Summary
Background
Plant body plans arise by the activity of meristematic growing tips during development and radiated independently in the gametophyte (n) and sporophyte (2n) stages of the life cycle during evolution. Although auxin and its intercellular transport by PIN family efflux carriers are primary regulators of sporophytic shoot development in flowering plants, the extent of conservation in PIN function within the land plants and the mechanisms regulating bryophyte gametophytic shoot development are largely unknown.
Results
We have found that treating gametophytic shoots of the moss Physcomitrella patens with exogenous auxins and auxin transport inhibitors disrupts apical function and leaf development. Two plasma membrane-targeted PIN proteins are expressed in leafy shoots, and pin mutants resemble plants treated with auxins or auxin transport inhibitors. PIN-mediated auxin transport regulates apical cell function, leaf initiation, leaf shape, and shoot tropisms in moss gametophytes. pin mutant sporophytes are sometimes branched, reproducing a phenotype only previously seen in the fossil record and in rare natural moss variants.
Conclusions
Our results show that PIN-mediated auxin transport is an ancient, conserved regulator of shoot development.
Highlights
-
•
PIN proteins have polar plasma membrane localizations in the moss Physcomitrella
-
•
PIN-mediated auxin transport drives gametophytic shoot development in Physcomitrella
-
•
PIN-mediated auxin transport suppresses branching in Physcomitrella sporophytes
Shooting systems have undergone 450 million years of independent evolution in flowering plant sporophytes and bryophyte gametophytes. Bennett et al. show that PIN-mediated auxin transport regulates shoot development in both life cycle stages in a moss and identify potential roles for PIN proteins in the evolution of plant body plans.
Introduction
Land plants evolved from freshwater algae with a haploid-dominant life cycle in which meiosis occurred straight after fertilization, and the colonization of land around 450 million years ago was accompanied by the innovation of a multicellular diploid body [1, 2, 3, 4]. Complex morphologies diversified independently in both the haploid (gametophyte) and diploid (sporophyte) life cycle stages in different plant groups during evolution [4, 5]. Bryophytes comprise a basal, gametophyte-dominant grade [6, 7, 8] with widely divergent thalloid, filamentous or shoot-like gametophytic forms, and the sporophyte comprises a single stem capped in a sporangium [2, 9, 10].The emergence of the vascular plant clade was associated with a shift to sporophyte dominance, a suite of sporophytic innovations including branching, and a gradual reduction in gametophyte size [4, 11, 12, 13]. The mechanisms underpinning architectural diversification in each life cycle stage are unknown, but the shared genetic toolkit available to land plants implicates conserved developmental mechanisms [14, 15].
One major candidate for such a conserved mechanism is the regulated intercellular transport of the plant hormone, auxin [16]. Most of our understanding of the key contribution of auxin transport to meristem function and shoot architecture comes from studies in flowering plants [17]. Pharmacological treatments that disrupt auxin transport across the multicellular apical dome inhibit leaf initiation [18], and in Arabidopsis, mutations in the auxin efflux carrier PIN-FORMED1 (PIN1) gene cause similar defects [19]. Local application of auxin to naked apices is sufficient to induce leaf initiation, and such auxin maximum formation usually occurs as a result of the dynamic polar transport of auxin by PIN1 to foci on the meristem [18, 20, 21]. Distinct patterns of leaf initiation arise as a consequence of the self-organizing properties of the auxin transport system [22, 23]. Patterns of leaflet initiation [24], vein insertion in leaves [25], marginal ornamentation [26], and leaf growth [27] are similarly regulated by PIN-dependent auxin transport. Thus, PIN-mediated auxin transport acts as a major contributor to architectural diversity in flowering plants by modulating meristem function and leaf development.
Auxin transport assays and auxin transport inhibitor applications in the lycophyte Selaginella kraussiana have shown that auxin transport has conserved roles in sporophytic meristem function within the vascular plants [28, 29, 30, 31]. Several recent papers have considered the contributions of auxin and its transport to bryophyte development, using mosses as model systems [32, 33, 34, 35]. Bulk basipetal polar auxin transport has been demonstrated in moss sporophytes, and application of polar auxin transport inhibitors (PATIs) causes severe disruptions in development, resulting in the formation of multiple sporangia [32, 33]. These data suggest that polar auxin transport is a conserved regulator of sporophyte development, but the extent of conservation between the sporophyte and gametophyte generation is unclear. Although gametophytic auxin transport has been reported in ferns [36], mosses [37, 38], liverworts [39, 40], and charophyte algae [41], it has proved undetectable in the gametophytic shoots of mosses [32, 33]. As sporophytic and gametophytic shoots (gametophores) evolved independently, the convergent shoot morphologies of each generation could have arisen through the recruitment of distinct genetic pathways to regulate development in plant evolution [32, 33].
One hypothesis to account for the divergent auxin transport properties of sporophytic and gametophytic shooting systems in mosses is a divergence in PIN function between mosses and vascular plants or between generations in mosses. In Arabidopsis, PIN function depends on subcellular protein localizations; whereas PIN1–PIN4 and PIN7 (canonical PINs) are plasma membrane targeted and function in many developmental processes by regulating intercellular auxin transport, PIN5, PIN6, and PIN8 (noncanonical PINs) are ER targeted and are thought to regulate auxin homeostasis within cells [42, 43, 44]. The apparent functional divergence between canonical and noncanonical PINs reflects differences in protein structure between the two classes, and canonical PINs have a predicted intracellular domain with characteristic motifs involved in membrane targeting, which is greatly reduced in noncanonical PINs [45, 46]. The genome of the model moss Physcomitrella patens encodes four PIN proteins (PINA–PIND), whose localization has been assayed by heterologous expression assays in tobacco protoplasts. These suggested that PINA localizes to the ER and that PIND localizes in the cytosol, implying roles in intracellular auxin homeostasis rather than intercellular transport [34]. Although these data support the hypothesis that the absence of bulk basipetal auxin transport in moss gametophores could reflect a divergence in PIN function between mosses and flowering plants, they cannot account for the divergent auxin transport properties of moss sporophytes and gametophores. Furthermore, we have recently shown that vascular plant PIN proteins diversified from a single canonical ancestor and that three Physcomitrella PINs (PINA–PINC) have canonical structure, placing canonical PINs one likely ancestral type within the land plants [45]. The data above raise questions about the evolution of land plant PIN functions and the roles of auxin transport and PIN proteins in moss gametophore development.
Here, we show that Physcomitrella PINs are plasma membrane targeted and that PIN-mediated auxin transport regulates many aspects of gametophore development. pin mutants have greatly impaired fertility and striking sporophytic defects that are similar to published defects arising from treatment with auxin transport inhibitors. Our results show that PIN proteins are conserved auxin transport facilitators.
Results
Exogenously Applied Auxins Affect Meristem Function and Leaf Development
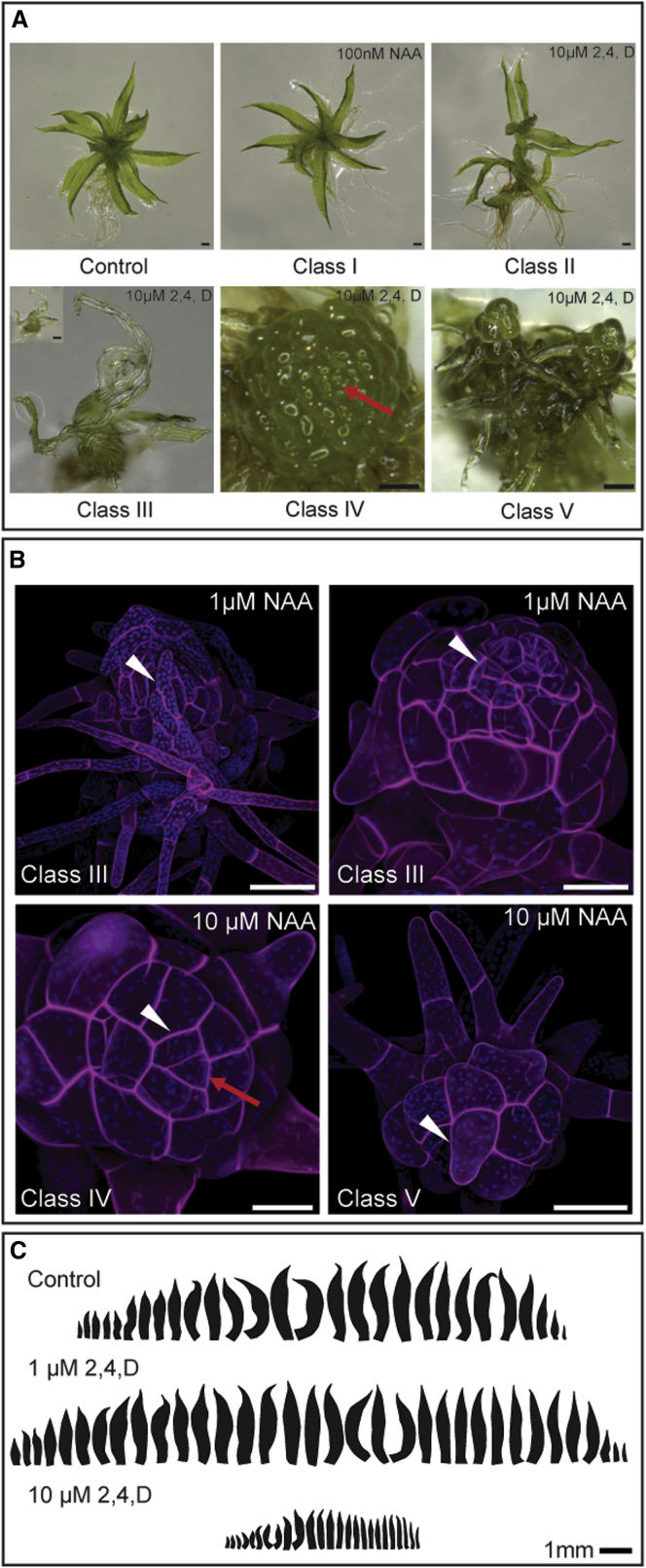
To clarify the roles of auxin in moss gametophore development, we grew colonies on medium supplemented with auxins that have different biochemical properties: indoleacetic acid (IAA), naphthylacetic acid (NAA), and 2,4-dichlorophenoxyacetic acid (2,4-D). Although weak effects were seen with the native auxin IAA (Figure S1 available online), a spectrum of phenotypes of lesser-to-greater severity was observed in treatments with NAA and 2,4-D and was classified into five phenotypic classes, classes I–V (Figures 1A and S1). An increased frequency of more-severe phenotypes correlated with increasing auxin concentrations (Figure S1C). When grown on lower auxin concentrations (e.g., 100 nM NAA, 1 μM 2,4-D), class I and class II shoots were prevalent. Class I shoots appeared similar to controls, but the zone of rhizoid emergence was displaced apically, as in previous reports [47, 48, 49]. Class II shoots (seen in 2,4-D treatments) were elongated and had more leaves than controls (Figures 1A, 1C, S1A, and S1D). Class III shoots were stunted, producing fewer leaves than untreated controls (Figures 1A, 1B, and S1D), and leaves were narrow with fewer, longer cells than untreated controls (Figures 1C, S1B, and S1D). In class IV shoots, leaf outgrowth was suppressed, and gametophores comprised a raspberry-like dome of cells above a zone of rhizoid emergence (Figure 1A). Confocal microscopy revealed a spiral of successively larger leaf progenitor cells emanating from the apical cell, thus demonstrating its continued activity (Figure 1B). The strongest effect of auxin was revealed in class V shoots, which lost apical cell function. Shoots terminated with irregularly shaped cells, or rhizoids, consistent with previous reports [47, 49] (Figure 1B). These data suggest that accumulation of auxin in shoots triggers diverse developmental effects at different threshold levels. Notably, auxin accumulation causes defects in meristem function, leaf initiation, and oriented leaf growth.
Figure 1.
Treatment with Auxins Perturbs Leaf Development and Can Cause Meristem Arrest
Plants were grown on BCD + ammonium tartrate (AT) medium for 3 weeks in continuous light at 23°C.
(A) Developmental defects arising as a result of auxin treatments. Scale bars in untreated control, class I and class II, and inset for class III represent 200 μm; scale bars in class IV and class V represent 100 μm. Red arrow indicates the apical cell.
(B) Confocal micrographs of class III–V buds showing severely stunted leaves (arrowheads in 1μM NAA treatments), a leaf progenitor cell and apical cell (arrowhead and arrow in class IV shoot), and a rhizoid terminating the shoot (arrowhead in class V shoot). Scale bars represent 50 μm.
(C) Leaf series of plants grown on different auxin treatments. 1 μM 2,4-D mildly promotes leaf initiation and development, whereas 10 μM 2,4-D inhibits leaf initiation and development relative to controls.
Treatment with Auxin Transport Inhibitors Phenocopies Auxin Treatment
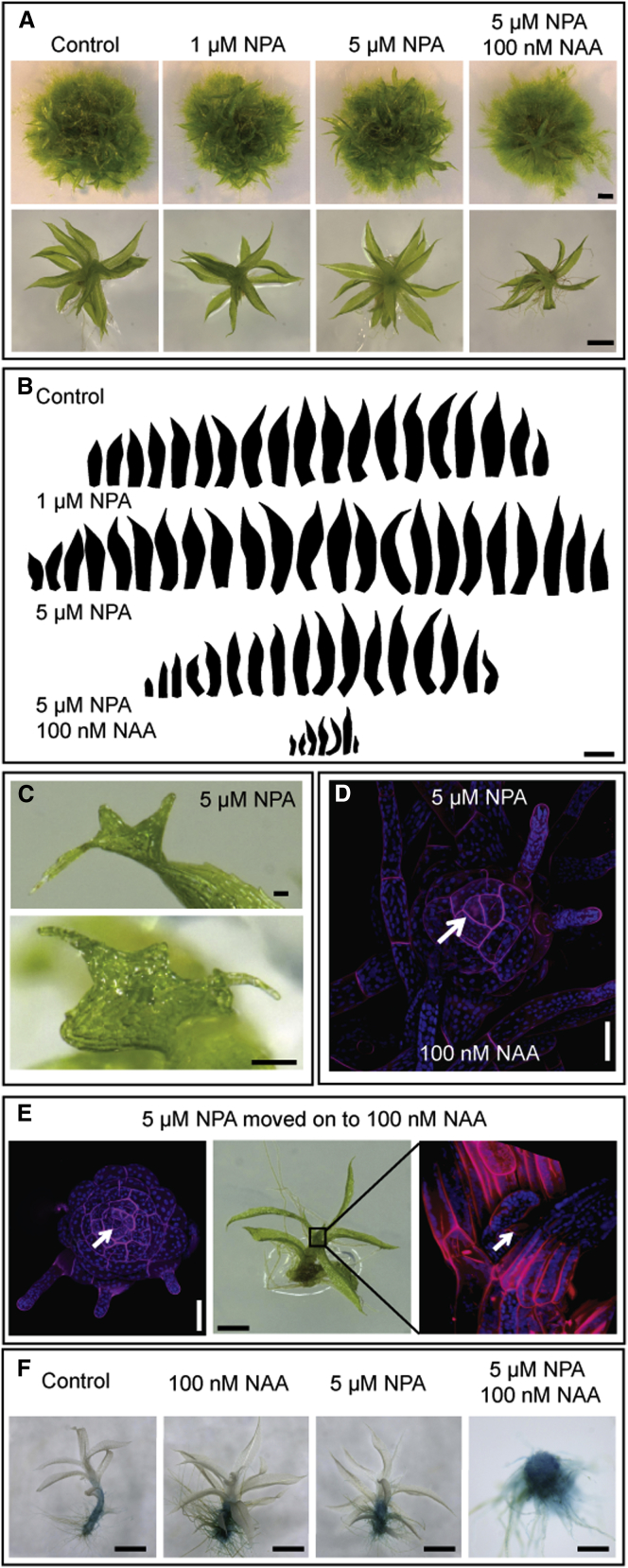
By analogy to flowering plants, we hypothesized that gametophore development is normally driven by changes in the auxin distribution within tissues, which was disrupted by adding exogenous auxin. We reasoned that such changes might occur by a conserved transport-dependent mechanism. To test this hypothesis, we analyzed the effect on gametophore development of the compounds 1-N-naphthylphthalamic acid (NPA) and naringenen (Nar), which are potent PATIs in angiosperms. Treatment with NPA caused mild developmental defects in leaves (Figure 2C), and both inhibitors had a similar effect to treatments with 2,4-D, which first promoted and then mildly suppressed leaf development (Figures 2A and 2B; Figure S2 in comparison to Figure 1; Figure S1D). However, class III–V phenotypes were not observed. Although the concentrations of NPA used here strongly inhibit auxin transport in Arabidopsis, the effect of PATIs is not well characterized in mosses, and we reasoned that our treatments might only partially inhibit auxin transport. We hypothesized that such partial inhibition might result in relatively mild phenotypes but might sensitize colonies to the addition of exogenous auxin. To test this hypothesis, we treated colonies with 5 μM NPA or Nar together with 100 nM NAA, which by itself only induces class I defects. These treatments gave rise to colonies with few visible gametophores that had class II and III defects (Figures 2A, 2B, S2B, and S2C): further investigation also revealed a number of class IV and V gametophores (Figures 2D and S2B). This response is similar to responses to higher concentrations of auxin applied alone, suggesting that transport normally relieves the effect of applying exogenous auxins.
Figure 2.
Pharmacological Polar Auxin Transport Inhibition Perturbs Leaf Development and Can Cause Meristem Arrest
Plants were grown on BCD + AT medium supplemented with auxin and transport inhibitors for 3 weeks in continuous light at 23°C.
(A) NPA treatment caused class I or II shoot defects, but used in combination with 100 nM NAA, it caused class III and IV defects. Scale bars represent 1 mm by row.
(B) Leaf series show that 1 μM NPA caused an increase in leaf number and size relative to untreated controls. 5 μM NPA mildly inhibits leaf initiation and development, and addition of 100 nM NAA strengthens the inhibition. The scale bar represents 1 mm.
(C) Treatment with 5 μM NPA caused mild perturbations to leaf development. Scale bars represent 100μm.
(D) Treatment with 5 μM NPA and 100 nM NAA generated class IV shoots. The scale bar represents 50 μM.
(E) If 100 nM NAA was added to plants treated with 5 μM NPA after 2 weeks, shoots that had already initiated arrested, revealing the apical cell (arrow). The scale bar represents 0.5 mm.
(F) Gametophores grown for 3 weeks on control medium and medium supplemented with 100 nM NAA or medium supplemented with 5 μM NPA, or both, were stained for β-glucuronidase activity.
Treatment with Auxin Transport Inhibitors Can Collapse Leaf Development and Meristem Function
The severity of class IV and V responses to auxin made it difficult to determine which aspects of development are disrupted. We therefore varied this treatment by allowing plants to form normal shoots while growing on 5 μM NPA for 2 weeks before adding 100 nM NAA. During the 2 weeks following auxin addition, gametophores underwent progressive developmental arrest. Recently initiated leaves toward the apex became shorter and more slender before initiation ceased, and the apical cell was exposed (Figure 2E). In conjunction with auxin treatments, which promoted or suppressed leaf initiation (Figure S1D), these data suggest that an appropriate auxin level is required for apical cell function and is attained by transport out of the apex.
Treatment with Auxin and Transport Inhibitors Alters the Distribution of a Marker for Auxin Response in Physcomitrella
The treatments with auxin and auxin transport inhibitors above suggest that the normal auxin distribution in moss gametophores is transport dependent. To evaluate this hypothesis, we analyzed the staining distribution pattern of an auxin-responsive GH3:GUS reporter [50] in untreated and pharmacologically treated plants (Figure 2F). As in previous reports [32, 50, 51, 52, 53, 54], untreated plants accumulated staining at the base of the shoot and in punctuated maxima at points of rhizoid initiation up the shoot. No staining was reproducibly detected in leaves. Treatment with 100 nM NAA increased the density of basal rhizoids and elevated the GUS staining intensity, a response that was phenocopied by treatment with 5 μM NPA. Plants that were grown on 5 μM NPA and 100 nM NAA and had class IV shoot defects accumulated stain at the shoot apex, supporting the inference that auxin transport maintains auxin levels at the apex to regulate its activity.
Physcomitrella PINs Are Plasma Membrane Localized
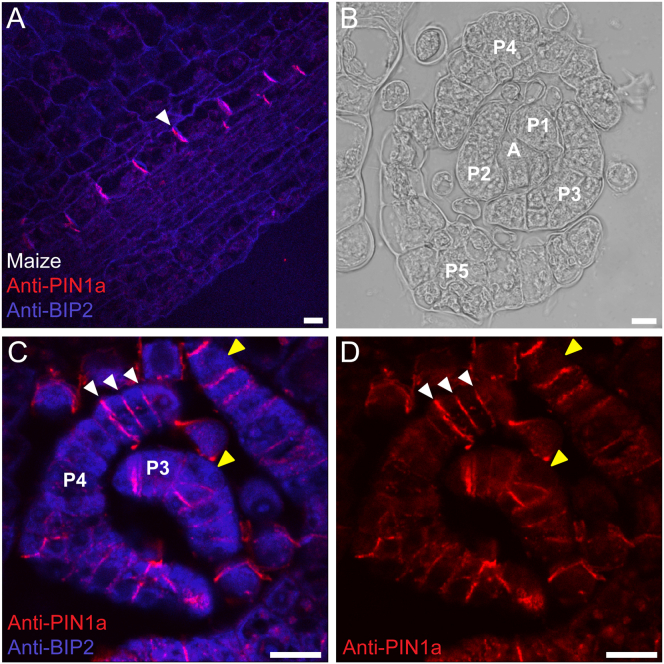
On the basis of the data above, we reasoned that the auxin distribution in gametophore apices and leaves might be PIN regulated. We therefore used an immunolocalization approach with transverse sections just above the apex to determine where Physcomitrella PINs localize (Figure 3B). We used antibodies raised in guinea pigs against residues 264–413 or 264–411 of maize PIN1-like variants PIN1a and PIN1b, respectively, and, as expected on the basis of published work [55], found that both antibodies gave strong polar plasma membrane-targeted signal in maize leaf sections used as a positive control (Figures 3A and S3). We used an antibody against an abundant ER-targeted protein, BIP2, as a control to test for ER colocalization. In our moss experiments, we found that the BIP2 signal (blue) localized broadly across the undifferentiated leaf tissues of P1–P5 (Figure 3C). In contrast, the PIN signal (red) was restricted mainly to narrow bands spanning the adaxial-abaxial leaf axis at the junctions between cells and did not colocalize with the BIP2 signal (Figures 3C and 3D). We also detected signal on the internal faces of cells around the presumptive midvein, but signal at the outermost cell edges was absent. Thus, Physcomitrella PINs are plasma membrane targeted, can polarize, and localize in tissues that are responsive to disruption of auxin levels.
Figure 3.
Physcomitrella PINs Are Plasma Membrane Targeted
(A) Maize anti-PIN1a antibodies detected a strong polar signal at the plasma membrane in developing maize leaves (arrowhead). The scale bar represents 17.5 μm.
(B) Physcomitrella leaves initiate in a spiral around the apical cell, and cell differentiation becomes apparent after P5. The scale bar represents 15 μm.
(C and D) Immunolocalization in Physcomitrella leaves showing that anti-BIP2 (blue) and anti-PIN (red) signals do not colocalize and that the PIN signal forms a transverse banding pattern across the youngest leaf primordia around the apex (white arrows). No signal was detected at the outer cell faces (yellow arrows). Scale bars represent 15 μm.
Physcomitrella pin Mutants Phenocopy Plants Treated with Auxin or Auxin Transport Inhibitors
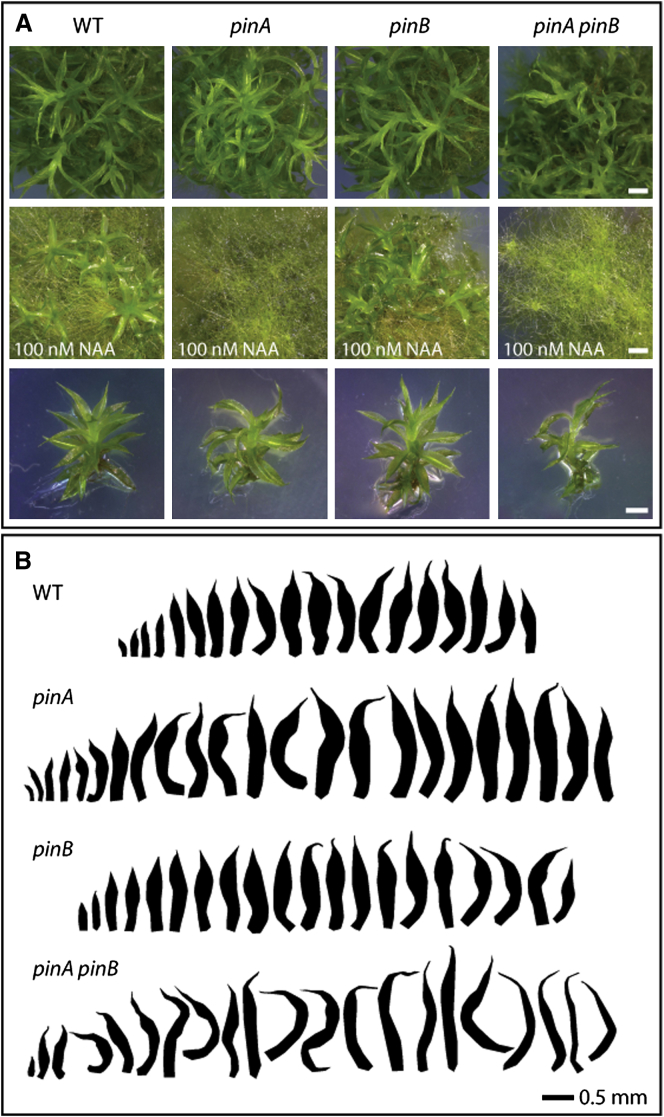
Physcomitrella PINs A–C are canonical and share many sequence motifs with Arabidopsis PIN1 in the central intracellular loop, whereas PIND is highly divergent [45], and PINA and PINB, but not PINC, were strongly expressed in gametophores (Figures S4A and S4B). Therefore, to analyze PIN function in Physcomitrella, we engineered targeted disruptants for PINA and PINB by homologous recombination [56] (Figures S4C–S4E). Several lines with the same phenotypes were recovered for each insertion, suggesting that mutant phenotypes were caused by lesions in targeted loci (Figure S4F). RT-PCR showed that disrupted PINA and PINB transcripts were present at low levels in pinA, pinB, and pinA pinB double mutants (Figures S4G and S4H), suggesting that the mutants may not be null. pinA and pinB single mutant shoots were not obviously different from wild-type (WT) (Figures 4A and 4B), but quantitative analysis showed that pinB gametophores were longer than WT (Figure S5). Double disruptants had class II shoot defects and defects in oriented leaf growth and cell division (Figures 4A and S5). pinA pinB double mutants therefore resemble plants treated with auxin (Figure S1), suggesting that they accumulate auxin as a result of a deficiency in auxin transport.
Figure 4.
Physcomitrella PIN Proteins Regulate Leaf Initiation and Development
Plants were grown on BCD + AT medium for 3 weeks in continuous light at 23°C.
(A) Whereas pinA and pinB mutants are not easily distinguished from WT, pinA pinB mutants have class II shoot defects. pinA mutants and pinA pinB mutants are sensitized to NAA. The scale bar represents 1 mm by row.
(B) Leaf series show subtle differences in leaf shape and size between WT and single mutants, whereas pinA pinB mutants have conspicuously irregularly shaped leaves that are longer and thinner than WT.
Physcomitrella pin Mutants Are Hypersensitive to Auxin
The pinA pinB double mutant phenotype comprises class II defects, but more-severe defects were not observed. We reasoned that this may be due to residual PINC activity or residual activity in other components of the auxin transport pathway, such as PGP or ABC transporters [57]. We also reasoned that if we had reduced the auxin transport capacity, mutants would be more sensitive to exogenous auxin treatment than WT plants. To test this hypothesis, we grew mutants on 100 nM NAA for 4 weeks. In pinA and pinA pinB mutants, this treatment generated gametophores with class III–V phenotypes (Figure 4A), phenocopying the effect of NAA and NPA cotreatment (Figure 2A). Our results suggest that PINA and PINB act redundantly to remove auxin from the apex and initiating leaves, allowing normal development to proceed. As shoot development is strongly affected in pinA single mutants treated with 100 nM NAA, but not in pinB mutants, we postulate that PINA plays the dominant role (Figure S4B). These data support the hypothesis that the apical auxin distribution in Physcomitrella regulates gametophore architecture and is modulated by PIN proteins.
A Marker for Auxin Response Is Redistributed in Physcomitrella pin Mutants
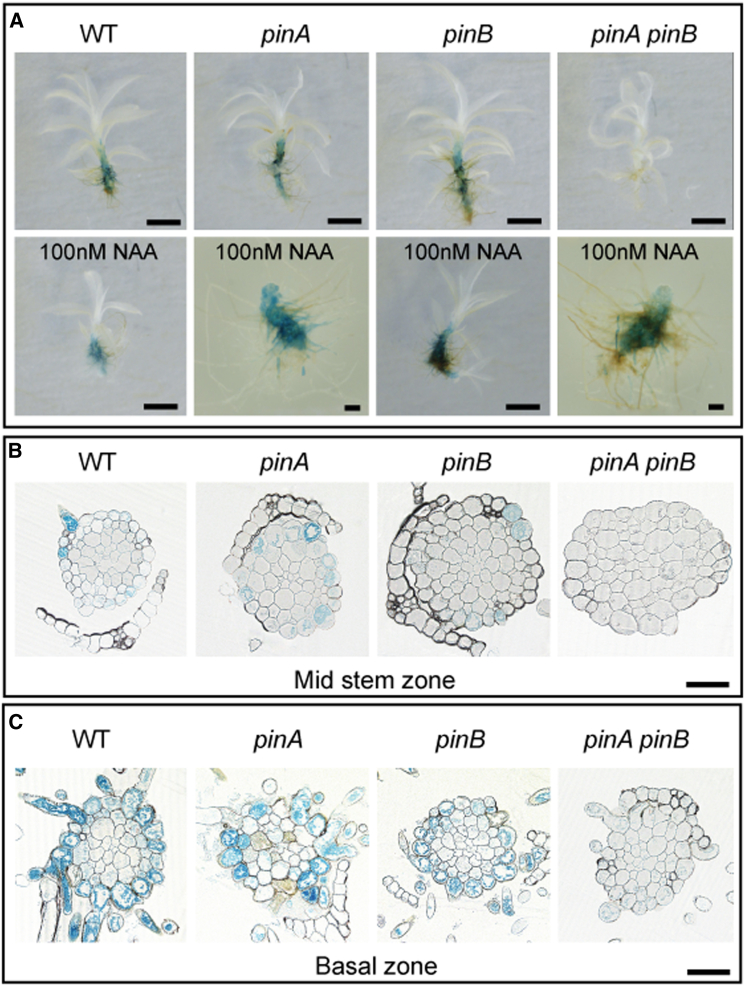
To further test the hypothesis that PIN proteins modulate the auxin distribution in Physcomitrella, we analyzed the staining distribution pattern of the GH3:GUS reporter [50] in WT and mutant plants (Figure 5A). In pinA and pinB single mutant shoots, staining was slightly stronger than in WT and displaced up the stem. In contrast, the staining intensity in pinA pinB mutants was strongly reduced with respect to WT and single mutants and, where present, was localized to the middle portion of the stem. Gametophores with the most-severe leaf phenotypes had the least signal and very few rhizoids initiated; no basal zone of rhizoid emergence was apparent (Figures 5A–5C). Transverse sections taken through the base and midstem region confirmed this inference, indicating a difference in the apical-basal auxin level and distribution as the main defect (Figures 5B and 5C). To test whether auxin-inducible phenotypic alterations to shoot development (Figure 3A) corresponded to an altered auxin response distribution, plants were grown on 100 nM NAA before staining. Whereas gametophores with a class I–III response showed only an upregulation in signal intensity, pinA and pinA pinB mutants with class IV and V phenotypes accumulated staining toward or at the apex (Figure 5A). These data support the hypothesis that PIN proteins modulate the auxin distribution in gametophores.
Figure 5.
A GH3:GUS Reporter Is Redistributed in pinA pinB Mutant Shoots
(A) GH3:GUS expression in the WT, pinA, pinB, and pinA pinB lines was assessed after 3 weeks of growth on control medium (top row) or medium supplemented with 100 nM NAA (bottom row). Gametophores were extracted and then stained for β-glucuronidase activity for 30 min. Scale bars (long) represent 1 mm; scale bars (short) represent 100 μm.
(B) Transverse sections through the midstem region showed a patterned distribution of epidermal staining in WT, pinA and pinB plants. In pinA pinB mutants, the staining intensity was much reduced. The scale bar (long) represents 1 mm
(C) Transverse sections through the basal region showed strong, evenly distributed epidermal staining in WT, pinA and pinB plants. In pinA pinB mutants, the staining intensity was much reduced or absent. The scale bar (long) represents 1 mm
Physcomitrella pin Mutants Have Disrupted Tropic Responses
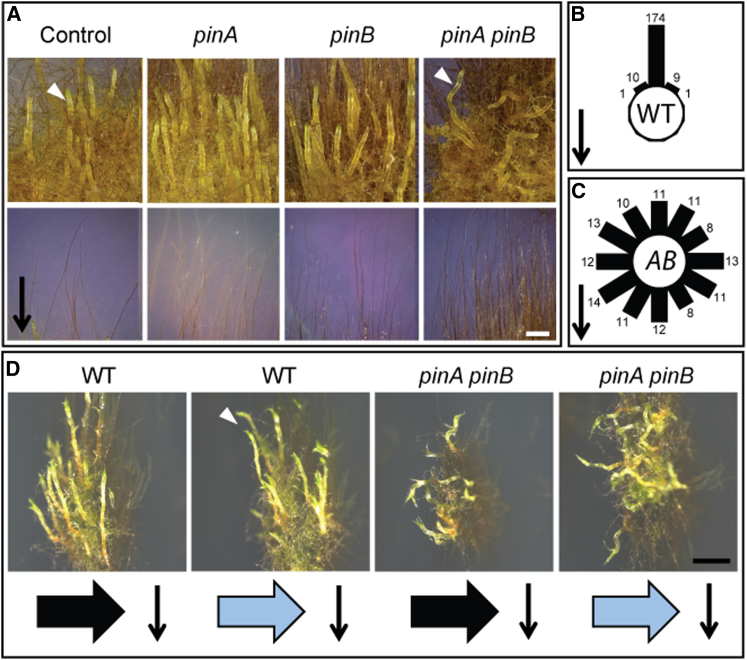
In angiosperms, PIN-mediated polar auxin transport drives phototropic and gravitropic responses in shoots and roots [58, 59]. Physcomitrella filaments and gametophores have strong negative gravitropism when grown in the dark [60]. Interestingly, moss mutants defective in filament gravitropism are not defective in shoot gravitropism, suggesting that two distinct tropism pathways may operate [60]. To assess a putative role for PIN-mediated auxin transport in gravitropism, we grew WT, single and double pin mutants for 2 weeks in the light and then grew them vertically in the dark on sucrose supplemented medium (0.5% w/v) for a further 2 weeks. In WT plants, this treatment induced a strong negative gravitropic response in both filaments and gametophores (Figures 6A–6C). Whereas pinA and pinB single mutants showed a normal gravitropic response, the pinA pinB double mutant had agravitropic gametophores. This result was phenocopied by treatment with 2,4-D (data not shown). To assess a putative role in phototropism, we grew plants as above but then exposed them to a unidirectional blue light stimulus for 24 hr. Whereas the tips of WT gametophores showed a clear reorientation toward the light stimulus (Figure 6D), pinA pinB colonies subjected to the same light stimulus continued to grow in a disoriented manner, showing no clear tropic growth toward the light stimulus (Figure 6D). These data suggest conservation of PIN-dependent, auxin transport-driven gravitropism and phototropism pathways between mosses and angiosperms and again highlight the importance of auxin transport-driven processes in Physcomitrella gametophore development.
Figure 6.
PIN Proteins Mediate Physcomitrella Shoot Tropism
Plants were grown on BCD + AT medium for 3 weeks horizontally in continuous light at 23°C before plates were wrapped in foil, oriented vertically, and allowed to grow for 2 more weeks.
(A) Whereas filaments reoriented away from the new gravity vector in all genotypes, shoot gravitopism was abolished in pinA pinB mutants. The scale bar represents 500 μm.
(B and C) For WT (B) and pinA pinB (C), the response was quantified by counting the number of shoot tips in 30° sectors relative to the gravity vector.
(D) Dark-grown colonies of WT and pinA pinB mutant plants were exposed to unidirectional blue light (blue arrows) for 24 hr to assess the phototropic response of etiolated gametophores. Whereas control shoots were kept in the dark and maintained their previous growth vectors (vertical arrows), WT shoots reoriented toward the light source (arrowhead). pinA pinB mutant shoots showed no obvious reorientation. The scale bar represents 500 μm.
Physcomitrella pin Mutants Have Disrupted Sporophyte Development
For reasons outlined in the introduction, this study has principally targeted recent controversy surrounding the roles of auxin transport in Physcomitrella gametophore development. However, as auxin transport has previously been detected in moss sporophytes and application of transport inhibitors perturbs sporophyte development [32], we also tested the hypothesis that PIN-mediated auxin transport regulates sporophyte development. We detected sporophytic expression of PINA and PINB (Figure S4B) and grew WT and pin mutant sporophytes to evaluate their phenotypes. Cultures were grown on four peat plugs in continuous light at 23°C for 6 weeks before transfer to a short-day 16°C regime for induction, and all the sporophytes present were harvested 4 weeks after induction. Whereas gametangia appeared normal (Figure 7A), PINA and PINB contributed synergistcially to fertility and development (Figures 7B and S6). Sporophytic defects were detected with variable penetrance: a low proportion (6 out of 208) on our GH3:GUS WT line had duplicated sporangia or dead sporophytes. Whereas pinA mutants had no obvious defects (1 out of 115 had duplicated sporangia; 3 out of 115 had an enlarged sporangium), a significant proportion of pinB mutants had duplicated sporangia (19 out of 89; 6 out of 89 were dead or had other defects), and around half of pinA pinB mutants had severe, sometimes lethal, developmental defects (5 out of 34 had duplicated sporangia; 7 out of 34 were dead or had other defects). The results suggest that PIN-mediated auxin transport regulates sporophytic shoot development, with a stronger contribution from PINB than from PINA.
Figure 7.
PIN Proteins Regulate Physcomitrella Sporophyte Development
Plants were grown on peat plugs in continuous light for 6 weeks at 23°C before transfer to a short-day regime at 16°C. All visible sporophytes were dissected out of gametophores after a further 4 weeks and photographed using a Keyence VHX-1000 microscope.
(A) Gametangium development appeared normal. Scale bars represent 75 μM.
(B) Gross phenotypic perturbations were rare in WT or pinA lines but occurred with variable penetrance in pinB and pinA pinB lines. The scale bar represents 100 μM.
Discussion
Physcomitrella PINs Can Polarize at the Plasma Membrane
On the basis of heterologous gene expression assays in tobacco, previous work suggested that Physcomitrella PINs A and D localize at the ER and cytosol, respectively, and land plant PINs were therefore postulated to have an ancestral role in regulating intracellular auxin homeostasis rather than intercellular transport [34, 35]. However, we have recently shown that Physcomitrella PINA–PINC are canonical, sharing sequence motifs that are required for plasma membrane targeting with Arabidopsis canonical PINs [45]. Our work suggested that canonical PINs are one ancestral type within the land plants and that Physcomitrella PINs A–C should have a capacity for plasma membrane targeting [45]. Using immunolocalization, we have found that Physcomitrella PINs A–C can indeed target the plasma membrane; we did not detect signal elsewhere in cells, and we did not detect signal colocalizing with an ER marker.
Physcomitrella PIN localization usually formed a conspicuous banding pattern traversing the adaxial-abaxial leaf axis, where two cells contact one another (Figures 3 and S3). Where leaves were thickened around the midvein, we also detected signal on the cell faces that were in contact with other cells, but the outermost cell faces were usually free from signal. Although we cannot rule out the possibility that each neighboring cell contributes to the high signal intensity at cell junctions, in our view, the localization is polarized. As auxin-treated gametophores and pinA pinB mutants have around half the number of cells in the mediolateral leaf axis than normal and the mediolateral leaf axis is elaborated by asymmetric cell divisions [61], a polar localization pattern perpendicular to the mediolateral axis is consistent with a role for PINA and PINB in promoting asymmetric cell division. These results suggest a role for canonical Physcomitrella PINs in intercellular polar auxin transport in leaf development.
PIN-Mediated Auxin Transport Drives Meristem Function and Leaf Development
Recent work was unable to detect polar auxin transport in gametophytic moss shoots, and no effect of treatment with transport inhibitors was observed, leading to the conclusion that auxin transport does not contribute to gametophore development [32, 33]. We were also unable to detect long-range polar auxin transport using radio-labeled IAA (data not shown). The discrepancy between the results that we obtained with NPA and previously published results arises from a difference in experimental approach. Whereas previous experiments immersed fully grown shoots in 50 μM NPA [32, 33], we grew colonies on NPA, exposing shoots to transport inhibition from the earliest developmental stages, and cotreatment with low auxin concentrations was needed to see strong developmental effects (Figure 2). We found that treatment of WT gametophores with NPA disrupted extension of proximodistal and mediolateral axes of leaf development and disrupted meristem function. The effects observed were similar to treatments with high concentrations of auxin or treatments of pinA mutants with low concentrations of auxin. Again, these results support a role for PIN-mediated auxin transport in the asymmetric cell divisions that drive leaf development and meristem function [61]. Consistent with PIN localization patterns, we hypothesize that auxin transport in moss gametophores occurs in a localized manner, to remove auxin from the leaves and meristem without detectable long-distance flux [62]. It is also possible that Physcomitrella PINs distribute auxin principally in the epidermis and, therefore, that the overall levels of transport involved are low.
PIN-Regulated Shoot Development Is a Deep Homology of Stomatophytes
Collectively, our data show that auxin transport regulates a suite of characteristics in Physcomitrella gametophore development that are similar to the developmental characteristics that are PIN regulated in angiosperm sporophytes, and our inferences are supported by data from Viaene et al. [63], published in this issue of Current Biology. PIN-mediated auxin transport in Physcomitrella regulates intrinsic developmental processes, such as asymmetric cell division, growth, meristem function, and leaf development, and dynamic responses to the environment, such as shoot tropisms. In conjunction with recently published results showing that charophytes have a capacity for long-range polar auxin transport [41], the regulation of these aspects of gametophore development in Physcomitrella raises the possibility that auxin transport could be a core mechanism for plant development that was recruited from the gametophyte to the sporophyte during land plant evolution. Alternatively, the roles of PIN-mediated auxin transport could have evolved convergently in moss gametophores. In either case, the recruitment of PIN-mediated auxin transport to regulate gametophore development is a clear instance of deep homology within the stomatophytes and the first that affects such general developmental programs.
PIN-Mediated Auxin Transport Is a Conserved Regulator of Sporophyte Development
Work in Selaginella has shown that the roles of polar auxin transport in regulating apical meristem function and shoot branching are conserved within the vascular plants [28, 29, 30, 31]. Previous work in mosses has shown that bulk polar auxin transport in sporophytes can be disrupted by NPA treatment, causing multiple sporangia to form [32, 33]. Our data also support the notion that sporophyte development in Physcomitrella is regulated by polar auxin transport [32, 33]. We have demonstrated that PINA and PINB are expressed in sporophytes and contribute synergistically to fertility and development (Figure 7); PIN-mediated auxin transport is a conserved regulator of sporophyte development in stomatophytes. We note that the duplicated sporangium phenotype of pinB and pinA pinB mutants reproduces branching morphologies of early prevascular fossils, such as Partitatheca [13], and speculate that this phenotype could arise by an early embryonic duplication of the apical cell, or bifurcation [64, 65, 66]. PIN-mediated auxin transport is a major driver of plant architecture in flowering plants [17], and changes in meristem function underpin architectural divergence between plant groups [4, 67]. The identification of conserved roles for auxin transport in land plant meristem function opens the possibility that PIN proteins played a key role in the radiation of plant form.
Experimental Procedures
A GH3:GUS reporter line [50] was used as the WT moss strain. Spot cultures were grown as described previously [61], and tissue for genetic analysis was prepared as in [50]. All lines were stored in the International Moss Stock Center (http://www.moss-stock-center.org; see Supplemental Information).
For immunolocalizations, tissue was grown for 4 weeks in continuous light, fixed in 3:1 methanol acetic acid, dehydrated, and embedded in PEG 1600. Eight-micrometer sections were interrogated with anti-maize PIN antibodies [55] at a 1/150 dilution and anti-BIP2 (Agrisera) at a 1/50 dilution. DyLight 594 and DyLight 405 were used as secondary antibodies at a 1/300 dilution.
pin disruptants were generated and screened for insertion as described in Supplemental Information.
GUS staining was carried out as elsewhere [32]. Light micrographs were compiled using a Keyence VHX-1000 series microscope with 50× and 200× objectives. Confocal imaging was undertaken as previously described [61], except for immunolocalizations; a Leica TCS 5 was used, with excitation from the Diode 405 and HeNe 594 laser lines, and emission was collected at 410–480 nm and 600–670 nm.
Author Contributions
E.L.D., R.R., and C.J.H. conceived this study. All authors contributed to experimental design. Foundational experiments were undertaken by T.A.B., M.M.L., T.A., N.M.B., M.B., X.Y.W., C.D.W., and C.J.H., with supervision from E.L.D., R.R., and C.J.H. T.A.B. contributed Figures 6B–6D, S1C, and S2B; M.M.L. contributed Figures 5B and 5C; Y.C. contributed Figure 7B; T.A. contributed Figures S4G and S4H; R.J.D. contributed Figures S1D, S2A, and S5; E.L.D. contributed Figure S4A; C.D.W. contributed Figure S4B; X.Y.W. contributed Figure S4F; and C.J.H. contributed the remainder. T.A.B., M.M.L., T.A., R.J.D., E.L.D., R.R., and C.J.H. contributed to data analysis and interpretation. The final manuscript was drafted by C.J.H., with help from T.A.B., T.A., E.L.D., and R.R. C.J.H. handled submission. D.O. contributed anti-PIN antibodies and technical help with immunohistochemistry.
Acknowledgments
We thank James Lloyd for a preliminary experiment. We thank Gertrud Wiedemann and Anna Beike for initial expression analyses and Ingrid Heger and Agnes Novakovic for technical assistance. We thank Jane Langdale and David Baulcombe for comments on the manuscript. C.J.H. is supported by a Royal Society University Research Fellowship, a Gatsby Charitable Foundation Fellowship (GAT2962), and the Biotechnology and Biological Sciences Research Council (BB/L00224811), and R.R. is supported by the Deutsche Forschungsgemeinschaft (SPP 1067, RE 837/6) and the Excellence Initiative of the German Federal and State Governments (EXC294).
Published: November 13, 2014
Footnotes
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/3.0/).
Supplemental Information includes Supplemental Experimental Procedures and six figures and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2014.09.054.
Supplemental Information
References
- 1.Harrison C.J., Alvey E., Henderson I.R. Meiosis in flowering plants and other green organisms. J. Exp. Bot. 2010;61:2863–2875. doi: 10.1093/jxb/erq191. [DOI] [PubMed] [Google Scholar]
- 2.Graham L.E., Cook M.E., Busse J.S. The origin of plants: body plan changes contributing to a major evolutionary radiation. Proc. Natl. Acad. Sci. USA. 2000;97:4535–4540. doi: 10.1073/pnas.97.9.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gensel P.G. The earliest land plants. Annu. Rev. Ecol. Evol. Syst. 2008;39:459–477. [Google Scholar]
- 4.Langdale J.A., Harrison C.J. In: Evolving Pathways: Key Themes in Evolutionary Developmental Biology. Fusco A.M.G., editor. Cambridge University Press; Cambridge: 2008. Developmental changes during the evolution of plant form; pp. 299–315. [Google Scholar]
- 5.Friedman W.E. Plant science. One genome, two ontogenies. Science. 2013;339:1045–1046. doi: 10.1126/science.1234992. [DOI] [PubMed] [Google Scholar]
- 6.Qiu Y.-L., Li L., Wang B., Chen Z., Knoop V., Groth-Malonek M., Dombrovska O., Lee J., Kent L., Rest J. The deepest divergences in land plants inferred from phylogenomic evidence. Proc. Natl. Acad. Sci. USA. 2006;103:15511–15516. doi: 10.1073/pnas.0603335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karol K.G., McCourt R.M., Cimino M.T., Delwiche C.F. The closest living relatives of land plants. Science. 2001;294:2351–2353. doi: 10.1126/science.1065156. [DOI] [PubMed] [Google Scholar]
- 8.Chang Y., Graham S.W. Inferring the higher-order phylogeny of mosses (Bryophyta) and relatives using a large, multigene plastid data set. Am. J. Bot. 2011;98:839–849. doi: 10.3732/ajb.0900384. [DOI] [PubMed] [Google Scholar]
- 9.Parihar N.S. Indian Universities Press; Allahabad: 1967. Bryophyta. [Google Scholar]
- 10.Kato M., Akiyama H. Interpolation hypothesis for origin of the vegetative sporophyte of land plants. Taxon. 2005;54:443–450. [Google Scholar]
- 11.Doyle J.A. In: The Evolution of Plant Form. Ambrose B.A., Purugganan M., editors. Wiley-Blackwell; Oxford: 2013. Phylogenetic analyses and morphological innovations in land plants; pp. 1–50. [Google Scholar]
- 12.Goffinet B., Buck W.R. In: The Evolution of Plant Form. Ambrose B.A., Purugganan M., editors. Wiley-Blackwell; Oxford: 2013. The evolution of body form in bryophytes; pp. 51–90. [Google Scholar]
- 13.Edwards D., Morris J.L., Richardson J.B., Kenrick P. Cryptospores and cryptophytes reveal hidden diversity in early land floras. New Phytol. 2014;202:50–78. doi: 10.1111/nph.12645. [DOI] [PubMed] [Google Scholar]
- 14.Floyd S.K., Bowman J.L. The ancestral developmental toolkit of land plants. Int. J. Plant Sci. 2007;168:1–35. [Google Scholar]
- 15.Rensing S.A., Lang D., Zimmer A.D., Terry A., Salamov A., Shapiro H., Nishiyama T., Perroud P.F., Lindquist E.A., Kamisugi Y. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science. 2008;319:64–69. doi: 10.1126/science.1150646. [DOI] [PubMed] [Google Scholar]
- 16.Cooke T.J., Poli D., Cohen J.D. In: The Evolution of Plant Physiology. Hemsley A.R., Poole I., editors. Academic Press; London: 2003. Did auxin play a crucial role in the evolution of novel body plans during the late silurian-early devonian radiation of land plants; pp. 85–107. [Google Scholar]
- 17.Reinhardt D., Kuhlemeier C. Plant architecture. EMBO Rep. 2002;3:846–851. doi: 10.1093/embo-reports/kvf177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinhardt D., Mandel T., Kuhlemeier C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell. 2000;12:507–518. doi: 10.1105/tpc.12.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gälweiler L., Guan C., Müller A., Wisman E., Mendgen K., Yephremov A., Palme K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- 20.Reinhardt D., Pesce E.-R., Stieger P., Mandel T., Baltensperger K., Bennett M., Traas J., Friml J., Kuhlemeier C. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- 21.Heisler M.G., Ohno C., Das P., Sieber P., Reddy G.V., Long J.A., Meyerowitz E.M. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 2005;15:1899–1911. doi: 10.1016/j.cub.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 22.Jönsson H., Heisler M.G., Shapiro B.E., Meyerowitz E.M., Mjolsness E. An auxin-driven polarized transport model for phyllotaxis. Proc. Natl. Acad. Sci. USA. 2006;103:1633–1638. doi: 10.1073/pnas.0509839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith R.S., Guyomarc’h S., Mandel T., Reinhardt D., Kuhlemeier C., Prusinkiewicz P. A plausible model of phyllotaxis. Proc. Natl. Acad. Sci. USA. 2006;103:1301–1306. doi: 10.1073/pnas.0510457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barkoulas M., Hay A., Kougioumoutzi E., Tsiantis M. A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nat. Genet. 2008;40:1136–1141. doi: 10.1038/ng.189. [DOI] [PubMed] [Google Scholar]
- 25.Scarpella E., Barkoulas M., Tsiantis M. Control of leaf and vein development by auxin. Cold Spring Harb. Perspect. Biol. 2010;2:a001511. doi: 10.1101/cshperspect.a001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilsborough G.D., Runions A., Barkoulas M., Jenkins H.W., Hasson A., Galinha C., Laufs P., Hay A., Prusinkiewicz P., Tsiantis M. Model for the regulation of Arabidopsis thaliana leaf margin development. Proc. Natl. Acad. Sci. USA. 2011;108:3424–3429. doi: 10.1073/pnas.1015162108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuchen E.E., Fox S., de Reuille P.B., Kennaway R., Bensmihen S., Avondo J., Calder G.M., Southam P., Robinson S., Bangham A., Coen E. Generation of leaf shape through early patterns of growth and tissue polarity. Science. 2012;335:1092–1096. doi: 10.1126/science.1214678. [DOI] [PubMed] [Google Scholar]
- 28.Williams S. Correlation phenomena and hormones in Selaginella. Nature. 1937;139:966. [Google Scholar]
- 29.Wochok Z.S., Sussex I.M. Morphogenesis in selaginella: auxin transport in the stem. Plant Physiol. 1973;51:646–650. doi: 10.1104/pp.51.4.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wochok Z.S., Sussex I.M. Morphogenesis in Selaginella. III. Meristem determination and cell differentiation. Dev. Biol. 1975;47:376–383. doi: 10.1016/0012-1606(75)90291-2. [DOI] [PubMed] [Google Scholar]
- 31.Sanders H.L., Langdale J.A. Conserved transport mechanisms but distinct auxin responses govern shoot patterning in Selaginella kraussiana. New Phytol. 2013;198:419–428. doi: 10.1111/nph.12183. [DOI] [PubMed] [Google Scholar]
- 32.Fujita T., Sakaguchi H., Hiwatashi Y., Wagstaff S.J., Ito M., Deguchi H., Sato T., Hasebe M. Convergent evolution of shoots in land plants: lack of auxin polar transport in moss shoots. Evol. Dev. 2008;10:176–186. doi: 10.1111/j.1525-142X.2008.00225.x. [DOI] [PubMed] [Google Scholar]
- 33.Fujita T., Hasebe M. Convergences and divergences in polar auxin transport and shoot development in land plant evolution. Plant Signal. Behav. 2009;4:313–315. doi: 10.4161/psb.4.4.8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mravec J., Skůpa P., Bailly A., Hoyerová K., Krecek P., Bielach A., Petrásek J., Zhang J., Gaykova V., Stierhof Y.D. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature. 2009;459:1136–1140. doi: 10.1038/nature08066. [DOI] [PubMed] [Google Scholar]
- 35.Viaene T., Delwiche C.F., Rensing S.A., Friml J. Origin and evolution of PIN auxin transporters in the green lineage. Trends Plant Sci. 2013;18:5–10. doi: 10.1016/j.tplants.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Albaum H.G. Inhibitions due to growth hormones in fern prothallia and sporophytes. Am. J. Bot. 1938;25:124–133. [Google Scholar]
- 37.Reski R. Development, genetics and molecular biology of mosses. Bot. Acta. 1998;111:1–15. [Google Scholar]
- 38.Decker E.L., Frank W., Sarnighausen E., Reski R. Moss systems biology en route: phytohormones in Physcomitrella development. Plant Biol (Stuttg) 2006;8:397–405. doi: 10.1055/s-2006-923952. [DOI] [PubMed] [Google Scholar]
- 39.Gaal D.J., Dufresne S.J., Maravolo N.C. Transport of 14C-indoleacetic acid in the hepatic Marchantia polymorpha. Bryologist. 1982;85:410–418. [Google Scholar]
- 40.Larue C.D., Narayanaswami S. Auxin inhibition in the liverwort Lunularia. New Phytol. 1957;56:61–70. [Google Scholar]
- 41.Boot K.J., Libbenga K.R., Hille S.C., Offringa R., van Duijn B. Polar auxin transport: an early invention. J. Exp. Bot. 2012;63:4213–4218. doi: 10.1093/jxb/ers106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benjamins R., Scheres B. Auxin: the looping star in plant development. Annu. Rev. Plant Biol. 2008;59:443–465. doi: 10.1146/annurev.arplant.58.032806.103805. [DOI] [PubMed] [Google Scholar]
- 43.Paponov I.A., Teale W.D., Trebar M., Blilou I., Palme K. The PIN auxin efflux facilitators: evolutionary and functional perspectives. Trends Plant Sci. 2005;10:170–177. doi: 10.1016/j.tplants.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Krecek P., Skupa P., Libus J., Naramoto S., Tejos R., Friml J., Zazímalová E. The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol. 2009;10:249. doi: 10.1186/gb-2009-10-12-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett T., Brockington S.F., Rothfels C., Graham S.W., Stevenson D., Kutchan T., Rolf M., Thomas P., Wong G.K.-S., Leyser O. Paralogous radiations of PIN proteins with multiple origins of noncanonical PIN structure. Mol. Biol. Evol. 2014;31:2042–2060. doi: 10.1093/molbev/msu147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ganguly A., Park M., Kesawat M.S., Cho H.-T. Functional analysis of the hydrophilic loop in intracellular trafficking of Arabidopsis PIN-FORMED Proteins. Plant Cell. 2014;26:1570–1585. doi: 10.1105/tpc.113.118422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashton N.W., Grimsley N.H., Cove D.J. Analysis of gametophytic development in the moss, Physcomitrella patens, using auxin and cytokinin resistant mutants. Planta. 1979;144:427–435. doi: 10.1007/BF00380118. [DOI] [PubMed] [Google Scholar]
- 48.Menand B., Yi K., Jouannic S., Hoffmann L., Ryan E., Linstead P., Schaefer D.G., Dolan L. An ancient mechanism controls the development of cells with a rooting function in land plants. Science. 2007;316:1477–1480. doi: 10.1126/science.1142618. [DOI] [PubMed] [Google Scholar]
- 49.Jang G., Dolan L. Auxin promotes the transition from chloronema to caulonema in moss protonema by positively regulating PpRSL1and PpRSL2 in Physcomitrella patens. New Phytol. 2011;192:319–327. doi: 10.1111/j.1469-8137.2011.03805.x. [DOI] [PubMed] [Google Scholar]
- 50.Bierfreund N.M., Reski R., Decker E.L. Use of an inducible reporter gene system for the analysis of auxin distribution in the moss Physcomitrella patens. Plant Cell Rep. 2003;21:1143–1152. doi: 10.1007/s00299-003-0646-1. [DOI] [PubMed] [Google Scholar]
- 51.Ludwig-Müller J., Jülke S., Bierfreund N.M., Decker E.L., Reski R. Moss (Physcomitrella patens) GH3 proteins act in auxin homeostasis. New Phytol. 2009;181:323–338. doi: 10.1111/j.1469-8137.2008.02677.x. [DOI] [PubMed] [Google Scholar]
- 52.Eklund D.M., Ståldal V., Valsecchi I., Cierlik I., Eriksson C., Hiratsu K., Ohme-Takagi M., Sundström J.F., Thelander M., Ezcurra I., Sundberg E. The Arabidopsis thaliana STYLISH1 protein acts as a transcriptional activator regulating auxin biosynthesis. Plant Cell. 2010;22:349–363. doi: 10.1105/tpc.108.064816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eklund D.M., Thelander M., Landberg K., Ståldal V., Nilsson A., Johansson M., Valsecchi I., Pederson E.R.A., Kowalczyk M., Ljung K. Homologues of the Arabidopsis thaliana SHI/STY/LRP1 genes control auxin biosynthesis and affect growth and development in the moss Physcomitrella patens. Development. 2010;137:1275–1284. doi: 10.1242/dev.039594. [DOI] [PubMed] [Google Scholar]
- 54.Jang G., Yi K., Pires N.D., Menand B., Dolan L. RSL genes are sufficient for rhizoid system development in early diverging land plants. Development. 2011;138:2273–2281. doi: 10.1242/dev.060582. [DOI] [PubMed] [Google Scholar]
- 55.O’Connor D.L., Runions A., Sluis A., Bragg J., Vogel J.P., Prusinkiewicz P., Hake S. A division in PIN-mediated auxin patterning during organ initiation in grasses. PLoS Comput. Biol. 2014;10:e1003447. doi: 10.1371/journal.pcbi.1003447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strepp R., Scholz S., Kruse S., Speth V., Reski R. Plant nuclear gene knockout reveals a role in plastid division for the homolog of the bacterial cell division protein FtsZ, an ancestral tubulin. Proc. Natl. Acad. Sci. USA. 1998;95:4368–4373. doi: 10.1073/pnas.95.8.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zazímalová E., Murphy A.S., Yang H., Hoyerová K., Hosek P. Auxin transporters—why so many? Cold Spring Harb. Perspect. Biol. 2010;2:a001552. doi: 10.1101/cshperspect.a001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haga K., Sakai T. PIN auxin efflux carriers are necessary for pulse-induced but not continuous light-induced phototropism in Arabidopsis. Plant Physiol. 2012;160:763–776. doi: 10.1104/pp.112.202432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friml J., Wiśniewska J., Benková E., Mendgen K., Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- 60.Jenkins G.I., Courtice G.R.M., Cove D.J. Gravitropic responses of wild-type and mutant strains of the moss Physcomitrella patens. Plant Cell Environ. 1986;9:637–644. doi: 10.1111/j.1365-3040.1986.tb01621.x. [DOI] [PubMed] [Google Scholar]
- 61.Harrison C.J., Roeder A.H.K., Meyerowitz E.M., Langdale J.A. Local cues and asymmetric cell divisions underpin body plan transitions in the moss Physcomitrella patens. Curr. Biol. 2009;19:461–471. doi: 10.1016/j.cub.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 62.Landberg K., Pederson E.R., Viaene T., Bozorg B., Friml J., Jönsson H., Thelander M., Sundberg E. The MOSS Physcomitrella patens reproductive organ development is highly organized, affected by the two SHI/STY genes and by the level of active auxin in the SHI/STY expression domain. Plant Physiol. 2013;162:1406–1419. doi: 10.1104/pp.113.214023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viaene T., Landberg K., Thelander M., Medvecka E., Pederson E., Feraru E., Cooper E.D., Karimi M., Delwiche C.F., Ljung K. Directional auxin transport mechanisms in early diverging land plants. Curr. Biol. 2014;24:2786–2791. doi: 10.1016/j.cub.2014.09.056. [DOI] [PubMed] [Google Scholar]
- 64.Harrison C.J., Corley S.B., Moylan E.C., Alexander D.L., Scotland R.W., Langdale J.A. Independent recruitment of a conserved developmental mechanism during leaf evolution. Nature. 2005;434:509–514. doi: 10.1038/nature03410. [DOI] [PubMed] [Google Scholar]
- 65.Harrison C.J., Rezvani M., Langdale J.A. Growth from two transient apical initials in the meristem of Selaginella kraussiana. Development. 2007;134:881–889. doi: 10.1242/dev.001008. [DOI] [PubMed] [Google Scholar]
- 66.Harrison C.J., Langdale J.A. Comment: the developmental pattern of shoot apices in Selaginella kraussiana (Kunze) A. Braun. Int. J. Plant Sci. 2010;171:690–692. [Google Scholar]
- 67.Philipson W.R. The significance of apical meristems in the phylogeny of land plants. Plant Syst. Evol. 1990;173:17–38. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.