Abstract
Purpose
To evaluate the ability of Systane® Balance (SYSB) administered four times per day for 4 weeks to increase noninvasive tear film break-up time (NITFBUT) over baseline compared with a saline (SAL) control in patients with lipid-deficient dry eye (DE).
Patients and methods
Patients aged ≥18 years with DE and evidence of meibomian gland dysfunction (ie, abnormal gland expression and missing meibomian glands) were included in this randomized, parallel-group, controlled, investigator-masked comparison study. Patients were randomized to SYSB or SAL four times daily for 4 weeks. The primary efficacy variable was mean change in NITFBUT from baseline at week 4. Ocular surface staining, goblet cell density, and meibomian gland expression were also assessed. Safety assessments included adverse events (AEs), best-corrected visual acuity, and ocular signs.
Results
A total of 49 patients received study treatments (SYSB, n=25; SAL, n=24). Most patients were women (67.4%) and Caucasian (63.3%); mean ± standard deviation (SD) age was 44±19 years. DE characteristics at baseline were similar between groups. After 4 weeks of treatment, the mean ± SD NITFBUT increase from baseline was significantly greater with SYSB (2.83±0.74 seconds) compared with SAL (0.66±0.55 seconds; P<0.001, t-test). Improvements in conjunctival and corneal staining, percentage of patients with increased goblet cell density, and meibomian gland expression were also observed with 4 weeks of SYSB over SAL. No AEs were reported for either treatment group; best-corrected visual acuity and ocular signs remained stable or improved compared with baseline.
Conclusion
SYSB restored tear film stability, improved ocular surface healing, and improved meibomian gland functionality after 4 weeks of use in patients with lipid-deficient DE. No AEs were reported with either SYSB or SAL.
Keywords: artificial tears, corneal staining, conjunctival staining, goblet cells
Introduction
Dry eye (DE) syndrome is associated with ocular discomfort, tear film instability (ie, decreased tear film break-up time [TFBUT]), increased corneal and conjunctival staining, and conjunctival goblet cell loss.1 Estimates of DE prevalence range from approximately 7% to 34% depending on the diagnosis criteria and population studied;2 symptoms of DE vary from patient to patient and are not always associated with clinical signs.3
DE stems from a disruption of the tear film caused by deficient aqueous, lipid, and/or mucin layers, either individually or in combination.4,5 Other sources may include epitheliopathy, lid abnormalities, or autoimmune disease, and risk factors include female sex, increasing age, and smoking.2,4 Meibomian gland dysfunction is the most common cause of evaporative DE and frequently occurs with aqueous-deficient DE.6,7 With decreased numbers of meibomian glands or decreased lipid excretion from existing glands, the lipid component that stabilizes the tear film becomes deficient, and tear film instability and evaporation increase.6 Decreased TFBUT, dehydration, exposure of ocular tissues such as the corneal epithelium, and inflammation can result in epithelial damage.8,9
DE is typically managed through the use of artificial tears to supplement or restore the natural tear film. Saline (SAL) eye drops are commonly used to manage DE by increasing aqueous volume, which improves corneal hydration, but SAL does not replace the lipid component of the precorneal tear film or enhance TFBUT.10 Systane® Balance (SYSB) ocular emulsion (Alcon Laboratories, Inc., Fort Worth, TX, USA) is a gellable lubricant eye drop containing microemulsions of oils (the LipiTech™ System) intended to restore lipid, aqueous, and mucin tear components to enhance tear film stability.9 SYSB contains propylene glycol 0.6%, hydroxypropyl-guar, borate, sorbitol, dimyristoylphosphatidylglycerol (a polar phospholipid surfactant), and mineral oil. In a recent study of patients with DE associated with meibomian gland dysfunction, SYSB was shown to produce significant improvements in DE symptoms, assessed using the Impact of Dry Eye on Everyday Life questionnaire.11 After 4 weeks of treatment, most patients reported that they agreed or strongly agreed that SYSB successfully treated their DE symptoms (64%) and that their DE symptoms were much better with SYSB compared with their habitual treatment (66%).11 The manner in which SYSB improves symptoms of DE remains to be fully determined.
The purpose of this study was to evaluate the ability of SYSB administered four times per day for 4 weeks to increase noninvasive tear film break-up time (NITFBUT) over baseline compared with SAL placebo control in patients with lipid-deficient DE.
Patients and methods
Patients
Eligible patients were aged ≥18 years with best-corrected visual acuity (BCVA) ≤0.6 logMAR in both eyes at screening and no use of topical ocular drops within approximately 24 hours before screening. Patients were required to meet all the following criteria for DE at screening: answered at least “some of the time” to the previously published symptom eligibility question “how often have your eyes felt dry enough to want to use eye drops (artificial tears)”,12 focusing on the past 24 hours; NITFBUT ≤7 seconds in one or both eyes; meibomian gland expression of grade 1 or higher in both eyes; and evidence of missing meibomian glands in both eyes. Key exclusion criteria included intolerance or hypersensitivity to any component of study treatments, ocular or intraocular surgery or serious ocular trauma ≤6 months before enrollment, current punctal occlusion of any type, use of concomitant topical ocular medications, use of systemic medications that may contribute to DE (unless on a stable regimen for ≥30 days before screening and throughout the study), ocular or systemic infections or conditions (eg, epithelial herpes simplex keratitis; vaccinia, varicella, or mycobacterial infection; fungal disease; iritis) that preclude safe administration of study treatment, use of contact lenses within 1 week before screening and throughout the study period, and participation in an investigational drug or device study ≤30 days before screening.
Study design and treatment
This was a randomized, two-arm, parallel-group, controlled, investigator-masked comparison of SYSB and the placebo control SAL conducted at a single site in Argentina from September to October 2012 (ClinicalTrials.gov identifier, NCT01718028). The study period consisted of three scheduled visits: a screening/baseline at days 0 and 2 and follow-up visits conducted after 2 and 4 weeks of treatment. At the conclusion of the screening/baseline assessments, patients were assigned a subject number in numerical sequence and were randomized 1:1 to receive either SYSB or SAL (0.9% NaCl; Larmabak®; Grupo de Empresas Farmacéuticas SIDUS, Buenos Aires, Argentina). Patients were instructed to instill the study treatment topically in both eyes four times per day corresponding to morning, early afternoon, evening, and bedtime for 4 weeks. Compliance with the assigned study treatment was assessed based on patient feedback and by counting the remaining eye drops in the product bottle.
This study was performed in compliance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice. The protocol was approved by the applicable independent ethics committees or institutional review boards, and all patients provided written informed consent before participating in the study.
Efficacy outcomes
The primary efficacy endpoint was mean NITFBUT change from baseline (day 0) at week 4. Secondary efficacy endpoints were NITFBUT by visit, NITFBUT change from baseline at week 2, and percent NITFBUT change from baseline after 2 and 4 weeks. Exploratory efficacy endpoints included meibomian gland expression and meibography (ie, evaluation of total and partial missing meibomian glands), conjunctival and corneal staining, and goblet cell density classification. Meibomian gland evaluation and ocular surface staining were assessed for mean scores and percent change from baseline after 2 and 4 weeks; goblet cell density was assessed for change from baseline in classification (ie, improved, maintained, or worse) after 2 and 4 weeks of treatment.
NITFBUT was assessed using a cold-light illumination interferometer (Tearscope®, Keeler Ltd., Windsor, UK) that enabled visualization of the precorneal tear film. The interferometer was attached to the slit lamp to allow recording of the tear film in real time. One reading was performed per patient, between 60 and 120 minutes after instillation of the assigned study treatment. Patients were instructed to blink naturally for 10 seconds and then to stare straight ahead without blinking. The tear film was observed until dry areas appeared.
Meibomian gland expression was observed by slit-lamp examination. Glands were expressed from the temporal to the nasal aspect using a cotton-tipped applicator. Expressed meibum was classified based on color and viscosity as follows: normal, clear oil (grade 0); opaque, diffusely turbid meibum with normal viscosity (grade 1); opaque meibum with increased viscosity (grade 2); or inspissated meibum or no excreted material (grade 3). Meibography of the lower eyelid was evaluated by slit-lamp examination aided by a clinical transilluminator (Welch Allyn Inc., Skaneateles Falls, NY, USA). Missing partial or whole meibomian glands in nasal, central, and temporal regions were counted by the examiner.
Surface staining of five corneal regions and six conjunctival regions was observed by slit-lamp examination after instillation of 5 μL of 2% preservative-free fluorescein or lissamine green, respectively. Surface staining was scored as 0 (normal, no staining), 1 (mild, superficial stippling or macropunctate staining), 2 (moderate, macropunctate staining with some coalescent areas), or 3 (severe, numerous coalescent macropunctate areas or patches). Scores were summed to yield total corneal and total conjunctival staining scores for each eye.
Goblet cell density was evaluated using impression cytology and Nelson’s classification system.13 After instillation of tetracaine topical anesthetic, polyvinylidene fluoride filters (22 μm pore) were applied to the inferior tarsal and bulbar conjunctiva for ~10 seconds and processed using standard methods. Periodic acid-Schiff reagent–positive areas were averaged across 15 random fields, and results were classified as 0 (normal), I (low number of goblet cells), II (absence of goblet cells), or III (absence of goblet cells plus squamous metaplasia).
Safety outcomes
Patient-reported and investigator-solicited adverse events (AEs), BCVA, and ocular signs were evaluated as safety endpoints. BCVA was assessed using an Early Treatment Diabetic Retinopathy Study chart at a distance of 10 ft, and results were calculated as logMAR scores. Ocular signs were assessed by slit-lamp examination at all study visits. Conditions and illnesses evaluated were signs of active inflammation or significant structural change or discharge (eyelids and conjunctiva); active inflammation or active structural change, including focal scarring and fine deposition (cornea); active inflammation (iris and anterior chamber); and level of lens opacity, pseudophakia, or aphakia (lens, with an emphasis on the visual axis).
Statistical analysis
Demographic data were summarized descriptively. Between-group differences in demographic data and baseline characteristics were analyzed using Mann–Whitney nonparametric tests, χ2 tests, or unpaired t-tests. Efficacy endpoints were analyzed in the intent-to-treat population. NITFBUT differences between groups were analyzed in the worse eye using Mann–Whitney tests and t-tests, with a significance level of 5%. The worse eye was defined as the eye with lower NITFBUT score at baseline; if NITFBUT was equal in both eyes at baseline, the right eye was used. Between-group differences in ocular staining were analyzed using t-tests; goblet cell density and meibomian gland expression were analyzed with χ2 tests; and meibography data were analyzed using Mann–Whitney tests or t-tests. AEs were summarized descriptively, BCVA was analyzed with Mann–Whitney tests, and ocular signs were analyzed using Fisher exact tests.
The sample size was based on results of an exploratory study of patients with meibomian gland dysfunction. The mean NITFBUT was 5.2 seconds, and the maximum standard deviation (SD) was ±2.88 seconds. A power calculation based on the assumption of a common SD of 2.88 seconds determined that a sample size of 50 patients would provide 90% power to detect a superiority margin of 2.7 seconds using a two-sided significance level of P<0.05.
Results
Patients
A total of 51 patients were screened and randomized to treatment. Two patients in the SAL placebo control group withdrew consent before receiving treatment; 49 patients received treatment and completed the study (SYSB, n=25; SAL, n=24). Most patients were women (67.4%, n=33/49) and Caucasian (63.3%, n=31/49). The mean ± SD age was 44±19 years (range, 21–85 years). A total of 47 patients received treatment for 28 days and two patients received treatment for 29 days. Most patients reported wanting to use eye drops because of dryness at least half of the time (SYSB, n=21/25 [84.0%]; SAL, n=14/24 [58.3%]). No enrolled patients had a diagnosis of a systemic condition associated with DE (eg, Sjögren’s syndrome). One patient in the SYSB group had an anxiety disorder, and one patient in the SAL group had both hypertension and a lipid metabolism and deposit disorder. Patient demographics and baseline DE characteristics for the intent-to-treat population are presented in Table 1. No patients were determined to be noncompliant with their assigned treatment.
Table 1.
Demographics and baseline characteristics (ITT population)
| Systane® Balance (n=25) | Saline (n=24) | P-value | |
|---|---|---|---|
| Age, years | 0.873a | ||
| Mean ± SD | 46±20 | 43±18 | |
| Range | 21–85 | 22–77 | |
| Sex, n (%) | 0.478b | ||
| Women | 18 (72.0) | 15 (62.5) | |
| Men | 7 (28.0) | 9 (37.5) | |
| Race, n (%) | 0.196b | ||
| White | 18 (72.0) | 13 (54.2) | |
| Hispanic | 7 (28.0) | 11 (45.8) | |
| Symptom question (How often have your eyes felt dry enough to want to use eye drops) response, n (%) | NA | ||
| Some of the time | 4 (16.0) | 10 (41.7) | |
| Half of the time | 19 (76.0) | 12 (50.0) | |
| Most of the time | 1 (4.0) | 2 (8.3) | |
| All of the time | 1 (4.0) | 0 | |
| NITFBUT (seconds), mean ± SD | 4.60±0.69 | 4.93±0.80 | 0.139c |
| Total corneal staining score, mean ± SD | 1.2±0.85 | 0.71±0.81 | 0.064a |
| Total conjunctival staining score, mean ± SD | 7.7±3.9 | 5.2±2.4 | 0.013a |
| Goblet cell density, n (%) | NA | ||
| Normal | 2 (8.0) | 3 (12.5) | |
| Reduced number of goblet cells | 9 (36.0) | 15 (62.5) | |
| Absence of goblet cells | 13 (52.0) | 6 (25.0) | |
| Absence of goblet cells plus squamous metaplasia | 1 (4.0) | 0 | |
| Meibomian gland expression, n (%) | NA | ||
| Normal, clear oil | 0 | 0 | |
| Opaque, diffusely turbid meibum with normal viscosity | 9 (36.0) | 12 (50.0) | |
| Opaque meibum with increased viscosity | 15 (60.0) | 12 (50.0) | |
| Inspissated meibum or no excreted material | 1 (4.0) | 0 | |
| Total number of missing meibomian glands, mean ± SD | |||
| Nasal | 2.94±1.22 | 3.38±1.20 | 0.214c |
| Temporal | 3.90±2.70 | 3.42±1.49 | 0.739a |
| Central | 3.12±2.81 | 3.04±1.20 | 0.111a |
Notes:
Mann–Whitney test.
χ2 test.
Unpaired t-test.
Abbreviations: ITT, intent-to-treat; NA, not assessed; NITFBUT, noninvasive tear film break-up time; SD, standard deviation.
Efficacy
Mean ± SD NITFBUT was similar between groups at baseline (Table 1). After 4 weeks of treatment, NITFBUT was increased from baseline by 2.83±0.74 seconds (range, 1.70–4.60 seconds) with SYSB and by 0.66±0.55 seconds (range, 0.00–2.00 seconds) with SAL (between-group difference, 2.17 seconds [95% confidence interval (CI), 1.79–2.54], P<0.001; Figure 1). At baseline, mean ± SD NITFBUT in the SYSB and SAL groups was 4.60±0.69 seconds and 4.93±0.80 seconds, respectively. At week 4, the mean ± SD NITFBUT was significantly greater with SYSB (7.43±0.51 seconds) compared with SAL (5.59±0.66 seconds, P<0.001). Mean NITFBUT by visit is shown in Figure 2. Percent change from baseline was significantly greater with SYSB compared with SAL at week 2 (45.0% vs 14.4%, respectively) and week 4 (65.0% vs 15.1%, respectively; P<0.001 for both).
Figure 1.
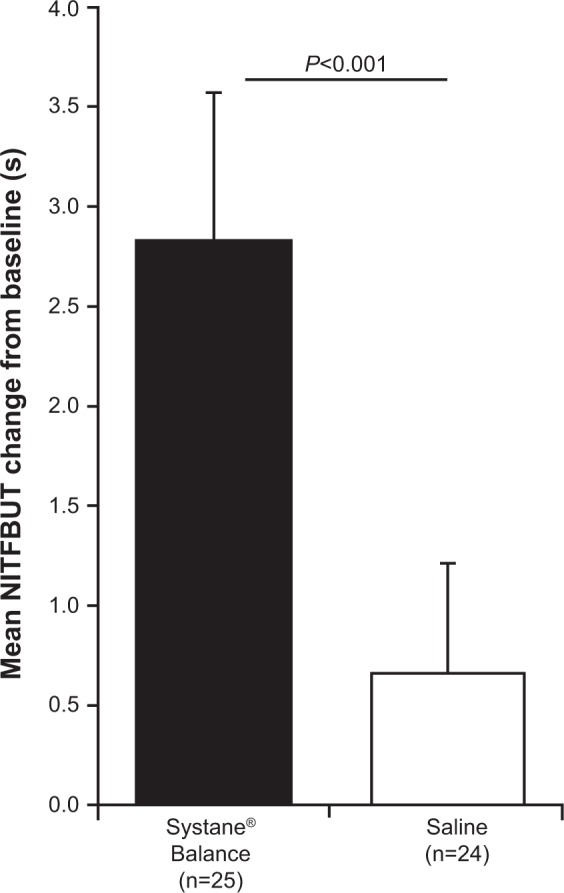
NITFBUT change from baseline at week 4.
Notes: Data are mean ± SD. P-value was determined using an unpaired t-test.
Abbreviations: NITFBUT, noninvasive tear film break-up time; SD, standard deviation.
Figure 2.
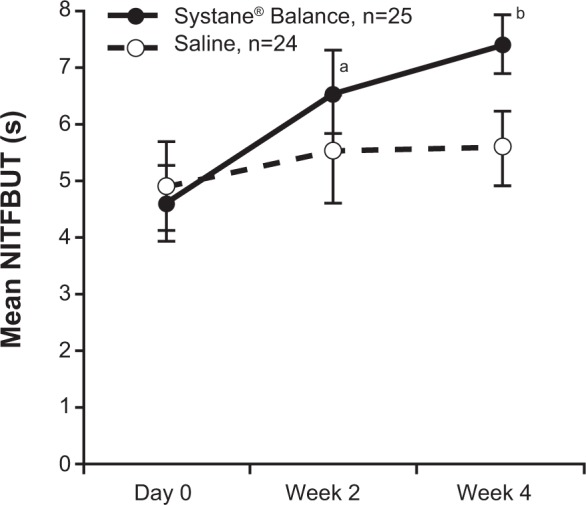
NITFBUT by visit.
Notes: Data are mean ± SD. aP<0.001 for SYSB vs SAL, Mann–Whitney test; bP<0.001 for SYSB versus SAL, unpaired t-test.
Abbreviations: NITFBUT, noninvasive tear film break-up time; SYSB, Systane® Balance; SAL, saline; s, seconds; SD, standard deviation.
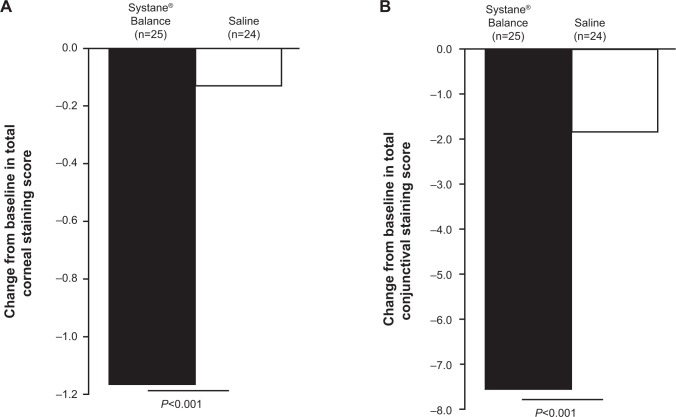
At baseline, the mean total corneal and conjunctival staining was greater in the SYSB group compared with the SAL group (Table 1). At the 4-week follow-up visit, the reduction in total ocular staining score from baseline (mean [percent]) was significantly greater with SYSB than with SAL for both cornea (SYSB, −1.16 [−80.0%]; SAL, −0.13 [−10.4%]; P<0.001; Figure 3A) and conjunctiva (SYSB, −7.52 [−98.2%]; SAL, −1.83 [35.8%]; P<0.001; Figure 3B). Mean treatment difference in corneal staining for SYSB vs SAL after 4 weeks of treatment was −1.04 (95% CI, −1.43 to −0.64); mean treatment difference in conjunctival staining for SYSB vs SAL was −5.69 (95% CI, −7.44 to −3.93). Total staining scores were also significantly improved with SYSB compared with SAL at week 2 for cornea (SYSB, −0.76 [−54.7%]; SAL, −0.08 [−8.3%]) and conjunctiva (SYSB, −5.36 [71.9%]; SAL, −1.00 [−21.3%]); group differences were statistically significant (P≤0.001 for both cornea and conjunctiva).
Figure 3.
Mean change in total ocular surface staining scores at week 4 for (A) cornea and (B) conjunctiva.
Notes: Data are mean ± standard deviation. P-values were determined using unpaired t-tests.
Impression cytology at baseline revealed that most patients had reduced numbers or absence of goblet cells (SYSB, n=22/25 [88.0%]; SAL, n=21/24 [87.5%]; Table 1). Conjunctival goblet cell density was improved after 4 weeks of treatment in 84.0% of patients (n=21/25) receiving SYSB compared with 33.3% of patients (n=8/24) receiving SAL (Figure 4). Compared with baseline classifications, goblet cell density classification at both weeks 2 and 4 worsened in 4.2% and 12.5% of patients receiving SAL, respectively, and in no patients receiving SYSB. In the SYSB treatment group, impression cytology findings were maintained or improved in all patients at both follow-up visits compared with baseline.
Figure 4.
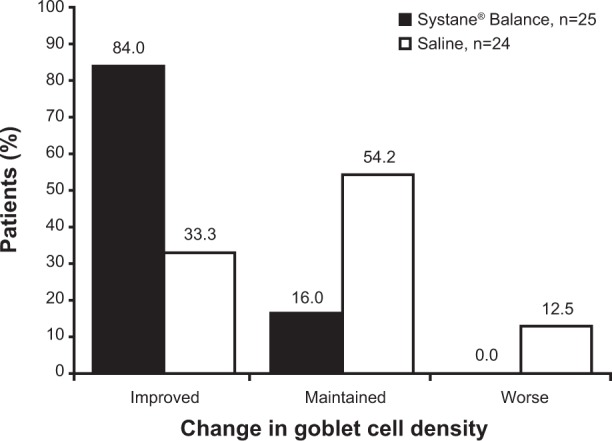
Classification of goblet cell density score change from baseline at week 4.
Note: The percentage of patients is indicated above bars.
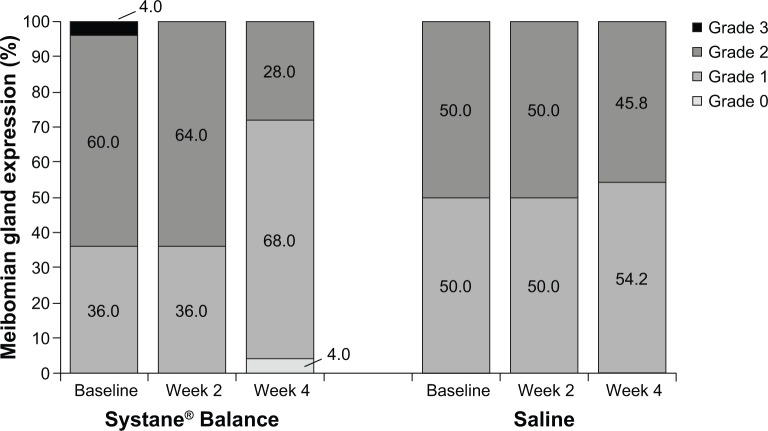
At baseline, expressed meibum was classified as grade 2 (opaque meibum with increased viscosity) in 60% of patients in the SYSB group (n=15/25) and 50% of patients in the SAL group (n=12/24); meibomian gland expression classified as grade 3 (inspissated meibum or no excreted material) was evident in one patient in the SYSB group (Table 1). No patients had normal meibomian gland expression. From baseline to week 4, the percentage of patients with meibomian gland expression classified as grade 2 or 3 decreased from 64.0% to 28.0% in the SYSB treatment group and from 50.0% to 45.8% in the SAL placebo control group (Figure 5). The percentage of patients with grade 0 or grade 1 expression at week 4 was 72.0% with SYSB and 54.2% with SAL. Overall, after 4 weeks of treatment with SYSB, the number of patients with grade 0 or grade 1 meibomian gland expression doubled; meibomian gland expression was largely unchanged from baseline with SAL. At week 2, meibomian gland expression was similar to baseline in both groups.
Figure 5.
Classification of meibomian gland expression by visit.
Notes: The percentage of patients with grades 0–3 meibum is indicated within bars. Data for the worse eye are shown. Grade 0= normal, clear oil; grade 1= opaque, diffusely turbid meibum with normal viscosity; grade 2= opaque meibum with increased viscosity; grade 3= inspissated meibum or no excreted material.
Safety
No AEs were reported for either the SYSB treatment group or the SAL placebo control group in this study. BCVA of the worse eye remained stable from baseline through the 4 weeks of treatment. There were no significant differences in BCVA between groups at any visit. Active inflammation, structural change, and discharge decreased markedly from baseline with both SYSB and SAL; no other changes in ocular signs were observed. There were no significant differences in eyelid/conjunctiva assessment between treatment groups at any assessment.
Discussion
Discomfort, tear film instability, and ocular surface damage are characteristic signs and symptoms of DE. Reduced meibomian gland secretion, caused by decreased number or function of glands, is associated with evaporative DE.6 Artificial tears are commonly used to manage DE symptoms by restoring the natural tear film. A frequently used artificial tear, SAL, replaces the aqueous component of the tear film lost to evaporation but does not restore the lipid component of the tear film that minimizes evaporation and promotes stability.10,14 SYSB, formulated to restore aqueous, lipid, and mucin components of the precorneal tear film, may bind to desiccated or damaged epithelial cells and improve tear film lipid layer thickness.9,15 The goal of this study was to assess the effectiveness of SYSB to increase NITFBUT compared with SAL in patients with lipid-deficient DE. At baseline, patients in both groups had signs of mild to moderate DE. After 4 weeks of treatment, NITFBUT increased by 65.0% (nearly 3 seconds) in the SYSB treatment group compared with 15.1% (<1 second) in the SAL group; NITFBUT improvement was significantly greater with SYSB compared with SAL. Improvements in corneal and conjunctival staining scores were also significantly greater with SYSB, and a greater percentage of patients in the SYSB group showed increased goblet cell density compared with the SAL group. Meibomian gland expression was also improved with 4 weeks of SYSB compared with SAL. Both study treatments were well tolerated by patients; no AEs were reported during the study period. BCVA remained stable in both groups, and ocular signs (conjunctiva/eyelid) improved from baseline with SYSB and SAL.
The improvement in NITFBUT with SYSB may be attributable to the demonstrated ability of SYSB to promote tear film stability and protect the ocular surface.9 The results of the current study also indicated that SYSB may help to manage DE by improving characteristics of DE beyond tear film instability, such as by reducing meibomian gland dysfunction, increasing mucin production, and promoting regeneration of the damaged ocular surface epithelium.
The results of the current study are similar to those of another open-label, multicenter study in patients with DE associated with meibomian gland dysfunction who switched to SYSB from various other therapies.11 After 4 weeks of SYSB treatment, TFBUT, meibomian expression, and corneal staining were significantly improved. Blurred vision was the most common AE (reported by 6% of patients). At week 4, nearly 80% of patients reported satisfaction with SYSB, and >60% preferred SYSB to their previous treatment. These improvements in DE signs and symptoms occurred with an average of <2 SYSB doses per day. In the current study, corneal staining decreased by nearly 100% after 4 weeks of SYSB administered four times daily compared with a previously noted decrease of approximately 25% after 4 weeks of SYSB administered approximately twice daily; the magnitude of NITFBUT increase from baseline in the current study was also larger than the previously reported increased TFBUT with SYSB.11
A potential limitation to this study was the relatively small study population (n=24–25 per group) and the use of SAL as a placebo control. Comparison of SYSB with SAL enabled comparison of study outcomes after treatment with a formulation that replaces only the aqueous component of the tear film vs a formulation that also restores the lipid and mucin components. Future studies comparing the efficacy of SYSB vs other lipid-containing artificial tears are needed. Additionally, treatment compliance was assessed by patients’ self-reports and by counting the number of instillations remaining in the treatment bottles. This approach did not allow monitoring of frequency or time of instillation, and potential differences in compliance between SYSB and SAL groups may have influenced study results. Previous work demonstrated that SYSB markedly improved both objective signs of DE and patient-reported DE symptoms,11 and a significant relationship between TFBUT (a DE sign) and Ocular Surface Disease Index scores of ocular discomfort (a DE symptom) has been reported.16 Although patient-reported symptoms of DE were not assessed in the current study, objective signs of DE, including NITFBUT and ocular surface staining, were markedly improved with SYSB.
Conclusion
In summary, SYSB restored long-term tear film stabilization and reduced ocular surface staining in patients with lipid-deficient DE. After 4 weeks of treatment with SYSB, NITFBUT increases from baseline and improvements in corneal and conjunctival staining scores were significantly greater than with SAL. Goblet cell density was improved in a greater percentage of patients who received SYSB compared with those who received SAL, and at the week 4 visit, meibomian gland expression was improved in the SYSB group compared with the SAL group. Both SYSB and SAL were safe and well tolerated; no AEs were reported.
Acknowledgments
Medical writing support was provided by Heather D Starkey, PhD, of Complete Healthcare Communications, Inc. (Chadds Ford, PA, USA).
Footnotes
Disclosure
Alcon Research, Ltd., sponsored this study and funded medical writing support. Dr Aguilar has commercial relationships with Poen Laboratories and Merck & Co., Inc. The other authors have no conflicts of interest to disclose.
References
- 1.Shimazaki-Den S, Dogru M, Higa K, Shimazaki J. Symptoms, visual function, and mucin expression of eyes with tear film instability. Cornea. 2013;32(9):1211–1218. doi: 10.1097/ICO.0b013e318295a2a5. [DOI] [PubMed] [Google Scholar]
- 2.Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405–412. doi: 10.2147/opth.s5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23(8):762–770. doi: 10.1097/01.ico.0000133997.07144.9e. [DOI] [PubMed] [Google Scholar]
- 4.McGinnigle S, Naroo SA, Eperjesi F. Evaluation of dry eye. Surv Ophthalmol. 2012;57(4):293–316. doi: 10.1016/j.survophthal.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–478. doi: 10.1097/ICO.0b013e318225415a. [DOI] [PubMed] [Google Scholar]
- 6.Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):1930–1937. doi: 10.1167/iovs.10-6997b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922–1929. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130(1):90–100. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benelli U. Systane lubricant eye drops in the management of ocular dryness. Clin Ophthalmol. 2011;5:783–790. doi: 10.2147/OPTH.S13773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters K, Millar T. The role of different phospholipids on tear break-up time using a model eye. Curr Eye Res. 2002;25(1):55–60. doi: 10.1076/ceyr.25.1.55.9965. [DOI] [PubMed] [Google Scholar]
- 11.Sindt CW, Foulks GN. Efficacy of an artificial tear emulsion in patients with dry eye associated with meibomian gland dysfunction. Clin Ophthalmol. 2013;7:1713–1722. doi: 10.2147/OPTH.S35833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davitt WF, Bloomenstein M, Christensen M, Martin AE. Efficacy in patients with dry eye after treatment with a new lubricant eye drop formulation. J Ocul Pharmacol Ther. 2010;26(4):347–353. doi: 10.1089/jop.2010.0025. [DOI] [PubMed] [Google Scholar]
- 13.Nelson JD, Havener VR, Cameron JD. Cellulose acetate impressions of the ocular surface. Dry eye states. Arch Ophthalmol. 1983;101(12):1869–1872. doi: 10.1001/archopht.1983.01040020871007. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien PD, Collum LM. Dry eye: diagnosis and current treatment strategies. Curr Allergy Asthma Rep. 2004;4(4):314–319. doi: 10.1007/s11882-004-0077-2. [DOI] [PubMed] [Google Scholar]
- 15.Christensen MT, Cohen S, Rinehart J, et al. Clinical evaluation of an HP-guar gellable lubricant eye drop for the relief of dryness of the eye. Curr Eye Res. 2004;28(1):55–62. doi: 10.1076/ceyr.28.1.55.23495. [DOI] [PubMed] [Google Scholar]
- 16.Unlü C, Güney E, Akçay Bİ, Akçalı G, Erdoğan G, Bayramlar H. Comparison of ocular-surface disease index questionnaire, tearfilm break-up time, and Schirmer tests for the evaluation of the tearfilm in computer users with and without dry-eye symptomatology. Clin Ophthalmol. 2012;6:1303–1306. doi: 10.2147/OPTH.S33588. [DOI] [PMC free article] [PubMed] [Google Scholar]