Abstract
Introduction
Mutant BRAF is a driver oncogene found in 2% of lung adenocarcinomas and represents a target for therapy. We examined the clinical characteristics and course of patients with lung adenocarcinomas harboring BRAF mutations.
Methods
We identified patients with lung adenocarcinomas harboring BRAF mutations between 2009 and 2013 detected using a mass spectrometry-based PCR genotyping assay of hot-spot mutations involving codons corresponding to amino acids V600, D594, and G469 of BRAF. Patient characteristics and treatment outcomes were analyzed. Overall survival was compared to stage-matched patients with KRAS and EGFR mutant lung adenocarcinomas.
Results
Sixty-three patients were diagnosed with BRAF mutant lung adenocarcinomas between 2009 and 2013 (V600, 36; non-V600, 27). The majority of patients with BRAF mutations were smokers (92%), although patients with V600 mutations were more likely to be light/never smokers compared to patients with non-V600 mutations (42% vs. 11%, p=0.007). Of the 32 patients with early stage disease, 6 (19%, 95% CI 7-36%) developed second primary lung cancers harboring KRAS mutations. Patients with advanced V600 mutant lung adenocarcinomas had a better survival from diagnosis as compared to those with non-V600 mutant lung adenocarcinomas (3-year OS: 24% vs. 0%, p<0.001).
Conclusions
This is the largest series of patients with BRAF mutant lung cancers described. Most patients were heavy smokers. Nineteen percent of patients with early stage BRAF mutant lung cancers developed second primary lung cancers harboring KRAS mutations. Patients with advanced lung adenocarcinomas harboring V600 mutations have an improved overall survival compared to those with non-V600 mutations.
Keywords: BRAF, NSCLCs, Lung Cancers
INTRODUCTION
The discovery of targetable driver mutations in a subset of patients with lung adenocarcinomas has transformed the therapeutic approach to patients with lung cancers. Treatment with epidermal growth factor receptor tyrosine kinase inhibitors (gefitinib, erlotinib, and afatinib) improves response rates and progression-free survival as compared to cytotoxic chemotherapy for patients with advanced stage lung adenocarcinomas harboring EGFR mutations.1-5 Similarly, in patients with lung cancers defined by the anaplastic lymphoma kinase gene rearrangement, crizotinib prolongs progression free survival compared to docetaxel or pemetrexed.6-7
Activating molecular alterations have also been identified in genes such as BRAF, KRAS, HER2, FGFR2, RET, ROS1, and PIK3CA that could potentially be targeted in lung cancers.8,9 BRAF is a serine/threonine kinase downstream of RAS in the RAS-RAF-MEKERK signaling pathway. When activated by mutations, BRAF phosphorylates MEK to promote cell growth, proliferation, and survival. Somatic mutations in BRAF are found in several different cancers, including melanoma, papillary thyroid cancers, colorectal cancers, ovarian carcinomas, and lung cancers. The clinical significance of V600 BRAF mutations is highlighted by the demonstrated activity of BRAF and/or MEK inhibitors in patients with BRAF mutant melanoma.10-12
In lung cancers, preclinical work has confirmed a role of mutant BRAF in the development and maintenance of lung adenocarcinomas.13,14 BRAF mutations are detected in 2% of lung cancers. Unlike melanomas in which the vast majority of BRAF mutations occur at V600, only approximately 50% of BRAF mutant lung adenocarcinomas harbor V600 mutations with the rest of the cases harboring non-V600 mutations in exons 11 and 15.15-19 This has clear therapeutic implications, as non-V600 mutant BRAF kinases appear to be resistant to BRAF targeted therapies but may be sensitive to pharmacologic inhibition of MEK via the transactivation of CRAF.20, 21 The prognostic significance of different BRAF mutations has not been evaluated in patients with lung cancers.
Several previous groups have begun to define the prevalence, distribution, and prognosis of BRAF mutations in patients with lung adenocarcinoma.16-19 These studies have been limited by relatively small numbers of patients. As part of a multiplex assay, we have routinely tested lung adenocarcinomas for the presence of hot-spot mutations in BRAF since 2009 and have collected the largest series of patients to date.22,23 In this paper, we report the characteristics of patients with lung adenocarcinomas harboring BRAF mutations and describe their clinical course. We hypothesized that patients with V600 mutant tumors would have a significantly prolonged survival as compared to patients with non-V600 mutant tumors.
MATERIALS AND METHODS
Study Patients
We identified patients with lung cancers harboring BRAF mutations detected between 2009 and 2013. Patient demographics and characteristics including age, sex, race, stage at initial diagnosis of BRAF mutant disease, date of resection, treatment history, and smoking history were recorded. Stage was determined according to the American Joint Committee on Cancer (AJCC) staging system, 7th edition. Patients were followed from the date of cancer diagnosis until date of death or last available follow-up. This cohort of patients includes the 18 patients described by Paik et al.16 A comparison group of consecutive EGFR and KRAS mutant patients diagnosed and treated at Memorial Sloan Kettering during the same calendar period was used for comparison.
Genotype Analysis
BRAF mutation analysis was performed using a MassARRAY system, a technique based on matrix assisted laser desorption/ionization time of flight mass spectrometry (Sequenom, San Diego, USA).24,25 Amplification and extension primers were designed using the Sequenom Assay Designer v3.1 software to target mutations involving codons V600, D594, and G469 of BRAF. The primer sequences are listed in Supplementary Table S1. EGFR exon 19 deletions and exon 21 L858R amino acid substitutions were identified by previously reported methods.26, 27 KRAS codon 12 and 13 mutations were identified by mass spectrometry based genotyping or direct sequencing.
Statistical Analysis
Fisher exact and Wilcoxon rank sum tests were used to compare the demographics and clinical characteristics between patients in the V600 and non-V600 mutated subgroups. Overall survival (OS) was either calculated from the date of resection (for early stage disease) or from date of pathologic diagnosis (for stage IIIb or stage IV disease) to death. Patients who did not die during the study time were censored at the time they were last confirmed alive.
Patients became eligible for the study at the time of their molecular diagnosis for BRAF mutation. In some cases, there was a non-negligible amount of time between resection/ pathologic diagnosis (when follow-up started) and BRAF status determination. In order to account for this delay and avoid any potential length-time bias associated, all analyses were performed using left truncation (or delayed entry) techniques. Consequently, overall survival was estimated using the Kaplan-Meier method, with survival probabilities calculated conditional on patients having survived until the date of their molecular testing. Group comparisons were performed using the log-rank test. A two-sided p-value of <0.05 was considered statistically significant. Statistical analyses were performed using the ‘survival’ package in R (version 3.0.1; R Development Core Team) and SAS statistical software (SAS Institute, Inc, Cary, NC).
RESULTS
Patient Characteristics
Sixty-three patients with BRAF mutant lung adenocarcinomas were identified with a median follow-up time from diagnosis of 42 months for early stage disease and 18 months for advanced stage disease. Thirty-six patients had a BRAF V600 mutation and 27 had a non-V600 mutation. Patient characteristics are summarized in Table 1. There was no significant difference in age, sex, or stage at initial diagnosis between patients with V600 and non-V600 mutations. Patients with V600 mutant tumors were more likely to be never/light smokers as compared to patients with tumors harboring non-V600 mutations (p=0.007).
Table 1.
Patient Characteristics
| Mutant BRAF | All (n=63) | V600 (n=36) | Non-V600 (n=27) | p-value |
|---|---|---|---|---|
| Median age, years Range | 65 (33-85) | 64 (48-79) | 66 (33-85) | 0.72 |
| Sex | 0.97 | |||
| Female | 34 (54%) | 19 (53%) | 15 (56%) | |
| Male | 29 (46%) | 17 (47%) | 12 (44%) | |
| Smoking history | 0.007 | |||
| Never smokers | 5 (8%) | 3 (8%) | 2 (7%) | |
| ≤15 pack year | 13 (21%) | 12 (33%) | 1 (4%) | |
| >15 pack year | 45 (71%) | 21 (58%) | 24 (89%) | |
| Histology Adenocarcinoma | 100% | 100% | 100% | |
| Stagea | 0.054 | |||
| I | 17 (27%) | 9 (25%) | 8 (30%) | |
| II | 4 (6%) | 2 (6%) | 2 (7%) | |
| IIIa | 11 (17%) | 3 (8%) | 8 (30%) | |
| IIIb | 4 (6%) | 2 (6%) | 2 (7%) | |
| IV | 27 (43%) | 20 (56%) | 7 (26%) | |
| Race | 0.48 | |||
| White, Non-Hispanic | 55 (87%) | 30 (83%) | 25 (93%) | |
| Asian | 3 (5%) | 2 (6%) | 1 (4%) | |
| Black | 3 (5%) | 2 (6%) | 1 (4%) | |
| White, Hispanic | 2 (3%) | 2 (6%) | 0 (0%) | |
Stage at initial NSCLC diagnosis, American Joint Committee on Cancer AJCC, staging system 7th edition
BRAF Genotypes
Five BRAF mutation genotypes were identified: V600E (57%), G469A (22%), D469V (13%), D594G (6%), and V600M (2%). Figure 1 demonstrates the distribution of BRAF genotypes based on early stage and advanced stage disease. No tumor with a BRAF mutation had a concomitant mutation in EGFR, KRAS, or a rearrangement in ALK.
Figure 1.
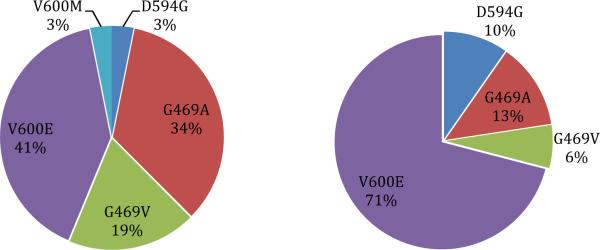
Frequency of BRAF mutations in NSLCC
Second Lung Cancers
Of the 32 patients with early stage disease, 6 (19%) developed metachronous or synchronous second primary lung cancers harboring KRAS mutations (Table 2), 1 patient developed metachronous squamous lung cancer, 1 patient developed EGFR L895R mutant lung cancer, and 1 patient developed a metachronous lung cancer for which molecular testing was not performed. All six patients with second primary KRAS mutant lung cancers were former or current smokers that smoked a median of 28 pack-years (range: 24-60).
Table 2.
Secondary Lung Cancer with KRAS Mutations
| Patient | BRAF mutation | Type of Second Lung Cancer | Months Between |
|---|---|---|---|
| 1 | G469A | Metachronous with KRAS G12D | 43 months |
| 2 | G469A | Metachronous with KRAS G13C | 15 months |
| 3 | V600E | Metachronous with KRAS G12V | 50 months |
| 4 | G469V | Synchronous with KRAS G12C | -- |
| 5 | V600E | Synchronous with KRAS G12C | -- |
| 6 | G469A | Synchronous with KRAS G12C | -- |
Clinical Outcomes of Patients with and without BRAF Mutant Lung Cancer
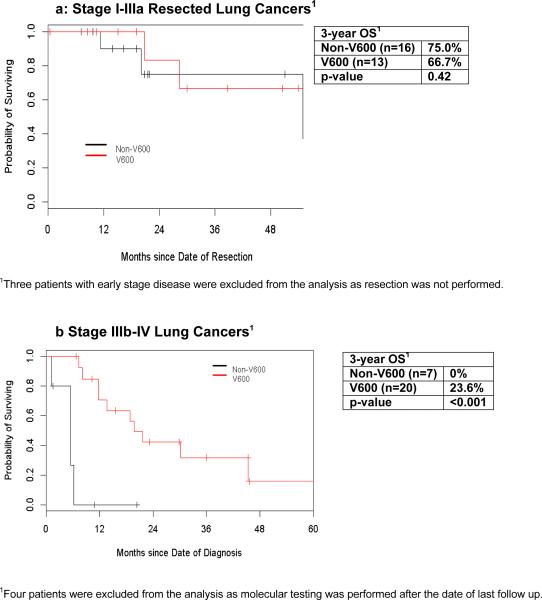
The 3-year overall survival after resection of early stage lung cancer was similar for patients with V600 mutant tumors compared to non-V600 mutant tumors (67% vs 75%, p=0.42, Figure 2a). Three patients with early stage disease were excluded from the analysis as they did not undergo resection. In patients with stage IIIb or IV BRAF mutant lung adenocarcinomas, those with V600 mutations had a longer 3-year overall survival as compared to patients with non-V600 mutations (24% vs. 0%, p<0.001, Figure 2b). Four patients with advanced stage BRAF mutant lung adenocarcinomas were excluded from this survival analysis as they had molecular testing after the date of their last follow up.
Figure 2.
Overall Survival of BRAF Mutant Lung Cancer
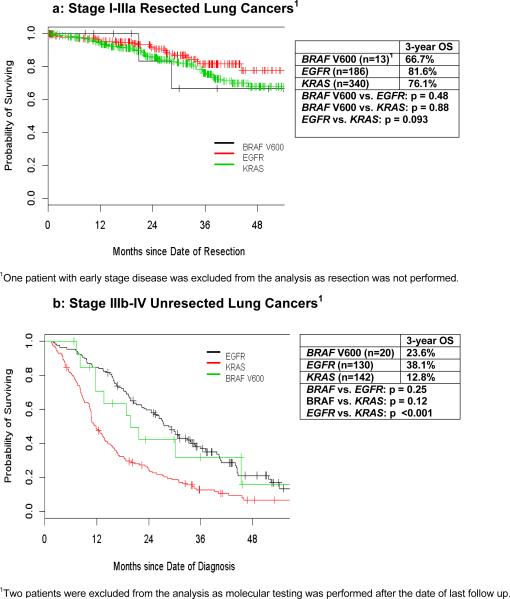
We then compared overall survival of patients with BRAF V600 mutant disease to patients with KRAS or EGFR mutations during the same time period. For early stage disease, no difference was found in overall survival based on genotype (p=0.23, Figure 3a). In patients with advanced stage disease, 3-year overall survival was significantly longer for the EGFR mutant group as compared to the KRAS group (38% vs. 13% for EGFR and KRAS patients, respectively, p<0.001, Figure 3b). The overall survival for patients with BRAF mutations was numerically intermediate between those with KRAS and EGFR mutations, but not statistically distinct from either genotype defined cohort. Only half of those patients with BRAF V600 mutations received BRAF-targeted therapy, while 94% of patients with EGFR mutations in this cohort received EGFR-tyrosine kinase inhibitors.
Figure 3.
Overall Survival of BRAF V600 vs. Other Lung Adenocarcinoma Genotypes
Response to BRAF inhibitors
Ten of the 20 patients with advanced stage BRAF V600E mutant lung cancers were treated with BRAF inhibitors at some point during their treatment course. Sixty percent had a partial response, 30% had stable disease, and 10% had progressive disease per RECIST. Half (5/10) of these patients have remained on therapy for over 6 months, while 3 remain on therapy for over 1 year.
DISCUSSION
Patients with BRAF mutant lung cancers represent a distinct subset of patients with lung cancers that may benefit from BRAF targeted therapy. Building on the success of BRAF inhibitors in patients with BRAF V600 melanomas, similar activity has recently been demonstrated in patients with lung cancers. An interim analysis of a phase II study of dabrafenib in patients with BRAF V600 mutant lung adenocarcinomas showed an overall response rate of 54% with the longest duration of response of 49 weeks thus far.28 To more fully understand the underlying biology of these patients, it is important to investigate the clinical characteristics of these patients and the prognostic significance of these mutations.
This is the largest series of patients with BRAF mutant lung cancers reported to date. Similar to previous studies, we found that BRAF mutations occurred most often in smokers. The large number of patients in this analysis allowed us to observe that smoking status differs significantly according to BRAF mutation type with V600 mutations occurring preferentially in never/light smokers. These results are similar to what was presented by Marchetti et al who found that BRAF V600 mutations were more frequent in never smokers/light smokers.17 No other clinical profile emerged in our study in association with BRAF positive tumors. We did not find an association between gender, age, race, or stage at first diagnosis of lung adenocarcinoma and BRAF mutation type.
In regards to BRAF mutation genotype, we confirmed the finding that non-V600 mutations are more common in lung cancers than in melanomas. The high incidence of non-V600 mutations has important clinical consequences as current second generation RAF inhibitors such as dabrafenib and vemurafenib are most active in V600 mutant kinases. Third generation BRAF inhibitors and MEK1/2 inhibitors may be more effective in non-V600 mutations, which represented 43% of the patents in our series and current clinical trials are ongoing. Unlike Cardarella et al and Marchetti et al, we did not have any tumors harboring concurrent BRAF mutations and KRAS or EGFR mutations, suggesting that these are likely rare events. This is consistent with the prospective Lung Cancer Mutation Consortium which showed that only 3 of 1007 tumors analyzed had a BRAF mutation and a second oncogenic driver.29
In our series, 19% of patients with early stage BRAF mutant disease had a metachronous or synchronous second primary lung cancers harboring a KRAS mutation. Each of the six patients with second primary lung cancers were heavy smokers, which may explain the co-occurrence of these cancers. This finding emphasizes the importance of repeating molecular studies to distinguish between “de novo” lung cancers and recurrence/metastasis as there may be critical treatment and prognostic implications.
We further showed that patients with advanced V600 mutant lung cancers have an improved overall survival as compared to non-V600 mutant lung cancers and similar survival to EGFR and KRAS mutant lung cancers. In contrast, it has been previously suggested by Marchetti et al that V600 mutations may have a worse prognosis as compared to BRAF wild-type tumors. It is interesting to note that in our series, 10/20 patients with stage IV BRAF V600 mutations received an agent targeting BRAF as part of routine care or as part of a clinical trial. This may have altered the natural history of this patient population and improved overall survival, although this is clearly speculative. The outcomes of patients treated with BRAF inhibitors are now being determined as part of ongoing studies. Furthermore, since patients with BRAF V600 tumors were more likely to be light/never smokers than those with non-V600 tumors, the effects of cigarette smoking may have had an impact on survival.30
As a retrospective study of patients pursing their care at a single site, there are some limitations to our analysis. BRAF mutations were detected using a platform that identified only a limited number of BRAF point mutations. We note that other BRAF mutations in lung adenocarcinomas have been identified including mutations in amino acids 421, 439, 459, 466, 471, 595, 597, 604, and 606.31,32 However, these individual mutations represent just 1-3% of all BRAF mutations reported. As the number of cases of BRAF mutant lung cancers is relatively small, larger studies are needed to extend and confirm our results.
In conclusion, our data shows that both BRAF non-V600 and V600 mutant lung adenocarcinomas are more common in smokers, but can be identified in never smokers and light smokers. Among patients with BRAF mutant lung cancers, the incidence of non-V600 BRAF mutations is 43%. Nineteen percent of patients with early stage BRAF mutant disease have a metachronous or synchronous second primary lung cancer harboring a KRAS mutation, possibly secondary to a similar risk factor of cigarette smoking. Repeat biopsies and molecular testing should be routine for such patients. Patients with advanced V600 mutant lung cancers have a prolonged overall survival as compared to non-V600 mutant lung cancers. BRAF directed therapies have promising clinical activity in these patients, and various agents are currently being tested in the clinic that can potentially expand the number of candidates eligible for targeted therapy.
Supplementary Material
Acknowledgments
Financial Support: Supported in part by NCI P30 CA008748
REFERENCES
- 1.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;36:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 3.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 4.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 5.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–34. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 6.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non–small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 8.Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–75. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weir BA, Woo MS, Getz G, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature. 2007;450:893–8. doi: 10.1038/nature06358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF- mutated melanoma. N Engl J Med. 2012;367:107–14. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 13.Ji H, Wang Z, Perera SA, et al. Mutations in BRAF and KRAS converge on activation of the mitogen-activated protein kinase pathway in lung cancer mouse models. Cancer Res. 2007;67:4933–4939. doi: 10.1158/0008-5472.CAN-06-4592. [DOI] [PubMed] [Google Scholar]
- 14.Dankort D, Filenova E, Collado M, et al. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 2007;21:379–384. doi: 10.1101/gad.1516407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 16.Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29:2046–51. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchetti A, Felicioni L, Malatesta S, et al. Clinical features and outcome of patients with non–small-cell lung cancer harboring BRAF mutations. J Clin Oncol. 2011;29:3574–9. doi: 10.1200/JCO.2011.35.9638. [DOI] [PubMed] [Google Scholar]
- 18.Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res. 2013;19:4532–40. doi: 10.1158/1078-0432.CCR-13-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinno T, Tsuta K, Shiraishi K, et al. Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Ann Oncol. 2014;25:138–42. doi: 10.1093/annonc/mdt495. [DOI] [PubMed] [Google Scholar]
- 20.Wan P, Garnett M, Roe M, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 21.Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–21. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Angelo SP, Park B, Azzoli CG, et al. Reflex testing of resected stage I through III lunadenocarcinomas for EGFR and KRAS mutation: report on initial experience and clinical utility at a single center. J Thorac Cardiovasc Surg. 2011;141:476–80. doi: 10.1016/j.jtcvs.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kris M, Lau C, Ang D, et al. Initial results of LC-MAP: An institutional program to routinely profile tumor specimens for the presence of mutations in targetable pathways in all patients with lung adenocarcinoma. J Clin Oncol. 2010;28(suppl):516s. abstr 7009. [Google Scholar]
- 24.Jurinke C, Oeth P, van den Boom D. MALDI-TOF mass spectrometry. Mol Biotechnol. 2004;26:147–164. doi: 10.1385/MB:26:2:147. [DOI] [PubMed] [Google Scholar]
- 25.Arcila M, Lau C, Nafa K, et al. Detection of KRAS and BRAF mutations in colorectal carcinoma: Roles for high-sensitivity locked nucleic acid-PCR sequencing and broad- spectrum mass spectrometry genotyping. J Mol Diagn. 2011;13:64–73. doi: 10.1016/j.jmoldx.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan Q, Pao W, Ladanyi M. Rapid polymerase chain reaction-based detection of epidermal growth factor receptor gene mutations in lung adenocarcinomas. The Journal of molecular diagnostics: JMD. 2005;7:396–403. doi: 10.1016/S1525-1578(10)60569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li AR, Chitale D, Riely GJ, et al. EGFR mutations in lung adenocarcinomas: clinical testing experience and relationship to EGFR gene copy number and immunohistochemical expression. The Journal of molecular diagnostics: JMD. 2008;10:242–248. doi: 10.2353/jmoldx.2008.070178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Planchard David, Mazieres Julien, Riely Gregory J., et al. Interim results of phase II study BRF113928 of dabrafenib in BRAF V600E mutation–positive non-small cell lung cancer (NSCLC) patients. J Clin Oncol. 2013;31(suppl) abstr 8009. [Google Scholar]
- 29.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. doi: 10.1001/jama.2014.3741. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janjigian YY, McDonnell K, Kris MG, et al. Pack-years of cigarette smoking as a prognostic factor in patients with stage IIIB/IV nonsmall cell lung cancer. Cancer. 2010;116:670–5. doi: 10.1002/cncr.24813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forbes SA, Tang G, Bindal N, et al. COSMIC (the Catalogue of Somatic Mutations in Cancer): A resource to investigate acquired mutations in human cancer. Nucleic Acids Res. 2010;38:D652–D657. doi: 10.1093/nar/gkp995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.