Abstract
The brain is the key organ of stress processes. It determines what individuals will experience as stressful, it orchestrates how individuals will cope with stressful experiences, and it changes both functionally and structurally as a result of stressful experiences. Within the brain, a distributed, dynamic, and plastic neural circuitry coordinates, monitors, and calibrates behavioral and physiological stress response systems to meet the demands imposed by particular stressors. These allodynamic processes can be adaptive in the short term (allostasis) and maladaptive in the long term (allostatic load). Critically, these processes involve bidirectional signaling between the brain and body. Consequently, allostasis and allostatic load can jointly affect vulnerability to brain-dependent and stress-related mental and physical health conditions. This review focuses on the role of brain plasticity in adaptation to, and pathophysiology resulting from, stressful experiences. It also considers interventions to prevent and treat chronic and prevalent health conditions via allodynamic brain mechanisms.
Keywords: brain-body medicine, brain plasticity, hippocampus, amygdala, prefrontal cortex
Introduction
Stress and stressful experiences have long been implicated in the etiology and pathophysiology of chronic physical and mental health conditions that now pose a great threat to public health (1). Historically, disciplinary variation in defining and studying stress and stressful experiences posed both methodological and conceptual challenges to the medical community's understanding of how an individual's health status could be affected by such complex processes over the life course. These challenges have been addressed by current perspectives, which build on recent advances in translational animal and human research and emphasize that the relationships between stressful experiences and health status depend on a dynamic interaction between genetic liability and exposure to environmental factors. This interaction begins in utero and continues until death (2).
Canonically, we can label a stressful experience as “good,” “tolerable,” or “toxic” depending on the extent to which an individual has control over a given stressor and has support systems and resources in place for coping with it (3, 4). Meeting the demands imposed by stressful experiences can lead to growth, adaptation, and beneficial forms of learning that promote resiliency and good health. By contrast, other stressful experiences can foster a proliferation of recursive neural, physiological, behavioral, cognitive, and emotional changes that increase vulnerability to ill health and premature death by several chronic medical conditions (5).
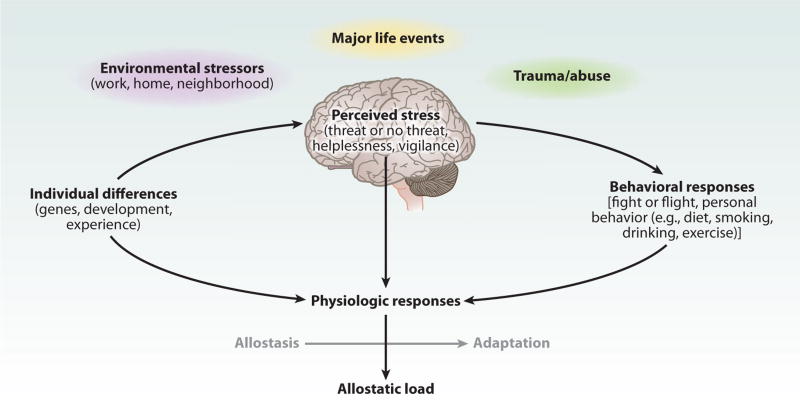
Here, we highlight translational animal and human evidence demonstrating that the brain is the central mediator and target of stress resiliency and vulnerability processes. We emphasize that the brain (a) determines what is threatening, and hence stressful, to the individual; (b) regulates the physiological, behavioral, cognitive, and emotional responses that an individual will deploy in order to cope with a given stressor; and (c) changes in its plasticity both adaptively and maladaptively as a result of coping with stressful experiences (Figure 1). We underscore the organizing concepts of allostasis and allostatic load, which can aid in our understanding of how forms of stress-related brain plasticity impact mental and physical health. We then consider the potential of social and personal interventions to prevent and treat chronic and prevalent health conditions via plastic and allodynamic brain mechanisms.
Figure 1.
Central role of the brain in allostasis and the behavioral and physiological response to stressors. Redrawn from Reference 5 with permission.
Stress, Brain Plasticity, and Health: the Roles of Allostasis and Allostatic Load
The brain processes not only external sensory inputs from the environment but also internal inputs from the body. This parallel processing enables the brain to control and coordinate behavioral and physiological adjustments engendered by external or internal challenges to homeostasis. These adjustments can promote adaptation, such as calibrating cardiac output and peripheral vascular resistance to provide hemodynamic and metabolic support for large muscle groups needed for immediate or anticipated action (e.g., escape from a predator). The biological systems that promote such adaptation include the hypothalamic-pituitary-adrenal (HPA) axis, the autonomic nervous system, the metabolic system, the gut, the kidneys, and the immune system (including the network of cytokine-producing cells throughout the body). The chief biomediators of these systems (e.g., cortisol, sympathetic and parasympathetic transmitters, cytokines, metabolic hormones) operate within a nonlinear, dynamic, and interactive network in which mediators down- and upregulate each other, depending on such factors as their concentration, location in the body, and sequential temporal patterning (6). Importantly, the activities of these systems and mediators are influenced by the genetic make-up, developmental history, and current behavioral and psychological states of the individual.
Adjustments of the aforementioned biological systems thus enable protection and adaptation of the individual to particular challenges by a process called allostasis (7). Whereas many physiological parameters, such as blood oxygen and pH, are maintained in a narrow homeostatic range, many other parameters vary in their functionality with time of day and in response to external and internal demands. Dynamic mediators of allostasis expressing marked variability, therefore, facilitate adaptation, whereas the parameters associated with homeostasis do not express comparable variability as a means of promoting adaptation. Allostasis is essential for maintaining homeostasis in the face of external and internal demands that are registered by the brain. Critically, however, allodynamic adaptation has a price, and the cost of this adaptation is called allostatic load—the wear-and-tear on the body and brain (5, 8).
Allostatic systems promote adaptation to stressful experiences and are generally most useful when rapidly mobilized and terminated. When they are prolonged or not terminated promptly, allostatic systems undermine mental and physical health—primarily because of their effects on brain plasticity (see below). The inability to engage allostatic systems when needed also produces a load on the body, because the normal protection afforded by these systems is lacking.
An important aspect of allostasis and allostatic load is the notion of anticipation. Here, anticipation implies psychological states, such as apprehension, worry, and anxiety, as well as cognitive preparation for a forthcoming event. Anticipation arising from neural activity within the brain can drive the output of allostatic biomediators, and it is likely that states of prolonged anxiety and anticipation can result in allostatic load (9). Other important aspects of individual responses to stress in relation to allostasis and allostatic load are health-damaging and health-promoting behaviors such as smoking, alcohol consumption, sleep, diet, and physical activity, collectively called lifestyle behaviors. These may be embodied within the overall notion of allostasis—i.e., how individuals adapt to and cope with a challenge—and they also contribute to allostatic load. For example, a Western diet accelerates atherosclerosis and progression to type II diabetes; smoking accelerates atherogenesis; exercise and restorative sleep promote cognitive functioning and health (6).
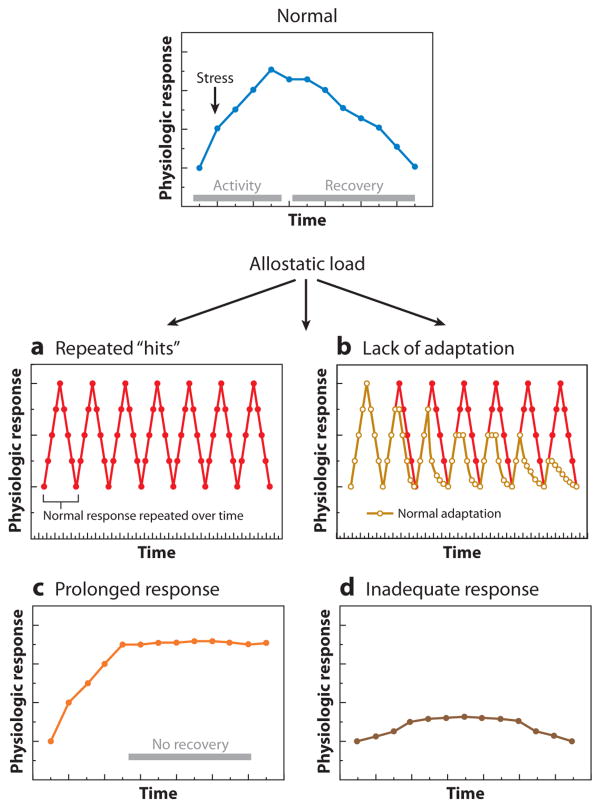
Within the framework presented above and detailed elsewhere (5), there are four types of allodynamic and physiological responses that may contribute to and reflect allostatic load (Figure 2). These include (a) repeated “hits” from multiple stressors, (b) a lack of adaptation or habituation, (c) prolonged response due to delayed shutdown, and (d) inadequate response that leads to compensatory hyperactivity of other mediators. As discussed next, these forms of allodynamic response, which can contribute to allostatic load, are mediated by the brain and target the brain. Importantly, conditions of allostatic load that adversely affect the brain are associated with forms of neural plasticity that are amenable to prevention and intervention.
Figure 2.
Types of allostasis and allostatic load. (a) Repeated “hits” from multiple stressors. Individuals who are repeatedly exposed to stressors over their life course and who also experience large surges in blood pressure and cardiovascular activity, which depend on the engagement of the hypothalamic-pituitary-adrenal (HPA) and autonomic axes, are more likely to show premature hypertension and atherosclerotic heart disease. (b) Lack of adaptation. A failure to habituate to a repetition of the same stressor results in a persistent elevation of mediators such as cortisol. This was first described in individuals who failed to habituate in their cortisol response to a public-speaking stressor (5, 71). (c) Prolonged response due to delayed shutdown. Adaptive autonomic and neuroendocrine responses are slow to terminate; e.g., blood-pressure elevations are sustained during repetitive, time-pressured work. (d) Inadequate response leads to compensatory hyperactivity of other mediators. For example, autoimmunity and inflammation can be associated with inadequate endogenous glucocorticoid responses, as in the Lewis rat and possibly also in chronic fatigue syndrome and fibromyalgia.
Brain Systems Mediating and Targeted by Allodynamic Processes
The brain is the central organ of stress processes and allodynamic adaptation, and it is a key target of allostatic load (Figure 1). Within the brain, a distributed, dynamic, and plastic neural circuitry coordinates, monitors, and calibrates behavioral and allodynamic response systems to cope with the demands of particular stressors. This circuitry includes the hippocampus and amygdala, which are limbic brain structures that process experiences by interfacing with lower vegetative brain areas (such as the hypothalamus and brainstem) and higher cortical areas, particularly the prefrontal cortex. Hence, the hippocampus, amygdala, and prefrontal cortex can be viewed as coordinating behavior with allodynamic response systems in the service of coping with external and internal challenges or perceived threats to homeostasis and well-being. They also serve important functions in cognition, emotions, and impulse control, and they help to interpret, on the basis of current and past experiences, whether an event is threatening or otherwise stressful—thus influencing allostatic responses. Next, we review translational animal and human studies focusing on these areas, particularly in the context of their importance for mediating allodynamic processes important for health.
The hippocampus has been found in animal models to show remarkable structural plasticity, including remodeling of dendrites and synaptic connections and a limited amount of neurogenesis. The hippocampus contains receptors for adrenal steroids and for major metabolic hormones that have functional effects on the hippocampus. Specifically, these biomediators can enhance cognitive processes, affect mood and motivation, and promote excitability and neuroprotection. Yet, these same biomediators can have deleterious effects on the hippocampus under conditions of chronic stress and allostatic load (2).
Animal studies of the hippocampus have revealed a mechanism by which repeated or chronic stress exposure causes a plastic remodeling of hippocampal circuitry: shortening of dendrites, loss of spine synapses, and suppression of the neurogenesis that occurs in the dentate gyrus region of the hippocampal formation up to young adulthood. This is a reversible response to stress exposure lasting a number of weeks, and it is mediated not only by circulating glucocorticoids but also by excitatory amino acid neurotransmitters and other endogenous mediators and modulators. Because of these two interrelated roles of the hippocampus— supporting aspects of memory and regulating HPA activity—impairment of hippocampal function through changes in excitability, reversible plasticity, or permanent damage may be expected to have two effects. The first is to impair hippocampal involvement in episodic, declarative, contextual, and spatial memory. Impairments of these functions are likely to debilitate an individual's ability to process information in new situations and to make decisions about how to deal with new challenges or stressors. The second effect is to impair hippocampal regulation of HPA activity, particularly the termination of the stress response, leading to elevated HPA activity and further exacerbating the actions of adrenal steroids in the long-term effects of repeated and chronic stress exposure. This concept, first called the “glucocorticoid cascade hypothesis” of hippocampal aging (10), is crucial to the notions of allostasis and allostatic load and the central role of the brain in stress plasticity processes.
Complementing animal studies of stress-related processes mediated by and affecting plasticity in the hippocampus, a growing number of human neuroimaging studies have begun to examine stress processes in association with aspects of gross hippocampal morphology. For example, individuals with stress-related psychiatric disorders, such as major depressive disorder and posttraumatic stress disorder, show volumetric reductions in the hippocampus (see 11, 12). Reduced hippocampal volume has also been found in Cushing's disease (13). Interestingly, in Cushing's disease, surgical correction of hypercortisolemia has been reported to partially reverse hippocampal volume reduction, as well as ameliorate mood and memory deficits (14, 15). In depression, there is evidence of volumetric increase in the hippocampus after antidepressant treatment (16), suggesting that the deficits in depression are potentially reversible. Moreover, there is increasing support for the notion that targeting the plasticity of the hippocampus in depression and mood disorders may underpin pharmacological and nonpharmacological treatment efficacy (17, 18).
In addition to disease studies, there is emerging evidence from otherwise healthy individuals for a relationship between chronic stressful experiences and changes in hippocampal morphology. Among postmenopausal women, for example, higher levels of chronic perceived stress, as measured over an approximate 20-year period of life, have been associated with reduced gray matter volume in the hippocampus and in a region of the lateral prefrontal cortex (19). Further, more than three years after the terrorist attacks on the World Trade Center buildings on September 11, 2001, otherwise healthy adults living near the site of the attacks showed a reduction in gray matter volume in the hippocampus, as well as in anatomically networked areas of the amygdala and prefrontal cortex (20).
In humans, as well as in animal models, there appears to be a heritable component of stress-related plasticity in the hippocampus. For example, human carriers of the methionine (met) allele of the valine (val) 66met brain-derived neurotrophic factor (BDNF) polymorphism express lower gray matter volume in the hippocampus and prefrontal cortex compared with carriers of the val/val allele (21–23). In animal models, chronic stress downregulates BDNF, possibly contributing to cellular remodeling (24). Given that the met allele is associated with relatively reduced activity-dependent secretion and intracellular trafficking of pro-BDNF, this allele could plausibly affect the contribution of BDNF to signaling cascades mediating synaptic or cellular plasticity and, potentially, neurogenesis in response to stress exposure. In aggregate, these studies reveal both vulnerability and experience-dependent patterns of hippocampal morphology relevant to stress-related risk for and resilience against ill health.
Within the context of the allostatic load model presented in Figure 2, there are several additional immune-mediated mechanisms involving bidirectional brain-body and body-brain communication patterns that may further account for individual differences in hippocampal plasticity. Growing evidence supports an association between peripheral immune activation and behavioral, affective, and cognitive disturbances. Peripheral proinflammatory cytokines, such as interleukin (IL)-6, represent plausible mediators of these effects, as they can penetrate the blood-brain barrier (25) feedback to the brain via visceral afferent transmission along the vagus nerve (26, 27) to stimulate the production of central proinflammatory cytokines, including IL-6, which are expressed in the hippocampus along with their receptors (28, 29).
Moreover, this central inflammation may adversely affect learning and memory through processes related to neurodegeneration and structural remodeling of the hippocampus in particular. In humans, there is evidence for an inverse association between peripheral levels of IL-6, a relatively stable marker of systemic inflammation, and memory function in midlife adults (30). In extension, peripheral levels of IL-6 have been found to covary inversely with hippocampal and prefrontal gray matter volume (31). However, the mechanisms by which peripheral IL-6 relates to hippocampal gray matter volume and cognition in humans are not yet known; nor are their implications for stress-related processes involved in mediating brain plasticity.
Related to inflammation are metabolic imbalance, oxidative stress (32), and the consequences of diabetes for cognitive function and the hippocampus. Studies of type II diabetes have revealed reduced hippocampal volume that is larger in those subjects with the greatest elevations of glycosylated hemoglobin, indicative of elevated blood glucose levels (33). Mild cognitive impairment in aging is also associated with hippocampal volume reduction that is in turn related to elevated glycosylated hemoglobin levels below the threshold for type II diabetes (34). One of the interventions that can prevent type II diabetes is physical activity, and a recent study shows that fit individuals have larger hippocampal volumes than unfit individuals (35).
The amygdala is also involved in and affected by allostatic processes. This region comprises cell groups in the medial anterior temporal lobe, adjacent to the hippocampus. One function of the amygdala in stressor-related processing is the rapid assignment of emotional salience to environmental events (36). Hence, the amygdala is thought to interrelate perceptual and cortical processes supporting the coordination of stressor-evoked changes in behavior and peripheral physiological reactivity, particularly within the context of adverse environmental conditions and stressors that negatively affect health (12).
Interestingly, the same stressors may affect the hippocampus and amygdala differently. For example, animal studies of amygdala plasticity have shown that chronic immobilization stress of the type that causes retraction of dendrites in the CA3 region of the hippocampus produces dendritic growth in neurons in the basolateral amygdala (37). Moreover, chronic stress of this type not only impairs hippocampus-dependent cognitive function but also enhances amygdala-dependent unlearned fear and fear conditioning processes (38), consistent with the opposite effects of stress on hippocampal and amygdala structures. Chronic stress also increases aggression between animals living in the same cage, and this is likely to reflect hyperactivity of the amygdala (39). Moreover, chronic corticosterone treatment in drinking water has an anxiogenic effect in mice (40), which could be due to the glucocorticoid enhancement of corticotrophin-releasing factor expression in the amygdala (41). Thus, animal studies on the amygdala reveal stress-induced plasticity that relates to aggression and anxiety.
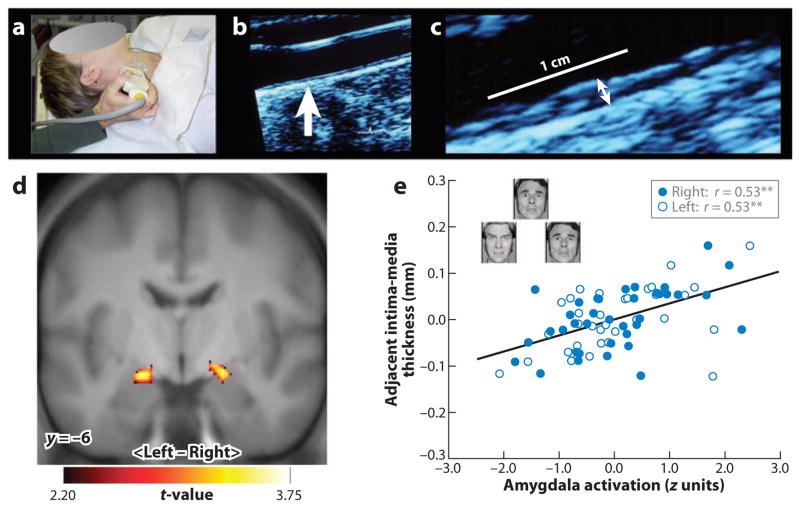
There is human neuroimaging evidence that the amygdala is involved in mediating forms of peripheral or allodynamic stress reactivity that have been linked to prevalent physical health outcomes. For example, individual differences in amygdala reactivity to emotionally salient stimuli have been shown to covary with physiological parameters associated with cardiovascular disease risk, including basal levels of autonomic-cardiac control (42), stressor-evoked changes in blood pressure (43), and diurnal variations in the secretion of the stress hormone, cortisol (44). Recently, it has been demonstrated that individuals who express greater amygdala reactivity to threatening social cues (angry and fearful facial expressions) also exhibit higher levels of preclinical atherosclerosis (Figure 3) (45). In that study, individuals who showed lower levels of preclinical atherosclerosis exhibited a pattern of dynamic functional connectivity (correlated activity) between the amygdala and prefrontal cortex that suggested a potentially greater regulation of amygdala activity by the prefrontal cortex during threat processing. These findings are noteworthy from a clinical perspective because the amygdala and its functional interactions with the prefrontal cortex have long been implicated in conferring risk for psychopathologies of mood and anxiety (46), which are highly comorbid with atherosclerotic cardiovascular disease (47). Further, functional aspects of the prefrontal cortex in particular have been recently implicated in atherogenesis in a primate model of comorbid depression and cardiovascular disease (48).
Figure 3.
In a functional neuroimaging study, a measure of preclinical atherosclerosis was related to activation of the amygdala in response to threatening facial expressions. Carotid intima-media thickness (IMT) was assessed by B-mode ultrasonography. (a) A trained vascular technologist imaged the carotid arteries. (b) An image of the carotid artery with the common carotid segment visible at right and the beginning of the carotid bulb visible at left; arrow indicates a point along the far wall of the common carotid artery, which is shown at higher magnification in panel c. (c) Magnified point of the far wall of the common carotid artery illustrating the lumen-intima and media-adventitia interfaces. IMT was measured by averaging the IMT (the distance between two lines tracking the lumen-intima and media-adventitia interfaces, not illustrated here) in 1-mm increments along the distal 1 cm of the far wall of the common carotid artery, the far wall of the carotid bulb, and the first centimeter of the internal carotid artery. As assessed by this ultrasound procedure, carotid IMT covaried positively with amygdala reactivity to angry and fearful facial expressions in an adult sample of otherwise healthy humans. (d) Statistical parametric maps from a regression analysis identifying regions of the left and right amygdala where carotid IMT covaried with amygdala reactivity after controlling for age, sex, resting systolic blood pressure, and family income. (e) Adjusted IMT values are shown as a function of amygdala activation values of the left (open circles) and right (closed circles) amygdala areas profiled in panel d. Insets in panel e illustrate sample trials from the facial-expression protocol designed to elicit amygdala reactivity. **p < 0.001.
Areas of the prefrontal cortex that are strongly implicated in allostatic processes are the orbital and medial prefrontal cortex and the anterior cingulate cortex. Along with many other brain regions, the prefrontal cortex has adrenal steroid receptors (12); however, the role of adrenal steroids, excitatory amino acids, and other mediators has not yet been studied in detail in the prefrontal cortex, in contrast to the hippocampus. Nevertheless, glucocorticoids do appear to play a role: Three weeks of chronic corticosterone treatment produced retraction of dendrites in medial prefrontal cortex (49), although there were subtle qualitative differences from the effect of chronic restraint stress (50). Another study determined the effect of adrenalectomy, compared to four weeks of chronic treatment with either corticosterone or dexamethasone, on volume and neuron number in the prefrontal cortex (51). Dexamethasone treatment at a dose that may have been high enough to enter the brain (although this was not directly measured) caused a loss of neurons in Layer II of the infralimbic, prelimbic, and cingulate cortex, whereas corticosterone treatment reduced the volume but not the neuron number of these cortical regions (51). Dexamethasone treatment was particularly effective in impairing working memory and cognitive flexibility, as measured by a working-memory task in a Morris water maze (51). Effects of chronic stress were not investigated in this study. These data notwithstanding, the cautions expressed above concerning differences between chronic stress and chronic glucocorticoid treatment must be kept in mind for the prefrontal cortex, as well as the amygdala, which has not been studied yet in this regard.
Behavioral correlates of remodeling induced by chronic restraint stress in the prefrontal cortex include impairments in cognitive flexibility and decision making, possibly reflecting structural remodeling in the medial prefrontal cortex (52, 53). Thus, animal studies on the prefrontal cortex reveal stress-induced changes in neuronal structure and connectivity. The medial prefrontal cortex shows reduced neuronal complexity and loss of synaptic connections as a result of repeated stress, whereas the orbitofrontal cortex shows greater neuronal complexity as a result of chronic stress (52).
From a translational perspective developed within the context of these animal findings, stress-related dimensions of human life could thus plausibly covary with changes in the morphology of the prefrontal cortex in humans. In support of this notion, there is structural neuroimaging evidence in humans that individuals who report holding a low social standing in the United States, which is thought to be a correlate of chronic stress, show a reduced gray matter volume in the anterior cingulate portion of the prefrontal cortex (54).
Interventions for Allostatic Load and Brain-Body Medicine
The concept that the brain is the central organ of stress, with downward influences on many physiological processes involved in adaptation, provides a basis for understanding how interventions that are integrative (or “holistic” insofar as they stimulate the entire body to help itself to function normally) can have enormous preventative and therapeutic benefits by targeting a person's interpersonal relationships and lifestyle. Other interventions can alter the social environment through policies of the government and private sector that provide groups of individuals with access to and control over environmental, social, and material resources that are important for health and well-being.
Two of the most important interventional approaches target physical activity and social integration. Whereas a sedentary lifestyle is a major risk factor for many of the diseases of modern life, including obesity, diabetes, cardiovascular disease, depression, and dementia, moderate physical activity can be beneficial for the brain and the cardiovascular and metabolic systems (see 12 for review). In animal models, voluntary physical activity has been shown to increase expression of neurotrophic growth factors in the cortex and hippocampus, as well as to increase neurogenesis in the dentate gyrus of both young and aging animals (see 2 for review). Besides improving memory, physical activity appears to have antidepressant effects, similar to the actions of antidepressant drugs (12).
An increasing number of human neuroimaging studies are beginning to examine these processes in relation to human cognition and brain structure and function (12). An aerobic exercise program, involving regular and brisk walking over 6–12 months, improved cognitive performance and postintervention patterns of brain activation in the prefrontal and parietal cortices that were comparable to those displayed by a much younger control group. More recent neuroimaging studies report an increase in the volume of gray matter in the prefrontal and temporal cortices (55), increased cerebral blood volume in the dentate gyrus of the hippocampus among middle-aged individuals who completed a three-month aerobic exercise program (56), and larger hippocampal volumes in fit than unfit individuals (35). In contrast to these beneficial effects of exercise, gaining greater amounts of weight in middle age is associated with reduced brain tissue volume (57).
In addition to aerobic exercise, dimensions of social relationships have long been linked to longevity and aspects of physical and mental health (58; for review, see 12). These include social network composition, social support, social interaction frequency and quality, and the experience of isolation and loneliness accompanying deficient or broken social relationships. One important dimension of social life is social integration. Epidemiological studies have associated measures of social integration with life-span, trajectories of cognitive aging and risk for dementia, severity of subclinical cardiovascular disease, risk for stroke, survival times in patients with cardiovascular disease, and the recurrence of cancer (12, 58). In addition to social integration, social support helps individuals cope more adaptively with acute and chronic stressors. For example, the social support provided by family or health professionals who offer emotional support and useful information has been shown to reduce an epidemiological summary measure of allostatic load, which encompasses key physiological markers related to chronic stress and a potentially health-damaging lifestyle (59). Social support also ameliorates reported levels of chronic stress in caregivers, who show a reduced length of telomeres in white blood cells (60).
For the individual, lifestyle behaviors and habits may be hard to change, and it is often necessary to turn to pharmacological interventions. Sleeping pills, anxiolytics, beta blockers, and antidepressants are all used to counteract problems associated with allostatic overload. Likewise, drugs that reduce oxidative stress or inflammation, block cholesterol synthesis or absorption, and treat insulin resistance or chronic pain can help deal with the metabolic and neurological consequences of chronically stressful experiences. All of these agents have value, but each one has side effects and limitations that are based in part on the fact that all of the systems that are dysregulated in allostatic overload interact with each other and perform normal functions when properly regulated.
In promoting interventions that affect brain-body health, employers have a powerful role. For example, businesses that encourage healthy lifestyle practices among their employees could have a dramatic impact on the prevalence and course of many costly medical conditions, thus reducing health insurance costs and perhaps even gaining a more loyal workforce (61, 62). Governmental policies regarding education, housing, taxation, a minimum wage, occupational health and safety, and environmental pollution may all affect brain-body health at a population level; these effects are likely to be especially powerful for the health of children, impacting cognitive development, future academic achievement, and physical health (see 12). For the elderly, community centers and activities that promote social interactions and physical activity have been demonstrated to be beneficial across a number of domains (63, 64).
A program that exemplifies combining education, physical activity, and social engagement—along with one other ingredient that is hard to quantify, namely, finding meaning and purpose in life—is the Experience Corps. This program trains elderly volunteers as teachers' assistants for younger children in neighborhood schools. Not only does this program improve the education of the children, it also benefits elderly volunteers and improves their physical and mental health. A pilot study (65) reports gains in executive function and prefrontal cortical activity in the older adult volunteers who are at elevated risk for cognitive impairment. Holistic programs, such as this, should serve as models of the kinds of interventions that can dramatically affect the course of chronic and prevalent health conditions via allodynamic brain mechanisms.
Summary Points.
Stressful experiences affect risk for and resilience against physical and mental illnesses that are both prevalent and often comorbid with one another. Stressful experiences are not uniformly health impairing. In the short term, they can lead to growth, adaptation, and new learning. In the long term, however, they become problematic for health when they are chronic, uncontrollable, unpredictable, and difficult to cope with because of a lack of supportive personal, social, and environmental resources.
Allostatic systems enable the individual to cope with stressful experiences. They are adaptive when rapidly mobilized and terminated. However, when the activity of allostatic systems is sluggish, ineffective, prolonged, or not terminated promptly, allostatic systems can impair mental and physical health through their maladaptive effects on brain plasticity and metabolic, immune, and cardiovascular pathophysiology (allostatic load).
The adult, as well as the developing, brain shows structural as well as neurochemical plasticity and resilience with all experiences, including those that are stressful. Animal models of brain plasticity are providing neuroanatomical details, as well as evidence for regional specificity and cellular and molecular mechanisms. Modern neuroimaging techniques are providing converging details and evidence in the human brain. Loss of resilience is a key feature of disorders of stress adaptation, e.g., anxiety, depression. Reversibility of maladaptive forms of stress-related brain plasticity is possible, and this reversibility may underpin many forms of treatment efficacy.
The brain is the central organ of stress and adaptation. It regulates and responds to the mediators of allostasis, which are normally involved in adaptation but which, when dysregulated and overused, lead to wear-and-tear on the brain and body (allostatic load). Interventions to alleviate allostatic load include improving diet, promoting regular physical activity, increasing access to social support and integration, and changing policies of the government and private sector to improve quality of life, particularly for the disadvantaged.
Specific areas of the brain that show several forms of plasticity, are involved in allostasis, are affected by allostatic load, and are implicated in stress-related vulnerability to chronic health conditions include regions of the prefrontal cortex, hippocampus, and amygdala. These brain areas represent the primary targets of preventative and intervention efforts to reduce the public health burden of mental and physical illnesses.
Future Issues.
As illustrated by its response to increased physical activity, the human brain has a considerable degree of plasticity and resilience. An important task for future research will be to delineate the biological pathways by which physical activity affects aging and health in the brain and body.
Cognitive behavioral therapy has been demonstrated to be as efficacious as several medication regimens aimed at treating disorders of mood, particularly depression; moreover, cognitive therapy and medication appear to affect many of the same or overlapping neural mechanisms (68). There is recent evidence that successful cognitive therapy can produce changes in brain morphology that parallel those of physical activity, particularly within the context of chronic fatigue syndrome—a brain-body disorder characterized by unabating or recurrent fatigue adversely affecting allostatic control systems (69). Studies on animal models reveal that the amygdala shows neuronal growth after chronic stress, and imaging studies on the human brain demonstrate hyperactive amygdala function in mood and anxiety disorders. A recent longitudinal magnetic resonance imaging study investigated the relationship between changes in the perceived stress scale and changes in amygdala gray matter density following a stress-reduction intervention (70). Reductions in perceived stress correlated positively with decreases in right basolateral amygdala gray matter density, a finding that is consistent with the reported ability of chronic stress to increase dendritic branching in the basolateral amygdala. Therefore, further longitudinal studies of how the brain is changed by behavioral, as well as by pharmaceutical, therapies are important future directions.
There are currently limited data on whether and how social integration, social support, or other social factors may benefit human brain circuits that are affected by chronic stress and allostatic load, although it is clear that these factors are linked to mood, overall mental health, and related brain-based processes (58). An important direction for future research will be to delineate the pathways by which dimensions of social relationships and networks may affect brain and bodily aging and health, and to design interventions impacting health-related aspects of social ties.
Related Resources.
Online documentation regarding available measurement methods for the study of health behavior, sleep, and behavioral and biological aspects of stress are available through the Pittsburgh Mind-Body Center, http://pmbcii.psy.cmu.edu/.
Online documentation regarding available measurement methods for the study of stress and health processes linked to socioeconomic status are available through the MacArthur Research Network on Socioeconomic Status and Health, http://www.macses.ucsf.edu/.
Online documentation regarding brain health in relation to healthy brain development and the lasting effects of adverse early life experiences may be found through the National Scientific Council on the Developing Child, http://developingchild.harvard.edu/initiatives/council/.
Acknowledgments
B.S.M. is supported by National Institutes of Health (NIH) grants R01 MH41256 and 5P01 MH58911. P.J.G. is supported by NIH grants K01 MH070616 and R01 HL089850. The authors are grateful for the support and comments of members of the John D. and Catherine T. MacArthur Research Network on Socioeconomic Status and Health.
Glossary
- Brain plasticity
the mutability of brain structure and function as a result of experience
- Homeostasis
a process wherein physiological parameters, such as blood oxygen and pH, are maintained in a narrow range
- HPA
hypothalamic-pituitary-adrenal
- Allostasis
the active process of responding to a challenge to the body by triggering chemical mediators of adaptation (HPA, autonomic, metabolic, immune) that operate in a nonlinear network. Allostasis is essential for maintaining homeostasis in the face of challenges or demands imposed by changes in (a) the environment and (b) an individual's behavioral state that are registered by the brain
- Allostatic load
the wear-and-tear on the body and brain that results from chronic dysregulation (overactivity or inactivity) of mediators of allostasis
- Hippocampus
a brain region in the medial temporal lobe that is instrumental for learning and remembering declarative and spatial information, processing the contextual aspects of emotional events, and regulating visceral functions, including the HPA axis
- Amygdala
a brain region in the medial anterior temporal lobe, adjacent to the hippocampus. It rapidly assigns emotional significance to environmental events, and it regulates physiological and behavioral responses to those events
- Prefrontal cortex
a large brain region occupying the anterior portion of the frontal lobe, connected with the hippocampus. It is broadly involved in higher cognitive functions (e.g., working memory and executive control), as well as the control of emotion, mood, stress functions, and impulsive actions
- Social integration
an individual's effortful engagement in social activities and relationships, cognitive construal of her or his communality, and identification with diverse social roles
- Social support
psychological and material resources provided by one's social ties
Footnotes
Disclosure Statement: The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Literature Cited
- 1.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–88. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 3.Lazarus RS, Folkman S, editors. Stress, Appraisal and Coping. New York: Springer-Verlag; 1984. [Google Scholar]
- 4.Knudsen EI, Heckman JJ, Cameron JL, et al. Economic, neurobiological, and behavioral perspectives on building America's future workforce. Proc Natl Acad Sci USA. 2006;103:10155–62. doi: 10.1073/pnas.0600888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–79. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dial Clin Neurosci Stress. 2006;8:367–81. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. New York: Wiley; 1988. pp. 629–49. [Google Scholar]
- 8.McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Arch Int Med. 1993;153:2093–101. [PubMed] [Google Scholar]
- 9.Schulkin J, McEwen BS, Gold PW. Allostasis, amygdala, and anticipatory angst. Neurosci Biobehav Rev. 1994;18:385–96. doi: 10.1016/0149-7634(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 10.Sapolsky R, Krey L, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 11.Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–52. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 12.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann NY Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starkman MN, Gebarski SS, Berent S, et al. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing's syndrome. Biol Psychiatry. 1992;32:756–65. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- 14.Starkman MN, Giordani B, Gebrski SS, et al. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing's disease. Biol Psychiatry. 1999;46:1595–602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- 15.Starkman MN, Giordani B, Gebarski SS, et al. Improvement in learning associated with increase in hippocampal formation volume. Biol Psychiatry. 2003;53:233–38. doi: 10.1016/s0006-3223(02)01750-x. [DOI] [PubMed] [Google Scholar]
- 16.Vermetten E, Vythilingam M, Southwick SM, et al. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 18.Lucassen PJ, Meerlo P, Naylor AS, et al. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Gianaros PJ, Jennings JR, Sheu LK, et al. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. NeuroImage. 2007;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganzel BL, Kim P, Glover GH, et al. Resilience after 9/11: multimodal neuroimaging evidence for stress-related change in the healthy adult brain. Neuroimage. 2008;40:788–95. doi: 10.1016/j.neuroimage.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bueller JA, Aftab M, Sen S, et al. BDNF Val66 Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59:812–15. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Szeszko PR, Lipsky R, Mentschel C, et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10:631–36. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- 23.Pezawas L, Meyer-Lindenberg A, Goldman AL, et al. Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Mol Psychiatry. 2008;13:709–16. doi: 10.1038/mp.2008.32. [DOI] [PubMed] [Google Scholar]
- 24.Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Science's STKE. 2004;225:1–11. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- 25.Banks WA, Kastin AJ. Blood to brain transport of interleukin links the immune and central nervous systems. Life Sci. 1991;48:PL117–21. doi: 10.1016/0024-3205(91)90385-o. [DOI] [PubMed] [Google Scholar]
- 26.Maier SF, Goehler LE, Fleshner M, et al. The role of the vagus nerve in cytokine-to-brain communication. Ann NY Acad Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- 27.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–59. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 28.Rosell DR, Nacher J, Akama KT, McEwen BS. Spatiotemporal distribution of GP130 cytokines and their receptors after status epilepticus: comparison with neuronal degeneration and microglial activation. Neuroscience. 2003;122:329–48. doi: 10.1016/s0306-4522(03)00593-1. [DOI] [PubMed] [Google Scholar]
- 29.Rosell DR, Akama KT, Nacher J, et al. Differential expression of suppressors of cytokine signaling-1, -2, and -3 in the rat hippocampus after seizure: implications for neuromodulation by GP130 cytokines. Neuroscience. 2003;122:349–58. doi: 10.1016/s0306-4522(03)00594-3. [DOI] [PubMed] [Google Scholar]
- 30.Marsland AL, Petersen KL, Sathanoori R, et al. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosom Med. 2006;68:895–903. doi: 10.1097/01.psy.0000238451.22174.92. [DOI] [PubMed] [Google Scholar]
- 31.Marsland AL, Gianaros PJ, Abramowitch SM, et al. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–90. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bierhaus A, Schiekofer S, Schwaninger M, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-κB. Diabetes. 2001;50:2792–808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 33.Gold SM, Dziobek I, Sweat V, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–19. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- 34.Convit A, Wolf OT, Tarshish C, et al. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc Natl Acad Sci USA. 2003;100:2019–22. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erickson KI, Prakash RS, Voss MW, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–39. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 37.Vyas A, Mitra R, Rao BSS, et al. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–18. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conrad CD, Magarinos AM, LeDoux JE, et al. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–13. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 39.Wood GE, Norris EH, Waters E, et al. Chronic immobilization stress alters aspects of emotionality and associative learning in the rat. Behav Neurosci. 2008;122:282–92. doi: 10.1037/0735-7044.122.2.282. [DOI] [PubMed] [Google Scholar]
- 40.Ardayfio P, Kim K-S. Anxiogenic-like effect of chronic corticosterone in the light-dark emergency task in mice. Behav Neurosci. 2006;120:249–56. doi: 10.1037/0735-7044.120.2.249. [DOI] [PubMed] [Google Scholar]
- 41.Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–12. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- 42.Neumann SA, Brown SM, Ferrell RE, et al. Human choline transporter gene variation is associated with corticolimbic reactivity and autonomic-cholinergic function. Biol Psychiatry. 2006;60:1155–62. doi: 10.1016/j.biopsych.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 43.Gianaros PJ, Sheu LK, Matthews KA, et al. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci. 2008;28:990–99. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupling during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gianaros PJ, Hariri AR, Sheu LK, et al. Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol Psychiatry. 2009;65:943–50. doi: 10.1016/j.biopsych.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 47.Grippo AJ, Johnson AK. Biological mechanisms in the relationship between depression and heart disease. Neurosci Biobehav Rev. 2002;26:941–62. doi: 10.1016/s0149-7634(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 48.Shively CA, Musselman DL, Willard SL. Stress, depression, and coronary artery disease: modeling comorbidity in female primates. Neurosci Biobehav Rev. 2009;33:133–44. doi: 10.1016/j.neubiorev.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–53. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 50.Radley JJ, Sisti HM, Hao J, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Cerqueira JJ, Pego JM, Taipa R, et al. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25:7792–800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liston C, Miller MM, Goldwater DS, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–74. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dias-Ferreira E, Sousa JC, Melo I, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–25. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 54.Gianaros PJ, Horenstein JA, Cohen S, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cogn Affect Neurosci. 2007;2:161–73. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–70. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 56.Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104:5638–43. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soreca I, Rosano C, Jennings JR, et al. Gain in adiposity across 15 years is associated with reduced gray matter volume in healthy women. Psychosom Med. 2009;71:485–90. doi: 10.1097/PSY.0b013e3181a5429d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen S, Janicki-Deverts D. Can we improve our health by altering our social relationships? Perspect Psychol Sci. 2009;4:375–78. doi: 10.1111/j.1745-6924.2009.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seeman TE, Singer BH, Ryff CD, et al. Social relationships, gender, and allostatic load across two age cohorts. Psychosom Med. 2002;64:395–406. doi: 10.1097/00006842-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 60.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312–15. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aldana SG. Financial impact of health promotion programs: a comprehensive review of the literature. Am J Health Promotion. 2001;15:296–320. doi: 10.4278/0890-1171-15.5.296. [DOI] [PubMed] [Google Scholar]
- 62.Pelletier KR. A review and analysis of the clinical- and cost-effectiveness studies of comprehensive health promotion and disease management programs at the worksite: 1998–2000 update. Am J Health Promotion. 2001;16:107–15. doi: 10.4278/0890-1171-16.2.107. [DOI] [PubMed] [Google Scholar]
- 63.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age or older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 64.Rovio S, Kareholt I, Helkala E-L, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4:705–11. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 65.Carlson MC, Erickson KI, Kramer AF, et al. Evidence for neurocognitive plasticity in at-risk older adults: the Experience Corps program. J Gerontol. 2009;64:1275–82. doi: 10.1093/gerona/glp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alves S, Weiland NG, Hayashi S, et al. Immunocytochemical localization of nuclear estrogen receptors and progestin receptors within the dorsal raphe nucleus. J Comp Neurol. 1998;391:322–34. [PubMed] [Google Scholar]
- 67.Acheson SD. Independent Inquiry into Inequalities in Health Report. London: Stationary Off.; 1998. [Google Scholar]
- 68.DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9:788–96. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Lange FP, Koers A, Kalkman JS, et al. Increase in prefrontal cortical volume following cognitive behavioural therapy in patients with chronic fatigue syndrome. Brain. 2008;131:2172–80. doi: 10.1093/brain/awn140. [DOI] [PubMed] [Google Scholar]
- 70.Holzel BK, Carmody J, Evans KC, et al. Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci. 2010;5:11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kirschbaum C, Pruessner JC, Stone AA, et al. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med. 1995;57:468–74. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]