++ |
| 100 mg/kg sylimarin + CCl4 | 27.01 ± 1.26++ | 7.22 ± 0.75++ | 0.072 ± 0.001++ |
| 200 mg/kg SME alone | 29.02 ± 2.49++ | 7.03 ± 0.67++ | 0.070 ± 0.006++ |
Mean ± SE (n = 6 number).
**indicate significance from the control group at P < 0.05 and P < 0.01 probability level.
++indicate significance from the CCl4 group at P < 0.05 and P < 0.01 probability level.
Lipids profile
Administration of CCl4 increased triglycerides, total cholesterol, LDL cholesterol while decreased the HDL cholesterol as shown in Table 2. Reduction of HDL cholesterol was significantly (P < 0.01) enhanced by SME while triglycerides, total cholesterol and LDL-cholesterol concentration was appreciably (P < 0.01) augmented to compensate the CCl4 group.
Table 2.
Effect of SME on liver markers enzymes
| Treatment | ALT(U/L) | AST(U/L) | ALP(U/L) | γ-GT(nM/min /mg protein) |
|---|---|---|---|---|
| Control | 45.8 ± 3.2++ | 53.8 ± 3.4++ | 148 ± 5.9++ | 105.5 ± 2.2++ |
| 3 ml/kg CCl4 | 102 ± 4.2** | 94.0 ± 4.7** | 340.3 ± 6.9** | 154.3 ± 3.2** |
| 100 mg/kg SME + CCl4 | 68 ± 3.8++ | 62.3 ± 4.9++ | 207.5 ± 4.9++ | 121.3 ± 3.4++ |
| 200 mg/kg SME + CCl4 | 91 ± 2.2++ | 56 ± 4.1++ | 167.7 ± 5.7++ | 108 ± 2.7++ |
| 100 mg/kg sylimarin + CCl4 | 89 ± 1.5++ | 57.5 ± 2.0++ | 157.8 ± 3.9++ | 110 ± 3.5++ |
| 200 mg/kg SME alone | 97 ± 2.4++ | 49.5 ± 3.6++ | 145.3 ± 5.1++ | 103 ± 2.7++ |
Mean ± SE (n = 6 number).
**indicate significance from the control group at P < 0.05 and P < 0.01 probability level.
++indicate significance from the CCl4 group at P < 0.05 and P < 0.01 probability level.
Genotoxicity studies
Exposure of CCl4 elicited the hepatic DNA damages (%fragmentation), number of AgNORs/cell. Treatment of rats with 100 mg/kg b.w. and 200 mg/kg b.w. SME restored the level of these markers (Table 3). DNA ladder assay showed conformity to the DNA fragmentation assay (Figure 1).
Table 3.
Effect of SME on liver markers enzymes
| Treatment | AgNORS (NORs/cell) | %DNA fragmentation |
|---|---|---|
| Control | 2.0 ± 0.33++ | 5.33 ± 2.46++ |
| 3 ml/kg CCl4 | 6.4 ± .29** | 22.50 ± 3.68** |
| 100 mg/kg SME + CCl4 | 3.1 ± 0.35*++ | 5.00 ± 1.83++ |
| 200 mg/kg SME + CCl4 | 3.5 ± 0.18**++ | 6.67 ± 2.08++ |
| 100 mg/kg sylimarin + CCl4 | 2.14 ± 0.23++ | 5.67 ± 3.12++ |
| 200 mg/kg SME alone | 1.9 ± 0.17**++ | 4.67 ± 2.23++ |
Mean ± SE (n = 6 number).
**indicate significance from the control group at P < 0.05 and P < 0.01 probability level.
++indicate significance from the CCl4 group at P < 0.05 and P < 0.01 probability level.
Figure 1.
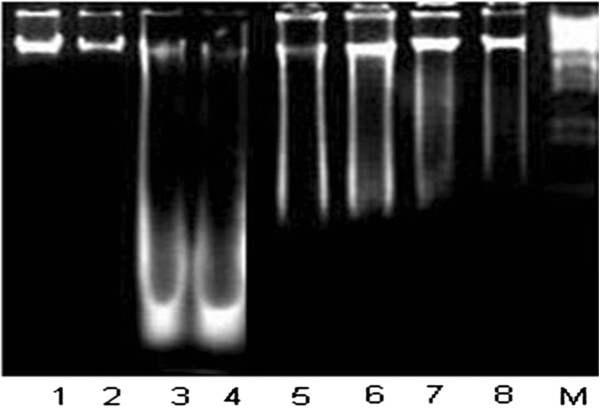
Protective effects of SME on DNA; Lane 1–2 (control), 3–4 (CCl4 treated rats), 5,6 (CCl4 + 100 mg/kg b.w. SME), 7,8 (CCl4 + 200 mg/kg b.w. SME).
Liver function profile
Administration of CCl4 markedly increased (P < 0.01) the activity of liver serum marker enzymes such as AST, ALT, ALP and γ-GT as compared with the control group. Elevations in the secretion of these enzymes were significantly decreased (P < 0.01) by 100 mg/kg b.w. and 200 mg/kg b.w. SME as compared with the CCl4 group are shown in Table 4.
Table 4.
Effect of SME on liver cholesterol profile
| Treatment | TG (mg/dl) | TC (mg/dl) | HDL (mg/dl) | LDL (mg/dl) |
|---|---|---|---|---|
| Control | 7.8 ± 0.45++ | 6.1 ± 0.25++ | 3.6 ± 0.21++ | 2.48 ± 0.32++ |
| 3 ml/kg CCl4 | 11.3 ± 0.58** | 11.2 ± 0.23** | 2.8 ± 0.18** | 8.4 ± 0.17** |
| 100 mg/kg SME + CCl4 | 8.5 ± 0.44++ | 5.7 ± 0.20**++ | 3.2 ± 0.23++ | 2.52 ± 0.28++ |
| 200 mg/kg SME + CCl4 | 9 ± 0.41**++ | 7.7 ± 0.21**++ | 3.08 ± 0.09++ | 4..2 ± 0.21**++ |
| 100 mg/kg sylimarin + CCl4 | 8.3 ± 0.18++ | 6.4 ± 0.27++ | 3.5 ± 0.20++ | 2..53 ± 0.35++ |
| 200 mg/kg SME alone | 7.2 ± 0.44++ | 5.7 ± 0.19++ | 3.7 ± 0.21++ | 2.21 ± 0.31++ |
Mean ± SE (n = 6 number).
**indicate significance from the control group at P < 0.05 and P < 0.01 probability level.
++indicate significance from the CCl4 group at P < 0.05 and P < 0.01 probability level.
Assessment of oxidative stress
CCl4 treatment in rats significantly decreased (P < 0.01) the activity of CAT, SOD, GST, GSH-Px, GSR, GSH while increased TBARS contents. The increase of lipid peroxidation caused; reduction in the activities of antioxidant enzymes and glutathione (GSH) contents were markedly attenuated (P < 0.01) by administration of 100 mg/kg and 200 mg/kg b.w. of SME in intoxicated rats (Table 5).
Table 5.
Effect of SME on antioxidant profile
| Treatment | CAT (U/min ) | SOD (U/mg protein) | GSH-Px nM/min/mg protein | GSH (μM /min/mg protein) | GSR nM/min/mg protein) | TBARS(nM /min/mg protein) |
|---|---|---|---|---|---|---|
| Control | 6.0 ± 0.5++ | 18.7 ± 2.8++ | 64.7 ± 3.9++ | 2.12 ± 0. 2++ | 121.7 ± 6.4++ | 29.3 ± 1.2++ |
| 3 ml/kg CCl4 | 2.9 ± 0.6** | 9.9 ± 0.7** | 34.2 ± 6.3** | 1.03 ± 0.3** | 67.3 ± 3.5** | 53.17 ± 1.2** |
| 100 mg/kg SME + CCl4 | 5.0 ± 0.7++ | 16.5 ± 0.7++ | 52.4 ± 7.8++ | 1.90 ± 0.1++ | 111.2 ± 12.4++ | 38.7 ± 2.6++ |
| 200 mg/kg SME + CCl4 | 5.8 ± 0.9++ | 17.5 ± 0.8++ | 62.7 ± 5.6 | 2.03 ± .07 | 122.33 ± 5.28++ | 31.17 ± 1.4++ |
| 100 mg/kg sylimarin + CCl4 | 5.7 ± 0.5++ | 19.4 ± 0.3++ | 60.2 ± 5.3++ | 2.17 ± 0.04++ | 115.3 ± 9.14+ | 30.0 ± 2.7++ |
| 200 mg/kg SME alone | 5.9 ± 0.6++ | 20.9 ± 0.5++ | 66.8 ± 3.3++ | 2.09 ± 0.2++ | 120.2 ± 6.3++ | 31.2 ± 2.7++ |
Mean ± SE (n = 6 number).
**indicate significance from the control group at P < 0.01 probability level.
++indicate significance from the CCl4 group at P < 0.01 probability level.
Discussion
Metabolism of various metabolites and exogenous toxic chemicals (pesticides, drugs, metals), are takes place inside the hepatic tissue causes the formation of free radicals which may be extensively toxic than the parent compound. CCl4, an extensively studied hepatotoxin is converted into its metabolites such as CCl3 radicals which are involved in the liver pathogenesis including cirrhosis, genotoxicity of hepatic tissue and hepatic carcinoma [8]. Our present results showed that exposure of rats to CCl4 caused significant increase in the secretion of ALT, AST, ALP, γ-GT and cholesterol profile due to hepatic injuries caused by their free radicals [22]. Co-administration of 100 mg/kg and 200 mg/kg b.wSME significantly improved the pathogenesis of liver, might be due to the presence of poly phenolic constituent as was reported by Xiao et al. [23]. SME might have the ability to chealat free radical which in turn lowering serum cholesterol, triglycerides and lipid peroxide were reported in other investigations while working on hepatoprotective effects of plant extract against CCl4 induced hepatic injury in rats [24, 25]. Super oxide dismutase and catalase are the main antioxidant enzymes which play an important role in oxidative dysfunction against free radicals induced oxidative stress. Results of our investigation showed that CCl4 administration in rats result in depletion of antioxidant activities of SOD and CAT, which is in close relationship with other reports [26, 27] and have an agreement with investigation following CCl4 intoxication [28]. GSH is an important protein thiol which coordinates body defense system against oxidative stress. GSH effectively scavenge free radicals and other reactive oxygen species (e.g., hydroxyl radical, lipid peroxy radical, peroxy nitrite and H2O2) directly or through GSHpx, GST and GSR [29]. Present study revealed that induction of CCl4 caused significant reduction in GSH contents as well as significant depletion in the activity of phase II metabolizing enzymes; GSH-px, GST and GSR [30]. Co-treatment of SME in rats markedly improved the activity of metabolizing enzymes as mentioned in literature. TBARS is a major reactive aldehyde resulting during the peroxidation of polyunsaturated fatty acids (PUFA) a useful indicator of oxidative damages [31–34]. Results revealed that 100 mg/kg and 200 mg/kg b.w. SME significantly improved lipid peroxidation products as was altered by treatment of CCl4 in rats, which has been well documented [35]. According to Marnett [36] the product of lipid peroxidation react with DNA to form adducts MIG, the mutagenic pirimedopurinone adduct of deoxyguanosine. Like other macromolecules such as lipids and proteins, nucleic acids are also attacked by free radicals to cause oxidative DNA damage. In the present study, carbon tetrachloride degrades the DNA of liver tissue of rats by generating free radicals. On the other hand, co-treatment of SME appreciably reduced the DNA fragmentation% which also exposed by DNA ladder assay banding pattern. Similar results were reported by Murugesan et al. [37] while studying the protective effects of Kombucha tea against CCl4 induced oxidative stress in kidneys of rats.
Conclusion
These results demonstrate that administration of SME may be useful in the treatment and prevention of hepatic genotoxicity and oxidative stress.
Acknowledgement
We are thankful to Prof. Dr. Muhammad Rashid Khan for supervision of the whole research work.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RAK (ORCID ID: 0000-0003-0453-2090) made a significant contribution to acquisition of and analyses of data and drafting of the manuscript. MRK, SS and HMA made a substantial contribution to the conception and design of the study, interpretation of data, as well as drafting and revising of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Huda Mohammad Alkreathy, Email: halkreathy@gmail.com.
Rahmat Ali Khan, Email: Rahmatgul_81@yahoo.com.
Muhammad Rashid Khan, Email: mrkhanqau@yahoo.com.
Sumaira Sahreen, Email: sumairasahreen@gmail.com.
References
- 1.Khan RA, Khan MR, Sahreen S. Evaluation of Launaea procumbens use in renal disorders: a rat model. J Ethanopharmacol. 2010;128:452–461. doi: 10.1016/j.jep.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 2.Preethi KC, Kuttan R. Hepato and reno protective action of Calendula officinalis L. flower extract. Exp Biol. 2009;47:163–168. [PubMed] [Google Scholar]
- 3.Adewole SO, Salako AA, Doherty OW, Naicker T. Effect of melatonin on carbon tetrachloride-induced kidney injury in Wistar rats. Afr J Biomed Res. 2007;10:153–164. [Google Scholar]
- 4.Adewole SO, Ojo SK, Adenowo TK, Salako AA, Naicker T, Ojewole JAO. Effects of Ficus exasperata Vahl. (Moraceae) leaf aqueous extract on the renal function of streptozotocin-treated rats. Folia Morphologica. 2012;71:1–9. [PubMed] [Google Scholar]
- 5.Sahreen S, Khan MR, Khan RA. Hepatoprotective effects of methanol extract of Carissa opaca leaves on CCl4-induced damage in rat. BMC Compl Alternative Med. 2011;11:48. doi: 10.1186/1472-6882-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan RA, Khan MR, Sahreen S: Protective effect ofSonchus asperextracts against experimentally-induced lung injuries in rats: a novel study.Exp Toxicol Pathol doi:10.1016/j.etp.2011.01.007 [DOI] [PubMed]
- 7.Bhadauria M, Nirala KS, Shukla S. Multiple treatment of Propolis ameliorates carbon tetrachloide induced liver injuries in rats. Food Chem Toxicol. 2008;46:2703–2712. doi: 10.1016/j.fct.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Khan RA, Khan MR, Sahreen S. CCl4-induced hepatotoxicity: protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat. BMC Compl Alternative Med. 2012;12:178. doi: 10.1186/1472-6882-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tirkey NG, Kaur G, Vij K, Chopra K. Hesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol. 2005;5:15–21. doi: 10.1186/1471-2210-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sreelatha S, Padma PR, Umadevi M: Protective effects ofCoriandrum sativumextracts on CCl4- induced hepatotoxicity in rats.Food Chem Toxicol doi:10.1016/j.fct.2009.12.022 [DOI] [PubMed]
- 11.Weber LW, Boll M, Stampfl M. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Revw Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 12.Ogeturk M, Kus I, Colakoglu N, Zararsiz I, Ilhan N, Sarsilmaz M. Caffeic acid phenethyl ester protects kidneys against carbon tetrachloride toxicity in rats J. Ethnopharmacol. 2005;97:273–80. doi: 10.1016/j.jep.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Chance B, Maehly AC. Assay of catalase and peroxidases. Methods Enzymol. 1955;11:764–775. doi: 10.1016/S0076-6879(55)02300-8. [DOI] [Google Scholar]
- 14.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 15.Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 16.Carlberg I, Mannervik EB. Glutathione level in rat brain. J Biol Chem. 1975;250:4475–4480. [PubMed] [Google Scholar]
- 17.Mohandas J, Marshal JJ, Duggin GG, Horvath JS, Tiller DJ. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney. Possible implications in analgesic nephropathy. Biochem Pharmacol. 1984;33:1801–1807. doi: 10.1016/0006-2952(84)90353-8. [DOI] [PubMed] [Google Scholar]
- 18.Jollow DJ, Mitchell JR, Zampaglione N, Gillete JR. Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as a hepatotoxic metabolite. Pharmacol. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 19.Iqbal M, Sharma SD, Zadeh HR, Hasan N, Abdulla M, Athar M. Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate (Fe-NTA) mediated hepatic injury. Redox Rep. 1996;2:385–391. doi: 10.1080/13510002.1996.11747079. [DOI] [PubMed] [Google Scholar]
- 20.Wu B, Ootani A, Iwakiri R, Sakata Y, Fujise T, Amemori S, Yokoyama F, Tsunada S, Fujimoto K. T cell deficiency leads to liver carcinogenesis in Azoxymethane-treated rats. Exp Biol Med. 2005;231:91–98. doi: 10.1177/153537020623100111. [DOI] [PubMed] [Google Scholar]
- 21.Trere D, Zilbering A, Dittus D, Kim P, Ginsberg PC, Daskal I. AgNOR quantity in needle biopsy specimens of prostatic adenocarcinomas: correlation with proliferation state, Gleason score, clinical stage, and DNA content. Clin Mol Pathol. 1996;49:209–213. doi: 10.1136/mp.49.4.M209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng HL, Hu YY, Wang RP, Liu C, Liu P, Zhu DY. Protective actions of salvianolic acid on hepatocyte injured by peroxidation in vitro. World J Gastroenterol. 2000;6:402–404. doi: 10.3748/wjg.v6.i3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao-hui H, Liang-qi C, Xi-ling C, Kai S, Yun-jian L, Long-juan Z. Polyphenol epigallocatechin-3-gallate inhibits oxidative damage and preventive effects on carbon tetrachloride–induced hepatic fibrosis. Nutr Biochem. 2007;3:511–515. doi: 10.1016/j.jnutbio.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Zhou JR, Erdman JW., Jr Phytic acid in health and disease. Crit Rev Food Sci Nutr. 1995;35:495–508. doi: 10.1080/10408399509527712. [DOI] [PubMed] [Google Scholar]
- 25.Al-Shabanah OA, Alam K, Nagi MN, Al-Rikabi AC, Al-Bekairi AM. Protective effect of aminoguanidine, a nitric oxide synthetase inhibiter against CCl4 induced hepatotoxicity in mice. Life Sci. 2000;66:265–270. doi: 10.1016/S0024-3205(99)00589-5. [DOI] [PubMed] [Google Scholar]
- 26.Khan MR, Rizvi W, Khan GN, Khan RA, Shaheen S. Carbon tetrachloride induced nephrotoxicity in rat: protective role of Digera muricata. J Ethnopharmacol. 2009;122:91–99. doi: 10.1016/j.jep.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Khan RA, Daud A. Protective effects of Digera muricata (L.) Mart. on testis against oxidative stress of carbon tetrachloride in rat. Toxicol. 2009;47:1393–1399. doi: 10.1016/j.fct.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Manna P, Sinha M, Sil PC. Aqueous extract of Terminalia arjuna prevents carbon tetrachloride induced hepatic and renal disorders. BMC Compl Altern Med. 2007;6:33–37. doi: 10.1186/1472-6882-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yadav P, Sarkar S, Bhatnagar D. Action of Capparis deciduas against alloxan-induced oxidative stress and diabetes in rat tissues. Pharmacol Res. 1997;36:221–228. doi: 10.1006/phrs.1997.0222. [DOI] [PubMed] [Google Scholar]
- 30.Gumieniczek A. Effects of repaglinide on oxidative stress in tissues of diabetic rabbits. Diab Res Clin Pract. 2005;68:89–95. doi: 10.1016/j.diabres.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 31.Cheeseman KH. Mechanisms and effects of lipid peroxidation. Mol Aspects Med. 1993;14:191–197. doi: 10.1016/0098-2997(93)90005-X. [DOI] [PubMed] [Google Scholar]
- 32.Aleynick SI, Leo MA, Ma X, Aleynick MK. Polyenoyl phasphatidylcholine prevents CCl4 induced lipid peroxidation while C.S. it attenuates liver fibrosis. J Hepatol. 1997;27:554–561. doi: 10.1016/S0168-8278(97)80361-3. [DOI] [PubMed] [Google Scholar]
- 33.Janbaz KH, Gilani AH. Evaluation of protective potential of Artemisia maritima extract on acetaminophen and CCl4 induced liver damage. J Ethnopharmacol. 1955;47:43–47. doi: 10.1016/0378-8741(95)01252-9. [DOI] [PubMed] [Google Scholar]
- 34.Chandan BK, Sharma AK, Anand KK. Boerhaavia diffusa: a study of its hepatoprotective activity. J Ethnopharmacol. 1991;31:299–307. doi: 10.1016/0378-8741(91)90015-6. [DOI] [PubMed] [Google Scholar]
- 35.Maritim AC, Sanders RA, Watkins JB. Effects of α-lipoic acid on biomarkers of oxidative stress in streptozotocin-induced diabetic rats. J Nutr Biochem. 2003;14:288–294. doi: 10.1016/S0955-2863(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 36.Marnett JL. Oxyridicals and DNA damage. Carcinogenesis. 2000;21:61–70. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 37.Murugesan GS, Sathishkumar M, Jayabalan R, Binupriya AR, Swaminathan K, Yun SEZ. Hepatoprotective and curative properties of Kombucha tea against carbon tetrachloride-induced toxicity. J Microbiol Biotechnol. 2009;19:397–402. doi: 10.4014/jmb.0806.374. [DOI] [PubMed] [Google Scholar]
Pre-publication history
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1472-6882/14/452/prepub


