Abstract
Composite bone models are increasingly used in orthopaedic biomechanics research and surgical education—applications that traditionally relied on cadavers. Cadaver bones are suboptimal for myriad reasons, including issues of cost, availability, preservation, and inconsistency between specimens. Further, cadaver samples disproportionately represent the elderly, whose bone quality may not be representative of the greater orthopaedic population. The current fourth-generation composite bone models provide an accurate reproduction of the biomechanical properties of human bone when placed under bending, axial, and torsional loads. The combination of glass fiber and epoxy resin components into a single phase has enabled manufacturing by injection molding. The high anatomic fidelity of the cadaver-based molds and negligible shrinkage properties of the epoxy resin results in a process that allows for excellent definition of anatomic detail in the cortical wall and optimized consistency of features between models. Recent biomechanical studies of composites have validated their use as a suitable substitute for cadaver specimens.
Cadaver specimens have long been used in orthopaedic research and education specifically as well as medical education more broadly.1 Biomechanical study of fracture fixation constructs and orthopaedic implants necessitates a substrate that reproduces the complex and anisotropic properties of organic human bone in order to produce clinically relevant insights. An accurate model of anatomy is also required in the educational setting for the instruction and practice of surgical technique. Accordingly, cadavers have remained a cornerstone of both research and education in orthopaedic surgery.
Although cadaver specimens have distinct advantages in these settings, their use is complicated by a variety of factors. First, they are costly. Femurs, tibiae, and humeri—long bones commonly trialed in the biomechanical study of fracture fixation constructs—cost approximately $500 per specimen (Platinum Training, personal communication, 2013), compared with $170 for a fourth-generation composite equivalent.2 A cadaver lower extremity segment including the knee joint and 4 inches of anatomy proximally and distally costs approximately the same amount as comparable composite bones used in a bench-top arthroscopy simulator.2 However, the composite can be used repeatedly with the replacement of inexpensive components. For elementary surgical skills development, such as increasing familiarity with surgical tools or learning the basics of orthopaedic implant instrumentation, inexpensive foam cortical shell models are also available. These inexpensive bones are not designed to accurately reproduce the biomechanical behavior of human bone.
Logistical considerations also add cost to cadaver specimen use. Specimens must be preserved before, during, and after use. Freezing of fresh specimens is sometimes adequate but requires facility space and limits the duration and episodes of specimen use.3 Use of traditional formalin-based embalming solutions may excessively stiffen soft tissues.4 Recently developed embalming solutions may preserve tissue-handling characteristics, but they are expensive and require even more specialized storage of specimens under vacuum refrigeration.4,5 Cadaver specimens are costly to ship and require specialized pathologic waste disposal procedures.
In addition, cadaver specimens are ethically, religiously, and culturally controversial. The concept of the anatomic gift is well established in Western societies, but much of the demand for cadaver specimens is met through the use of the unclaimed deceased—persons, especially the elderly and homeless, who are wards of the state at the time of their passing.6,7 Cadaver utilization is also hotly debated by religious scholars of many faiths, including Judaism, Islam, and Christianity. Although most religions make concessions for legally required autopsy, the elective violation of the body during educational dissection or biomechanics research is problematic for many groups.8,9 Procurement of cadaver specimens is challenging throughout much of the Middle East and India, where the practice of making an anatomic gift may be less culturally compatible .10,11
Available cadaver tissue is skewed toward the elderly and infirm population due to the overwhelming tendency of these specimens to be procured as unclaimed deceased in nursing homes.7 Accordingly, cadaver specimens may not accurately represent the behavior of fracture fixation constructs and orthopaedic implants in young, healthy patients who present with orthopaedic trauma. Furthermore, there is a high degree of variation in biomechanical properties between cadaver specimens, reportedly up to 100% of the mean in some parameters.12
The challenges and costs associated with the use of cadaver specimens, in addition to inconsistencies between specimens, has prompted the development of a high-fidelity synthetic replacement that accurately recreates the complex properties of natural human bone. Since their introduction in the late 1980s, composite bone models have been the subject of intense investigation as replacements for cadaver specimens in orthopaedic research and education. Unlike cadaver specimens, contemporary composite bone models are relatively inexpensive, ubiquitously available, have minimal variability between specimens, are not ethically controversial, and require no Institutional Review Board oversight. No special storage or preservation techniques are required. Composites are available in various formulations to optimize desirable properties for specific applications, such as enhanced radiopacity or ease of cutting, reaming, or drilling.2 Additionally, composite models may be incorporated into surgical training simulators or obtained singly for use in surgical skills laboratories.
Material Properties and the Development of Composites
Composite bone models were initially introduced in the late 1980s.13 These first-generation models represented the first biomechanically relevant composite bone and consisted of a rigid polyurethane foam core surrounded by an epoxy-reinforced, braided glass sleeve. However, mismatch between the glass fiber size and epoxy component resulted in delamination of the cortical material.13 First-generation models are poorly represented in the biomechanics literature.
Second-generation composites, which were introduced in the early 1990s, were constructed from layers of woven fiberglass matting that were solidified into the cortical matrix by the pressure injection of epoxy resin.14,15 These fiberglass-fabric-reinforced (FFR) composites had no intramedullary canal and were limited by the need for manual craftsmanship; technicians had to layer fiberglass sheets over the rigid polyurethane foam core.13 Despite manufacturing challenges, the FFR cortical matrix of these models successfully replicated the diaphyseal flexural stiffness of cadaver bone. Cristofolini and Viceconte12 found no statistical difference in the lateral bending stiffness of second-generation composite tibias compared with cadaver tibias. However, the FFR composites achieved a 15-fold reduction in variability of this parameter.
Innovation of second-generation models firmly established a role for composites in biomechanics research, but limitations of the FFR cortical material were noted. Although the 45° orientation of glass fibers in the FFR matrix excelled at reproducing physiologic lateral bending rigidity, this geometry bolstered material strength in the rotational plane. Although Cristofolini and Viceconte12 validated the lateral bending rigidity of composites, they demonstrated diaphyseal torsional stiffness values for composite tibias nearly double those of the cadaver specimens. Heiner and Brown13 reported similar data for second-generation FFR tibias.
The late 1990s marked a significant change in composite bone models with the introduction of new materials and manufacturing processes for a third-generation composite. Whereas prior iterations relied on the manual craftsmanship of fiberglass fabric material, third-generation models were manufactured with an entirely pressure-injected technique by which short glass fiber reinforced (SGFR) epoxy was injection-molded around the polyurethane foam core to form the cortical wall.13 Tooling for this process was created using direct castings of cadaver bones from an adult male donor; thus, the composites maintained a high level of anatomic fidelity with regard to topography of the cortical wall and gross specimen size. To reproduce anisotropy in thickness of the cortical wall along the length of the model, the donor specimens underwent serial sectioning to determine physiologic values. This heterogeneity was then reproduced in the models.
Including the glass fiber and epoxy resin components in the same material phase reduced the labor required to manufacture composites, while also improving the consistency in bone shape and anatomic detail within and between specimens.14,16,17 Heiner and Brown13 reported improved consistency of fit in preformed axial compression and torsional rigidity testing molds with third-generation composites compared with second-generation composites.
The properties of the new SGFR material and behavior of the short glass fibers under suspension in the epoxy carrier resulted in better approximation of organic bone when stressed in the rotational plane. Third-generation composite tibias were found to be 140% as stiff as cadaver specimens under torsion, whereas second-generation models were 240% as stiff as cadaver specimens.13,18
Even with the comparative reduction in torsional stiffness, the third-generation composites retained the physiologic bending properties of second-generation composites. This is attributed to the distribution of short glass fibers within the epoxy matrix, which tend to orient longitudinally through the diaphyseal region and more randomly at the metaphyses during injection molding.
The most recent, fourth-generation, composite bone models use the same SGFR construction and injection molding manufacturing process as the third-generation models and therefore have similar reproduction of anatomic detail and consistency of geometry of the cortical wall. The new models, however, benefit from an optimized epoxy component, resulting in incremental improvement in torsional and bending stiffness. Biomechanical testing data for select fourth-generation composite long bones and cadaver comparisons are summarized in Table 1. Photographic examples of biomechanics testing methodology are depicted in Figure 1.
Table 1.
Biomechanical Properties of Select Human Cadaver and Fourth-generation Composite Bonesa
| Bone Type |
Study | Material Type (size) |
Flexural Rigidity, AT (Nm2) |
Flexural Rigidity, LT (Nm2) |
Axial Stiffness (N/μm) |
Torsional Stiffness (Nm/deg) |
Torsional Rigidity (Nm2/deg) |
|---|---|---|---|---|---|---|---|
| Femur | Heiner14,b | 4th-generation composite (medium) |
241 (4.5%) |
273 (5.8%) |
1.86 (7.5%) |
— | 3.21 (2.6%) |
| Gardner et al19,c | 4th-generation composite (large) |
291 (2.1%) |
305 (5.6%) |
1.23 (16.3%) |
4.14 (5.3%) |
||
| Heiner14,b | Cadaver | 317 (23%) | 290 (42%) | 2.48 (25%) |
— | 4.41 (37%) |
|
| Cristofolini et al20,d |
Cadaver | 369 (42.78%) |
277 (29.2%) |
1.39 (14.4%) |
— | 3.35 (32.2%) |
|
| Tibia | Heiner14,b | 4th-generation composite (medium) |
199 (5.0%) |
146 (3.5%) |
7.48 (9.3%) |
1.93 (3.6%) |
|
| Gardner et al19,c | 4th-generation composite (large) |
252 (3.6%) |
202 (2%) | — | 1.9 (5.3%) |
||
| Heiner14,b | Cadaver | 233 (30%) | 205 (23%) | 2.42 (33%) |
|||
| Cristofolini and Viceconti12,d |
Cadaver | 217 (43.9%) |
193 (58%) | — | — | ||
| Humerus | Dunlap et al21,e | 4th-generation composite (large) |
85.6 (10%) |
113.7 (3%) |
— | 3.22 (1.2%) |
— |
| Grover et al22 | 4th-generation composite (large) |
84.1 (1.8%) |
92.7 (1.9%) | — | — | — | |
| Lin et al23 | Cadaver | 130.6 (43.2%) |
118.4 (47.6%) |
— | — | — |
All studies used a four-point bending apparatus to assess flexural rigidity, with the exception of Lin et al,23 who employed a three-point bending device. The standard deviation for each metric is expressed as a percentage. P < 0.05 for all values.
Axial stiffness testing was performed with the femoral condyles or tibial plafond bedded in preformed Cerrobend molds and with the femoral head or tibial plateau situated in a fitted recess that interfaced with the actuator stage. Torsional rigidity testing was performed with bones potted both proximally and distally in preformed Cerrobend molds.
Axial stiffness of the femurs was tested with the model seated in preformed recesses shaped for the femoral head (proximally) and the femoral condyles (distally). For torsional testing, models were secured in preformed, aluminum-filled epoxy molds proximally and distally.
Axial stiffness of the femur was tested with the femoral head seated in a formed recess and the condyles bedded in epoxy. Torsional stiffness testing of femurs used concave clamps proximally and distally. Tibias were bedded in epoxy proximally and distally for torsional stiffness testing.
Torsional stiffness of humeri was tested with models secured in preformed molds proximally and distally.
Figure 1.
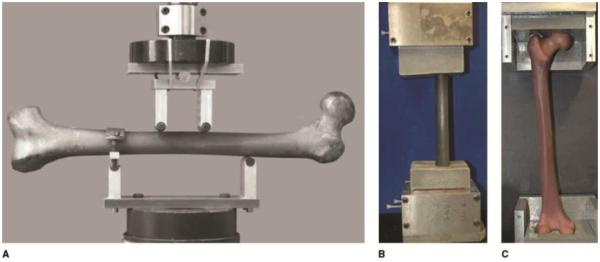
Photographs of composite bone models during biomechanical testing. A, A four-point bending apparatus is used to test the flexural rigidity of a fourth-generation composite femur, with the lateral surface in tension. B, Torsional stiffness is tested by the application of a rotational force to this fourth-generation composite femur bedded in preformed molds crafted from an aluminum-filled epoxy resin. C, Appearance of a fourth-generation composite femur resting in shaped recesses proximally and distally during axial stiffness testing. A compressive load was applied through the formed interface to simulate a single-leg stance. (Panel A reproduced with permission from Heiner AD, Brown TD: Structural properties of a new design of composite replicate femurs and tibias. J Biomech 2001;34[6]:773-781. Panels B and C reproduced with permission from Gardner MP, Chong AC, Pollock AG, Wooley PH: Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng 2010;38[3]:613-620.)
The fourth-generation resin also improves the new composites in metrics critical in orthopaedic implant testing.17,24 Chong et al24 compared third- and fourth-generation SGFR femurs in an in vitro cemented total hip arthroplasty (THA) construct. The investigators meticulously replicated the in vivo conditions sustained by a THA implant, including instrumentation with a cemented THA femoral component and submersion in a body-temperature water bath during a high activity loading protocol designed to simulate the transmission of axial forces sustained through a single-leg stance at a loading frequency of 5hz. The fourth-generation femurs were found to be superior to third-generation models in fatigue performance, avoiding total construct failure out to at least 10 million loading cycles with little actuator deflection. Under the same testing parameters, the third-generation models failed catastrophically at an average of 3.16 million cycles, with significant crazing and deformation at the tip of the stem occurring before the threshold of complete failure. These data demonstrate that fourth-generation composites have a high fatigue threshold, improved thermal stability, and improved solvent stability relative to third-generation models, making them ideal for repeat loading applications and biomechanical testing under physiologic conditions.
Zdero et al25 compared the pullout force, shear stress, and energy-to-pullout of cancellous screws placed in fourth-generation composite femurs with cellular urethane foam cancellous matrix, solid urethane cancellous matrix, and cadaver femurs. No statistical difference was found in any of these metrics between the three materials tested, and the authors concluded that either composite was an appropriate substitute for cadaver specimens. In a study testing cortical screw purchase, Zdero et al26 found that fourth-generation composite model femurs with a 20-mm canal best approximated the bicortical screw purchase observed in cadaver femurs at proximal, midshaft, and distal placement sites. The authors, however, hesitated to conclude that composite bones were a suitable replacement for cadaver samples because composites demonstrated uncharacteristic interspecimen variability in this parameter and produced overlap with physiologic values only at the lower limit of a considerable range for each metric.
Fourth-generation Composite Bone Models in the Orthopaedic Literature
Composite bone models have been used extensively in the study of fracture fixation constructs, where their consistency in most biomechanical properties permits careful testing of orthopaedic implants. Although considerable evidence exists validating fourth-generation composite bone models in comparison with cadaver specimens, many investigators still prefer to perform small-scale cadaver validation studies when testing previously unscrutinized composites.
Lower Extremity Applications
The physiologic replication of most biomechanical properties by fourth-generation composite bone models and the generally minimal variation between specimens has facilitated the study of fixation methods for complex fracture geometries involving precise placement of multiple bicortical screws. Wilkens et al27 used fourth-generation synthetic composite bones as a substrate to trial the integrity of uniaxial and polyaxial locking plates in simulated comminuted supracondylar femur fractures. Under cyclic axial compression and torsional loads, the polyaxial plating construct demonstrated improved stiffness and load to failure, which allows user-defined angles of bicortical screw insertion.
Eberle et al26 used proximal femur fracture models of varying stability (pertrochanteric, lateral neck, and subtrochanteric) with an intramedullary implant to show that the implant allowed all femur constructs to have similar values for stiffness under a hip load. The implant in the subtrochanteric fracture, however, had to bear a larger load share as compared to the implants from the other more stable fractures. A finite element model was then developed that was validated by these mechanical experiments.
Distal femur fracture has been reported as a postoperative complication of ACL reconstruction. Han et al29 sought to determine whether femoral tunnels created during ACL reconstruction serve as stress risers, resulting in this fracture type. They drilled variously sized holes into fourth-generation composite models, then placed the repair constructs under axial load equal to that generated in a partially flexed knee. Reproducible fracture patterns consistent with the stress riser hypothesis were found.
Upper Extremity Applications
The use of composite bone models to study fracture fixation constructs in the hand and upper extremity has recently received increased interest. The interspecimen consistency of contemporary composite bone models is particularly useful when studying the fixation of complex fracture patterns in the small and often oddly shaped bones of the hand and wrist.
In a study of three plating constructs in fourth-generation composite metacarpals with a transverse midshaft fracture, Sohn et al30 found better stiffness and load to failure under cantilever bending stress with a three-dimensional nonlocking plate than with nonlocking linear plates. This study showed the utility of a plate that requires less tissue dissection to achieve placement, which likely will reduce complications due to extensor tendon irritation while providing improved construct bending strength. A subsequent study demonstrated the superiority of a three-dimensional, double-row style plate over traditional linear plates for the stabilization of comminuted metacarpal shaft fractures.31 The authors osteotomized a 3-mm section of material from the midshaft of fourth-generation composite metacarpal models. Double-row plates conferred greater stiffness under axial compression and torsional loads than did traditional linear plates, regardless whether locking or nonlocking screws were used.
Using fourth-generation composite scaphoid models, Gokce et al32 studied various geometries of scaphoid waist fractures and the biomechanical stability of described Kirschner wire (K-wire) fixation methods. Transverse, dorsal oblique, and volar oblique fracture geometries were created in the models, which were then instrumented with K-wires oriented parallel to the long axis, 25° oblique to the long axis, or 20° crossed. Each of the six conditions was subjected to a vertical load at the distal pole of each epoxy-bedded specimen. Crossed K-wire fixation was superior for stabilization of transverse and volar oblique fractures, and oblique K-wire orientation was superior for fixation of the dorsal oblique fracture pattern.
Budoff et al33 investigated a similarly fragile fracture geometry, modeling O’Driscoll type 3 coronoid fractures in fourth-generation composite ulnas. Models were osteotomized to remove 70% of the coronoid before fixation with either an Acutrak Mini screw (Acumed), an Acumed coronoid plate, or both constructs. Servohydraulic testing under cyclic posterior axial loading was used to assess energy to failure, force at failure, stiffness of the first cycle, and stiffness at failure. For each metric, combined screw and plate fixation was found to be superior to either method alone.
Sokol et al34 examined volar plating constructs using all-nonlocking, all-locking, or combination locking and nonlocking screw configurations in the fixation of distal radius fracture created by a 1-cm dorsal wedge osteotomy in the radius of fourth-generation composite models. The authors applied these fixation constructs to three simulated bone stock conditions, using normal-density fourth-generation composites, low-density simulated osteoporotic models, and an overdrilled low-density model, to replicate decreased screw purchase in osteoporotic bone.34 Predictably, the normal-density specimens were stiffer under axial load and sustained greater loads to failure than either of the simulated osteoporotic conditions; however, no screw configuration conferred any significant benefit within a bone density group.34 Figure 2 illustrates differences in the cross-sectional profile of low- and normal-density fourth-generation composite bone models.
Figure 2.
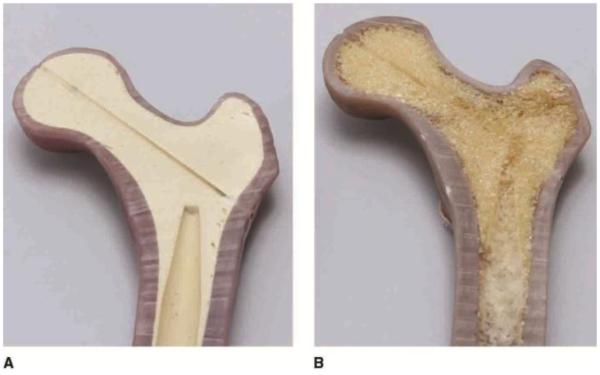
Photographs of normal-density (A) and low-density osteoporotic (B) bone models. A, The normal-density fourth-generation composite bone model has a rigid urethane foam core that mimics cancellous bone found in cadaver specimens. B, Fourth-generation low-density model with open cell urethane foam and reduced thickness of the cortical short glass fiber reinforced material, which replicates osteoporotic changes. Low-density bone models have not yet been validated against cadaver specimens. (Reproduced with permission from Pacific Research Laboratories, Vashon, Washington.)
Composite Bone Models in Surgical Education
Apprenticeship is the fundamental educational tool of any residency program; however, the surgical specialties have a particular obligation to employ lower-stakes training modalities to foster the development of fundamental surgical skills of physicians early in their training. One study estimated that the average increases in anesthesia and general operating room time attributable to resident cases at one institution amounted to more than $600 per operation.35
Current trends threaten the longstanding methodology of surgical education due to a combination of work hour restrictions, institutional cost-cutting measures, and patients’ increasing self-advocacy and demand for excellence from the residents participating in their care.36 At the same time as clinical learning opportunities are diminishing, the demand for additional surgical competencies and skill sets is broadening with the explosive pace of innovation in orthopaedic surgery. Consequently, the search for safe, cost-effective, and evidence-driven training tools has received renewed interest.
Cadaver specimens have been the cornerstone of medical education for centuries, and the many advantages of their use in surgical training are evident.1 Cadaver anatomy is essentially a duplicate of anatomy encountered in the operating room. Some preservation methods ensure tissue-handling characteristics of cadaver muscles and tendons that are immediately comparable to those in living patients.4,5 In addition, because of the anatomically complete nature of cadaver specimens, all aspects of a particular surgical approach may be practiced in sequence, from incision to closure, including the management of soft tissues. Privately run contract-driven, cadaver-based surgical training centers offer a realistic operating room experience to trainees in orthopaedic surgery.3,4
Cadaver specimens present unique challenges in the academic setting. For example, a recent situation analysis of cadaver source sustainability suggests that availability may become increasingly tenuous as the rising demand further strains the already inefficient method of acquiring the bodies of unclaimed deceased from the state.7 Likewise, the relative expense of acquiring, shipping, storing, and disposing of specimens must be considered.
Cadavers may be deep-frozen for preservation; however, repeat freezing and thawing can not only negatively affect tissue characteristics and disrupt fine anatomy but requires the use of costly storage facilities.3 Traditional fixation methods using variations on a formalin solution reduce the need for refrigerated storage, but such methods require specially ventilated space, pose environmental hazards and exposure risks to trainees, and stiffen the soft tissues. The Thiel preservation method addresses many of these detriments, but it requires special refrigerated vacuum storage, reduces the longevity of cadavers exposed to open air, and involves more costly embalming solutions than conventional methods.5 The benefits and pitfalls of other preservation methods have been reported elsewhere.37
Inexpensive and less biomechanically accurate composite bone models made with either a plastic or foam cortical material are available for use in surgical skills laboratories.2 Foam cortical shell models are easily sawn, drilled, or broached and are preferred for creating osteotomies or placing arthroplasty components. Plastic cortical shell models are more difficult to saw but are easily drilled and durably retain plate-and-screw constructs. Both models have a lower-density cancellous foam material in the medullary space and can be reamed for intramedullary fixation. The presence of low-density foam in the medullary space flanked by more rigid cortical material also reproduces the feel of bicortical drilling. Such models are frequently used in isolation and are simply attached to bench-top clamp assemblies. Although this setup does not provide for the management of soft-tissue structures or the consideration of formal surgical approaches, it does enable trainees the opportunity to develop dexterity with surgical tools, familiarity with construct-specific orthopaedic instrumentation, and learn important bony landmarks for implant placement.
Composite bone models and the bench-top simulators built around them have received some consideration in the literature as the need to develop more standardized and cost-effective training aids has been realized. Anastakis et al38 found similar improvements in the ability of postgraduate year 1 surgical residents to perform basic orthopaedic and general surgical procedures regardless whether they trained on a low-fidelity bench-top model or a cadaver specimen. Composite bone models also have been used to demonstrate the benefit of active mentorship during simulation training. Kirkpatrick39 reported the merits of a mentorship component in a training exercise involving the placement of C1-C2 transarticular screws into composite cervical spine models by orthopaedic residents.
Owing to the steep learning curve and difficult acquisition of complex psychomotor and visual-spatial abilities necessary for competency, knee arthroscopy has recently become a popular target for the development of simulators. Contemporary bench-top knee arthroscopy trainers cost nearly as much as a soft-tissue complete cadaver knee specimen, but the trainers may be used iteratively with the replacement of inexpensive modular components.2 These devices consist of an articulated composite tibia and femur joined by synthetic ligamentous structures, which is enclosed in a pliable soft-tissue envelope. Instrument ports are molded into the casing at routine positions, and additional ports can be added with a scalpel and trocar. Not only do these models demonstrate physiologic flexion and extension, but they can be stressed with varus and valgus maneuvers to recreate the challenges of accessing the posteromedial and posterolateral compartments. Surgically relevant intra-articular structures, such as the menisci and cruciates are specifically molded with an element of distention incorporated to simulate the effects of irrigation.
Howells et al40 used a bench-top knee arthroscopy simulator to demonstrate the transfer validity of skills practiced on the model to the operating room. Residents who were trained on the simulator produced superior scores on a subjective global rating scale when evaluated by an experienced surgeon in the surgical theater. Alvand et al41 used the same device to demonstrate that a novel assessment of visuospatial ability accurately discriminated between the technical skill level of expert faculty and novice residents on a knee arthroscopy task.
Virtual reality–based simulators are being used to teach the surgical skills needed to perform arthroscopy and other orthopaedic procedures. In contrast with entirely physical bench-top models instrumented with actual surgical tools, these simulators are manipulated by representative instruments that transduce movement to computed on-screen changes. The development of these technologically sophisticated devices was initially hampered by the technical limitations and high cost of the haptic feedback components needed to provide realistic tactile sensation to the user.
In their description of the challenges associated with developing the Sheffield Knee Arthroscopy Training Simulator (SKATS), McCarthy et al42 examined the cost benefits of combining physical anatomic models with an otherwise virtual experience. With SKATS, a computer model of the knee joint was temporally and spatially calibrated to a physical model to enable realistic haptic feedback on instrument encounter with physical composite bone models and to provide a digital display that rendered exquisite visual detail and assisted in recording performance metrics. The authors demonstrated that the SKATS simulator could discriminate between experienced and novice surgeons using the parameters of task time to completion and probe path length, and they reported that SKATS merited further investigation with regard to the training of orthopaedic residents.
Composite bone models have been compared with animal cadavers, as well. Leong et al43 studied the construct, content, face, and predictive validity of three training exercises: (1) dynamic compression plating of an oblique fracture in a soft-tissue–complete cadaver porcine tibia, (2) insertion of an unreamed intramedullary nail into a composite model tibia with a transverse fracture, and (3) placement of a small external fixator device on a composite ulna with a transverse midshaft fracture. The composite bone model exercises were found to be deficient in discriminating between novice and expert surgeons using an objective motion capture analysis and a modified Objective Structured Assessment of Technical Skill global rating scale. The latter was assessed on video recordings.
Summary
Composite bone models have evolved from rudimentary approximations of human anatomy to a high-fidelity, biomechanically relevant replacement for cadaveric bone in many settings. Advancements in the composition and manufacture of the SGFR cortical analogue material have resulted in the current fourth-generation composite bone model, which has been shown to replicate physiologic or near physiologic values for torsional, axial compressive, and lateral bending stiffness, as well as cancellous screw pullout strength.14,18,19,25,26 In each of these dimensions, fourth-generation composite bone models demonstrate marked reductions in interspecimen variability compared with their organic counterparts. For these reasons, fourth-generation composite bone models represent an acceptable alternative to cadaveric specimens for most biomechanics studies.
Sparse evidence exists regarding the comparative effectiveness of composite bone models to cadaver specimens. Although few studies have indicated that surgical techniques learned on simple bench-top models are transferrable to the operating room, this has not been unanimously observed.38,40,41,43 Additionally, the trend of orthopaedic surgical education appears to be toward more complex computer simulations of complete procedures.36 Even so, anecdotal reports suggest that simple composite bone models may foster familiarity with surgical tools and procedures in a nonthreatening, low-risk environment. Further, there is evidence to suggest that bench-top trainers, particularly in the setting of knee arthroscopy, do develop skill sets with transfer validity to the operating room.40 These devices may also be helpful in the reliable assessment of the abilities of surgical trainees, which is an area of current investigation.41
Historically, one detraction from the educational and research value of composite bone models was the lack of soft-tissue components, which provide realism in the setting of surgical education and potentially stabilizing properties in the context of biomechanics testing. However, soft-tissue–complete models are now being developed and marketed.2 Further, outside arthroscopy, there are few rigorous studies examining the potential benefits of simple bone-only surgical skills workshops, and it is similarly unclear whether the addition of soft tissues is necessary to a successful training experience in this setting. The use of single bone models in basic surgical skill acquisition merits continued investigation because these products may represent a particularly cost-effective training opportunity.
Although this article focused on studies that used only the most recent third- and fourth-generation composite bone models, an abundance of literature exists on trialing fixation constructs in earlier iterations. These studies should be interpreted in the context of the limitations of each generation of model design and manufacture as presented here.
Acknowledgments
The authors would like to thank Amy Johnson, John James, and Lori Lawrence of Pacific Research Laboratories, Vashon, Washington, for providing information on the manufacture, properties, and marketing of composite bone models. The authors received no benefits of any form directly or indirectly from the makers of the subject of this article.
Footnotes
Dr. Elfar or an immediate family member has received research or institutional support from Synthes and Arthrex, and serves as a board member, owner, officer, or committee member of the American Society for Surgery of the Hand and the J. Robert Gladden Orthopaedic Society. None of the following authors or any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article: Mr. Menorca, Mr. Reed, and Dr. Stanbury.
References
Evidence-based Medicine: Levels of evidence are described in the table of contents. In this article, References printed in bold type are those published within the past 5 years.
- 1.Ferrari G. Public anatomy lessons and the carnival: The anatomy theatre of Bologna. Past Present. 1987;(117):50–106. doi: 10.1093/past/117.1.50. Medline. [DOI] [PubMed] [Google Scholar]
- 2.Sawbones . Product Catalogue. Pacific Research Laboratories; Vashon, WA: 2012. 2012. Available at: http://www.sawbones.com/products/productlist.aspx?111. Accessed December 6, 2013. [Google Scholar]
- 3.Holland JP, Waugh L, Horgan A, Paleri V, Deehan DJ. Cadaveric hands-on training for surgical specialties: Is this back to the future for surgical skills development? J Surg Educ. 2011;68(2):110–116. doi: 10.1016/j.jsurg.2010.10.002. Medline. [DOI] [PubMed] [Google Scholar]
- 4.Kerckaert I, Van Hoof T, Pattyn P, D’Herde K. Endogent: Centre for Anatomy and Invasive Techniques. International Journal of Experimental and Clinical Anatomy. 2008;2:28–33. [Google Scholar]
- 5.Thiel W. The preservation of the whole corpse with natural color [German] Ann Anat. 1992;174(3):185–195. Medline. [PubMed] [Google Scholar]
- 6.Hulkower R. From sacrilege to privilege: The tale of body procurement for anatomical dissection in the United States. The Einstein Journal of Biology and Medicine. 2011;27(1):23–26. [Google Scholar]
- 7.Singh AK, Sharma RC, Sharma RK, Musmade DM. Challenges in cadaver availability for learning and research in medical sciences. International Journal of Medical and Clinical Research. 2011;2(2):67–71. [Google Scholar]
- 8.Rispler-Chaim V. The ethics of postmortem examinations in contemporary Islam. J Med Ethics. 1993;19(3):164–168. doi: 10.1136/jme.19.3.164. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Notzer N, Zisenwine D, Oz L, Rak Y. Overcoming the tension between scientific and religious views in teaching anatomical dissection: The Israeli experience. Clin Anat. 2006;19(5):442–447. doi: 10.1002/ca.20312. Medline. [DOI] [PubMed] [Google Scholar]
- 10.Suganthy J, Francis DV. Plastination using standard S10 technique: Our experience in Christian Medical College, Vellore. Journal of the Anatomic Society of India. 2012;61(1):44–47. [Google Scholar]
- 11.Harris PF, Abu-Hijleh MF, Moqattash S. Teaching anatomy in the Middle East: Opportunities and challenges at a new medical school. Clinical Anatomy. 1994;7(3):152–155. [Google Scholar]
- 12.Cristofolini L, Viceconti M. Mechanical validation of whole bone composite tibia models. J Biomech. 2000;33(3):279–288. doi: 10.1016/s0021-9290(99)00186-4. Medline. [DOI] [PubMed] [Google Scholar]
- 13.Heiner AD, Brown TD. Structural properties of a new design of composite replicate femurs and tibias. J Biomech. 2001;34(6):773–781. doi: 10.1016/s0021-9290(01)00015-x. Medline. [DOI] [PubMed] [Google Scholar]
- 14.Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282–3284. doi: 10.1016/j.jbiomech.2008.08.013. Medline. [DOI] [PubMed] [Google Scholar]
- 15.MatWeb Material Property Data. Sawbones Second-Generation Simulated Cortical Bone. Available at: http://www.matweb.com/search/DataSheet.aspx?MatGUID=82d1e15c662d4c97b75df5e9d9171da9&ckck=1. Accessed December 6, 2013.
- 16.MatWeb Material Property Data. Sawbones Third-Generation Simulated Cortical Bone. Available at: http://www.matweb.com/search/DataSheet.aspx?MatGUID=d622f0334d7c4d10987783ab235ed379. Accessed December 6, 2013. [Google Scholar]
- 17.Chong AC, Friis EA, Ballard GP, Czuwala PJ, Cooke FW. Fatigue performance of composite analogue femur constructs under high activity loading. Ann Biomed Eng. 2007;35(7):1196–1205. doi: 10.1007/s10439-007-9284-z. Medline. [DOI] [PubMed] [Google Scholar]
- 18.Heiner AD, Brown TD. Transactions of the 29th Society for Biomaterials. Society for Biomaterials; Mount Laurel, NJ: 2003. Structural properties of an improved re-design of composite replicate femurs and tibias; p. 702. [Google Scholar]
- 19.Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613–620. doi: 10.1007/s10439-009-9887-7. Medline. [DOI] [PubMed] [Google Scholar]
- 20.Cristofolini L, Viceconti M, Cappello A, Toni A. Mechanical validation of whole bone composite femur models. J Biomech. 1996;29(4):525–535. doi: 10.1016/0021-9290(95)00084-4. Medline. [DOI] [PubMed] [Google Scholar]
- 21.Dunlap JT, Chong AC, Lucas GL, Cooke FW. Structural properties of a novel design of composite analogue humeri models. Ann Biomed Eng. 2008;36(11):1922–1926. doi: 10.1007/s10439-008-9568-y. Medline. [DOI] [PubMed] [Google Scholar]
- 22.Grover P, Albert C, Wang M, Harris GF. Mechanical characterization of fourth generation composite humerus. Proc Inst Mech Eng H. 2011;225(12):1169–1176. doi: 10.1177/0954411911423346. Medline. [DOI] [PubMed] [Google Scholar]
- 23.Lin J, Inoue N, Valdevit A, Hang YS, Hou SM, Chao EY. Biomechanical comparison of antegrade and retrograde nailing of humeral shaft fracture. Clin Orthop Relat Res. 1998;(351):203–213. Medline. [PubMed] [Google Scholar]
- 24.Chong AC, Miller F, Buxton M, Friis EA. Fracture toughness and fatigue crack propagation rate of short fiber reinforced epoxy composites for analogue cortical bone. J Biomech Eng. 2007;129(4):487–493. doi: 10.1115/1.2746369. Medline. [DOI] [PubMed] [Google Scholar]
- 25.Zdero R, Olsen M, Bougherara H, Schemitsch EH. Cancellous bone screw purchase: A comparison of synthetic femurs, human femurs, and finite element analysis. Proc Inst Mech Eng H. 2008;222(8):1175–1183. doi: 10.1243/09544119JEIM409. Medline. [DOI] [PubMed] [Google Scholar]
- 26.Zdero R, Elfallah K, Olsen M, Schemitsch EH. Cortical screw purchase in synthetic and human femurs. J Biomech Eng. 2009;131(9):094503. doi: 10.1115/1.3194755. Medline. [DOI] [PubMed] [Google Scholar]
- 27.Wilkens KJ, Curtiss S, Lee MA. Polyaxial locking plate fixation in distal femur fractures: A biomechanical comparison. J Orthop Trauma. 2008;22(9):624–628. doi: 10.1097/BOT.0b013e31818896b3. Medline. [DOI] [PubMed] [Google Scholar]
- 28.Eberle S, Gerber C, von Oldenburg GV, Hungerer S, Augat P. Type of hip fracture determines load share in intramedullary osteosynthesis. Clin Orthop Relat Res. 2009;467(8):1972–1980. doi: 10.1007/s11999-009-0800-3. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han Y, Sardar Z, McGrail S, Steffen T, Martineau PA. Peri-anterior cruciate ligament reconstruction femur fracture: A biomechanical analysis of the femoral tunnel as a stress riser. Knee Surg Sports Traumatol Arthrosc. 2011;19(suppl 1):S77–S85. doi: 10.1007/s00167-011-1527-8. Medline. [DOI] [PubMed] [Google Scholar]
- 30.Sohn RC, Jahng KH, Curtiss SB, Szabo RM. Comparison of metacarpal plating methods. J Hand Surg Am. 2008;33(3):316–321. doi: 10.1016/j.jhsa.2007.11.001. Medline. [DOI] [PubMed] [Google Scholar]
- 31.Gajendran VK, Szabo RM, Myo GK, Curtiss SB. Biomechanical comparison of double-row locking plates versus single- and double-row non-locking plates in a comminuted metacarpal fracture model. J Hand Surg Am. 2009;34(10):1851–1858. doi: 10.1016/j.jhsa.2009.07.005. Medline. [DOI] [PubMed] [Google Scholar]
- 32.Gokce V, Oflaz H, Dulgeroglu A, Bora A, Gunal I. Kirschner wire fixation for scaphoid fractures: An experimental study in synthetic bones. J Hand Surg Eur Vol. 2011;36(4):325–328. doi: 10.1177/1753193410394525. Medline. [DOI] [PubMed] [Google Scholar]
- 33.Budoff JE, Meyers DN, Ambrose CG. The comparative stability of screw versus plate versus screw and plate coronoid fixation. J Hand Surg Am. 2011;36(2):238–245. doi: 10.1016/j.jhsa.2010.10.022. Medline. [DOI] [PubMed] [Google Scholar]
- 34.Sokol SC, Amanatullah DF, Curtiss S, Szabo RM. Biomechanical properties of volar hybrid and locked plate fixation in distal radius fractures. J Hand Surg Am. 2011;36(4):591–597. doi: 10.1016/j.jhsa.2010.12.032. Medline. [DOI] [PubMed] [Google Scholar]
- 35.Farnworth LR, Lemay DE, Wooldridge T, et al. A comparison of operative times in arthroscopic ACL reconstruction between orthopaedic faculty and residents: The financial impact of orthopaedic surgical training in the operating room. Iowa Orthop J. 2001;21:31–35. Medline. [PMC free article] [PubMed] [Google Scholar]
- 36.Atesok K, Mabrey JD, Jazrawi LM, Egol KA. Surgical simulation in orthopaedic skills training. J Am Acad Orthop Surg. 2012;20(7):410–422. doi: 10.5435/JAAOS-20-07-410. Medline. [DOI] [PubMed] [Google Scholar]
- 37.Jaung R, Cook P, Blyth P. A comparison of embalming fluids for use in surgical workshops. Clin Anat. 2011;24(2):155–161. doi: 10.1002/ca.21118. Medline. [DOI] [PubMed] [Google Scholar]
- 38.Anastakis DJ, Regehr G, Reznick RK, et al. Assessment of technical skills transfer from the bench training model to the human model. Am J Surg. 1999;177(2):167–170. doi: 10.1016/s0002-9610(98)00327-4. Medline. [DOI] [PubMed] [Google Scholar]
- 39.Kirkpatrick JS. A comparison C1-C2 transarticular screw placement after self-education and mentored education of orthopaedic residents. J Spinal Disord Tech. 2012;25(6):E155–E160. doi: 10.1097/BSD.0b013e31825bd0f6. Medline. [DOI] [PubMed] [Google Scholar]
- 40.Howells NR, Gill HS, Carr AJ, Price AJ, Rees JL. Transferring simulated arthroscopic skills to the operating theatre: A randomised blinded study. J Bone Joint Surg Br. 2008;90(4):494–499. doi: 10.1302/0301-620X.90B4.20414. Medline. [DOI] [PubMed] [Google Scholar]
- 41.Alvand A, Khan T, Al-Ali S, Jackson WF, Price AJ, Rees JL. Simple visual parameters for objective assessment of arthroscopic skill. J Bone Joint Surg Am. 2012;94(13):e97. doi: 10.2106/JBJS.K.01437. Medline. [DOI] [PubMed] [Google Scholar]
- 42.McCarthy AD, Moody L, Waterworth AR, Bickerstaff DR. Passive haptics in a knee arthroscopy simulator: Is it valid for core skills training? Clin Orthop Relat Res. 2006;442:13–20. doi: 10.1097/01.blo.0000194678.10130.ff. Medline. [DOI] [PubMed] [Google Scholar]
- 43.Leong JJ, Leff DR, Das A, et al. Validation of orthopaedic bench models for trauma surgery. J Bone Joint Surg Br. 2008;90(7):958–965. doi: 10.1302/0301-620X.90B7.20230. Medline. [DOI] [PubMed] [Google Scholar]


