Abstract
Human primary placental explant culture is well established for cytokine signaling and toxicity, but has not been validated for steroidogenic or metabolic toxicology. The technique has never been investigated in the mouse. We characterized human and mouse placental explants for up to 96hr in culture. Explant viability (Lactate dehydrogenase) and sex steroid levels were measured in media using spectrophotometry and ELISA, respectively. Expression and activities of the steroidogenic (3β-hydroxysteroid dehydrogenase, Cytochrome P45017A1, Cytochrome P45019), conjugation (UDP-glucuronosyltransferase, sulfotransferase (SULT)), and regeneration (β-glucuronidase, arylsulfatase C (ASC)) enzymes were determined biochemically in tissues with fluorimetric and spectrophotometric assays, and western blot. Explants were viable up to 96hr, but progesterone, estrone, and 17β-estradiol secretion decreased. Steroidogenic enzyme expression and activities were stable in mouse explants and similar to levels in freshly isolated tissues, but were lower in human explants than in fresh tissue (P<0.01). Human and mouse explants exhibited significantly less conjugation after 96hr, SULT was not detected in the mouse, and neither explants had active ASC, although proteins were expressed. Mouse explants may be useful for steroid biochemistry and endocrine disruption studies, but not metabolic conjugation. In contrast, human explants may be useful for studying conjugation for <48hr, but not for steroid/endocrine studies.
Keywords: steroidogenesis, placenta, mouse, ex vivo culture, conjugation
Introduction
Placental function is often studied in fresh human placentas at term, as this is a readily available tissue that is most often discarded after birth. However, for many underlying pregnancy syndromes where obstetric or fetal manipulation/exposure is required (e.g. in vitro fertilization, developmental biology, and reproductive toxicology) animal models provide the ethical and appropriate alternatives. The ideal models of human pregnancy are non-human primates or guinea pigs (Carter, 2007). Despite this, mice are the most commonly used laboratory model for human pregnancy (Cockburn and Rossant, 2010; Dilworth and Sibley 2013; Ratajczak and Muglia, 2008).
Comparatively, the gestational period in humans is 40 weeks, significantly longer than 20 days gestation in mice. Placentation in mice takes around half of gestation, while in humans the placenta is fully functional after one-third of the gestational period (12 weeks) (Malassiné et al., 2003). In both mice and humans, the site of chemical and gas exchange is the trophoblast. In humans, trophoblasts form a single cell layer separating fetal and maternal circulations (Gude et al., 2004), but in mice the analogous region is comprised of syncytiotrophoblasts, chorionic trophoblasts, fetal blood vessels and stroma forming the maternal decidua, junctional and labyrinth zones (Malassiné et al., 2003; Niemand et al., 2003). Even so, the placenta performs similar metabolic and endocrine functions in both species.
Steroidogenesis in pregnancy differs in mice and humans. In humans, the ovaries are responsible for progesterone secretion to establish and maintain pregnancy for 8 weeks (Gude et al., 2004), after which progesterone production is performed by the placenta. The placenta, in combination with the fetal adrenals, function as the main sites of steroidogenesis and steroid elimination for the remainder of gestation (Evain-Brion and Malassine, 2003). In contrast, the mouse ovarian corpus luteum is responsible for progesterone secretion throughout gestation and is main site of steroidogenesis (Ben-Zimra et al., 2002; Malassiné et al., 2003), with minor contributions from the placenta and fetal tissues in the first and second half of gestation, respectively (Ben-Zimra et al., 2002; Malassiné et al., 2003). Despite these structural and physiological differences, the placenta plays similar metabolic and clearance roles in both species.
This study was undertaken to characterize primary villous placental explant culture of human and mouse tissues for the study of endocrine and chemical toxicology ex vivo. A widely used technique in autocrine, paracrine and inflammatory studies (Benyo et al., 1997; Keelan et al., 2000; Mitchell et al., 2000), it was hypothesized that placental explants may be useful for reproductive toxicology studies investigating endocrine disruptors and direct chemical teratogens. By comparing and contrasting mouse and human explants, the utility of mouse placental explants for these studies was also examined under a secondary hypothesis, that mouse explants could be an effective surrogate model for human placental functions. To establish the appropriateness of the explants, levels of sex steroids as well as placental enzymes involved in steroidogenesis, steroid clearance and steroid regeneration were determined for both human and mouse. We demonstrated that mouse placental explants are suitable for studying steroidogenesis, and human explants may be appropriate for short-term metabolic clearance studies. However, due to the effects of culture on native systems, neither model is suitable for studying the totality of steroid endocrinology or drug/chemical biotransformation.
Materials and Methods
All chemicals and reagents were from Sigma-Aldrich (St Louis, MO) unless stated otherwise.
Primary tissue culture – Human and Mouse
Human placental villous explants were prepared from normal, term (≥38 weeks) placentas from patients undergoing repeat caesarean delivery. Samples were collected from labor and delivery at Kapi’olani Medical Center for Women and Children, (Honolulu, HI) with IRB approval granted to Claire Wright by Hawaii Pacific Health and the Western IRB, and transported within 30 min to the John A. Burns School of Medicine (University of Hawaii, Honolulu, HI). Human placental trophoblast explants were prepared and cultured as previously described (Benyo et al., 1997). Briefly, trophoblast tissue was excised by blunt dissection and stripped of connective tissue. Trophoblastic tissue samples from the same placenta were plated to a density of 30–40% of the well in small pieces using 6 well plates (n = 3 wells per placenta). Explant culture proceeded at 37°C in 5% CO2/air in 3 mL of Ham’s/F-12 media with 10% fetal bovine serum, 1% penicillin/streptomycin, 2mM glutamine and phenol red. The sizes of the tissue pieces (2–3 mm) were similar within wells, between wells and also between placentas collected on different days, due to the mechanical and procedural similarities in the technique. Time “zero” was the time of plating, and all explants were left for 24 hr to equilibrate before measurements were taken. Medium for the explants was renewed every 24 hr, and the collected media stored at −80°C. The same placentas were used for each time point, in triplicate. A total of n=9 wells were seeded from the same placenta at the beginning of the time course, then at each of the time points (24, 48, 72 hr), explants from a set of n = 3 wells were collected and lysate prepared. Media was similarly taken each day from the same wells that were being harvested. Therefore, both the media and lysates used for each time point are consistent across LDH and steroid levels (e.g. the media used for 24 hr time point comes from the same culture for all LDH and steroid concentrations) and consistent with the lysates. At the end of each experiment, explant tissues were weighed and stored at −80°C until used.
Murine placentas were harvested from B6D2F1 (C57BL/6 x DBA/2, National Cancer Institute, Raleigh, NC) mice on Day 18.5 of gestation by caesarian section. Mice were fed ad libitum with a standard diet and maintained in a temperature and light-controlled room (22°C, 14 hr light/10 hr dark). Animal handling and treatment procedures were reviewed and approved by the Animal Care and Use Committee at the University of Hawaii. Tissues were harvested within minutes (<10 min) of mouse caesarian section, washed briefly in Dulbecco phosphate buffered saline (DPBS) and placed in microcentrifuge tubes with 1 mL of DPBS for transport to the John A. Burns School of Medicine for culture preparation; tissues were delivered within 2 hr of harvest. Each placenta was cut in half, blunt dissected and plated in 96-well plates with 100 µL of the same media as human explants. Mouse explants were treated in the same manner as human placental villous explants. Time “zero” was the time of plating, and all explants were left for 24 hours to equilibrate before measurements were taken. Media and explant tissues were collected and stored at −80°C until use.
Each individual placenta was cultured in three wells, with each well assayed in triplicate. However, only geometric means were used for statistical analysis (i.e. for each placenta, the mean of triplicates from well one was replicate one, the mean of triplicates from well two was replicate two, and so forth). The N values given refer to individual placentas, not culture wells or triplicate determinations.
Media samples were collected each day when media was refreshed. Therefore time-points for every day (up to 96 hr) are included in analyses of lactate dehydrogenase (LDH) and for sex steroid quantification, performed in media.
Tissue processing
Placental tissues were homogenized 1:3 (w:v, human) and 1:4 (w:v, mouse) in 0.1 M Tris-HCl, 5 mM MgCl2, 2 mM phenylmethylsulfonylfluoride (PMSF) and then centrifuged at 10,000 x g for 10 min. The supernatant was further centrifuged at 100,000 × g for 1 hr at 4°C to produce microsomes and cytosol. Subcellular fractions were resuspended in homogenization buffer, aliquoted and stored at −80°C until use. Control tissues from freshly collected human and mouse placentas (n = 5 each, with triplicate determinations) were pooled and used to determine culture effects. All samples were normalized to protein content utilizing the Bicinchoninic acid method with bovine serum albumin as the protein standard (Smith et al., 1985).
Tissue viability
Placental explants were evaluated for viability following culture using a LDH assay adapted from Zewe and Fromm (Zewe and Fromm, 1965). Briefly, 90 µL of buffer consisting of 0.2 M Tris-HCl (pH 7.3), 3.3 µL of 6.6 mM NADH, and 3.3 µL of 30 mM sodium pyruvate were incubated in optically clear 96-well microtiter plates at 25°C for 5 min. After incubation, 3.3 µL of explant medium was added to the wells. Change in absorbance, was monitored every 5 sec for 10 min at 340 nm (Spectra Max, Molecular Devices, Sunnyvale, CA). Rates were transformed to OD/min/mL of medium.
Sex steroid levels
The levels of the sex steroids progesterone, estrone, and 17β-estradiol were assessed in the media of explant cultures for both humans and mice, normalized to wet weight of tissue. Although wet weight was only measured at the end of the experimental period, term placenta explant cultures are non-proliferative, so the same amount of placenta was present throughout. Commercial ELISAs were performed per manufacturer’s instructions (ALPCO Diagnostics, Salem, NH). These ELISAs have been previously validated for use in mouse and human tissues (Raunig et al., 2011).
Steroidogenic enzyme activity
Steroidogenic enzymes were measured at 48 hr (“acute”) and 96 hr (“chronic”) time-points in the human explants. The 72 hr time-point was added for mouse 3β-HSD, CYP17 and CYP19 after observing significant differences in enzyme activity for human explants between 48 hr and 96 hr points. The purpose of the 72 hr time-point was to characterize whether enzymes declined progressively, or whether there was an initial decline, that was sustained in culture. All human placental enzyme metabolism assays were optimized for linearity over a series of substrate concentrations (0 – 200 µM) and several time points (0 – 30 min) using freshly isolated placental tissues. Subsequently, the shortest time point, where substrate turnover was ≤ 10% of initial substrate concentration and enzyme activity was linear, within the standard curve and measurably greater (3X) than baseline were used. For mouse tissues, optimization of these assays was previously published in freshly isolated mouse tissues from our laboratory (Raunig et al., 2011), and the published conditions were used. Explants from three wells for each placenta were collected at each time point, generating enough material that all of the enzyme assays were performed in the same set of placental lysates.
3β-Hydroxysteroid Dehydrogenase (3β-HSD)
Activity of 3β-HSD was determined as previously described in placental lysates incubated with pregnenolone (Raunig et al., 2011; Sugawara et al., 2012). Incubations were stopped after 10 min then precipitated of protein and progesterone product measured in supernatant using a commercial ELISA kit (ALPCO Diagnostics, Salem, NH) as per the manufacturers’ instructions.
Cytochrome P450 17A1 (CYP17)
The conversion of 17 α-hydroxypregnenolone to dehydroepiandrosterone (DHEA) was measured as an index of CYP17 activity (Raunig et al., 2011; Sugawara et al., 2012). Quantification of DHEA produced in the incubations was measured after 10 min, when reactions were stopped, precipitated of protein and the supernatant measured in a microplate spectrophotometer at λ = 520 nm. Quantification of DHEA was by comparison to a standard curve generated from pure standard.
Cytochrome P450 19 (CYP19)
Activity of CYP19 was determined using a modified version (Sugawara et al. 2012) of the method originally described by Gore-Langton, et al. (Gore-Langton et al., 1980). The substrate testosterone was incubated for 10 min in explant lysate, then the reaction stopped and protein precipitated. The 17β-estradiol product in the supernatant was detected using a commercial ELISA kit (ALPCO Diagnostics, Salem, NH) as per manufacturers’ instructions.
Assays for steroid metabolism/clearance and regeneration enzyme activity
Uridine Diphosphate Glucuronosyltransferase (UGT)
Total UGT activity was determined as previously described with 4-methylumbelliferone (4MU) as the substrate and alamethicin (50 µg/mg protein) as the activator (Collier et al., 2000). Fluorescence was detected continuously at λex = 355 nm, λem = 460 nm and results were transformed to nmol · min−1 · mg protein−1 using a standard curve generated with 4MU.
Beta-glucuronidase
The β-glucuronidase activity was determined by a method adapted from Trubetskoy and Shaw (Trubetskoy and Shaw, 1999) with 4-methylumbelliferone-glucuronide as the substrate. Fluorescence was then read continuously at λex = 355 nm, λem = 460 nm and results were transformed to nmol · min−1 · mg protein−1 using a standard curve generated with 4MU.
Sulfotransferase (SULT)
The activity of SULT (SULT1A1) was measured using a modification of the method of Frame et al (Frame et al., 2000). Briefly, placental cytosolic fractions (human: 10 µg; mouse: 2.5 µg), 50 mM potassium phosphate supplemented with 5 mM MgCl2 (pH 6.5), 5 mM p-nitrophenylsulfate, and 100 µM β-naphthol were added to a clear microtiter plate on ice. Plates were incubated for 5 min at 37°C in the microplate reader, followed by addition of 60 µM adenosine 3'-phosphate 5'-phosophosulfate PAPS to initiate the reaction and absorbance was read at λ = 405 nm every 30 sec for 1 hr. Results were calculated using the molar extinction coefficient ε = 182000 M−1 (Frame et al., 2000).
Arylsulfatase C (ASC)
Arylsulfatase C activity was determined as previously described using the substrate p-nitrophenyl sulfate (Collier et al., 2009; Sugawara et al., 2012). Absorbance was measured at λ = 420 nm for 45 min. Results were transformed to pmol · min−1 · mg protein−1 using a standard curve generated with p-nitrophenol.
Western blot analysis
Proteins from human placental explants (n = 3 for 48 and 96 hr), mouse placental explants (n = 6, 5, 5 for 48, 72, 96 hr, respectively) and pooled (n = 50) human liver positive control (Xenotech, KS, 4µg, 4µg and 5 µg, respectively) were separated by SDS-PAGE using 10% gels under reducing conditions (Laemmli, 1970). Each placental protein extract was electrophoresed in an individual lane and the pooled human liver also electrophoresed in one lane per gel. Proteins were transferred to PVDF membrane and these were blocked in 2% fish skin gelatin powder in phosphate buffered saline (PBS) with 0.1% Tween (PBST) overnight. Total SULT1A1 detection occurred with primary antibody (1:100 polyclonal, 2 hr, Abcam, Cambridge, MA), donkey-anti-rabbit biotinylated (1:5000, 1 hr, Jackson ImmunoResearch, PA) and streptavidin-biotinylated-horseradish peroxidase (HRP) (1:3000, 1 hr, GE Healthcare, Piscataway, NJ), before chemiluminescent detection using Western Lightning Plus ECL (Perkin Elmer, Waltham, MA). Total ASC was detected using primary antibody (1:100 polyclonal, 2 hr, Abnova, Taipei, Taiwan), donkey-anti-mouse HRP (1:3000, 1 hr, Jackson ImmunoResearch, Westgrove, PA), before visualization with chemiluminescence. Protein band intensity for samples were analyzed using ImageJ software (National Institute of Health, Bethesda, MD) and normalized to the relative intensity of pooled human liver samples on the corresponding membrane, with subtraction of background from film for each result.
Statistical methodology
Statistical analyses were performed using GraphPrism 5.0 (GraphPad Software, Inc., San Diego, CA) with statistical significance set at α = 0.05. Normality of the data was verified using the D’Agostino-Pearson test. If the data met normality, a one-way analysis of variance (ANOVA) was used for three or more groups, and Tukey’s multiple comparison post hoc test was performed to determine differences between the groups. Unpaired Student’s t-test was used when comparing two groups. Mean ± standard deviation (SD) are presented for n = 3, while mean ± standard error of the mean (SEM) are presented for an n > 3.
Results
Culture effects on placental explant viability
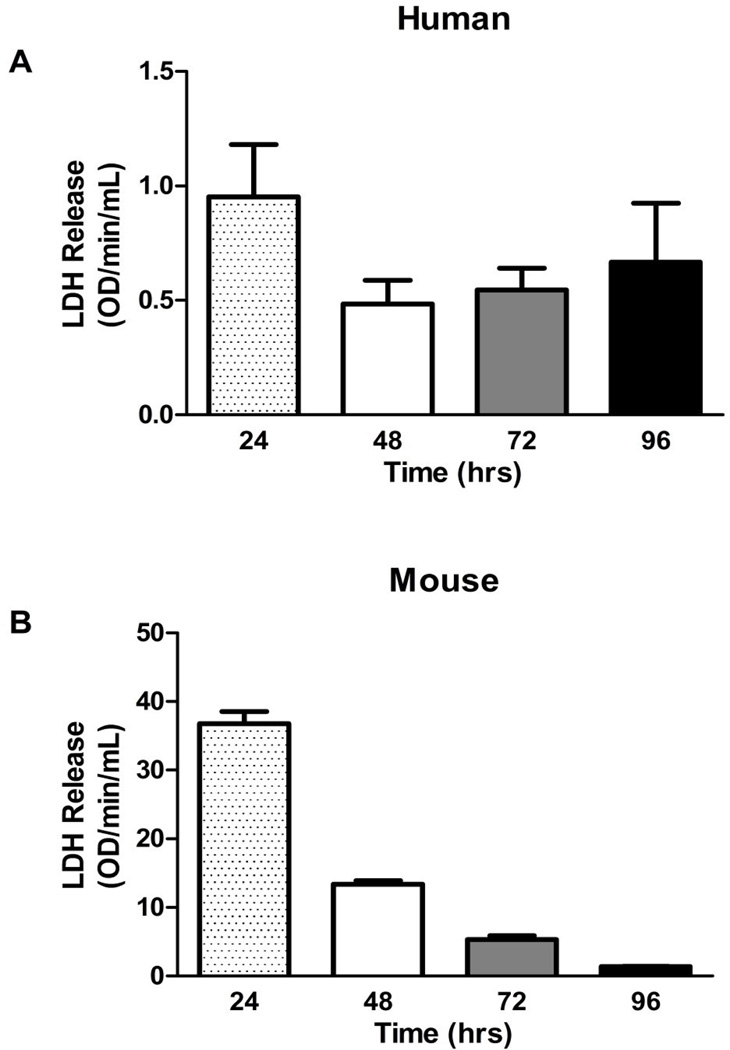
Levels of LDH, which are indicative of cell death, were measured in media collected from human and mouse placental explant culture at the different time-points. In both humans and mice LDH release was highest at 24 hr, likely representing tissue damage from the blunt dissection process. Although human explants did exhibit a slight increase in LDH secretion near 96 hr as compared to 48 and 72 hr, the differences were not significant and, in general, LDH release was consistent. In mice, the LDH levels decreased over the culture period, implying that the cultured tissue had recovered from the initial damage (Figure 1A & B). Overall, human and mouse placental explants did not exhibit significant losses of viability between 24 and 96 hr of culture.
Figure 1. Viability of enriched primary explant culture over four days. LDH release is indirectly indicative of cell death.
There was no significant loss of viability of A. human and B. mouse primary explants between 24 and 96 hr of culture. Bars are means ± SD for humans (n = 3 individual placentas cultured for each time point, with every placenta cultured in three wells and wells assayed in triplicate); bars are means ± SEM for mice (n = 12, 12, 11, 12 individual placentas for each time point, with every placenta cultured in three wells and wells assayed in triplicate)‥
Culture effects on placental sex steroid secretion
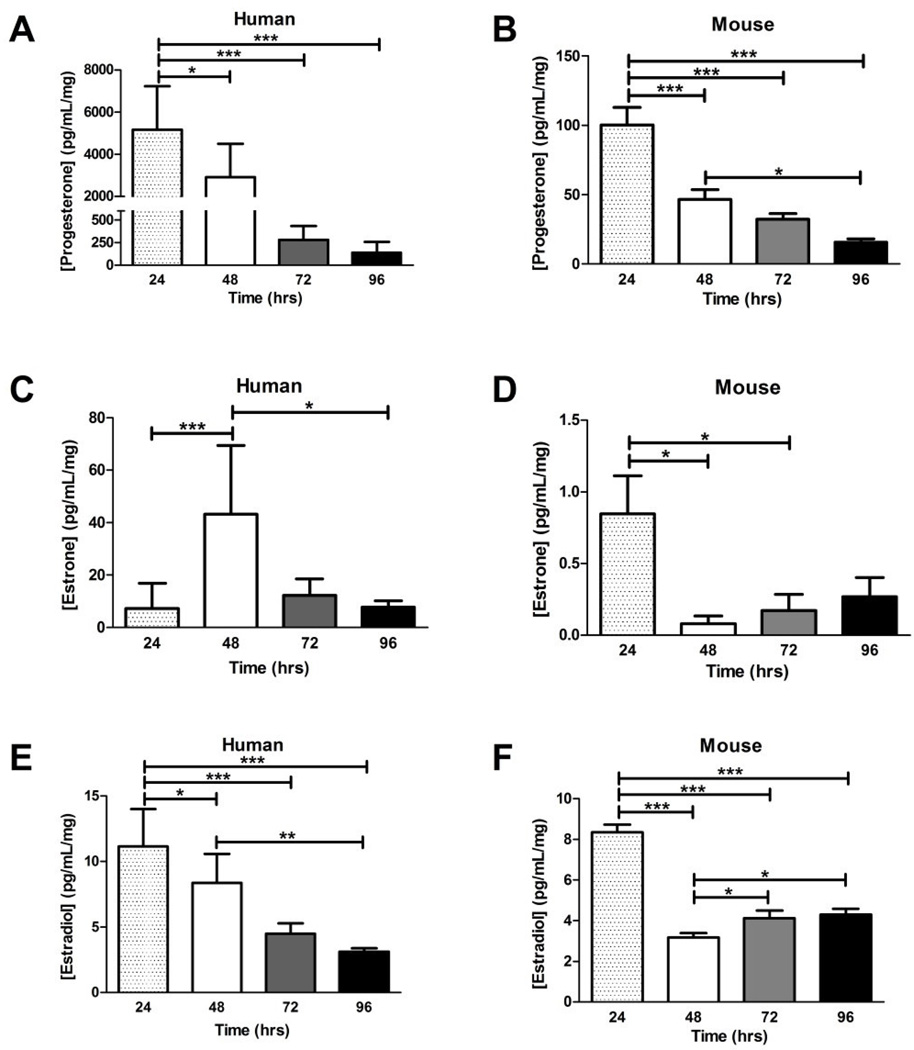
The levels of sex steroid hormones secreted into the culture media by placental explants were quantified. Progesterone levels secreted into culture media by human and mouse explants were significantly decreased at all culture time-points compared to the 24 hr time-point (Figure 2A & B, ANOVA: P < 0.001), with humans having higher levels of progesterone than mice per mg of tissue as well as a larger reduction in progesterone levels (~37-fold change in humans and ~6-fold change in mice) over the 96 hr culture period. Estrone was highest at 48 hr in humans and decreased significantly by 96 hr of culture (Figure 2C, ANOVA: P < 0.001). Conversely, estrone levels (Figure 2D) in mouse explant supernatants exhibited a ~90% reduction at 48 hr when compared to 24 hr (0.848 pg/mL/mg to 0.0820 pg/mL/mg, respectively; ANOVA: P < 0.05), followed by a slight, but not significant increase in later time-points. Levels of estrone were significantly higher in human culture medium compared to that measured in the mouse. Human 17β-estradiol levels decreased significantly in a time-dependent manner (ANOVA: P < 0.001, Figure 2E), while mice 17β-estradiol declined initially (~62%) within 48 hr in culture and remained low when measured at subsequent time-points (ANOVA: P < 0.001, Figure 2F). Unlike with progesterone and estrone, the levels of 17β-estradiol were in the similar range for both human and mouse explant culture media. Overall, explant culture demonstrated altered sex steroid secretion in both human and mouse placental explants.
Figure 2. Levels of sex steroid hormones secreted into culture medium over four days of primary explant culture.
A. Progesterone levels measured in the media of human primary explants significantly decreased from 24 to 96 hr, P < 0.001, ANOVA. B. Significant decreases were observed in progesterone levels of mouse primary explants. C. Estrone levels in human primary explant media peaked at 48 hr before declining significantly, P < 0.001. D. Mouse estrone levels declined in the media after the initial 24 hr of culture, P < 0.05. E. The 17β-estradiol levels in the media of human primary explant culture displayed a significant time-dependent decrease, P < 0.001. F. The 17β-estradiol measured in mouse explant media was significantly reduced after the initial 48 hr of culture, with a slight increase at 48 and 72 hr, P < 0.001. Bars are means ± SD for human (n = 11, 12, 3, 3 placentas cultured at each time point, each well assayed in triplicate); and means ± SEM for mice (n = 12, 12, 11, 12 placentas cultured at each time point, each well assayed in triplicate). Horizontal bars indicate significance between individual groups determined with Tukey’s post hoc test. * = P < 0.05, ** = P < 0.01, *** = P < 0.001. The ANOVA results for total trend are P values reported in the legend.
Culture effects on placental steroidogenic enzymes
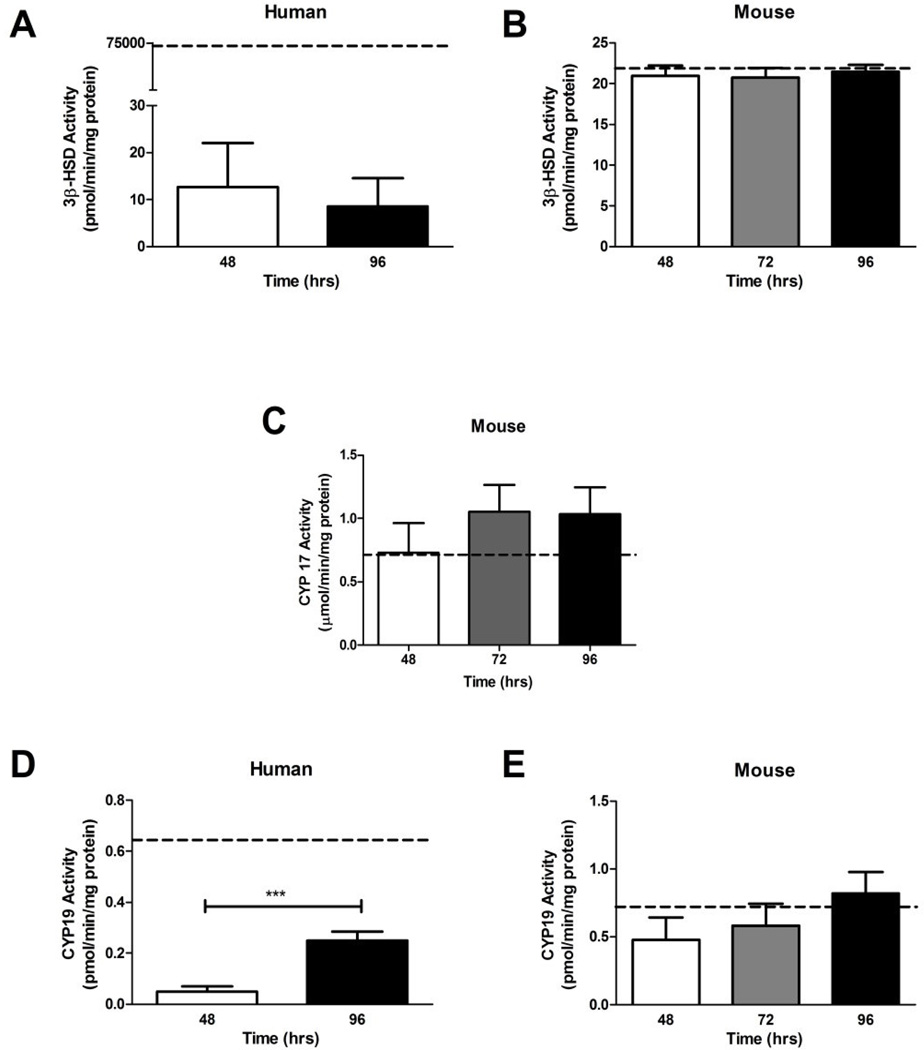
While there was no change in 3β-HSD (Figure 3A), activity of CYP19 in human placental explants increased 5-fold from 48 to 96 hr (T-test: P < 0.001, Figure 3D). The activity of the isolated pooled human placentas not subjected to culture was 7,000-fold and 4- fold higher, for 3β-HSD and CYP19 respectively, than the levels detected in human explants. Activity for CYP17 was not performed in humans, since the human placenta does not express CYP17 (Evain-Brion and Malassine, 2003).
Figure 3. The activities of steroidogenic enzymes over four days of primary explant culture.
A. The activity of 3β-HSD was not significantly altered in human primary explants between 48 and 96 hr; however levels were significantly lower than 3β-HSD activity in freshly isolated human placental tissue, depicted by dotted line (n = 3 human placentas cultured in individual wells at each time point, with each well assayed in triplicate). 5). B. 3β-HSD activity in mouse placental explants did not change between 48 and 96 hr of culture, nor differ from activity levels in freshly isolated mouse placental tissue (n = 6, 6 and 7 placentas cultured in individual wells at each time point, with each well assayed in triplicate). 5). C. Levels of CYP17 activity in mouse placental explants did not significantly differ between 48 and 96 hr, nor vary from isolated mouse placental tissue (n = 6, 6, 7, individually cultured placentas, each assayed in triplicate). D. Activity of CYP19 in human placental significantly increased between 48 and 96 hr of culture and was significantly lower than activity measured in isolated placental tissue (n = 5 and 3 individual placentas cultured separately for each timepoint, then assayed in triplicate). E. CYP19 activity levels were similar in isolated mouse placental tissue and mouse placental explants cultured between 48 and 96 hr (n = 6, 6, 7 individual placentas cultured separately for each timepoint, then assayed in triplicate). The dotted line represents mean of isolated pooled placenta (n = 5 for humans and mice), bars are means ± SD for humans; bars are means ± SEM for mice. *** = P < 0.001 between time-points.
None of the activities of steroidogenic enzymes (3β-HSD, CYP17, CYP19) differed significantly in explant tissues compared to freshly isolated mouse placenta during the culture period for mice placental explants (Figure 3B, C, and E).
Culture effects on steroid clearance and regeneration enzymes
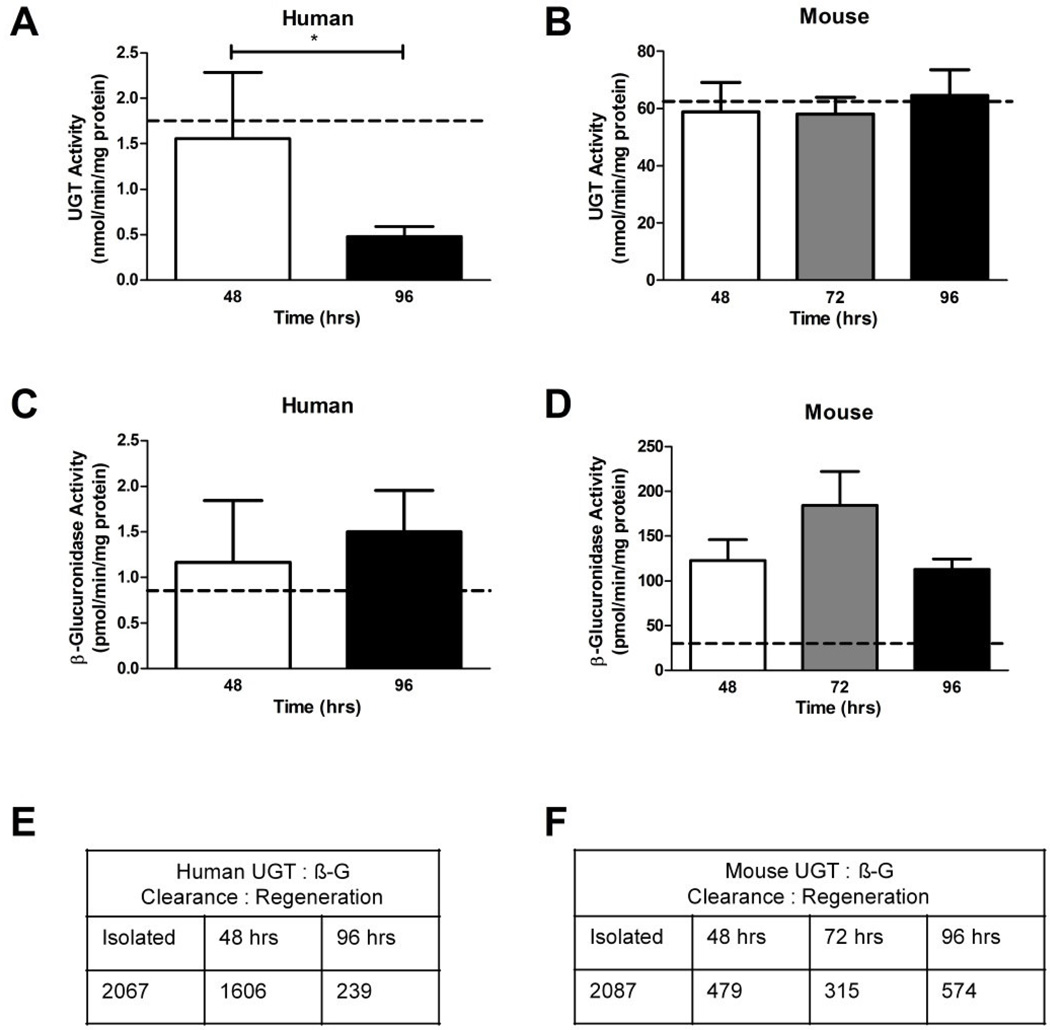
General UGT activity significantly decreased from 48 to 96 hr culture for human explants (Figure 4A), yet remained constant during culture in mice explants (Figure 4B). Activity of β-glucuronidase (β-G), for both human and mice, did not differ significantly during culture between 48 and 96 hr (Figure 4C, D). The UGT activity in freshly isolated human placenta was not different than that in explant culture at 48 hr, but was higher than at 96 hr. The β-G activity in freshly isolated, pooled (n = 5) mouse placental tissue was significantly lower than that of the 48, 72 and 96 hr time-points in cultured explants (29.995 ± 11.278 pmol/min/mg, 122.684 ± 57.552 pmol/min/mg, 184.347 ± 84.556 pmol/min/mg, respectively, T-test: P < 0.01 all). This indicated that the dissection and culture conditions were causing greater β-G activity than the in vivo condition in mice.
Figure 4. The activities of UGT (steroid clearance) and β-G (steroid regeneration) enzymes over four days of primary explant culture.
A. Activity levels of UGT enzymes significantly decreased between the 48 to 96 hr in human primary explant cultures, P < 0.05. B. Mouse placental explant did not experience any alterations to UGT activity between 48 and 96 hr of culture, nor differ from levels measured in the freshly isolated mouse placental tissue. C. Levels of β-G activity were not altered between 48 and 96 hr of culture in human primary explants and were similar to freshly isolated tissue. D. Mouse placental explant β-G activity was consistent between 48 and 96 hr of culture, but at all time-points was significantly higher than activity in isolated mouse placental tissue. E. Ratios of UGT: β-G showing progressive decline of clearance by glucuronidation in human cultures. F. Ratios of UGT: β-G showing immediate loss of glucuronidation that is maintained at ~25% of fresh tissue levels during culture of mouse explants. Dotted line in A–D represents mean of isolated pooled placenta (n = 5 for humans and mice), bars are means ± SD for humans (n = 5, 3, 5); bars are means ± SEM for mice (n = 6, 5, 6, 5).
The clearance (UGT) to regeneration (β-G) ratio in human explants was almost 7-fold higher at 48 hr compared to 96 hr (Figure 4E). In mice explants, the UGT: β-G ratio did not differ amongst the culture time points (Figure 4F). When compared to freshly isolated placental tissue, the ratios of UGT and β-G at 96 hr were 8.6-fold and 3.6-fold lower for humans and mice, respectively. This indicates that explant culture has an overall effect of reduced elimination by glucuronidation within the culture system.
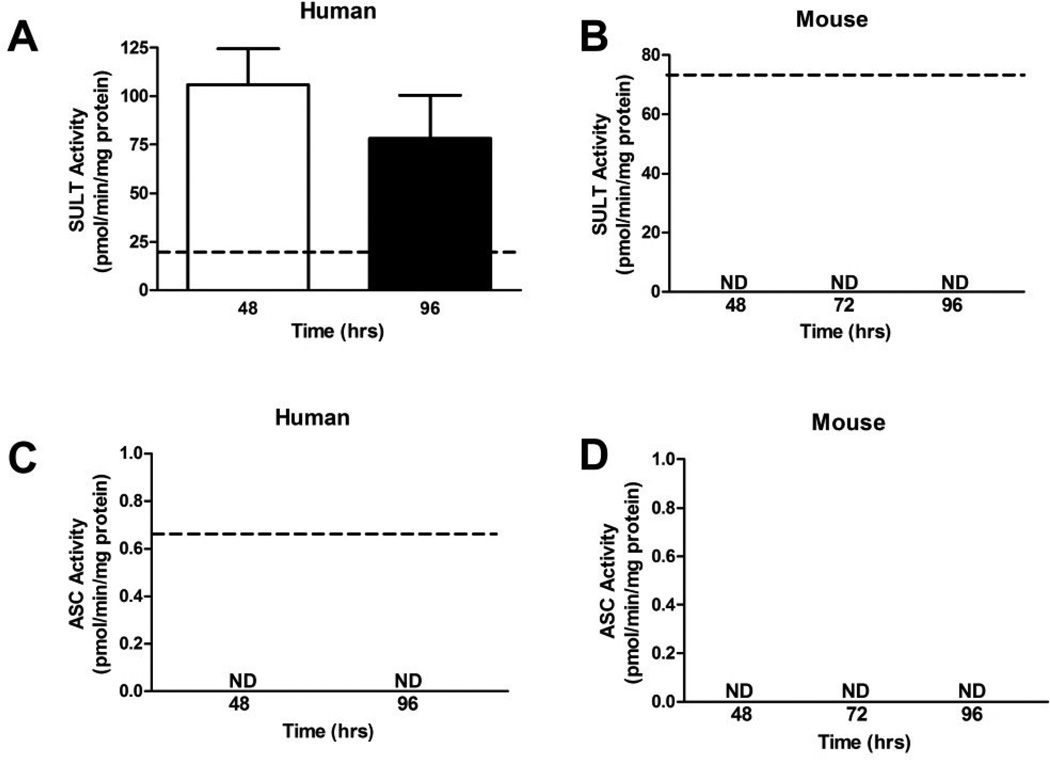
The activity of SULT (SULT1A1) in human explants was not significantly different between 48 and 96 hr of culture, but was 5.5- and 4-fold higher than the isolated pooled human placental control, respectively (Figure 5A). In the mouse placental explants, SULT activity could only be detected in freshly isolated mouse placental tissue but not in explants (Figure 5B). Activity of ASC was measurable only in isolated human placental tissue (Figure 5C), but not in human explants, mouse isolated placental tissue, or mouse explants (Figure 5B, 5C and 5D).
Figure 5. The activities of SULT (steroid clearance) and ASC (steroid regeneration) enzymes over four days of primary explant culture.
A. SULT activity was similar after 48 and 96 hr of culture in human placental explants. Explants activity levels were significantly higher than levels measured in isolated human placental tissue, dotted line (n = 3, placentas cultured at each time point, then assayed in triplicate). B. SULT activity was only detectable in isolated mouse placental tissue and not in mouse placental explants. C. ASC activity was only detectable in isolated human placental tissue, but not detectable in human placental explants. D. ASC activities were not detected in fresh mouse tissue or placental explants. The dotted line represents a pool of n = 5 freshly isolated mouse or human placentas for SULT, and n = 10 for ASC, with the pool assayed once in triplicate. Enzyme activities were determined in triplicate for each sample. Bars are means ± SD.
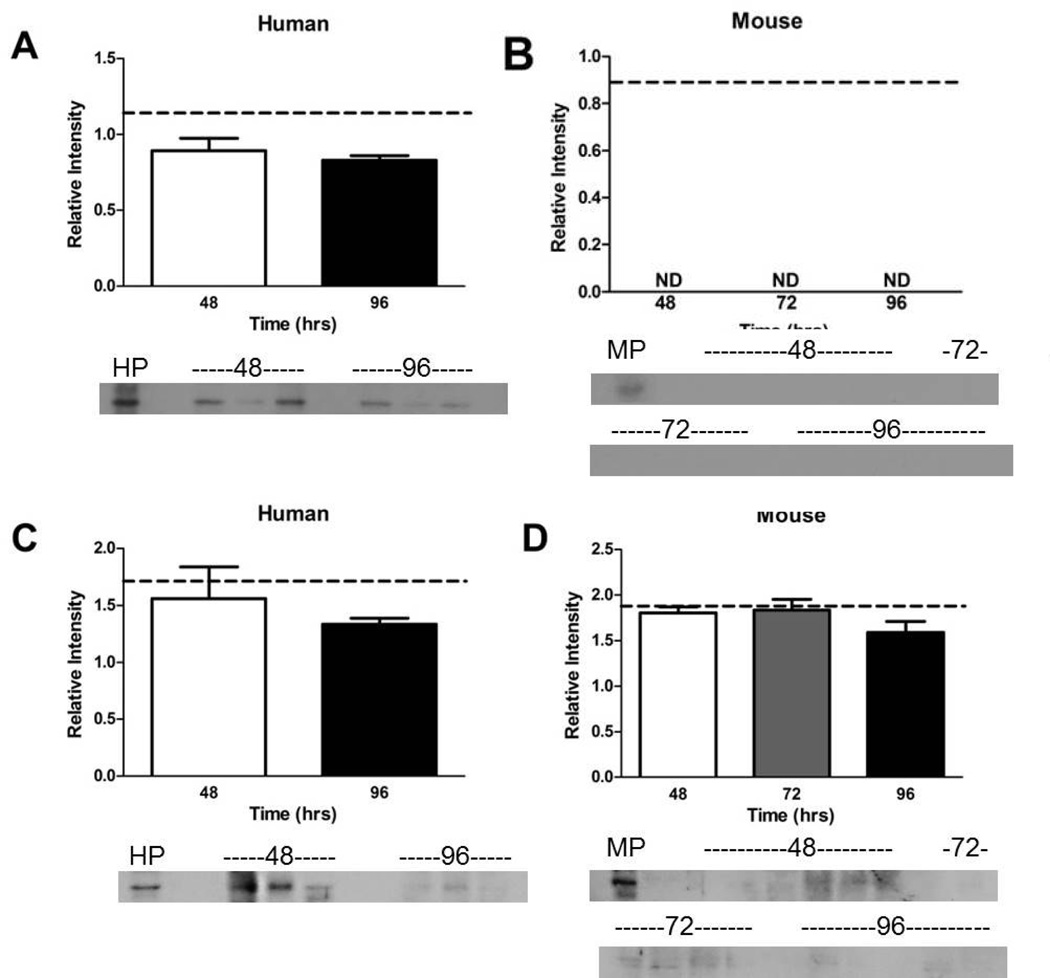
Due to the lack of measurable activity of SULT and ASC, Western immunoblotting was used to confirm their presence. Relative protein levels of SULT and ASC in humans were not significantly different between the 48 and 96 hr culture time points and freshly isolated mouse placental tissue (Figure 6A & C). The SULT1A1 protein was detected in isolated mouse placental tissue (Figure 6B), but not in mouse explant tissues. The ASC protein was detected in mice explants and isolated mouse placental tissue and protein levels were not significantly different (Figure 6D). The SULT proteins and activity were observed in humans, but neither was observed in mouse tissues (Figure 5A & B). The ASC protein results are more interesting as its activity was not observed in humans or mice (Figure 5C & D), despite the detection of the protein in both species by Western blot (Figure 6C & D). This indicates that culture conditions may be inhibiting ASC enzymatic activity but not protein production.
Figure 6. The expression of SULT and ASC over four days of primary explant culture.
A. The expression of SULT proteins in human placental explants did not change between 48 and 96 hr and was similar to expression of SULT in isolated human placental tissue. B. The expression of SULT proteins was only detectable in isolated mouse placental tissue but not in mouse explants. C. Expression of ASC proteins was similar in isolated human placental tissue and human placental explants. D. Although ASC activity was undetectable in mouse placental explants (Fig. 5D), expression of the proteins was measurable across the cultured time points and isolated mouse placental tissue. Dotted line represents mean of isolated pooled placenta (n = 5 in the pool for humans and mice, assayed once in triplicate), bars are means ± SD for humans (n = 3 individual placentas cultured, then each culture assayed in triplicate); bars are means ± SEM for mouse (n = 6, 5, 5 individual placentas cultured, then each culture assayed in triplicate). HP and MP are pooled human (n = 5) and pooled mouse (n = 5) freshly isolated placental tissue for western blot.
Discussion
This is the first study to characterize human primary placental explant culture for applications to sex steroid and metabolic toxicity studies. It is also the first to test the mouse placenta as an explant model, and to compare mouse explants to humans. Several differences between the human and mouse tissues were observed. The ex vivo stability of murine enzymes involved in placental steroid production suggests that the mouse explants may be appropriate for studying placental steroidogenesis, including basic endocrinology, pharmacological and toxicological studies. This was not true for human tissue explants where steroidogenic enzyme activities decreased rapidly in culture. In contrast, the mouse model showed immediate dysregulation of conjugation enzymes, which in the human explant system were stable for up to 48 hours. Both mouse and human placental explants remained viable throughout the culture period, based on the assays used. Therefore, the differences observed between the explants and freshly isolated tissues were not caused by cell death.
In human pregnancy, cholesterol from the maternal circulation is the initial substrate for steroidogenesis, which occurs throughout pregnancy through coordination between placental enzymes and enzymes in the fetal adrenal glands (Evain-Brion and Malassine, 2003). Because there is no production of cholesterol in the explant system, an argument can be made that cholesterol supplementation in the medium would have maintained steroid production and resolved our findings. We contend that while cholesterol supplementation would allow steroid production, quantification of the steroid levels would not be reflective of the in vivo situation because the 3β-HSD and CYP19 enzyme activities were significantly lower in the explants compared to freshly isolated tissues. Hence, even with cholesterol supplementation, findings regarding effects on steroid hormone levels would be artificially low, confounding results in, for example, endocrine disruption studies. These data are in agreement with Begum-Hasan, et al. (Begum-Hasan et al., 1993) who reported a 10-fold reduction of progesterone secretion in cultured human placental explants over 96 hours. This phenomenon does not occur with all endocrine functions of human placental explants because Rubin et al (1993) demonstrated that Vitamin D3 can be hydroxylated consistently to a pro-hormone involved in bone development for up to 96 hours, in similar culture conditions as used here (Rubin et al., 1993). Moreover gonadotropin releasing hormone and dehydroepiandrosterone can stimulate human chorionic gonadotropin release in term explants up to 8 days (Ahmed and Murphy, 1988). These prior studies indicate that some endocrine pathways are preserved. However, specifically for sex steroids (progesterone, androstenedione, testosterone, 17β-estradiol and estrone), we have shown that production is inherently compromised.
In contrast, the steroidogenic enzymes are expressed and active in mouse placental explants at levels similar to fresh tissue and remain so throughout culture. Although mouse placental enzymes play a minor role compared to the murine maternal ovaries (Malassiné et al., 2003), they make a measurable contribution to transplacental sex steroid homeostasis. In this case, cholesterol supplementation in the media would maintain steroid production in mouse explants at in vivo levels and the model is suitable for up to four days.
With reference to metabolic elimination, we observed that in both species UGT/β-G and SULT/ASC enzymes were compromised in this culture. This was more pronounced in the mouse where conjugation pathways were immediately deregulated, while in humans conjugation did not significantly decline for up to 48 hours. In general, sulfation is more critical for clearance of endogenous compounds such as sex steroids, and glucuronidation is more critical for xenobiotics, although in the absence of either enzyme due to pathological or environmental conditions the other can often compensate due to overlapping substrate affinities. Since it has recently been demonstrated that the mRNA expression of Sult and Ugt genes is not altered in mouse maternal liver, kidney, uterus or placenta across gestation (Shuster et al., 2013) we contend changes in conjugation enzyme expression and activity are a function of the harvesting and/or culture conditions. Additionally, because assays were optimized in fresh human and mouse tissues and the components of the biochemical reactions (e.g. co-factors, salt, and thermodynamic requirements) for these enzymes are conserved in mammals, our inability to detect activity is not due to limitations in the biochemical techniques used. Ultimately, murine placental explants are not appropriate for studies where conjugation is the primary path of elimination. However, short-term studies of conjugation in human explant culture can be accurate. A single report in human placental explants supports our findings by showing that both term and first trimester explants glucuronidate the drug AZT for up to 24 hours (Collier et al., 2004). Insofar as the rapid loss of metabolic enzymes in culture is concerned, this is also observed in other commonly used primary cultures such as hepatocytes and kidney cells (Trevisanuto et al., 2013), but the precise cellular mechanisms for this have not been elucidated.
In addition to cellular down-regulation of enzyme activities, the human placental explant system is known to slough syncytial cells within the first few days of culture (Redline, 2004). Because most of the Phase I and II metabolizing enzymes, including the steroidogenic enzymes, are localized in the placental syncytium (Collier et al., 2002), there is an inherent limitation to using placental explant culture for endocrine and metabolic studies. These known physiological characteristics of the culture both support, and are supported by, our results that show metabolism studies are only useful in humans for 48 hours – before the sloughing event takes place. Continuing culture to 8–10 days, when the syncytium regenerates (Redline, 2004), might provide another chance to assess metabolism but, because explant viability in culture is compromised around this time (Simán et al., 2001), these longer time-points may be confounded by cell death. In contrast, the length of viability for mouse placental cultures, which were a novel part of this study design, has not been determined apart from this report of viability for up to 4 days. Since the mouse model seems more robust for steroidogenic investigation, future ultrastructural studies of the characteristics of mouse placental explants up to 10–12 days of culture should be conducted. This can determine whether mouse explants behave similarly to the human in sloughing their syncytia or whether the mouse explants, being composed of a greater variety of tissues, show a different physiological pattern.
Over-and-above biological phenomena, two physical parameters in culture that may contribute to the decline of enzyme activities are oxygen tension and pH. With regards to oxygen tension, primary explant models have greater volume than monolayer cultures, hence the use of 95% air/5% CO2 for culture (which is standard) may be questioned. One recent study contends that the oxygen tension inside placental explants when using ambient (21%) oxygen actually constitutes a hyperoxic state (Reti et al., 2007) while others have reported that ambient oxygen falls into the realms of hypoxia since the pericellular oxygen tension in culture is as low as 0.6% (Chen et al., 2014). Because both hyperoxia and hypoxia in vitro have been associated with down-regulation of enzymes and transporters and altered hormone synthesis (Javam et al., 2014; Ma et al., 2001; Myatt, 2010), either of these could be a mechanism behind loss of enzyme function observed here. Despite these findings, several other investigators report good results in explant cultures and other placental models (such as placental perfusion), with oxygen tensions ranging 5 – 21% associated with normal markers of placental function (Chen et al., 2011; Peltier et al., 2011; Soydemir et al., 2011). Hence, hypoxia or hyperoxia may or may not be the reason for decreased enzymatic activity in this model. Indeed the effects of oxygen tension in placental culture and other ex vivo systems is an area of some controversy in the field of placental biology (Ernst et al., 2009). More definitive studies on the effects of oxygen tension on explant response, as well a more international consensus in the field, will be required for us to understand true in vivo oxygenation levels as well as optimal in vitro oxygen supplementation for placental tissues.
In culture, pH can also affect enzyme expression and activity. Although we did not directly measure pH, the media used contained phenol red as an indicator, which was permissible since enzymes were measured in tissue after harvesting and washing. Phenol red did not indicate that large changes in pH were occurring, and the media was refreshed at 24 hour intervals.
One limitation of this study is that it was performed exclusively in term placental tissues from both mice and humans. There is evidence in both species that Cytochromes P450 (including steroidogenic enzymes) as well as phase II conjugation enzymes, differ in their expressions and activities across gestation (Ben Zimra et al., 2002, Collier et al., 2002, Malassiné et al., 2003). At term, the placenta has begun to structurally and functionally senesce, hence obtaining placental tissues from earlier gestational periods may reveal better or different properties of the enzymes in culture. This would be an interesting area for further investigation.
Primary placental explants are well accepted and validated for studying inflammation, cytokines, placental differentiation and growth in human tissues (Benyo et al., 1997; Keelan et al., 2000; Mitchell et al., 2000). However, some limitations in their application to sex steroid endocrinology, pharmacology and toxicology have been demonstrated here. Human placenta is an accessible tissue and explants may be useful for studying acute (but not chronic) transplacental conjugation and clearance of compounds, but they are not recommended for sex steroid studies. Conversely, with further characterization, careful adoption of a murine placental explant model may help to study and define the mechanisms by which endocrine toxicants affect pregnancy and development.
Human placental explants are validated for cytokine but not sex steroid studies
Mouse placental explant cultures have not previously been examined
We assessed ex vivo culture of both species for steroidogenic and clearance traits
Human explants may be used for metabolism and clearance, but not endocrine studies
Mouse explants may be used for steroidogenesis, but not phase II metabolism studies
Acknowledgements
We thank the nurses and staff of the labor and delivery ward of Kapi‘olani Medical Center for Women and Children for their help with the tissue collection.
The study was supported by NIH G12 MD007601 and P20GM103457 (project 4) to A.C.C. as well as P20 GM103457 (project 2), HD072380 and Hawaii Community Foundation to M.A.W. Support was for research only and did not influence study design, interpretation or decision to publish.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors have nothing to declare.
Part of this work was presented to the Annual Society for Reproductive Investigation Meeting, Florence Italy, March 26–29, 2014.
References
- Ahmed N, Murphy B. The effects of various hormones on human chorionic gonadotropin production in early and late placental explant cultures. American Journal of Obstetrics and Gynecology. 1988;159:1220–1227. doi: 10.1016/0002-9378(88)90453-x. [DOI] [PubMed] [Google Scholar]
- Begum-Hasan J, Senterman M, Gillett P, LaPlante Branchaud C, Murphy BE. Effect of maternal serum on viability and function of early human placental explants. In Vitro Cellular and Developmental Biology - Animals. 1993;29A:505–511. [PubMed] [Google Scholar]
- Ben-Zimra M, Koler M, Melamed-Book N, Arensburg J, Payne AH, Orly J. Uterine and placental expression of steroidogenic genes during rodent pregnancy. Molecular and Cellular Endocrinology. 2002;187:223–231. doi: 10.1016/s0303-7207(01)00713-4. [DOI] [PubMed] [Google Scholar]
- Benyo DF, Miles TM, Conrad KP. Hypoxia Stimulates Cytokine Production by Villous Explants from the Human Placenta. Journal of Clinical Endocrinology and Metabolism. 1997;82:1582–1588. doi: 10.1210/jcem.82.5.3916. [DOI] [PubMed] [Google Scholar]
- Carter AM. Animal models of human placentation - a review. Placenta. 2007;28(Supplement):S41–S47. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Chen B, Longtine M, Nelson D. Pericellular oxygen concentration of cultured primary human trophoblasts. Placenta. 2014;34:106–109. doi: 10.1016/j.placenta.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Liversidge X, Liu B, Stone P, Chamley L. Does oxygen concentration affect shedding of trophoblastic debris or production of inflammatory mediators from first trimester human placenta? Placenta. 2011;32:362–366. doi: 10.1016/j.placenta.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Cockburn K, Rossant J. Making the blastocyst: lessons from the mouse. J Clin Invest. 2010;120:995–1003. doi: 10.1172/JCI41229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A, Keelan J, Van Zijl P, Paxton J, Mitchell M, Tingle M. Human placental glucuronidation and transport of 3'azido-3'-deoxythymidine and uridine diphosphate glucuronic acid. Drug Metabolism and Disposition. 2004;32:813–820. doi: 10.1124/dmd.32.8.813. [DOI] [PubMed] [Google Scholar]
- Collier AC, Ganley NA, Tingle MD, Blumenstein M, Marvin KW, Paxton JW, Mitchell MD, Keelan JA. UDP-glucuronosyltransferase activity, expression and cellular localization in human placenta at term. Biochemical Pharmacology. 2002;63:409–419. doi: 10.1016/s0006-2952(01)00890-5. [DOI] [PubMed] [Google Scholar]
- Collier AC, Miyagi SJ, Yamauchi Y, Ward MA. Assisted reproduction technologies impair placental steroid metabolism. The Journal of Steroid Biochemistry and Molecular Biology. 2009;116:21–28. doi: 10.1016/j.jsbmb.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AC, Tingle MD, Keelan JA, Paxton JW, Mitchell MD. A Highly Sensitive Fluorescent Microplate Method for the Determination of UDP-Glucuronosyl Transferase Activity in Tissues and Placental Cell Lines. Drug Metabolism and Disposition. 2000;28:1184–1186. [PubMed] [Google Scholar]
- Dilworth MR, Sibley CP. Review: Transport across the placenta of mice and women. Placenta. 2013;34(Suppl):S34–S39. doi: 10.1016/j.placenta.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Ernst LM, Gonzalez J, Ofori E, Elovitz M. Inflammation-Induced Preterm Birth in a Murine Model Is Associated with Increases in Fetal Macrophages and Circulating Erythroid Precursors. Pediatric and Developmental Pathology. 2009;13:273–281. doi: 10.2350/09-05-0649-OA.1. [DOI] [PubMed] [Google Scholar]
- Evain-Brion D, Malassiné A. Human placenta as an endocrine organ. Growth Hormone & IGF Research. 2003;13:S34–S37. doi: 10.1016/s1096-6374(03)00053-4. [DOI] [PubMed] [Google Scholar]
- Frame LT, Ozawa S, Nowell SA, Chou H-C, DeLongchamp RR, Doerge DR, Lang NP, Kadlubar FF. A Simple Colorimetric Assay for Phenotyping the Major Human Thermostable Phenol Sulfotransferase (SULT1A1) Using Platelet Cytosols. Drug Metabolism and Disposition. 2000;28:1063–1068. [PubMed] [Google Scholar]
- Gore-Langton R, McKeracher H, Dorrington J. An Alternative Method for the Study of Follicle-Stimulating Hormone Effects on Aromatase Activity in Sertoli Cell Cultures. Endocrinology. 1980;107:464–471. doi: 10.1210/endo-107-2-464. [DOI] [PubMed] [Google Scholar]
- Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thrombosis Research. 2004;114:397–407. doi: 10.1016/j.thromres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Javam M, Audette M, Iqbal M, Bloise E, Gibb W, Matthews SG. Effect of oxygen on multidrug resistance in term human placenta. Placenta. 2014;35:324–330. doi: 10.1016/j.placenta.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Keelan JA, Zhou RL, Mitchell MD. Activin A Exerts both Pro- and Anti-inflammatory Effects on Human Term Gestational Tissues. Placenta. 2000;21:38–43. doi: 10.1053/plac.1999.0451. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ma T, Yang S, Kniss D. Oxygen tension influences proliferation and differentiation in a tissue-engineered model of placental trophoblast-like cells. Tissue Engineering. 2001;7:495–506. doi: 10.1089/107632701753213129. [DOI] [PubMed] [Google Scholar]
- Malassiné A, Frendo JL, Evain-Brion D. A comparison of placental development and endocrine functions between the human and mouse model. Human Reproduction Update. 2003;9:531–539. doi: 10.1093/humupd/dmg043. [DOI] [PubMed] [Google Scholar]
- Mitchell MD, Goodwin V, Mesnage S, Keelan JA. Cytokine-induced coordinate expression of enzymes of prostaglandin biosynthesis and metabolism: 15-hydroxyprostaglandin dehydrogenase. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2000;62:1–5. doi: 10.1054/plef.1999.0117. [DOI] [PubMed] [Google Scholar]
- Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31:S66–S69. doi: 10.1016/j.placenta.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R, Heinrich PC, Müller-Newen G. Activation of STAT3 by IL-6 and IL-10 in Primary Human Macrophages Is Differentially Modulated by Suppressor of Cytokine Signaling 3. The Journal of Immunology. 2003;170:3263–3272. doi: 10.4049/jimmunol.170.6.3263. [DOI] [PubMed] [Google Scholar]
- Peltier M, Gurzenda E, Murthy A, Chawala K, Lerner V, Kharode I, Arita Y, Rhodes A, Maari N, Moawad A, Hanna N. Can oxygen tension contribute to an abnormal placental cytokine milieu? American Journal of Reproductive Immunology. 2011;66:279–285. doi: 10.1111/j.1600-0897.2011.00998.x. [DOI] [PubMed] [Google Scholar]
- Ratajczak CK, Muglia L. Insights into parturition biology from genetically altered mice. Pediatric Research. 2008;64:581–589. doi: 10.1203/PDR.0b013e31818718d2. [DOI] [PubMed] [Google Scholar]
- Raunig JM, Yamauchi Y, Ward MA, Collier AC. Assisted reproduction technologies alter steroid delivery to the mouse fetus during pregnancy. The Journal of Steroid Biochemistry and Molecular Biology. 2011;126:26–34. doi: 10.1016/j.jsbmb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline R. Placental inflammation. Seminars in Neonatology. 2004;9:265–274. doi: 10.1016/j.siny.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Reti N, Lappas M, Huppertz B, Riley C, Wlodek M, Henschke P, Permezel M, Rice GE. Effect of high oxygen on placental function in short-term explant cultures. Cell and Tissue Research. 2007;328:607–616. doi: 10.1007/s00441-006-0375-1. [DOI] [PubMed] [Google Scholar]
- Rubin L, Yeung B, Vouros P, Vilner L, Reddy G. Evidence for human placental synthesis of 24,25-dihydroxyvitamin D3 and 23,25-dihydroxyvitamin D3. Pediatric Research. 1993;34:98–104. doi: 10.1203/00006450-199307000-00023. [DOI] [PubMed] [Google Scholar]
- Shuster D, Bammler T, Beyer R, Macdonald J, Tsai J, Farin F, Hebert M, Thummel K, Mao Q. Gestational age-dependent changes in gene expression of metabolic enzymes and transporters in pregnant mice. Drug Metabolism and Disposition. 2013;41:332–342. doi: 10.1124/dmd.112.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simán CM, Sibley CP, Jones CJ, Turner MA, Greenwood SL. The functional regeneration of syncytiotrophoblast in cultured explants of term placenta. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2001;280:R1116–R1122. doi: 10.1152/ajpregu.2001.280.4.R1116. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Analytical Biochemistry. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Soydemir F, Kuruvilla S, Brown M, Dunn W, Day P, Crocker I, Baker P, Sibley C, Brownbill P. Adapting in vitro dual perfusion of the human placenta to soluble oxygen tensions associated with normal and pre-eclamptic pregnancy. Laboratory Investigation. 2011;91:181–189. doi: 10.1038/labinvest.2010.171. [DOI] [PubMed] [Google Scholar]
- Sugawara A, Sato B, Bal E, Collier AC, Ward MA. Blastomere Removal from Cleavage-Stage Mouse Embryos Alters Steroid Metabolism During Pregnancy. Biology of Reproduction. 2012;87:1–9. doi: 10.1095/biolreprod.111.097444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisanuto D, Peruzzetto C, Cavallin F, Vedovato S, Cosmi E, Visentin S, Chiarelli S, Zanardo V. Fetal placental inflammation is associated with poor neonatal growth of preterm infants: a case-control study. Journal of Maternal-Fetal and Neonatal Medicine. 2013;26:1484–1490. doi: 10.3109/14767058.2013.789849. [DOI] [PubMed] [Google Scholar]
- Trubetskoy OV, Shaw PM. A Fluorescent Assay Amenable to Measuring Production of β-d-Glucuronides Produced from Recombinant UDP-Glycosyl Transferase Enzymes. Drug Metabolism and Disposition. 1999;27:555–557. [PubMed] [Google Scholar]
- Zewe V, Fromm HJ. Kinetic Studies of Rabbit Muscle Lactate Dehydrogenase. II. Mechanism of the Reaction*. Biochemistry. 1965;4:782–792. doi: 10.1021/bi00880a024. [DOI] [PubMed] [Google Scholar]