Abstract
Introduction
The aim of this study was to evaluate a new thyroidectomy difficulty scale (TDS) for its inter-rater agreement, correspondence with operative times, and correlation with complications.
Methods
We developed a four item, 20-point TDS. Following cases where two board-certified surgeons participated, each surgeon completed a TDS, blinded to the other’s responses. Paired sets of TDS scores were compared. The relationship between operative time and TDS scores was analyzed with linear regression. Multiple regression evaluated the association of TDS scores and other clinical data with operative times.
Results
A total of 119 patients were scored using TDS. In this cohort, 22.7% suffered from hyperthyroidism, 37.8% experienced compressive symptoms, and 58.8% had cancer. The median total TDS score was 8, and both surgeons’ total scores exhibited a high degree of correlation. 87.4% of both raters’ total scores were within one point of each other. Patients with hyperthyroidism received higher median scores compared to euthyroid patients (10 vs. 8, p<0.01). Similarly, patients who suffered a complication had higher scores compared to those patients without complications (10 vs. 8, p= 0.04). TDS scores demonstrated a linear relationship with operative times (R2 = 0.36, p<0.01, Figure 1). Cases with a score of 14 or greater took 41.0% longer compared to cases with scores of five or less (p<0.01). In multiple regression analysis, TDS scores independently predicted operative time (p<0.01).
Conclusion
The TDS is an accurate tool, and scores correlate with more difficult thyroidectomies as measured by complications and operative times.
Keywords: thyroidectomy, difficulty, operative time, hyperthyroidism
Introduction
During the late 19th and early 20th century, thyroidectomy was associated with a 50% mortality and equally high morbidity. This led many medical experts to consider thyroid surgery barbaric. Samuel Gross referred to thyroidectomy as “horrid butchery,” and it was banned by the French medical society due to its high mortality (1).
With the advent of aseptic technique and an improved understanding of thyroid physiology, thyroid surgery has become much safer. Theodor Kocher achieved a mortality rate of 1% and won the Nobel Prize in 1909 for advancing thyroid surgery (1). Today, thyroidectomy is associated with virtually zero mortality and an extremely low morbidity when performed by high volume surgeons (2–4). Yet, complications from thyroidectomy can be life-altering. These include injury to the recurrent laryngeal nerves causing hoarseness and dysphagia, injury to the parathyroid glands causing hypocalcemia, in addition to neck hematoma. Fortunately, the risk of permanent complications range from 1–2% in experienced hands (5, 6). Rates of temporary complications are much higher, but vary depending on the indication for thyroidectomy, patient factors, and concomitant lymph node dissection. For example, the reported rates of transient hypocalcemia after total thyroidectomy range from 5 to 22% (7–9).
Thyroid surgeons associate certain thyroid disease processes with a more difficult resection and higher complication rates. These include thyroidectomy for Graves’ disease, Hashimoto’s thyroiditis, thyromegaly, or widely invasive thyroid carcinomas (10–14). For example, transient complications range from 12 – 38% for patients with Hashimoto’s thyroiditis (11, 12, 15), and 11–28% (10, 16, 17) for Graves’ disease. Permanent complications are also reported to be higher in these subsets. Complication rates, blood loss, and operative time serve as surrogates for difficulty. Difficulty scales have been developed for other types of procedures, often as a means to quantify the learning curve (18–21). The notion of difficulty in thyroid surgery literature remains subjective, and is limited to case reports, opinion, and technique papers (22–26). Traditionally cited factors contributing to difficulty in thyroid surgery include increased vascularity, inflammation, friability, fibrosis, and large gland size (12, 13, 27).
Currently, there are no measures of thyroidectomy difficulty as there are for other operations. This requires a more objective measurement of difficulty and evidence-based identification of patient and disease factors associated with difficulty. The purpose of this study was to evaluate a novel thyroidectomy difficulty scale (TDS) for its inter-rater agreement, correspondence with operative times, and correlation with complications.
Methods
This was a prospective study performed at a single, tertiary care academic center between 2012–2013. We developed a four item (vascularity, friability, mobility/fibrosis, gland size), 20-point TDS where each item is graded on a scale from 1–5 (Figure 1). Following cases where two board-certified surgeons participated, each surgeon completed a TDS, blinded to the other’s responses. Participating surgeons were at the fellowship level of training or attending faculty members. Paired sets of TDS scores were compared using Spearman’s rank correlation coefficient and inter-rater agreement was evaluated with the kappa statistic. Patient co-morbidities, disease characteristics, and preoperative labs were obtained from an existing endocrine surgery database. Operative times were obtained from the electronic medical record and calculated as the time from incision until wound closure.
Figure 1. Thyroidectomy Difficulty Scale (TDS).

Scoring sheet for the Thyroidectomy Difficulty Scale (TDS).
Patients with any type of thyroid carcinoma greater than 1 cm on final histopathology were considered to have a diagnosis of cancer. Any patients with an undetectable TSH on preoperative labs or treated with anti-thyroid medications were considered to have hyperthyroidism. Compressive symptoms included pain or pressure in the neck, dysphagia, dysphonia, or respiratory symptoms. Complications included hoarseness, hypoparathyroidism, and neck hematoma. Hypoparathyroidism was defined as a PTH < 10 pg/ml. These complications were categorized as transient if they resolved within six months and permanent if they persisted beyond 6 months.
The relationship between operative time and TDS scores was analyzed with linear regression. Binary comparisons were made using the student’s t-test, Chi-squared test, or the Mann-Whitney U test where appropriate. Multiple regression evaluated the association of TDS scores and other clinical data with operative times. All analyses were performed using STATA v. 12.1 (StataCorp, College Station, TX). The University of Wisconsin’s Institutional Review Board approved this study.
Results
Pre-operative characteristics
There were 119 patients included in this study. The mean age was 51.6 ± 3.8 years, and 75.6% were female (Table 1). This cohort had a median of two co-morbidities, and 10.9% were current smokers. The thyroid pathology included hyperthyroidism (22.7%), Hashimoto’s thyroiditis (20.2%), and thyroid cancer (21.0%), although these categories were not mutually exclusive (Table 1). 45 patients (37.8%) reported compressive symptoms from their thyroid disease (Table 1).
Table 1.
Patient Characteristics (n = 119)
| Variable | Number (%) |
|---|---|
| Age | 51.6 ± 3.8 |
| Female | 90 (75.6%) |
| BMI (kg/m2) | 29.5 ± 2.6 |
| Smoker | 13 (10.9) |
| Pregnant | 1 (0.8%) |
| Median # co-morbidities | 2 ± 1 |
| Cancer | 25 (21.0%) |
| Hyperthyroidism | 27 (22.7%) |
| Hashimoto’s thyroiditis | 24 (20.2%) |
| Compressive symptoms | 45 (37.8%) |
Data are expressed as a mean ± standard error of the mean for continuous variables and the count with the percentage in parentheses for categorical variables. The categories cancer, hyperthyroidism, Hashimoto’s thyroiditis are not mutually exclusive.
BMI = body mass index
TDS Scores
The mean total TDS score was 8.6 with a range from 4 to 17 (Table 2). When examining the score profiles for patients with various thyroid pathologies, patients with hyperthyroidism tended to score higher in the vascularity (3.0 ± 1.1) and had the highest overall scores (10.0 ± 2.8, Table 2). Those with Hashimoto’s thyroiditis tended to score higher in the mobility/fibrosis category (2.5 ± 1.1, Table 2). Compared to euthyroid patients, those with hyperthyroidism received higher median scores (10.1 vs. 8.1, p = 0.02).
Table 2.
TDS Scores
| TDS Item | Mean Score | Standard Deviation |
|---|---|---|
| Overall Cohort | ||
| Vascularity | 2.2 | 1.1 |
| Friability | 1.9 | 1.0 |
| Mobility/Fibrosis | 2.0 | 1.0 |
| Gland Size | 2.5 | 1.1 |
| Total Score | 8.6 | 2.9 |
| Hashimoto’s | ||
| Vascularity | 2.3 | 1.1 |
| Friability | 1.7 | 0.8 |
| Mobility/Fibrosis | 2.5 | 1.1 |
| Gland Size | 2.1 | 0.9 |
| Total Score | 8.5 | 3.1 |
| Hyperthyroidism | ||
| Vascularity | 3.0 | 1.1 |
| Friability | 2.5 | 1.0 |
| Mobility/Fibrosis | 2.2 | 1.0 |
| Gland Size | 2.5 | 0.9 |
| Total Score | 10.0 | 2.8 |
Inter-rater agreement
For individual items on the TDS, exact agreement between surgeons ranged from 62.2 to 68.9%. Inter-rater agreement as measured by Cohen’s Kappa (κ) ranged from 0.44 to 0.59, indicating moderate to good agreement between raters (Table 3). Importantly, the agreement was significantly better than chance alone for each item on the TDS (Table 3).
Table 3.
Inter-Rater Agreement
| TDS Item | Median Score | Agreement (%) | Expected Agreement (%) | Inter-Rater Agreement (κ) | p |
|---|---|---|---|---|---|
| Vascularity | 3 | 62.2 | 26.7 | 0.48 | <0.01 |
| Friability | 1 | 62.2 | 32.5 | 0.44 | <0.01 |
| Mobility/Fibrosis | 2 | 63.1 | 30.2 | 0.46 | <0.01 |
| Gland Size | 3 | 68.9 | 24.1 | 0.59 | <0.01 |
For each item on the TDS scale, the average score between the two surgeons is displayed along with measures of agreement. Cohen’s kappa (κ) is a statistic that evaluates the agreement between two raters where zero indicates an agreement no better than chance and 1 indicates perfect agreement. Also displayed is the percentage of exact agreement (i.e, the exact same score) and the percentage of expected agreement due to chance alone.
TDS = thyroidectomy difficulty scale
While the above measures account for instances where the two surgeons gave the exact same score, the two surgeons’ scores correlated well, even if the two scores were not identical. Both surgeons’ total scores exhibited a high degree of correlation (Spearman’s rho = 0.92, p<0.001). 87.4% of both raters’ total scores were within one point of each other (Figure 2).
Figure 2. Cross Tabulation of Each Surgeon’s TDS Scores.
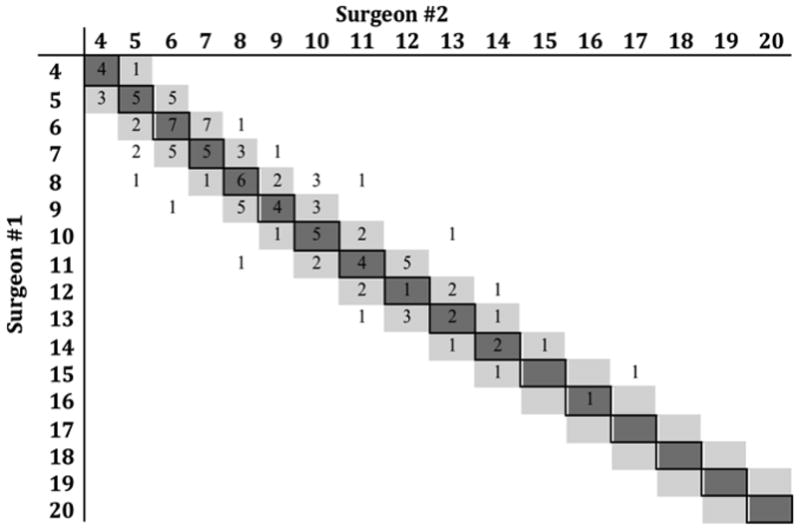
Cells with dark shading indicate exact correlation while lighter shaded cells indicate scores within one point of each other. Numbers in each cell indicate the number of patients with each set of scores.
Correlation with Operative Time
TDS scores demonstrated a linear relationship with operative times (R2 = 0.36, p<0.01, Figure 3). Cases with a score of 14 or greater took 41.0% longer compared to cases with scores of five or less (p<0.01).
Figure 3. TDS Scores Correlate with Operative Time.
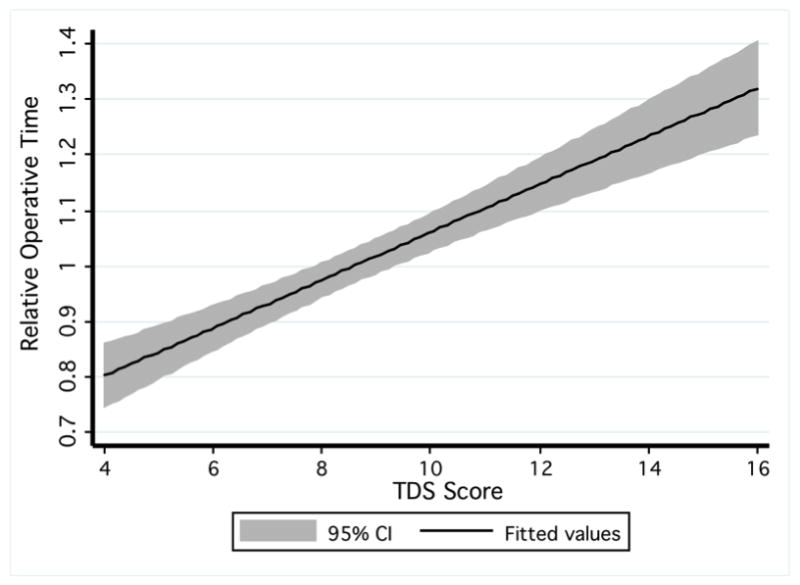
The line represents the linear regression between TDS scores and the adjusted operative times. The shaded area indicates the 95% confidence interval for the regression equation.
In multiple regression analysis, TDS scores were independently associated with operative time when controlling for other clinical, laboratory, and patient factors (p<0.01). Preoperative thyroglobulin levels were also significant in this model (p<0.01).
Complications
There were 23 complications (19.33%) that occurred in this cohort. Of these 23 patients, 8 (34.8%) experienced temporary hoarseness, 14 (60.9%) had temporary hypocalcemia, and 1 patient (4.3%) was re-operated on for neck hematoma. None of the patients in this cohort experienced a permanent complication. Patients who suffered a complication had higher TDS scores compared to those patients without complications (10 vs. 8, p= 0.04). There was a step-wise increase in the percentage of complications as TDS scores increased (Figure 4).
Figure 4. TDS Scores Correlate with Complications.
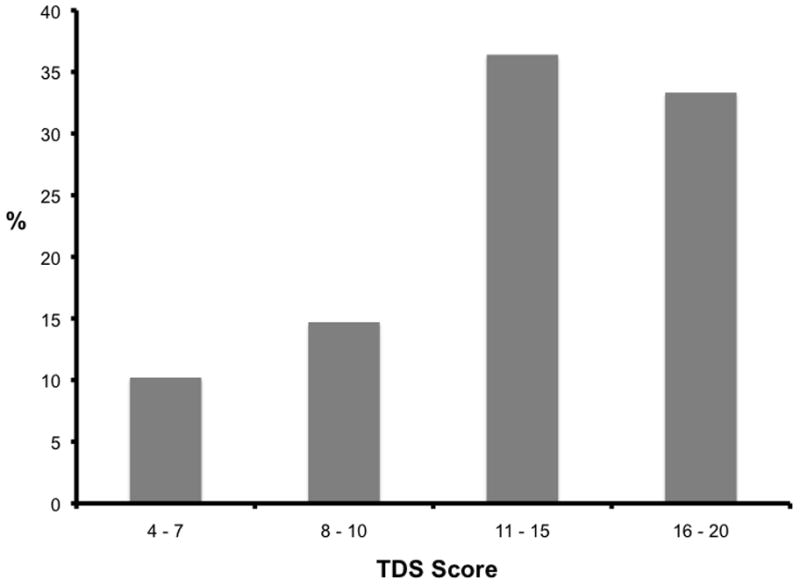
The percentage of complications within each Thyroidectomy Difficulty Scale (TDS) score range is shown.
Discussion
The TDS presented here had good inter-rater agreement for a variety of thyroid diseases. Importantly, TDS scores correlated with operative times and the number of complications. Therefore, TDS can serve as a useful tool to identify the patient and disease characteristics associated with a difficult thyroidectomy. This will enable future research on factors contributing to operative difficulty.
TDS is novel. Several difficulty scales exist for other operations such as laparoscopy, cataract surgery, or aneurysm repair (18, 19, 28). In thyroid surgery, literature on difficulty is somewhat sparse. The thyroid surgery literature does, however, include numerous examples of each individual item that comprises the TDS. For example, the benefit of Lugol’s potassium iodide drops in decreasing the thyroid’s vascularity and friability in Graves’ disease is still debated (29, 30). Fibrosis, another component of the TDS, is seen in Hashimoto’s thyroiditis, and contributes to higher complication rates (12). Finally, larger gland size contributes to higher complication rates, specifically airway complications and transient hypocalcemia (13, 15, 24). The TDS incorporates all of these aspects of difficulty—size, vascularity, friability, and gland size—to create a single composite score of difficulty.
Measuring difficulty is important since it correlated with increased complication rates (Figure 4). We have also shown that thyroidectomy was more difficult in patients with hyperthyroidism (Table 2). Since measurement of difficulty can only occur intra- or post-operatively, the surgeon cannot immediately use TDS as a preoperative prediction tool. Further analysis of the specific factors contributing to difficulty will allow improved preoperative risk counseling for patients undergoing thyroidectomy. In order to do this, we first needed a valid, objective measurement of difficulty; this report supports the use of TDS as such a validated measure of difficulty. The next step in this research will be to identify preoperative lab values, medications, co-morbidities, or patient demographics that predict more difficult operations (higher TDS scores), and therefore, a greater chance for complications. Further study of the factors contributing to difficulty using the TDS tool can allow a more personalized assessment of the perioperative risks. TDS is a valuable tool that will allow this type of research.
TDS scores also correlated with operative times. If TDS can improve our understanding of the specific factors contributing to difficulty, then it can be used to better predict operative times. This is especially important as more thyroid surgeons perform thyroidectomy on an outpatient basis (6, 31). Increased efficiency and timeliness characterize most outpatient surgery centers (32). Future use of TDS can help identify cases that would require more time, and allow better appropriation of OR time. Safety of outpatient thyroidectomy remains a concern. Although there are newer methods for managing hypocalcemia as an outpatient (33, 34), safety of outpatient thyroidectomy is still questioned due to the risk of life-threatening neck hematoma (35, 36). Since TDS measures the degree of difficulty, and this score correlated with operative times and complications, future research can now use TDS scores to identify the patient and disease factors associated with greater difficulty, or higher risk.
Although much of the thyroid surgery literature uses operative time, blood loss, and complications as surrogates for difficulty (11, 13, 29, 37, 38), these items alone may not capture the entire notion of difficulty. For example, Consorti and colleagues examined the factors influencing operating time for total thyroidectomy. They found that factors such as thyroid volume or neck circumference explained very little of the variance in operative time (39). Increased gland size is traditionally viewed as a factor contributing to difficulty. Although the TDS did correlate with operative times, we tested the TDS on a variety of thyroid diseases, and the correlation would likely improve with a more homogenous cohort (Figure 3).
This study was performed at a high volume tertiary referral center. Although we did express operative time in relation to average time rather than actual minutes, these results along with the complication rates may not be generalizable to other centers, especially those with less thyroid volume. We also recognize that the specific ratings in each category remain somewhat subjective despite providing guidelines and examples for each range of scores (Figure 1). Since this cohort only included cases where two surgeons of at least the fellow level participated, it may be biased toward more difficult cases. Validation by other institutions will determine if TDS applies to a wide case mix. The range of disease processes and scores implies that this cohort included a range of diseases and difficulty level.
TDS is a novel tool with high inter-rater agreement for scoring the difficulty of thyroid operations. Since TDS scores correlated with operative times and complications, TDS can be utilized in future research to identify risk factors for more difficult, risky, and lengthier cases.
Acknowledgments
This study was supported by NIH T32 CA009614-23.
References
- 1.Giddings AE. The history of thyroidectomy. J R Soc Med. 1998;91( Suppl 33):3–6. doi: 10.1177/014107689809133s02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sosa JA, Bowman HM, Tielsch JM, Powe NR, Gordon TA, Udelsman R. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998 Sep;228(3):320–30. doi: 10.1097/00000658-199809000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider DF, Ojomo KA, Chen H, Sippel RS. Remnant Uptake as a Postoperative Oncologic Quality Indicator. Thyroid. 2013 Jul 17; doi: 10.1089/thy.2012.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duclos A, Peix JL, Colin C, Kraimps JL, Menegaux F, Pattou F, et al. Influence of experience on performance of individual surgeons in thyroid surgery: prospective cross sectional multicentre study. BMJ. 2012;344:d8041. doi: 10.1136/bmj.d8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider DF, Chen H. New developments in the diagnosis and treatment of thyroid cancer. CA Cancer J Clin. 2013 Jun 24; doi: 10.3322/caac.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazeh H, Khan Q, Schneider DF, Schaefer S, Sippel RS, Chen H. Same-day thyroidectomy program: eligibility and safety evaluation. Surgery. 2012 Dec;152(6):1133–41. doi: 10.1016/j.surg.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 7.Sippel RS, Chen H. Controversies in the surgical management of newly diagnosed and recurrent/residual thyroid cancer. Thyroid. 2009 Dec;19(12):1373–80. doi: 10.1089/thy.2009.1606. [DOI] [PubMed] [Google Scholar]
- 8.Thomusch O, Machens A, Sekulla C, Ukkat J, Brauckhoff M, Dralle H. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery. 2003 Feb;133(2):180–5. doi: 10.1067/msy.2003.61. [DOI] [PubMed] [Google Scholar]
- 9.Kikuchi S, Perrier ND, Cheah WK, Siperstein AE, Duh QY, Clark OH. Complication of thyroidectomy in patients with radiation-induced thyroid neoplasms. Arch Surg. 2004 Nov;139(11):1185–8. doi: 10.1001/archsurg.139.11.1185. [DOI] [PubMed] [Google Scholar]
- 10.Wilhelm SM, McHenry CR. Total thyroidectomy is superior to subtotal thyroidectomy for management of Graves’ disease in the United States. World J Surg. 2010 Jun;34(6):1261–4. doi: 10.1007/s00268-009-0337-3. [DOI] [PubMed] [Google Scholar]
- 11.Shih ML, Lee JA, Hsieh CB, Yu JC, Liu HD, Kebebew E, et al. Thyroidectomy for Hashimoto’s thyroiditis: complications and associated cancers. Thyroid. 2008 Jul;18(7):729–34. doi: 10.1089/thy.2007.0384. [DOI] [PubMed] [Google Scholar]
- 12.McManus C, Luo J, Sippel R, Chen H. Is thyroidectomy in patients with Hashimoto thyroiditis more risky? J Surg Res. 2012 Dec;178(2):529–32. doi: 10.1016/j.jss.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McHenry CR, Piotrowski JJ. Thyroidectomy in patients with marked thyroid enlargement: airway management, morbidity, and outcome. Am Surg. 1994 Aug;60(8):586–91. [PubMed] [Google Scholar]
- 14.Wagner HE, Seiler C. Recurrent laryngeal nerve palsy after thyroid gland surgery. Br J Surg. 1994 Feb;81(2):226–8. doi: 10.1002/bjs.1800810222. [DOI] [PubMed] [Google Scholar]
- 15.Wormer BA, McHenry CR. Hashimoto’s thyroiditis: outcome of surgical resection for patients with thyromegaly and compressive symptoms. Am J Surg. 2011 Mar;201(3):416–9. doi: 10.1016/j.amjsurg.2010.08.021. discussion 9. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Bargren A, Schaefer S, Chen H, Sippel RS. Total thyroidectomy: a safe and effective treatment for Graves’ disease. J Surg Res. 2011 Jun 1;168(1):1–4. doi: 10.1016/j.jss.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittendorf EA, McHenry CR. Thyroidectomy for selected patients with thyrotoxicosis. Arch Otolaryngol Head Neck Surg. 2001 Jan;127(1):61–5. doi: 10.1001/archotol.127.1.61. [DOI] [PubMed] [Google Scholar]
- 18.Jamali FR, Soweid AM, Dimassi H, Bailey C, Leroy J, Marescaux J. Evaluating the degree of difficulty of laparoscopic colorectal surgery. Arch Surg. 2008 Aug;143(8):762–7. doi: 10.1001/archsurg.143.8.762. discussion 8. [DOI] [PubMed] [Google Scholar]
- 19.Dooley IJ, O’Brien PD. Subjective difficulty of each stage of phacoemulsification cataract surgery performed by basic surgical trainees. J Cataract Refract Surg. 2006 Apr;32(4):604–8. doi: 10.1016/j.jcrs.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 20.Van Oldenrijk J, Schafroth MU, Rijk E, Runne WC, Verheyen CC, van Egmond C, et al. Learning curve analysis of the Collum Femoris Preserving total hip surgical technique. Hip Int. 2013 Mar-Apr;23(2):154–61. doi: 10.5301/hipint.5000013. [DOI] [PubMed] [Google Scholar]
- 21.van Oldenrijk J, Schafroth MU, Bhandari M, Runne WC, Poolman RW. Time-action analysis (TAA) of the surgical technique implanting the collum femoris preserving (CFP) hip arthroplasty. TAASTIC trial identifying pitfalls during the learning curve of surgeons participating in a subsequent randomized controlled trial (an observational study) BMC Musculoskelet Disord. 2008;9:93. doi: 10.1186/1471-2474-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gil-Carcedo E, Menendez ME, Vallejo LA, Herrero D, Gil-Carcedo LM. The Zuckerkandl tubercle: problematic or helpful in thyroid surgery? Eur Arch Otorhinolaryngol. 2013 Aug;270(8):2327–32. doi: 10.1007/s00405-012-2334-7. [DOI] [PubMed] [Google Scholar]
- 23.Upile T, Jerjes W, Mahil J, Tailor H, Balakumar R, Rao A, et al. How to do it: the difficult thyroid. Head Neck Oncol. 2011;3:54. doi: 10.1186/1758-3284-3-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal A, Agarwal S, Tewari P, Gupta S, Chand G, Mishra A, et al. Clinicopathological profile, airway management, and outcome in huge multinodular goiters: an institutional experience from an endemic goiter region. World J Surg. 2012 Apr;36(4):755–60. doi: 10.1007/s00268-012-1447-x. [DOI] [PubMed] [Google Scholar]
- 25.Shindo ML. Considerations in surgery of the thyroid gland. Otolaryngol Clin North Am. 1996 Aug;29(4):629–35. [PubMed] [Google Scholar]
- 26.O’Sullivan MD, McAnena KS, Egan C, Waters PS, McCann PJ, Kerin MJ. Enlarging neck masses in the elderly - Histological and surgical considerations. Int J Surg Case Rep. 2013;4(4):378–81. doi: 10.1016/j.ijscr.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schussler-Fiorenza CM, Bruns CM, Chen H. The surgical management of Graves’ disease. J Surg Res. 2006 Jun 15;133(2):207–14. doi: 10.1016/j.jss.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Ahanchi SS, Carroll M, Almaroof B, Panneton JM. Anatomic severity grading score predicts technical difficulty, early outcomes, and hospital resource utilization of endovascular aortic aneurysm repair. J Vasc Surg. 2011 Nov;54(5):1266–72. doi: 10.1016/j.jvs.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Shinall MC, Jr, Broome JT, Baker A, Solorzano CC. Is potassium iodide solution necessary before total thyroidectomy for graves disease? Ann Surg Oncol. 2013 Sep;20(9):2964–7. doi: 10.1245/s10434-013-3126-z. [DOI] [PubMed] [Google Scholar]
- 30.Ansaldo GL, Pretolesi F, Varaldo E, Meola C, Minuto M, Borgonovo G, et al. Doppler evaluation of intrathyroid arterial resistances during preoperative treatment with Lugol’s iodide solution in patients with diffuse toxic goiter. J Am Coll Surg. 2000 Dec;191(6):607–12. doi: 10.1016/s1072-7515(00)00755-9. [DOI] [PubMed] [Google Scholar]
- 31.Snyder SK, Hamid KS, Roberson CR, Rai SS, Bossen AC, Luh JH, et al. Outpatient thyroidectomy is safe and reasonable: experience with more than 1,000 planned outpatient procedures. J Am Coll Surg. 2010 May;210(5):575–82. 82–4. doi: 10.1016/j.jamcollsurg.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 32.Clark N, Schneider DF, Vrabec S, Bauer PS, Chen H, Sippel RS. Increased efficiency of endocrine procedures performed in an ambulatory operating room. J Surg Res. 2013 May 9; doi: 10.1016/j.jss.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youngwirth L, Benavidez J, Sippel R, Chen H. Postoperative parathyroid hormone testing decreases symptomatic hypocalcemia and associated emergency room visits after total thyroidectomy. Surgery. 2010 Oct;148(4):841–4. doi: 10.1016/j.surg.2010.07.038. discussion 4–6. [DOI] [PubMed] [Google Scholar]
- 34.Wang TS, Roman SA, Sosa JA. Postoperative calcium supplementation in patients undergoing thyroidectomy. Curr Opin Oncol. 2012 Jan;24(1):22–8. doi: 10.1097/CCO.0b013e32834c4980. [DOI] [PubMed] [Google Scholar]
- 35.Samson PS, Reyes FR, Saludares WN, Angeles RP, Francisco RA, Tagorda ER., Jr Outpatient thyroidectomy. Am J Surg. 1997 Jun;173(6):499–503. doi: 10.1016/s0002-9610(97)00019-6. [DOI] [PubMed] [Google Scholar]
- 36.Hessman C, Fields J, Schuman E. Outpatient thyroidectomy: is it a safe and reasonable option? Am J Surg. 2011 May;201(5):565–8. doi: 10.1016/j.amjsurg.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 37.Pollei TR, Barrs DM, Hinni ML, Bansberg SF, Walter LC. Operative time and cost of resident surgical experience: effect of instituting an otolaryngology residency program. Otolaryngol Head Neck Surg. 2013 Jun;148(6):912–8. doi: 10.1177/0194599813482291. [DOI] [PubMed] [Google Scholar]
- 38.McManus C, Luo J, Sippel R, Chen H. Should patients with symptomatic Hashimoto’s thyroiditis pursue surgery? J Surg Res. 2011 Sep;170(1):52–5. doi: 10.1016/j.jss.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Consorti F, Milazzo F, Notarangelo M, Scardella L, Antonaci A. Factors influencing the length of the incision and the operating time for total thyroidectomy. BMC Surg. 2012;12:15. doi: 10.1186/1471-2482-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


