Abstract
RNA interference (RNAi) is well known for its ability to regulate gene expression in the cytoplasm of mammalian cells. In mammalian cell nuclei, however, the impact of RNAi has remained more controversial. A key technical hurdle has been a lack of optimized protocols for the isolation and analysis of cell nuclei. Here we describe a simplified protocol for nuclei isolation from cultured cells that incorporates a method for obtaining nucleoplasmic and chromatin fractions and removing cytoplasmic contamination. Cell fractions can then be used to detect the presence and activity of RNAi factors in the nucleus. We present a protocol for investigating an early step in RNAi, Argonaute protein loading with small RNAs, which is enabled by our improved extract preparations. These protocols facilitate characterization of nuclear RNAi and can be applied to the analysis of other nuclear proteins and pathways. From cellular fractionation to analysis of Argonaute loading results, this protocol takes 4–6 d to complete.
Keywords: RNAi, subcellular fractionation, Ago2 loading, RNAi programming, nucleus, nucleoplasmic, chromatin, endoplasmic reticulum, microscopy, mammalian, extracts
INTRODUCTION
RNA interference (RNAi) is a regulatory mechanism in which cellular proteins associate with small RNAs. The resultant ribonucleoprotein complexes recognize complementary sequences within cellular RNA targets to control gene expression1. First recognized in C. elegans2, in 2001 Elbashir and Tuschl demonstrated that RNAi could also be programmed with small RNAs to function in mammalian cells3. Since that discovery, RNAi in the mammalian cytoplasm has become a ubiquitous research tool and has been widely recognized as an important mechanism for controlling the expression of endogenous proteins.
Transcription and splicing are critical processes that involve RNA and occur in cell nuclei. If the cellular RNAi machinery that drives recognition in the cytoplasm also operates in the nucleus, transcription or splicing might also be affected by RNAi. For example, recognition of a sequence near a splice site might alter splicing. Recognition of a nascent transcript near a gene promoter or enhancer region might affect transcription. Such events would represent a previously unappreciated layer of biological control and imply that microRNAs (miRNAs) have a much wider range of cellular roles.
Several groups have made experimental observations suggesting that RNAi factors might be involved in regulating gene expression in the nucleus of mammalian cells. These include observations of RNA-mediated inhibition of transcription4–9, activation of transcription10–13, and control of alternative splicing14,15. Transcriptional activation and silencing has been reported to be dependent on RNAi factors like Argonaute9,12,16,17 and GW18212.
Surprisingly, given the broad potential of nuclear RNAi to impact gene expression in mammalian cells, relatively little research on the topic has been published, and few researchers have entered the field. Our impression from informal discussions at meetings and from anonymous peer review led us to conclude that a significant number of RNA biologists believed that RNAi factors are either not present or not active in mammalian cell nuclei. This belief has helped discourage research on nuclear RNAi in mammalian cells and dampened the chances for significant progress.
Motivated by this unofficial consensus among many RNA biologists that nuclear RNAi was, at best, an uncertain phenomenon, we re-examined published evidence. Some studies had concluded that RNAi factors are inactive in mammalian cell nuclei18,19. Localization studies had shown the presence of RNAi factors in cytoplasmic p-bodies or associated with ER20,21. In contrast, other data suggested the presence or activity of RNAi factors in cell nuclei22–28. As noted above, RNAi factors appeared to be involved in RNA-mediated control of transcription and splicing. These conflicting data were insufficient to conclusively determine whether the necessary RNAi factors were also nuclear and, if so, whether they were active.
Here we describe improved protocols for obtaining nuclear fractions from cultured human cells and an in vitro assay for investigating Argonaute loading activity using these nuclear fractions. Combining these protocols with complementary approaches, we have shown that RNAi factors are present and active in the human cell nucleus but loading of Argonaute-2 (Ago2) occurs in the cytoplasm29. These protocols will be useful for investigating RNAi activity in human cell nuclei. Our approach to obtaining cleaner nuclei and subnuclear fractions will also facilitate biochemical investigation of other nuclear processes where rigorous exclusion of organelle contamination, such as the ER, is necessary.
Protocol Development
We concluded that it was necessary to re-examine the techniques used to evaluate nuclear localization and activity of RNAi factors. We immediately identified a key technical challenge. Any experiment designed to clarify whether or not a cellular activity exists in cell nuclei must build a strong case that nuclear extracts are free of cytosolic or cytoplasmic organelle contamination30. This is especially true for RNAi studies since Ago proteins are known to be associated with the endoplasmic reticulum (ER)21. The ER is attached to the outer nuclear membrane and can be difficult to dissociate31. The implications of ER contamination for interpreting Ago localization had been previously noted30.
We found that standard methods often do not adequately or reliably remove ER proteins from purified nuclei29. These include protocols where complicated sucrose cushions are used to separate nuclei from other cellular organelles or large cell debris32,33. To optimize nuclei isolation, we took advantage of the ability of non-ionic detergents to strip membrane proteins from the endoplasmic reticulum while keeping the nuclear membrane intact29,32,33. Differential centrifugation speeds were also explored for the separation of nuclei from contaminating organelles and cell debris without resorting to sucrose cushions. We developed the protocol by systematically varying the identity and concentration of the detergent, nuclei washing conditions, and centrifugation speeds used to separate and wash nuclei. To evaluate purifications, we examined nuclei purity using fluorescence microscopy to detect ER integral membrane protein and western blot analysis to detect ER components as well as other cytoplasmic contaminants like mitochondria. The resulting protocol removes ER proteins and other cytoplasmic contaminants while keeping nuclei intact.
The presence of RNAi factors in cell nuclei does not address whether they will be active. To answer this question we used our nuclei purification protocol to obtain extracts suitable for biochemical studies. These studies included sequencing of small RNAs bound to Ago2 and assays to monitor Ago2-mediated cleavage, Dicer cleavage and small RNA loading of Ago229. Since no published protocols were found for directly evaluating in vitro loading of Ago proteins in cell extracts, a key early step in RNAi, we developed our own. It is important to directly assess Argonaute loading of small RNAs since this step in RNAi is distinct from target RNA engagement and cleavage and the activities of these steps may not directly correlate. Using our loading assay, we demonstrated that RNAi programming via Ago2 loading was deficient in nuclear extracts due to the absence of the known loading factors C3PO and Hsp90 and its co-chaperones. Ago2 loading only occurred in the cytoplasm, suggesting a novel layer of RNAi regulation in the nucleus29.
Applications of the method
The need for nuclear preparations free of ER protein contamination, as well as other cytoplasmic contaminants, is shared by many experimental approaches. In addition, there is often a need to simultaneously assay RNA, protein and enzyme activities from these fractions and to physically separate soluble nuclear and insoluble chromatin-associated nuclear fractions. Our subcellular fractionation approach is flexible and we have demonstrated its application for checking RNA levels by qPCR, RNA cleavage products by 5′-RACE, RNA-protein interactions by sequencing, protein levels by western blot, and enzyme activities by in vitro biochemical assays29.
In this protocol, we present fractionation techniques for multiple endpoint assays and demonstrate their use in a direct biochemical assay, Ago2 small RNA loading. While we focus on RNAi factors, this approach is readily adapted for RNA and general protein isolation and we describe protocols to obtain fractions for these purposes. These protocols should find wide application for a variety of experimental investigations of function within the nuclear compartment of mammalian cells.
Overview of the Procedure
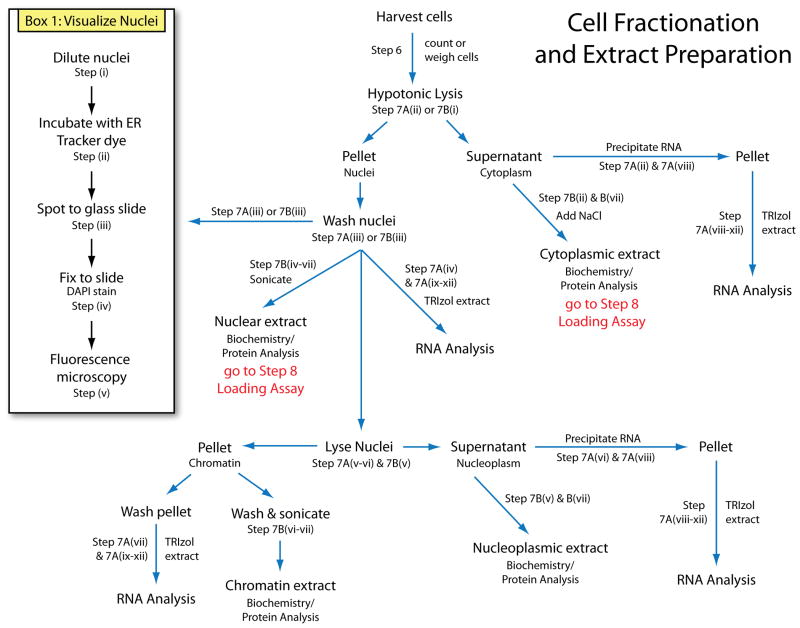
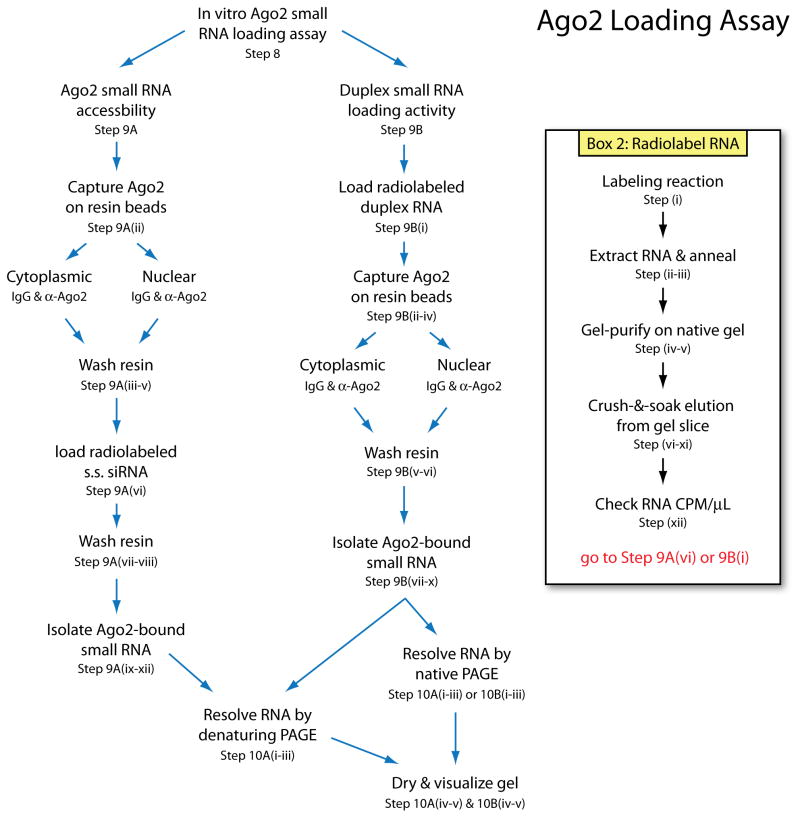
A schematic of the major endpoints in the cell fractionation and extract preparation is provided in Figure 1. Briefly, cells are harvested and weighed or counted, then lysed to generate cytoplasmic extract and nuclei. Nuclei are washed and directly lysed by sonication or incubation with TRIzol reagent, or further fractionated to collect soluble nuclear (nucleoplasmic) or insoluble nuclear (chromatin-associated) fractions. From each fraction, protein or RNA can be isolated, or fractions can be used for biochemical assays. A schematic of the Ago2 loading assay is shown in Figure 2. The accessibility of Ago2 for binding small RNA is evaluated by incubation of radiolabeled siRNA with isolated Ago2. Ago2 duplex RNA loading activity is assessed by incubation of duplex small RNA with extract followed by immunoprecipitation of Ago2. Radiolabeled RNA that co-purifies with Ago2 represents Ago2-bound and loaded siRNA, which is then extracted and resolved by gel electrophoresis for visualization.
Figure 1.
Schematic of cell fractionation and extract preparation steps of this protocol.
Figure 2.
Schematic of the in vitro Ago2 loading assay described in this protocol.
Harvest tissue culture cells (Step 1–6)
We have successfully used adherent HeLa, T47D, A549 and untransformed fibroblast cells for the protocols described here29. Based on our experience, we expect that these protocols will be applicable to a wide variety of mammalian tissue culture cells. It is expected that the user will have some experience with tissue culture and existing protocols for how to grow and maintain healthy cell cultures. For the protocol described here, we recommend HeLa cells as they are straightforward to culture. HeLa cells are cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 5% fetal bovine serum (FBS) and 0.5% non-essential amino acids (NEAA). Cells are grown in sterile incubators at 37°C in 5% CO2.
The time required to grow a sufficient number of cells varies from 3 to 7 days depending on the number of cells initially seeded. For production of sufficient quantities of cell extract, seeding and growth of cells in multiple large flasks, such as ten or more 15 cm dishes, is recommended. Cells are collected when they are close to 90% confluency to maximize cell numbers. However, some tissue culture cell types are more sensitive to crowding and may need to be harvested sooner, depending on future application of the extract and determined by the user. For experiments like in vitro Ago2 loading and RNAi factor localization, cell confluency did not appear to significantly affect our results.
Subcellular fractionation (Step 7 & Box 1)
Box 1. Fluorescence Microscopy of Isolated Nuclei, TIMING 2–3 h.
Visualizing isolated nuclei is a qualitative method for determining if nuclei are intact and if the purification procedure was sufficient to remove contaminants such as endoplasmic reticulum membrane protein from the surface of nuclei. This protocol complements Western analysis results.
Dilute the nuclei aliquots taken at step 7Aiii or step 7Biii 10-fold by adding 90 μL of PBS. Spin at 500 g for 1 min to pellet
Gently resuspend nuclei in 10 μL of PBS containing 1 μM ER Tracker Red dye and incubate on ice 20 min.
Dilute 10-fold by adding 90 μL of 4% PFA in PBS. Spot 5 μL of nuclei onto a glass slide and allow to partially air dry.
Add 1 drop of Vectashield Hard Set Mounting Medium with DAPI and carefully place a coverslip on top. Allow mounting media to harden for 15 min at room temp.
-
Visualize nuclei at 60x magnification with blue (DAPI) and red (TRITC) filters on a wide-field fluorescence microscope. Collect z-sections through the nuclei at 0.15 μm thickness. Deconvolute images by blind deconvolution. We use AutoQuant X3 (Media Cybernetics) for deconvolution and process stacked and pseudo-colored images in ImageJ for visualization. Alternatively, nuclei can be visualized using a confocal microscope with appropriate DAPI and TRITC filters. Z-sectioning is optional for this analysis, but enhances image clarity and assessment of nuclei purity. An example of nuclei imaged by this procedure is shown in Figure 3B.
TROUBLESHOOTING.
A key to successful fractionation is gentle pipetting so as not to lyse nuclei during the initial cell lysis, then low speed centrifugation during wash steps to separate cytoplasmic debris and organelles from the isolated nuclei. The varying properties of different cell types means that some protocol optimization may be required. For example, fibroblasts are larger cells, and therefore have larger nuclei, and also have a stronger cell membrane compared to HeLa cells. Accordingly, we have found that efficient fibroblast lysis required more pipetting and an additional five minute incubation on ice. However, during nuclei washing, lower speeds of 100 g were sufficient to pellet and wash nuclei. We also observed that T47D cell nuclei usually need to be spun at higher speeds, up to 300 g, for efficient washing.
Nuclei purity can be directly visualized by fluorescence microscopy using a DAPI stain (nuclear chromatin) and ER Tracker dye (ER integral membrane protein)29. Visualization of nuclei integrity and efficient ER protein removal is an important quality control checkpoint when beginning these experiments for the first time (see Box 1). After washing, nuclei are incubated with ER Tracker dye, then diluted and spotted to glass coverslips and set with mounting media containing DAPI. For imaging, a confocal or widefield microscope with blue and red fluorescence capability is sufficient. Once nuclei have been thoroughly washed, they are disrupted to make nuclear extract. Extracts should be kept on ice and flash frozen in liquid nitrogen for storage at −80°C. Extract quality is typically assessed by western blot analysis of subcellular marker proteins.
The subcellular fractionation protocol described here is quite flexible and can be adapted for various downstream assays. Here we detail how to obtain cytoplasmic, nuclear, nucleoplasmic and chromatin fractions and either isolate RNA or protein for analysis, or make extracts suitable for biochemical study. Analysis of RNA levels or identity often needs to be performed in replicate. It is important, therefore, to count cells and aliquot equivalent numbers for each sample or treatment.
During the isolation of RNA, both nuclei and chromatin can be directly dissolved in TRIzol reagent. However, cytoplasmic and nucleoplasmic fractions are liquid phase. To efficiently extract RNA, both RNA and protein are first precipitated, then the pellet is dissolved in TRIzol and heated. As an alternative to precipitation, TRIzol designed for liquid samples or phenol may be used to directly extract RNA. However, the efficiency of these alternatives has not been tested. A heating step is necessary, especially for the chromatin sample, to completely dissolve the precipitate or chromatin. Base-pairing of genomic DNA is not effectively denatured in TRIzol so heating releases bound histones, denatures the hybridized strands, and efficiently releases associated RNA. Sonication of the chromatin pellet is not recommended since it will substantially increase levels of DNA contamination in downstream assays. Subsequent analysis of RNA must include a DNA removal step, such as DNase treatment, to ensure analysis of RNA only.
Preparation of subnuclear extracts for biochemical assays may require further processing for subsequent use. Nucleoplasmic fractions contain 1 M urea. Many enzyme assays will be sensitive to chaotropic agents like urea and the extract may need to be dialyzed or diluted beforehand. Chromatin fractions will contain fragmented genomic DNA from sonication and may inhibit some activity assays. This DNA can be removed by treatment with DNase. If preparing chromatin fractions for western blot analysis, the chromatic fraction can be directly boiled in SDS loading buffer rather than sonicated. After cooling, genomic DNA will reanneal but extracted protein can be recovered, largely free of any genomic DNA, by filtering through spin filtration columns since the genomic DNA will stick to the spin filter and not flow through. If DNA is not removed, bands on the gel will tend to smear. The filtering step helps remove DNA and results in sharper gel images.
Radiolabeling RNA (Box 2)
Box 2. Prepare Radiolabeled siRNA, TIMING 2 d.
-
Prepare the following 30 μL reaction and incubate at 37°C for 2 h.
Component Volume (μL) Final siLuc siRNA guide strand (100 μM) 2 200 pmol [γ]-32P-ATP 6 1 mCi SUPERase-In (40 U/μL) 1 1.33 U/μL 10x T4 PNK Buffer 3 1x T4 PNK enzyme (10U/μL) 3 1 U/μL Nuclease-free water 15 --- CAUTION. 32P is radioactive and poses serious health risks. Follow accepted safety procedures, such as plexi-glass shielding, when handling radioactive materials.
Phenol-chloroform extract radiolabeled RNA using standard methods. PAUSE POINT. Extracted RNA can be stored at −20°C for a few days.
Divide radiolabeled siLuc guide RNA equally into 2 tubes and add 120 pmols of siLuc passenger strand RNA to one tube and heat to 90°C for 3 min. Remove from heating block and let cool at room temp for 15 min. This step generates a duplex siLuc siRNA from the half of the sample that was mixed with passenger strand RNA. One sample is used for the single stranded RNA probe for step 9A(vi) and the other as a duplex RNA probe for use in step 9B(i)
Add 5 μL of 4x native loading buffer and resolve samples on a native 15% TBE-buffered 19:1 polyacrylamide gel. As an alternative to pouring a gel, native TBE-buffered gels can often be purchased commercially. CRITICAL STEP. Use water cooling and low current to maintain native conditions and prevent denaturation of the annealed siRNA duplex. Run gel at 35 mA (gel should stay below 30°C) until lower dye (bromophenol blue) band reaches 2/3 of the way down the gel.
Remove gel from apparatus. Open the gel cassette and lay a sheet of Saran wrap over the gel. At three corners of the gel, spot 1 μL of radioactive black india ink and allow to air dry (~10 min). Cover dried ink spots with clear tape. CAUTION. The buffer in the anode tank (positive electrode that contacts buffer at the bottom of the gel) will be radioactive due to unincorporated [γ]-32P-ATP. Discard carefully in a designated liquid 32P waste container. Use a Geiger counter to monitor contamination and cleanup.
Expose in darkroom to autoradiography film placed on top of the gel. Expose for 15–60 sec and develop.
Slide the film under the glass that the gel is on. Orient and align the spots at the three corners of the gel to identify the location of the RNA bands. Gently peel back the Saran wrap and cut out the band with a flamed razor (RNase-free).
Crush the gel slice with a 1 mL plastic pipette tip (RNase-free and flame-sealed at tip), pipette 300 μL of nuclease-free water down the side of the tip and into a microfuge tube so as to collect all the gel bits and rotate at 4°C 4–16 h.
Cut ~2 mm off the end of a 1 mL pipette tip and use it to move gel bits and solution to an RNase-free filter spin column (~10 μm cutoff). Spin at 2,000 g for 2 min to collect eluted RNA in solution. Discard gel bits.
Add 10 μg of tRNA and split the sample evenly into two microfuge tubes to accomodate the total volume required during precipitation. Precipitate RNA from solution by adding 9 vol of 2% LiClO4 in acetone and incubating at −20°C for >15 min. CRITICAL STEP. The radiolabeled RNA is extremely dilute and will not precipitate well by standard ethanol precipitation. CAUTION. LiClO4 is a dangerous oxidizer. Handle with caution.
Spin at 12,000 g 10 min, wash pellet with ice-cold acetone, and spin again at 12,000 g for 2 min. Let pellet air dry at room temperature. PAUSE POINT. Dried RNA pellet can be stored at −20°C for a few days.
-
Resuspend RNA pellet in 30 μL of RNase-free water. Measure the radioactivity by scintillation counting of 1 μL (do not use scintillation fluid). PAUSE POINT Label the date and CPM/μL on the side of the tube and store RNA frozen at −20°C or −80°C. RNA radioactivity should range from several hundred thousand to a few million CPMs/μL. The half-life of 32P is 14.2 d.
TROUBLESHOOTING.
Although 5′-end radiolabeling of RNA is a standard procedure, we described our method here because it required special considerations, including the differential labeling of the siRNA guide strand, annealing to the passenger strand and purification on a native gel, high specific activity labeling, and precipitation with an uncommon reagent. Differential labeling of the guide strand is achieved by labeling it prior to mixing with passenger strand. The success of the subsequent Ago2 loading assay in cell extracts requires purified siRNA of very high specific radioactivity, on the order of 0.5–2×106 counts per minute (CPM) per loading reaction. Key aspects of this method are the use of [γ]-32P ATP with high specific activity, gel purification on a native gel to preserve native duplex siRNA structure, and a highly efficient precipitation method after gel purification using lithium perchlorate in acetone. Gel-purification of radiolabeled RNA of low quantity requires visualization and orientation of the RNA bands since they cannot be stained. To do this, we describe a simple use of radioactive dye spotted asymmetrically at corners of the gel then exposure to a film, which can then be slid underneath the gel to identify bands and extract them.
Ago2 Loading Assay (Steps 8–10)
For in vitro Ago2 loading assays, loading needs to be performed in the extract before Ago2 is subsequently captured with immuno-affinity resin. If Ago2 is bound to the resin first, duplex siRNA loading is inefficient. Thus, resin and antibody should not be mixed with the siRNA and extract together, but only added after an initial time of Ago2 loading in solution. It is important to centrifuge samples prior to addition of resin to remove any precipitation that has occurred during incubation. Otherwise, the precipitated material will co-pellet with the resin in later steps and increase contaminating background levels during analysis.
During wash steps, it can be difficult to prevent loss of resin. Loss of resin will skew results, making comparison across samples less reliable. To avoid this potential problem, users may want to perform washes in small spin columns with a molecular weight cutoff below that of the resin, such as 10 microns. If using this approach, do not spin over 2,000 g for more than 30 sec and immediately add fresh buffer after centrifugation to prevent the resin from drying.
MATERIALS
Reagents
Dulbecco’s phosphate-buffered saline (PBS) (Sigma, D8537)
Trypsin-EDTA solution (0.1%) (Sigma, T4174)
Tissue culture medium: Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma D5796)
Non-essential amino acid solution (NEAA) (100x) (Sigma, M7145)
Fetal bovine serum (FBS) (Sigma, F6178)
Tris-HCl, pH 7.5 (Invitrogen, 15567-027)
5 M Sodium chloride (Ambion, AM9759)
1 M Magnesium chloride (Ambion, AM9530G)
2 M Potassium chloride (Ambion, AM9640G)
3 M Sodium Acetate pH 5.5 (Ambion, AM9740)
Glycerol (molecular biology grade) (Fisher Scientific, AC15892-2500)
NP-40 or Igepal CA-630 non-ionic detergent (Sigma, 18896) CRITICAL! cannot be substituted with other detergents!
Nuclease-free water (Sigma, W4502)
Protease inhibitor (PI), Cocktail Set I (Calbiochem, 539131)
Sodium fluoride (Sigma, S-6776)
Sodium orthovanadate (New England Biolabs, P0758S)
Paraformaldahyde (PFA) solution, 4% in PBS (Santa Cruz Biotechnology, sc-281692) CRITICAL! order or prepare fresh and use within 6 months.
ER Tracker Red dye (Life Technologies, E34250)
Vectashield Hard Set Mounting Medium with DAPI (Vector Laboratories, H-1500)
Liquid nitrogen (Airgas, NI NF240LT22)
-
Synthetic siLuc siRNA guide strand (siLuc_as) (IDT):
rUrGrUrUrCrArCrCrUrCrGrArUrArUrGrUrGrCTT
-
Synthetic siLuc siRNA passenger strand (siLuc_ss) (IDT):
rGrCrArCrArUrArUrCrGrArGrGrUrGrArArCrATT
[γ]-32P-ATP (7,000 Ci/mmol) (MP Biomedicals, 13502002)
SUPERase-In RNase inhibitor (Ambion, AM2696)
T4 polynucleotide kinase (PNK) (New England Biolabs, M0201S)
Redistilled phenol, pH 4.3 (Sigma, P4682)
TRIzol Reagent (Life Technologies, 15596-026)
Chloroform:isoamyl alcohol (24:1) (Fluka Analytical, 25666)
Acetone (Fisher, A949-1)
Ethanol (Pharmco-Aaper, 111000200)
Isopropanol (Sigma, I9516)
Acrylamide:bis-acrylamide solution (19:1) (Bio-Rad, 161-0154)
10x TBE (Ambion, AM9863)
Bromophenol blue (Sigma, B0126)
Xylene cyanol (Sigma, X4126)
Ammonium persulfate (APS) (Sigma, A4418)
Tetramethylethylenediamine (TEMED) (Bio-Rad, 161-0801)
Black India ink (Higgins, local art supply store)
Yeast tRNA (Roche, 10109517001)
Lithium perchlorate (LiClO4) (Acros Organics, 194715000)
Protein G Plus/Protein A agarose (EMD Millipore, IP05-1.5ML)
α-Ago2 antibody (Abcam, ab57113) CRITICAL! substituting with an different Ago2 antibody may alter quality of results.
Mouse IgG antibody (Millipore, 12-371)
Adenosine triphosphate (ATP) (100 mM) (Sigma, A6559-25UMO)
Dithiothreitol (DTT) (1 M) (Life Technologies, P2325) CRITICAL! prepare fresh.
0.5 M EDTA, pH 8.0 (Ambion, AM9260)
Urea (Sigma, U5378)
Phosphocreatine (1 M) (Sigma, P7936)
Creatine kinase (4U/μL) (Sigma, C3755)
Deionized formamide (Sigma, F9037)
anti-Tubulin antibody (Sigma, T5201)
anti-OxPhos antibody (Invitrogen, A21351)
anti-Calreticulin antibody (Cell Signaling, 2891S)
anti-Lamin A/C antibody (Abcam, ab8984)
Equipment
Micro-pipettors
Automatic pippettor
Disposable RNase-free plastic pippette tips
Conical screw-top tubes (15 and 50 mL)
1.5 mL microfuge tubes
Refrigerated table-top centrifuge (15 or 50 mL conical tube adaptors)
Room termperature bench-top centrifuge (1.5 mL tube rotor)
Refrigerated bench-top centrifuge (1.5 mL tube rotor)
Cell counter
Digital scale
Widefield fluorescent deconvolution microscope (i.e. Deltavision) with blue and red filters
Sonicator
Microscope slides (Globe Scientific Inc., 1324)
Premium Cover Glass (Fisher Scientific, 12-548-A)
Light microscope
Variable temperature heating block
Plexi-glass shields
Liquid and solid 32P radioactive waste containers
Geiger counter
Scintillation counter
Water-cooled polyacrylamide gel electrophoresis apparatus
Denaturing polyacrylamide electrophoresis apparatus
Electrophoresis power supply
Glass plates (19 × 20 × 0.3 cm and 16 × 20 × 0.3 cm) and gel spacers and combs (0.75 cm)
Large binder clips (2 in wide) (local business supply store)
Saran wrap
Clear scotch tape
Dark room
Autoradioagraphy film
Razor blades
Bunsen burner
Microfuge tube rotator (room temp and 4°C)
RNase-free mini-spin filtration columns (<10 μm) (Pierce® Spin Cups-Paper Filter, Thermo Scientific, Prod# 69700)
Vortexer
Microfuge tube Cap Locks, 1.5 mL (Starlab, I1415-1508)
Whatman 3M paper
Vacuum gel dryer
Phosphorimager screen and cassette
Phosphorimager
Reagent Setup
Tissue culture medium for HeLa cells
Mix 10 mL of 100x NEAA and 100 mL of FBS with 1 L of DMEM media in a sterile hood. Store at 4°C in the dark for up to 3 months.
Hypotonic lysis buffer (HLB)
Final component concentrations of HLB are 10 mM Tris, pH 7.5, 10 mM NaCl, 3 mM MgCl2, 0.3% NP-40 (vol/vol), and 10% glycerol (vol/vol). Prepare using nuclease-free water. Store buffer solution at 4°C for up to 1 year.
Nuclear lysis buffer (NLB)
Final component concentrations of NLB are 20 mM Tris, pH 7.5, 150 mM KCl, 3 mM MgCl2, 0.3% NP-40 (vol/vol), and 10% glycerol (vol/vol). Prepare using nuclease-free water. Store buffer solution at 4°C for up to 1 year.
Modified Wuarin-Schibler buffer (MWS)
Final component concentrations of MWS are 10 mM Tris-HCl, pH 7.0, 4 mM EDTA, 0.3 M NaCl, 1 M urea, and 1% NP-40 (vol/vol). Prepare using nuclease-free water. Store buffer solution at 4°C for up to 6 months.
Protease inhibitor cocktail (PI)
Prepare a 100x solution by dissolving lyophilized powder with 1 mL of nuclease-free water. Keep solution on ice and store at −20°C for up to 6 months.
Phosphatase inhibitor solution (PhI) (100x)
PhI solution at 100x concentration contains 0.1 M sodium orthovanadate and 0.1 M sodium fluoride in nuclease-free water. Keep solution on ice and store at −20°C for up to 1 year.
15% Denaturing polyacrylamide gel solution (500 mL)
Prepare stock solution by dissolving 210 g of urea in 50 mL of 10x TBE and 187.5 mL of 40% (19:1) acrylamide. Add 10 mL of glycerol and bring up to 500 mL with nuclease-free water. Filter through a 0.22 μm bottle filter. Store at 4°C in the dark for up to 6 months.
15% Native polyacrylamide gel solution (500 mL)
Prepare stock solution by mixing 187.5 mL of 40% (19:1) acrylamide, 50 mL of 10x TBE, and 10 mL of glycerol. Bring up to 500 mL with nuclease-free water. Filter through a 0.22 μm bottle filter. Store at 4°C in the dark for up to 6 months.
10% APS (wt/vol)
Dissolve 1 g of ammonium persulfate in a final volume of 10 mL of nuclease-free water. Aliquot 0.5 mL into 1.5 mL microfuge tubes and store at −20°C for up to 6 months.
Radioactive black India ink
Mix 1 μL of fresh [γ]-32P-ATP into 100 μL of black India ink. Vortex to mix, spin down and store behind a plexi-glass shield at room temperature. Can be used until radioactivity decays below useful signal (~1–3 months). Can be regenerated by addition of fresh [γ]-32P-ATP.
tRNA solution (10 mg/mL)
Dissolve 5 mg of yeast tRNA in 0.5 mL of nuclease-free water. Phenol-chloroform extract and ethanol precipitate the RNA. Wash the RNA pellet with 70% ethanol (vol/vol), air dry the RNA pellet, and dissolve again in 0.5 mL of nuclease-free water. Store at −20°C for up to 2 years.
2% LiClO4 in acetone
Dissolve 1 g of LiClO4 in 50 mL acetone. Store at room temperature indefinitely.
RNA precipitation solution (RPS)
Mix 0.5 mL of 3 M sodium acetate, pH 5.5 with 9.5 mL of ethanol. Store at −20°C indefinitely.
IPEQ buffer
Final component concentrations of IPEQ buffer are 20 mM Tris, pH 7.5, 0.15 M NaCl, 2 mM MgCl2, 0.05% NP-40 (vol/vol), and 0.1% PVP (wt/vol). Prepare using nuclease-free water. Store buffer solution at room temperature for up to 1 year.
IP150 buffer
Final component concentrations of IP150 buffer are 50 mM Tris, pH 7.5, 0.15 M NaCl, 4 mM MgCl2, and 0.05% NP-40 (vol/vol). Prepare using nuclease-free water. Store buffer solution at room temperature for up to 1 year.
IP500 buffer
Final component concentrations of IP500 buffer 50 mM Tris, pH 7.5, 0.5 M NaCl, 4 mM MgCl2, 0.05% NP-40 (vol/vol). Prepare using nuclease-free water. Store buffer solution at room temperature for up to 1 year.
10x RNAi buffer
Final component concentrations of 10x RNAi buffer are 0.2 M Tris, pH 7.5, 0.8 M NaCl, 40 mM MgCl2, 5 mM DTT, 5 mM EDTA, and 0.2 M KCl. Prepare using nuclease-free water. Store buffer solution at −20°C for up to on year.
5x Native loading buffer
Mix 1 mL of 10x TBE, 5 mL of glycerol, and 4 mL nuclease-free water. Add 2 mg of xylene cyanol and 2 mg of bromophenol blue. Mix well to dissolve dyes. Store at room temperature indefinitely.
Formamide loading buffer
Mix 1 mL of 10x TBE with 9 mL of formamide. Store at room temperature indefinitely.
PROCEDURE
CAUTION! All experiments should be performed in accordance with relevant guidelines and regulations.
Harvest Tissue Culture Cells, TIMING 1–2 h
-
1
Grow cells to <90% confluency. We have successfully used HeLa, T47D, A549 and fibroblast cells for these experiments. Wash cells with 1x PBS at room temperature.
-
2
To detach adherent cells, add just enough trypsin-EDTA solution to lightly bathe cells and incubate at 37°C for 5 min. For non-adherent cells, proceed to step 5.
-
3
Add several mL of full media (containing 10% FBS) and pipette cells to stop trypsin proteolysis and to detach cells and breakup cell clumps.
-
4
Move cell suspension to conical tubes on ice. Pool dishes of cells as needed. Typical extract preparations start with a few to several 15 cm dishes, amounting to tens to hundreds of millions of cells. We recommend starting with 10 dishes.
-
5
Centrifuge cells at 500 g at 4°C for 5 min. Decant supernatant and gently suspend cells in ice-cold 1x PBS to wash.
-
6
Count the cells (or weigh the cell pellet). If cells were counted, aliquot into separate tubes at the desired number of cells and centrifuge at 500 g at 4°C for 5 min. CRITICAL STEP At this point it is important to either count cells or weigh the cell pellet for accurate comparison of results across separate experiments. CRITICAL STEP. Keep cell pellets on ice to slow metabolic processes and inhibit RNA or protein degradation.
Perform Subcellular Fractionation, TIMING 2–4 h
-
7
Cells can be fractionated for at least three different purposes - RNA isolation, biochemical assays, or protein analysis. This protocol describes how to prepare cytoplasmic, nucleoplasmic (soluble nuclear) and chromatin (insoluble nuclear) fractions for each of these particular purposes. Follow option A for preparation of RNA from cellular fractions. Follow option B for preparation of extracts for biochemical assays and protein analysis. This protocol does not provide downstream protocols for analysis of RNA or protein, but does provide a biochemical assay to evaluate extracts prepared in option B.
Option A: Isolation of RNA from cytoplasmic, total nuclear, nucleoplasmic and chromatin fractions
For each sample, aliquot 10 million cells into 1.5 mL microfuge tubes. Centrifuge at 500 g at 4°C for 5 min to pellet. Decant supernatant. CRITICAL STEP. Keep cell pellets on ice to slow metabolic processes and inhibit RNA or protein degradation.
-
Resuspend cells by gently pipetting up and down in 380 μL of ice-cold HLB supplemented with 100 U of SUPERase-In. CRITICAL STEP. As a negative control for ER contamination removal, consider lysing cells with no detergent or 0.5% Tween-20, which will lyse the cell membrane but leave ER integral membrane proteins intact. Prepare and visualize nuclei from NP-40 and control treatments in parallel following Box 1 procedure. CRITICAL STEP. HLB contains NP-40, a detergent that is critical to successful removal of ER contamination. Incubate on ice for 10 min. Vortex briefly. Centrifuge at 1,000 g at 4°C for 3 min. Carefully transfer the supernatant by pipette to a new tube and keep the pellet on ice. The supernatant is cytoplasmic fraction. Immediately add 1 mL of RNA precipitation solution (RPS) and store at −20°C for at least 1h and until step 7A(viii).
PAUSE POINT. Fractions in RPS are stable at −20°C overnight.
Wash the pellet from step 7A(ii) (semi-pure nuclei) with 1 mL of ice-cold HLB 3 times by gently pipetting up and down and centrifuging at 300 g at 4°C for 2 min. CRITICAL STEP. Collect a sample of nuclei for analysis of nuclei purity following Box 1 procedure.
-
To directly isolate total nuclear RNA from the nuclei pellet from step 7A(iii), add 1 mL TRIzol, vortex briefly, and store at −20°C until step 7A(ix). Alternatively, to further fractionate nuclei into nucleoplasmic and chromatin-associated RNA fractions, proceed with steps 7A(v)–(vii).
PAUSE POINT. Fractions in Trizol are stable at −20°C overnight.
Add 380 μL MWS supplemented with 100 units of SUPERase-In, vortex for 30 s, and set on ice 5 min. CRITICAL STEP. Do not pipette the sample! It will stick to the inside of the pipette tip.
-
Vortex nuclei in MWS for 30 s, then incubate on ice for an additional 10 min. Centrifuge at 1,000 g at 4°C for 3 min. Carefully transfer the supernatant by pipette to a new tube and keep the pellet on ice. The supernatant is nucleoplasmic fraction. Immediately add 1 mL of RPS and store at −20°C for at least 1h and until step 7A().
PAUSE POINT. Fractions in RPS are stable at −20°C overnight.
-
Wash the pellet (chromatin) with 1 mL of ice-cold MWS 3x by vortexing for 30 s and centrifuging at 500 g at 4°C for 2 min. Add 1 mL TRIzol to the chromatin pellet, vortex briefly, and store at −20°C until ready to proceed with step 7A(ix).
PAUSE POINT. Fractions in Trizol are stable at −20°C overnight.
For samples that have been incubated in RPS at −20°C >1 h (cytoplasmic and nucleoplasmic fractions from steps 7A(ii) and 7A(vi) respectively), vortex for 30 sec then centrifuge at 18,000 g at 4°C for 15 min. Discard supernatant and wash pellet by vortexing in ice-cold 70% ethanol and centrifugation at 18,000 g at 4°C for 5 min. Discard supernatant. Let pellet partially air dry. Add 1 mL TRIzol to semi-dry pellets. PAUSE POINT. Fractions in TRIzol are stable at −80°C for several weeks.
For samples in TRIzol from previous steps, add 10 μL of 0.5 M EDTA and heat to 65°C with vortexing until pellet is dissolved (~10 min). CRITICAL STEP. Without heating in TRIzol, RNA may not be efficiently released from chromatin or insoluble RNA-protein complexes. CAUTION. Sample tube tops can pop open when TRIzol is heated. TRIzol is caustic and proper lab safety, including eye goggles, should be followed. Microfuge tube cap locks should be used and samples should be watched closely.
Let samples cool to room temperature, add 200 μL chloroform: isoamyl alcohol (1:24), vortex for 30 s, then centrifuge at 18,000 g at room temperature 10 min.
Pipette the aqueous supernatant to new tubes and add 1 vol of isopropanol. Incubate at −20°C for >1 h, vortex, then centrifuge at 18,000 g at room temperature 15 min.
-
Wash RNA pellets with 70% ethanol by vortexing and centrifugation at 18,000 g at room temperature 5 min. Remove supernatant and air dry the pellet. Typical RNA yields from 100 mg of cells can range from 50–100 μg, 25–75 μg, and 15–30 μg for cytoplasmic, nucleoplasmic and chromatin-associated fractions, respectively.
PAUSE POINT. Purified and dry RNA is stable at −80°C for long periods of time.
CRITICAL STEP. RNA purified from nuclear and chromatin fractions will contain DNA contamination. Treatment with DNase may be required for downstream analysis. A procedure for DNase treatment and downstream analysis of chromatin-associated RNA are not described here.
Option B Preparation of cytoplasmic, total nuclear, nucleoplasmic and chromatin fractions for biochemical assays or protein analysis
Resuspend cell pellet by gentle pipetting with 1 mL of ice-cold HLB for every 75 mg of cells or every 10 million cells. To this cell suspension add protease inhibitor (PI) cocktail and phosphatase inhibitor (PhI) solution to a final concentration of 1x. Incubate cells on ice 10 min. Vortex briefly. CRITICAL STEP. As a negative control for ER contamination removal, consider lysing cells with no detergent or 0.5% Tween-20, which will lyse the cell membrane but leave ER integral membrane proteins intact. Prepare and visualize nuclei from NP-40 and control treatments in parallel following Box 1 procedure. CRITICAL STEP. HLB contains NP-40, a detergent that is critical to the success of ER contamination removal.
Centrifuge cell suspension at 800 g at 4°C for 8 min. Move supernatant (cytoplasmic fraction) to new tubes on ice. Keep nuclei pellet on ice to slow metabolic processes, inhibit RNA protein degradation, and maintain enzyme activity. Add enough 5 M NaCl to this cytoplasmic fraction to equal a concentration of 140 mM (final NaCl concentration is 150 mM) and mix well by gentle inversion. Set on ice until step 7B(vii).
-
Wash nuclei pellet from step 7B(ii) 4 times by adding HLB, pipetting, and centrifuging at 200 g at 4°C for 2 min. CRITICAL STEP. Collect a sample of nuclei for analysis of nuclei purity following Box 1 procedure. To prepare total nuclear extract, carry out step 7B(iv). Otherwise proceed directly to step 7B(v) to prepare nucleoplasmic and chromatin extracts. CRITICAL STEP. These conditions work well for HeLa cells. Wash steps need to be optimized for different cell types. In general, low speed spins for short periods are sufficient to wash most nuclei. The nuclei of some cell types can withstand vigorous pipetting while others cannot. Centrifugation speeds can range from 100–300 g.
TROUBLESHOOTING.
-
To prepare total nuclear extract, resuspend nuclei from step 7B(iii) in 0.5 mL of ice-cold NLB for every 75 mg or 10 million cells of original cell pellet. Add PI cocktail and PhI solution to the nuclei suspension to a final concentration of 1x. Aliquot nuclei suspension into 15 mL conical tubes at 2–4 mL/tube. Sonicate nuclei 3 times at 20% power for 15 s in an ice bath with 2 min cooling between each sonication. Sonication success can be checked by observing several μL of lysed nuclei on a glass coverslip with a light microscope. Place sonicated nuclei extract on ice until step 7B(vii). CRITICAL STEP. Over-sonication can damage proteins and their native complexes. Sonication instruments vary in power and efficiency.
TROUBLESHOOTING
To prepare nucleoplasmic and chromatin extracts, resuspend nuclei in 0.5 mL ice-cold MWS buffer for every 75 mg or 10 million cells of original cell pellet. Add PI cocktail and PhI solution to the nuclei suspension to a final concentration of 1x. Vortex gently and incubate on ice 15 min. Vortex again and centrifuge at 1,000 g at 4°C for 5 min. Collect supernatant as nucleoplasmic fraction and move to a new tube. Place nucleoplasmic extract on ice until step 7B(vii). CRITICAL STEP. Do not pipette the chromatin pellet! It will stick to the inside of the pipette tip.
Wash chromatin pellet 2 times with MWS buffer by vortexing, incubating on ice for 5 min, and centrifugation at 500 g at 4°C for 3 min. Add 0.5 mL ice-cold NLB to chromatin pellet for every 75 mg or 10 million cells of original cell pellet. Sonicate chromatin 3 times at 20% power for 15 s in an ice bath with 2 min cooling between each sonication. Place sonicated chromatin on ice until step 7B(vii). CRITICAL STEP. Over-sonication can damage proteins and their native complexes. Sonication instruments vary in power and efficiency. CRITICAL STEP. Chromatin extracts contain large amounts of DNA, which may need to be minimized or removed by DNase treatment for some downstream assays. A procedure for DNase treatment and downstream analysis of the chromatin fraction are not described here.
-
Centrifuge cytoplasmic, nuclear, nucleoplasmic and chromatin extracts in 1.5 mL microfuge tubes at 18,000 g for 15 min at 4°C. Pool supernatant of respective samples in new conical tube, invert to mix, and aliquot in 1 mL volumes into 1.5 mL microfuge tubes. Incubate on ice and continue to step 8 or flash-freeze samples in liquid nitrogen and store at −80°C. CRITICAL STEP. Typical protein concentrations can range from 3–6 mg/mL and 1–3 mg/mL for cytoplasmic and nuclear extracts, respectively.
PAUSE POINT. Extracts can be stored at −80°C for several weeks.
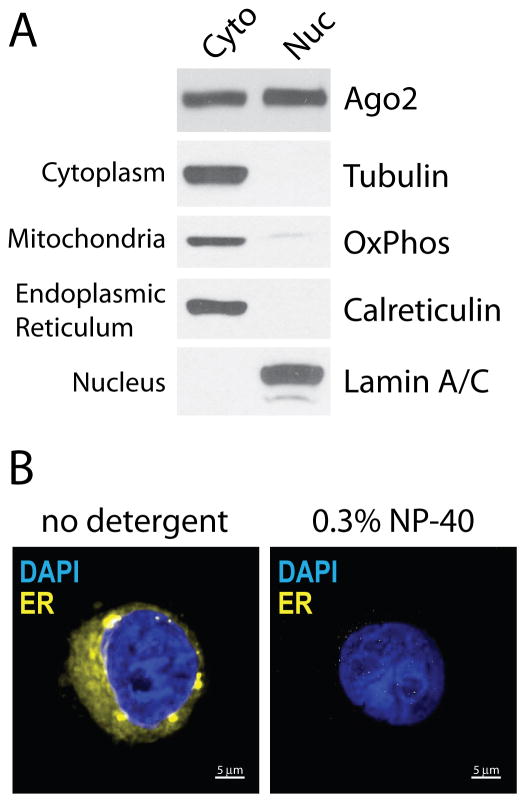
Perform western analysis. Western analysis should be performed to evaluate extract quality and success. Typical results are shown in Figure 3A. A protocol for Western analysis is not described here. Standard procedures should be followed using antibodies that detect subcellular markers like Tubulin (cytoplasm), OxPhos (mitochondria), Calreticulin (endoplasmic reticulum) and Lamin A/C (nucleus)29. CRITICAL STEP. Note that nuclear, nucleoplasmic and chromatin fractions are 1/2 the volume of corresponding cytoplasmic extracts. This makes application in biochemical assays more convenient since the cytoplasm usually has 2-4x more total protein than the nucleus. For western blot analyses, be sure to load 1/2 the volume of nuclear versus cytoplasmic extracts. This will give total protein of the same cell equivalents.
Figure 3.
Quality assessment of subcellular fractionation. (A) Western blot analyses of cytoplasmic (Cyto) and nuclear (Nuc) fractions. Western analysis protocol followed is from Gagnon and colleagues29. (B) Fluorescence microscopy of isolated nuclei. Blue is DAPI stain, which binds chromatin DNA. Yellow is ER Tracker Red dye pseudocolored yellow, which binds sulfonlyurea receptors on the ER membrane surface. Scale bar = 5 μm.
In Vitro Ago2 Loading Assay, TIMING 2–3 d
-
8
If cell extracts were frozen, thaw on ice. Centrifuge at 18,000 g for 10 min at 4°C. Move supernatant to a new tube. Keep on ice to avoid degradation or inactivation of enzymes in the extract.
-
9
Perform the Ago2 loading assay according to either Option A or Option B. Option A evaluates the accessibility of Ago2 for loading. Ago2 is first immunoprecipitated then mixed with radiolabeled single-stranded siLuc guide RNA. Single-stranded RNA cannot be directly mixed with extract since it is rapidly degraded, even with the addition of RNase inhibitors. However, Ago2 can bind and load single-stranded small RNAs without the need for accessory proteins. Option B determines the ability of cell extract to efficiently load a duplex siRNA into Ago2, which requires accessory proteins in mammalian cells.
Option A: Ago2 accessibility for small RNA loading
Vortex Protein G Plus/Protein A resin to resuspend, then aliquot 40 μL into four 1.5 mL microfuge tubes. Equilibrate resin by adding 1 mL of IPEQ buffer and rotating 5 min at room temp. Pellet resin by centrifugation at 2,000 g for 30 sec at room temp. Add 1 mL of IP150 buffer, rotate for 5 min and centrifuge again.
-
Prepare the following four reactions and rotate at room temp for 1 h.
Component Volume (μL) Final Tube 1 Tube 2 Tube 3 Tube 4 Cytoplasmic extract 300 300 --- --- Nuclear extract --- --- 300 300 300 μL α-Ago2 (1 μg/μL) 2 --- 2 --- 2 μg IgG antibody (1 μg/μL) --- 2 --- 2 Equilibrated resin from step 9A(i) 40 40 40 40 40 μL Centrifuge resin at 2,000 g for 30 s at room temp.
Wash resin 3x by adding 0.5 mL of IP500 buffer, rotating for 5 min at room temp, and centrifuging.
Wash again 1x with 0.5 mL of IP150 buffer.
-
Prepare the following 20 μL reaction and rotate at 4°C for 4 h.
Component Volume (μL) Final Washed resin from step 9A(v) --- --- tRNA (10 μg/μL) 1.5 0.75 μg/μL SUPERase-In (40 U/μL) 1.5 3 U/μL ATP (100 mM) 0.3 1.5 mM siLuc guide strand RNA (1×106 CPM/μL) 1 1×106 CPM 10x RNAi buffer 3 1x Nuclease-free water 12.7 --- Wash resin 3x by adding 0.5 mL of IP500 buffer, rotating for 5 min at room temp, and centrifuging at 2,000 g for 30 s at room temp.
Wash again 1x with 0.5 mL of IP150 buffer.
-
Stop the reaction by adding the following components:
Component Volume (μL) Final Nuclease-free water 40 --- EDTA (0.5 M) 0.5 1.8 mM tRNA (10 μg/μL) 0.5 35 ng/uL Phenol 80 --- Vortex for 30 sec, then phenol extract by centrifuging and keeping the top aqueous layer (move to a new tube).
Precipitate eluted RNA by adding 9 vol of 2% LiClO4 in acetone and incubating at −20°C for >15 min. CAUTION. LiClO4 is a dangerous oxidizer. Handle with caution.
-
Spin at 12,000 g 10 min, wash pellet with ice-cold acetone, and spin again at 12,000 g for 2 min. Let pellet air dry at room temperature.
PAUSE POINT. Dried RNA pellet can be stored at −20°C for a few days.
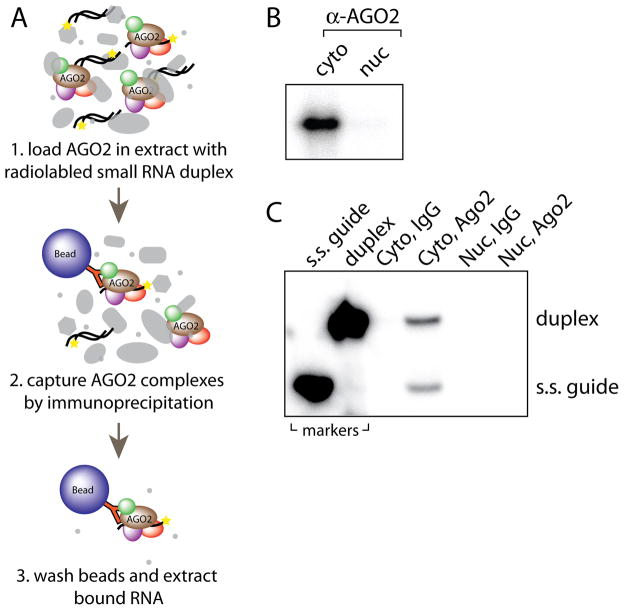
Option B: Ago2 duplex RNA loading activity assay (Figure 4A)
Figure 4.
In vitro Ago2 loading assay and typical results from Step 9B. (A) Illustration of in vitro Ago2 loading assay procedure described in Step 9B. (B) Denaturing polyacrylamide gel electrophoresis of Ago2-bound small RNAs from typical cytoplasmic (cyto) and nuclear (nuc) loading reactions. (C) Native polyacrylamide gel electrophoresis of small RNAs that co-purified with IgG or Ago2 antibody. Markers used are single-stranded guide siRNA (s.s. guide) and duplex siRNA (duplex) loaded in separate lanes to the left.
-
Prepare the following four loading reactions and rotate at room temperature for 1 h.
Component Volume (μL) Final Tube 1 Tube 2 Tube 3 Tube 4 Cytoplasmic extract 300 300 --- --- 300 μL Nuclear extract --- --- 300 300 siLuc duplex siRNA (1×106 CPM/μL) 1 1 1 1 1×106 CPM ATP (100 mM) 3 3 3 3 1 mM Phosphocreatine (1 M) 3 3 3 3 10 mM Creatine Kinase (4 U/μL) 3 3 3 3 0.04 U/ μL While loading reactions are rotating, prepare resin for immunoprecipitation. Vortex Protein G Plus/Protein A resin to resuspend, then aliquot 40 μL into four 1.5 mL microfuge tubes. Equilibrate resin by adding 1 mL of IPEQ buffer and rotating 5 min at room temp. Pellet resin by centrifugation at 2,000 g for 30 sec at room temp. Add 1 mL of IP150 buffer, rotate for 5 min and centrifuge again.
Centrifuge loading reactions from step 9B(i) at 12,000 g at room temp for 5 min. Move supernatant to 1.5 mL microfuge tube containing equilibrated resin (from step 9B(ii)).
Add 2 μg of α-Ago2 antibody into tubes 1 and 3 and 2 μg IgG antibody (control) into tubes 3 and 4 from step 9B(i). Rotate at room temp for 1 h.
Wash resin 3x by adding 0.5 mL of IP500 buffer, rotating for 5 min at room temp, and centrifuging at 2,000 g for 30 sec at room temp.
Wash again 1x with 0.5 mL of IP150 buffer.
-
Stop the reaction by adding the following components.
Component Volume (μL) Final Nuclease-free water 40 --- EDTA (0.5 M) 0.5 1.8 mM tRNA (10 μg/μL) 0.5 35 ng/uL Phenol 80 --- Vortex for 30 sec, then phenol extract by centrifuging and keeping the top aqueous layer (move to a new tube).
Precipitate eluted RNA by adding 9 vol of 2% LiClO4 in acetone and incubating at −20°C for >15 min. CAUTION. LiClO4 is a dangerous oxidizer. Handle with caution.
Spin at 12,000 g 10 min, wash pellet with ice-cold acetone, and spin again at 12,000 g for 2 min. Let pellet air dry at room temperature. PAUSE POINT. Dried RNA pellet can be stored at −20°C for a few days.
-
10
Samples can be resolved by denaturing or native gel electrophoresis. Denaturing gel electrophoresis will not distinguish duplex versus single-stranded guide RNA bound to Ago2, but will reveal a single band for radioactive RNA bound to Ago2. Native electrophoresis conditions will separate duplex from single-stranded small RNAs, which may suggest formation of premature and mature RISC complexes, respectively. For denaturing gel electrophoresis, follow option A. For native gel electrophoresis, follow option B.
Option A: Assessing Ago2 loading by denaturing gel electrophoresis
-
Cast a denaturing 15% polyacrylamide gel. Mix the following components then pour into a gel cassette assembled from glass plates, spacers, binder clips and a comb. As an alternative to casting a gel, denaturing TBE-buffered gels can often be purchased commercially.
Component Volume (mL) Final 15% denaturing polyacrylamide gel solution 35 15% 10% APS 0.21 0.0006% TEMED 0.035 0.001% Pre-run the gel at 40 mA with 1x TBE running buffer for 30 min without cooling. Gel should become very warm to the touch and reach a temperature of approximately 45–50°C for optimal denaturation and resolving conditions. CAUTION. If denaturing gels become too hot, the glass plates will crack. Monitor temperature and current to maintain a safe operating range.
Add 6–10 μL formamide loading buffer to dried RNA from step 9A(xii) or 9B(x), boil 5 min at 90°C, vortex, centrifuge at 2,000 g 30 sec, then load onto pre-run gel. Run gel at 40 mA until bottom blue dye band (bromophenol blue) is 1/2 to 2/3 down the gel. The RNA will run between the two blue dye bands on the gel.
Stop the gel, separate the glass plates, and lay Saran wrap over the top of the gel. Flip the gel over and peel the glass plate off so that the gel is now adhered to the Saran wrap. Press a sheet of Whatman 3M paper to the gel and dry in a vacuum gel dryer at 80°C for 2 h.
-
Expose dried gel to a phosphorimager screen overnight. Develop screen to visualize radioactive RNA that co-eluted with Ago2 in the in vitro loading assay. Typical results for Step 9B samples resolved by native gel electrophoresis are shown in Figure 4B.
TROUBLESHOOTING.
Option B: Assessing Ago2 loading by native gel electrophoresis
-
Cast a native 15% polyacrylamide gel. Mix the following components then pour into a gel cassette assembled from glass plates, spacers, binder clips and a comb. As an alternative to pouring a gel, native TBE-buffered gels can often be purchased commercially
Component Volume (mL) Final 15% native polyacrylamide gel solution 35 15% 10% APS 0.21 0.0006% TEMED 0.035 0.001% Pre-run the gel at 30 mA with 1x TBE running buffer for 30 min with cooling. Gel should stay cool with an ideal running temperature of less than 30°C.
Add 6–10 μL 1x native loading buffer to dried RNA from step 9B(x), vortex, centrifuge at 2000 g 30 sec, then load onto pre-run gel. Run gel at 35 mA until bottom blue dye band (bromophenol blue) is 2/3 to 3/4 down the gel. The RNA will run between the two blue dye bands on the gel.
Stop the gel, separate the glass plates, and lay Saran wrap over the top of the gel. Flip the gel over and peel the glass plate off so that the gel is now adhered to the Saran wrap. Press a sheet of Whatman 3M paper to the gel and dry in a vacuum gel dryer at 80°C for 2 h.
-
Expose dried gel to a phosphorimager screen overnight. Develop screen to visualize radioactive RNA that co-eluted with Ago2 in the in vitro loading assay. Typical results for Step 9B samples resolved by native gel electrophoresis are shown in Figure 4C.
TROUBLESHOOTING.
TROUBLESHOOTING
Troubleshooting advice can be found in Table 1.
Table 1.
Troubleshooting Advice
| STEP | PROBLEM | POSSIBLE REASON | SOLUTION |
|---|---|---|---|
| 7A(iii) & 7B(iii) | Nuclei do not pellet well | Cells are not completely lysed | Incubate on ice longer and use more vigorous pipetting to resuspend cells in Step 7A(ii) or 7B(ii). |
| Nuclei are smaller than typical | Centrifuge at higher speed or for longer duration. | ||
| Washes are incomplete | Wash additional times or with more vigorous pipetting during resuspension. | ||
| 7B(iv) | Nuclei are not completely lysed | Sonication ineffective | Increase sonication time or sonicator power output. |
| 7B(iv) | Nuclear extract is foamy | Oversonication | Decrease sonication time or sonicator power output. |
| 7B(iv) | Nuclear extract is viscous | genomic DNA not sufficiently sheared | Increase sonication time or sonicator power output. |
| Box 1 | Nuclei are not oval or rounded | nuclear membrane damaged | wash nuclei with fewer washes or gentler pipetting in Step 7A(ii) or 7B(ii). |
| Box 1 | DAPI staining outside of nuclei | nuclear membrane damaged | wash nuclei with fewer washes or pipette more gently in Step 7A(ii) or 7B(ii). |
| check to ensure NP-40 concentrations are correct during Reagent Setup. | |||
| Box 1 | Signal from ER tracker dye | ER membrane proteins not efficiently removed | increase wash times, NP-40 concentration, or pipetting stringency in Step 7A(ii) or 7B(ii). |
| tracker dye concentration too high | check tracker dye concentration, ensure nuclei are diluted after incubation. | ||
| Box 1 | No ER tracker signal from controls | ER tracker dye concentration too low | increase tracker dye concentration incubation or time. |
| Box 2 | RNA radioactivity is low | Inefficient labeling | Increase [λ]-32P-ATP, PNK enzyme, or reaction duration in Step (i) of Box 2. |
| Inefficient gel extraction | Use more water during elution from gel in Step (viii) of Box 2. | ||
| Recover gel bits off of tip by pipetting buffer down the tip Step (viii) of Box 2. | |||
| Add more tRNA and LiClO4 in acetone to RNA solution during precipitation in Step (x) of Box 2. | |||
| 10A(v) & 10B(v) | Bands are not visible | Inefficient siRNA loading | Add higher amounts of radioactive RNA to the loading reaction in Step 9A(vi) or 9B(i). |
| Increase extract volume or use more antibody and resin in Step 9A(ii) or 9B(ii–iv). | |||
| Incubate loading reactions longer in Step 9A(vi) or 9B(i) & 9B(iv). | |||
| Inefficient Ago2 immunoprecipitation | Increase amount of antibody or resin during immunoprecipitation in Step 9A(ii) or 9B(ii–iv). | ||
| Incubate antibody and resin with extract longer in Step 9A(ii) or 9B(iii–iv). | |||
| Reduce wash duration or wash buffer NaCl concentration in Step 9A(vii) or 9B(v). | |||
| Extract quality poor | Optimize fractionation in Step 7B. | ||
| Ago2 expression low | Increase extract volume during loading reactions in Step 9A(ii) and 9B(i). | ||
| 10A(v) & 10B(v) | Bands are blurry or heterogeneous | Poor gel quality | Optimize gel pouring conditions in Step 10A(i) and 10B(i) or purchase commercial gels. |
| Gel ran too hot or too fast | Reduce current or improve cooling in Step 10A(ii–iii). | ||
| Gel ran too cool or too slow | Increase current in Step 10B(ii–iii). | ||
| Gel ran too long | Reduce run time in Step 10A(ii) or 10B(ii). | ||
| 10A(v) & 10B(v) | Bands are visible in IgG controls | Inefficient resin washing | Wash longer, wash with more buffer, wash more times or wash with a higher NaCl concentration in the wash buffer in Step 9A(vii) or 9B(v). |
| Overexposed phosphorimager screen | Reduce exposure time to phosporimager screen in Step 10A(v) or 10B(v). |
TIMING
ANTICIPATED RESULTS
The overall aim of this procedure is to generate nuclear extracts from mammalian cells of high quality and minimal contamination from the cytoplasm or other cellular organelles, especially the endoplasmic reticulum. These extracts enable investigation of nuclear-specific processes, such as the presence and activity of RNAi. Extracts are then used to test Ago2 loading with small RNAs, an activity that we have found to be deficient in human nuclear extracts.
Western blot analysis
It is essential that the purity of subcellular fractionations be confirmed prior to any subsequent experiments and that efficient exclusion of potential cyoplasmic contaminants be demonstrated. When analyzed by western blot analysis, nuclear fractions produced by this protocol should show no evidence of cytoplasmic markers like tubulin, ER markers like calreticulin, or mitochondrial markers like Oxphos (Figure 3A). The nuclear fraction should reveal the presence of nuclear-specific proteins like lamin A/C or histone H3 while the cytoplasmic fraction should not contain these proteins. Abundant Ago2 should be seen in both cytoplasmic and nuclear fractions. Nucleoplasmic fractions should show a strong depletion of chromatin-associated proteins like histone H3. It is important to load protein from equal numbers of cells because the cytoplasm contains substantially more protein per cell. It will be impossible to compare fractions directly by western blot if equal cell equivalents are not used.
Fluorescence microscopy
Fluorescence microscopy is a useful tool for assessing the quality of isolated nuclei29. Key indicators of high quality nuclei include evidence of intact nuclear membrane and removal of membrane-associated ER proteins. Staining nuclei with DAPI should show no leakage of chromatin, which would appear as strings or wisps of blue staining outside of the oval nuclei. DAPI staining should also show sharp, well-defined boundaries. After isolation using 0.3% NP-40 for washes and incubation with ER tracker dye, there should be little or no fluorescence from the tracker dye in contrast to abundant staining when NP-40 detergent is not used (Figure 3B). There is no special requirement for confocal microscopy or other three-dimensional imaging technologies other than the ability to measure fluorescence with blue (ie. DAPI) and red (ie. TRITC) filters.
In vitro Ago2 loading assays
For the loading of Ago2 with duplex small RNA (Figure 4A), the first quality check is to observe a single band on a denaturing gel after immunoprecipitation of Ago2 from cytoplasmic extracts incubated with radiolabeled duplex (Figure 4B). No band should appear in the samples immunoprecipitated with non-specific IgG antibody when used. Running on a native, non-denaturing gel might be helpful in discriminating between duplex loading and passenger strand removal. If the passenger strand has not been completely removed two distinct bands will be observed (Figure 4C). The faster migrating band represents guide RNA that was bound to Ago2 whereas the slower migrating band represents duplex RNA that was bound to Ago2. In the cell lines used in our studies, Ago2 loading is observed in cytoplasmic extracts but not nuclear extracts29.
Acknowledgments
Funding was provided by the National Institutes of Health (1F32HD060377/KTG, GM 73042/DRC, GM85080/BAJ), the Welch Foundation (I-1244/DRC), and the Cancer Prevention and Research Institute of Texas (RP120311/BAJ).
Footnotes
AUTHOR CONTRIBUTIONS
K.T.G. and L.L. designed and performed experiments, including optimization of subcellular fractionation and development of the in vitro Argonaute loading assay. K.T.G, L.L., B.A.J. and D.R.C all participated in the writing of this manuscript.
COMPETING FINANCIAL INTERESTS
None declared.
References
- 1.Wilson RC, Doudna JA. Molecular mechanisms of RNA interference. Annu Rev Biophys. 2013;42:217–239. doi: 10.1146/annurev-biophys-083012-130404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 3.Elbashir SM, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 4.Morris KV, Chan SW, Jacobsen SE, Looney DJ. Small interfering RNA-induced transcriptional gene silencing in human cells. Science. 2004;305:1289–1292. doi: 10.1126/science.1101372. [DOI] [PubMed] [Google Scholar]
- 5.Janowski BA, et al. Inhibiting gene expression at transcription start sites in chromosomal DNA with antigen RNAs. Nat Chem Bio. 2005;1:216–222. doi: 10.1038/nchembio725. [DOI] [PubMed] [Google Scholar]
- 6.Castanotto D, et al. Short hairpin RNA-directed cytosine (CpG) methylation of the RASSF1A gene promoter in HeLa cells. Mol Ther. 2005;12:179–183. doi: 10.1016/j.ymthe.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Ting AH, Schuebel KE, Herman JG, Baylin SB. Short double-stranded RNA induces transcriptional gene silencing in human cancer cells in the absence of DNA methylation. Nat Genet. 2005;37:906–910. doi: 10.1038/ng1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Napoli S, Pastori C, Magistri M, Carbone GM, Catapano CV. Promoter-specific transcriptional interference and c-myc gene silencing by siRNAs in human cells. EMBO J. 2009;28:1708–1719. doi: 10.1038/emboj.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim DH, Villeneuve LM, Morris KV, Rossi JJ. Argonaute-1 directs siRNA-mediated transcriptional gene silencing in human cells. Nat Struct Mol Biol. 2006;13:793–797. doi: 10.1038/nsmb1142. [DOI] [PubMed] [Google Scholar]
- 10.Li LC, et al. Small dsRNAs induce transcriptional activation in human cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17337–17342. doi: 10.1073/pnas.0607015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janowski BA, et al. Activating gene expression in mammalian cells with promoter-targeted duplex RNAs. Nat Chem Biol. 2007;3:166–173. doi: 10.1038/nchembio860. [DOI] [PubMed] [Google Scholar]
- 12.Matsui M, et al. Promoter RNA links transcriptional regulation of inflammatory pathway genes. Nucl Acids Res. 2013;41:10086–10109. doi: 10.1093/nar/gkt777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang V, et al. RNAa is conserved in mammalian cells. PLoS One. 2010;5:e8848. doi: 10.1371/journal.pone.0008848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Hu J, Corey DR. Expanding the action of duplex RNAs into the nucleus: redirecting alternative splicing. Nucl Acids Res. 2012;40:1240–1250. doi: 10.1093/nar/gkr780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allo M, et al. Control of alternative splicing through siRNA-mediated transcriptional gene silencing. Nat Struct Mol Biol. 2009;16:717–724. doi: 10.1038/nsmb.1620. [DOI] [PubMed] [Google Scholar]
- 16.Janowski BA, et al. Involvement of AGO1 and AGO2 in mammalian transcriptional silencing. Nat Struct Mol Biol. 2006;13:787–792. doi: 10.1038/nsmb1140. [DOI] [PubMed] [Google Scholar]
- 17.Chu Y, Yue X, Younger ST, Janowski BA, Corey DR. Involvement of argonaute proteins in gene silencing and activation by RNAs complementary to a non-coding transcript at the progesterone receptor promoter. Nucl Acids Res. 2010;38:7736–7748. doi: 10.1093/nar/gkq648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vickers TA, et al. Efficient reduction of target RNAs by small interfering RNA and RNase H-dependent antisense agents: a comparative analysis. J Biol Chem. 2003;278:7108–7118. doi: 10.1074/jbc.M210326200. [DOI] [PubMed] [Google Scholar]
- 19.Zeng Y, Cullen BR. RNA interference in human cells is restricted to the cytoplasm. RNA. 2002;8:855–860. doi: 10.1017/s1355838202020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda K, et al. Detection of the argonaute protein Ago2 and microRNAs in the RNA induced silencing complex (RISC) using monoclonal antibody. J Immunol Meth. 2006;317:38–44. doi: 10.1016/j.jim.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stalder L, et al. The rough endoplasmatic reticulum is a central nucleation site of siRNA-mediated RNA silencing. EMBO J. 2013;32:1115–1127. doi: 10.1038/emboj.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ando Y, et al. Nuclear pore complex protein mediated nuclear localization of dicer protein in human cells. PLoS ONE. 2011;6:E23385. doi: 10.1371/journal.pone.0023385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu Y, Yue X, Younger ST, Janowski BA, Corey DR. Involvement of argonaute proteins in gene silencing and activation by RNAs complementary to a non-coding transcript at the progesterone receptor promoter. Nucleic acids research. 2010;38:7736–7748. doi: 10.1093/nar/gkq648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doyle M, et al. The double-stranded RNA binding domain of human Dicer functions as a nuclear localization signal. RNA. 2013;19:1238–1252. doi: 10.1261/rna.039255.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohrt T, Muetze J, Svoboda P, Schwille P. Intracellular localization and routing of miRNA and RNAi pathway components. Curr Top Med Chem. 2012;12:79–88. doi: 10.2174/156802612798919132. [DOI] [PubMed] [Google Scholar]
- 26.Rudel S, Flatley A, Weinmann L, Kremmer E, Meister G. A multifunctional human Argonaute2-specific monoclonal antibody. RNA. 2008;14:1244–1253. doi: 10.1261/rna.973808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Till S, et al. A conserved motif in Argonaute-interacting proteins mediates functional interactions through the Argonaute PIWI domain. Nature structural & molecular biology. 2007;14:897–903. doi: 10.1038/nsmb1302. [DOI] [PubMed] [Google Scholar]
- 28.Weinmann L, et al. Importin 8 is a gene silencing factor that targets argonaute proteins to distinct mRNAs. Cell. 2009;136:496–507. doi: 10.1016/j.cell.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Gagnon KT, Li L, Chu Y, Janowski BA, Corey DR. RNAi factors are present and active in human cell nuclei. Cell Rep. 2014;6:211–221. doi: 10.1016/j.celrep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holding C. RNAi active in the nucleus? Genome Biol. 2004:6. http://dx.doi.org/10.1186/gb-spotlight-20050112-20050101.
- 31.Hetzer MW. The nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2:a000539. doi: 10.1101/cshperspect.a000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michelson U, von Hagen J. Isolation of subcellular organelles and structures. Meth Enzym. 2009;463:306–328. doi: 10.1016/S0076-6879(09)63019-6. [DOI] [PubMed] [Google Scholar]
- 33.Greenberg ME, Bender TP. Identification of newly transcribed RNA. Curr Protoc Mol Biol. 2007;78:4.10.11–14.10.12. doi: 10.1002/0471142727.mb0410s78. [DOI] [PubMed] [Google Scholar]