Abstract
Propensity to develop acute functional (or within session) tolerance to alcohol (ethanol) may influence the amount of alcohol consumed, with higher drinking associated with greater acute functional tolerance (AFT). The goal of the current study was to assess this potential correlated response between alcohol preference and AFT in second and third replicate lines of mice selectively bred for high (HAP2&3) and low (LAP2&3) alcohol preference drinking. Male and female mice were tested for development of AFT on a static dowel task which requires that animals maintain balance on a wooden dowel in order to prevent falling. On test day, each mouse received one (1.75g/kg; Experiment 1) or two (1.75g/kg and 2.0g/kg; Experiment 2) injections of ethanol; an initial administration before being placed on the dowel and in Experiment 2, an additional administration after the first regain of balance on the dowel. Blood samples were taken immediately after loss of balance (when BECs were rising) and at recovery (during falling BECs) in Experiment 1, and after first and second recovery in Experiment 2. It was found that HAP mice fell from the dowel significantly earlier and at lower BECs than LAP mice following the initial injection of ethanol and were therefore more sensitive to its early effects. Furthermore, Experiment 1 detected significantly greater AFT development (BECfalling - BECrising) in HAP mice as compared to LAP mice which occurred within ~30 min, supporting our hypothesis. However, AFT was not different between lines in Experiment 2, indicating that ~30–60 min following alcohol administration, AFT development was similar in both lines. These data show that high alcohol drinking genetically associates with both high initial sensitivity and very early tolerance to the ataxic effects of ethanol.
There is mounting evidence for genetic contribution to the development of alcohol (ethanol) use disorders (Ducci & Goldman 2008; Mayfield et al. 2008; Schuckit 2009). Alcohol dependence develops after sustained, excessive consumption, and an important part of genetic diathesis may concern responses to alcohol in pre-dependent individuals (Ducci & Goldman 2008; Enoch 2003; Newlin & Thomson 1990; Schuckit 1994). One such pre-dependent response is tolerance, an important component for clinical diagnosis of alcohol dependence (APA, 2000). Pre-dependent individuals with enhanced propensity to develop alcohol tolerance may be more prone to abuse alcohol and later become dependent (Newlin & Thomson 1990; Schuckit et al. 2008).
Tolerance to ethanol can be pharmacokinetic and/or pharmacodynamic. Pharmacodynamic (or functional) tolerance is characterized by neurobiological/neurochemical adaptation that reduces functional impairment (i.e. cognitive and motor faculties). Functional tolerance may be an important factor in escalating alcohol consumption due to a reduction in perceived impairment (Brumback et al. 2007; Nicholson et al. 1992) and may develop over a single alcohol exposure; referred to as acute functional tolerance (AFT) (Mellanby 1919).
All of the human studies cited above utilized subjects that consumed alcohol at some point in their lives before testing. Some important deviant responses to alcohol that could contribute to abuse liability may only be present during the initiation of alcohol consumption behavior. Due to a myriad of ethical issues, we cannot initiate excessive alcohol consumption in alcohol-naïve humans. Therefore, the development of good animal models of AFT will be key to our eventual understanding of how this phenomenon influences alcohol consumption. Lines of rodents have been selectively bred for high (HAFT) and low (LAFT) AFT (Erwin & Deitrich 1996), although the relationship between AFT and alcohol consumption in these lines is ill-defined as neither consumes significant amounts of alcohol (Erwin et al. 2000). Investigating AFT in lines selected for alcohol preference, such as the high (HAP) and low (LAP) alcohol-preferring selected mouse lines (Grahame et al. 1999), may therefore offer a more complete picture of the relationship between the two phenotypes. Although no difference in AFT development between lines was observed in the first replicate of HAP and LAP lines (Grahame et al. 2000), AFT has yet to be investigated in the second and third replicate lines. Moreover, replicate line 2 mice drink much more alcohol now than did replicate 1 mice at the time of their earlier testing (Oberlin et al, 2010). Larger phenotypic differences between lines may allow more reliable assessment of correlated responses to selection, such as AFT, due to the recruitment of more trait-relevant alleles. It is important to measure the same behaviors and responses across all replicate lines due to potential differences/similarities resulting from genetic drift (Crabbe et al. 1990). Based on the aforementioned literature, as well as a revised operational definition of ataxia described in the methods below, we hypothesized a genetically correlated response in that HAP mice would exhibit greater AFT to ethanol than their LAP counterparts.
Materials and methods
Subjects
Naïve adult (postnatal day 60–95) male and female mice from the second and third replicate lines selected for high (HAP2 and HAP3) and low (LAP2 and LAP3) alcohol preference versus water in two-bottle tests were bred on site at the IUPUI School of Science, Indianapolis, IN (for an in depth description of the selection process and characterization of these lines, see Oberlin et al., 2011). Three replicates of the HAP/LAP lines now exist and there are extreme differences in alcohol intake and preference with the replicate HAP lines consuming high intoxicating amounts of alcohol, and replicate LAP lines demonstrating relative avoidance (Oberlin et al., 2011). Selected lines are particularly useful animal models to study genetic risk for alcohol abuse because they allow the researcher to theoretically fix only alleles that are specific to the phenotype of interest (in this case, alcohol preference) through selective mating. This process may allow the maintenance of heterogeneity at all other loci, thereby allowing simpler determination of correlated responses to selection than with inbred strains (Crabbe et al., 1990). The HAP and LAP lines therefore provide a powerful model with which to determine whether predisposition for high alcohol consumption is associated with greater propensity to develop AFT. In other words, selection for high alcohol preference may have also resulted in greater AFT ability due to common underlying genes; producing a genetically correlated response (Crabbe et al. 1990; Tabakoff & Hoffman 1988). If this is, in fact, observed, AFT may be an important contributor to the development of extremely high daily alcohol intake in HAP mice; a possible explanation being that these animals must continue to consume high quantities of alcohol over the course of the dark cycle, consistently increasing BEC, to maintain a desired level of intoxication.
All animals from replicate line 2 were from generations 38–39 of selection and all replicate line 3 mice were from generation 16 of selection. Mice were housed 3–4 per cage and maintained on a 12-hour light/dark cycle with lights on at 0700 and testing took place between 0900 and 1800. Temperature and humidity were held constant near 20° C and 50% respectively. Food and water were available ad libitum. All experiments were performed under a protocol approved by the IUPUI Institutional Animal Care and Use Committee.
Ethanol Administration
One-hundred-ninety proof ethanol was purchased from Pharmco Inc. (Brookfield, CT) and diluted in sterile 0.9% physiological saline to a concentration of 15% v/v. The solution was mixed fresh each test day. Ethanol was administered via intraperitoneal (i.p.) injection in weight-based doses of 1.75 g/kg and 2.0 g/kg.
Static Dowel Task
The static dowel task (Grahame et al. 2000) was employed to assess differences in initial sensitivity (IS) and AFT to the ataxic effects of ethanol. This task required animals to balance on an elevated wooden dowel horizontally centered in a Plexiglas box (32×32×60 cm; l × w × h) to prevent falling. The dowel was 1.58 cm in diameter, positioned 50 cm from the floor of the box which was covered in pine chip bedding to cushion the fall, and was placed on a level surface for testing. The time taken for the animal to lose its balance on the dowel following ethanol administration was recorded and interpreted as a loss of function and the BEC value at this behavioral endpoint was used as an index of IS. In both of these experiments, loss of function (LOF) was defined as the majority of an animal’s body falling below the imaginary horizontal plane which bisects the dowel, indicating that it no longer retained sufficient balance capacity to remain erect. When initially described by Gallaher and colleagues (1982), behavioral intoxication assessed by the static dowel task was ambiguously defined as ‘loss of balance’. Where it has been directly explained in the subsequent literature, ‘loss of balance’ is noted when the animal falls from the dowel to the floor of the box (Gehle & Erwin 2000; Hu et al. 2008; Kirstein et al. 2002; Wallace et al. 2006; Wu et al. 2001). Where no direct description is given and only previous literature is cited, it is reasonable to assume that the same definition of intoxication is adopted. As we were interested in AFT as it may develop over the short time course from LOF to recovery (described below as the Mellanby Approach), our revised definition accounts for the rapid rate at which ethanol is absorbed following i.p. administration while still assessing balance performance. If the classic definition of falling were to be used, the BEC at fall may be inaccurate due to a period of the animal clutching to or hanging from the wooden dowel. Therefore, we used the revised criterion for both LOF and recovery of function, although the exact numbers of animals that exhibited hanging vs. falling was not separately tracked. While this distinction would allow for the determination of LOF and BEC differences between hanging vs. falling, the new definition nevertheless afforded the opportunity for better temporal discrimination concerning the animals’ ability to perform the task. As a side note, the vast majority of animals in the current study were observed to hang from the dowel rather than fall for LOF.
After LOF, each animal was retested on the dowel every 5 min after falling until meeting recovery criterion which required that the mouse maintain its balance for 60 seconds. Periorbital sinus blood samples (25 μl) were taken at certain behavioral endpoints specified below to calculate IS and AFT.
Mellanby (Single Recovery) Approach
The procedure was similar to that described by Mellanby (1919) in his work with canines in that AFT was assessed in the current study by comparing the BEC of a mouse at LOF versus recovery. A significant increase in BEC value from LOF to recovery is an indication that AFT developed as the mouse is able to recover performance on the task at a higher BEC than that at LOF. One reason we chose to incorporate this approach is that AFT may develop more rapidly than is detectable by the classic Two-Recovery Approach described below, which often takes mice nearly 3 hours to complete.
After being moved into the testing room, animals were allowed at least 30 min for acclimation. Mice were then given three drug-free, 60 second training trials to ensure they could balance on the dowel. All mice were able to remain upright on the dowel for the entire duration of the last two training trials. Each animal was then given a 1.75 g/kg i.p. injection of ethanol and immediately placed on the dowel. The time point following injection for LOF was recorded and a 25 μl blood sample was immediately taken from the periorbital sinus to determine IS. Animals were retested on the dowel every 5 minutes until meeting the recovery criterion of successfully balancing for 60 seconds. Upon recovery, a second blood sample was collected. AFT using this approach was calculated by subtracting the BEC value at LOF (the animal’s IS) from that at recovery and will hereon be referred to as Mellanby Acute Functional Tolerance, or ‘M-AFT’; distinguishing this measure from the Two-Recovery assessment which will remain, ‘AFT’. One reason the Two-Recovery approach has been used is that accurate BEC determination at LOF is rather difficult to assess due to the steep rate at which ethanol is absorbed into blood and tissue following injection. It is therefore noteworthy to point out that after experimenter practice, all blood samples in the current study were collected within 8 sec of LOF.
Two-Recovery Approach
For detailed description of procedure, see Erwin & Dietrich (1996). This procedure is similar to that of the M-AFT approach described above, except no blood sample was taken at LOF and a second 2.0 g/kg injection of ethanol was administered following the first recovery. After a 1 hour rest period following the second injection, retesting ensued at 5 minute intervals until recovery criterion was met again whereupon another periorbital sinus blood sample was taken. AFT using this two-recovery approach was calculated by subtracting the blood ethanol concentration (BEC) value at Recovery 1 from the BEC value at Recovery 2.
Blood-Ethanol Concentration Determination
Blood samples were spun down in a centrifuge and the plasma supernatant was siphoned off and transferred to 0.5 ml microcentrifuge tubes. Samples were stored at −80° C until determination of BEC in mg/dl by an Analox Alcohol Analyzer (Analox Instruments, Lunenburg, MA).
Statistical Analysis
Data were analyzed as suggested by Crabbe and colleagues (1990) for studies involving correlated responses in selected lines using Statistica 7 sofware (StatSoft); detected main effects for line or replicate × line interactions were deemed sufficient evidence for a correlated response. ‘Correlated’, in this sense, is referring not directly to a statistical correlation, but rather the existence of a significant difference between selected lines on another phenotype of interest. The language of a correlation, therefore, is more an illustration of how the fixation of alleles related to a certain phenotype (alcohol preference in this case) may also have an effect on another related phenotype (pleiotropy); implicating shared genes between the two. For this assessment, a correlation is not computed in a classical sense, but rather the existence of a main effect of line is determined for the comparative phenotype of interest (AFT or IS in this case). If the lines are found to significantly differ, this is determined a correlated response because bidirectional selection produced significant differences in the phenotype of interest. As per the Crabbe et al. (1990) guidelines, post-hoc testing was only used where Line significantly interacted with Replicate to determine if one replicate in particular was driving the effect.
For the Mellanby experiment, LOF, DOI (duration of impairment), IS, and M-AFT were analyzed by three-way ANOVAs with Replicate, Sex, and Line as factors. For the Two-Recovery experiment, LOF, BEC value at Recovery 1, and AFT were analyzed by three-way ANOVAs with the same factors listed above. DOI for this study was analyzed by a four-way repeated measures ANOVA with Measurement (Recovery 1/Recovery 2) as the added factor. BEC in either experiment was not analyzed using such a four-way ANOVA as the computed AFT score represents the difference between both samples. Results were deemed significant at p < .05. Tukey-Kramer post-hoc statistics are reported where applicable. Main effects or potential interactions that are not explicitly mentioned below were not found to be statistically significant. However, the interested reader may find complete ANOVA tables identifying the F and P values for each possible comparison in the Mellanby and Two-Recovery experiments in Tables 4 and 5 as supplementary material.
For the single-injection Mellanby approach experiments, 7 animals (2 LAP2 M, 1 HAP3 F, 2 HAP3 M, 1 LAP3 F, 1 LAP3 M) were removed from the analysis due to improper injection or failure to obtain a blood sample within 8 sec after LOF. For the Two-Recovery experiments, 4 animals (2 LAP2 M, 1 HAP3 M, 1 HAP3 F) were removed from the analysis due to failure to lose balance on the dowel within 5 min following the initial 1.75 g/kg injection.
Results
Sensitivity and AFT - Mellanby Approach
LOF, Recovery Times, and BEC values at relevant behavioral endpoints using the single-injection Mellanby approach for HAP2/LAP2 and HAP3/LAP3 mice are shown in Figures 1 and 2, respectively. All graphs are displayed collapsed on Sex. However, we refer the interested reader to Table 1 for group means broken down by sex.
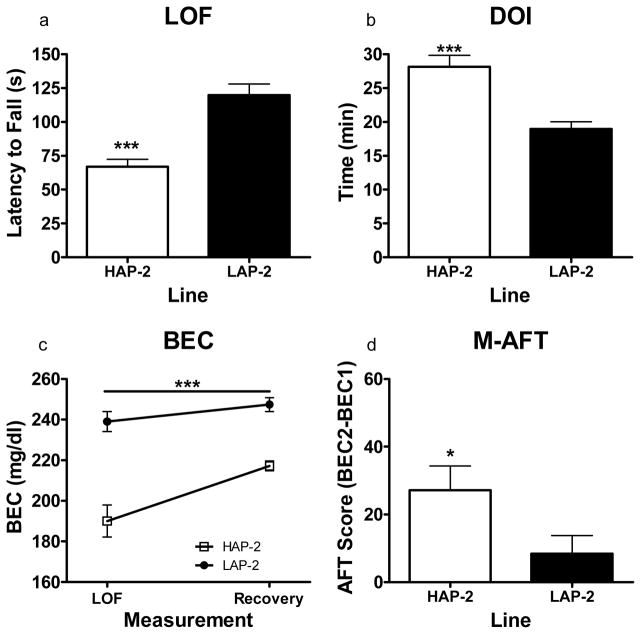
Figure 1.
Static dowel assessment of sensitivity and development of AFT to the ataxic effects of ethanol in HAP/LAP-2 mice (N = 24–26 per line) using the Mellanby approach. (a) Time taken to lose upright position on the dowel immediately following the 1.75 g/kg injection of ethanol. HAP-2 mice lost the ability to stay on the dowel significantly earlier than LAP-2 mice (P < .001) (b) Duration of impairment (DOI) following the injection of ethanol (1.75 g/kg) to reach recovery criterion. HAP-2 mice took significantly more time to recover than LAP-2 mice (P < .001) (c) BEC measurement at both fall and recovery. BEC values for HAP-2 mice were significantly lower than those for LAP-2 mice at both fall and recovery (P < .001). (d) Development of AFT. This was calculated by subtracting the BEC measurement at fall from the BEC measurement at recovery. HAP-2 mice developed greater AFT than LAP-2 mice (P < .05). *P < .05, **P < .01, ***P < .001
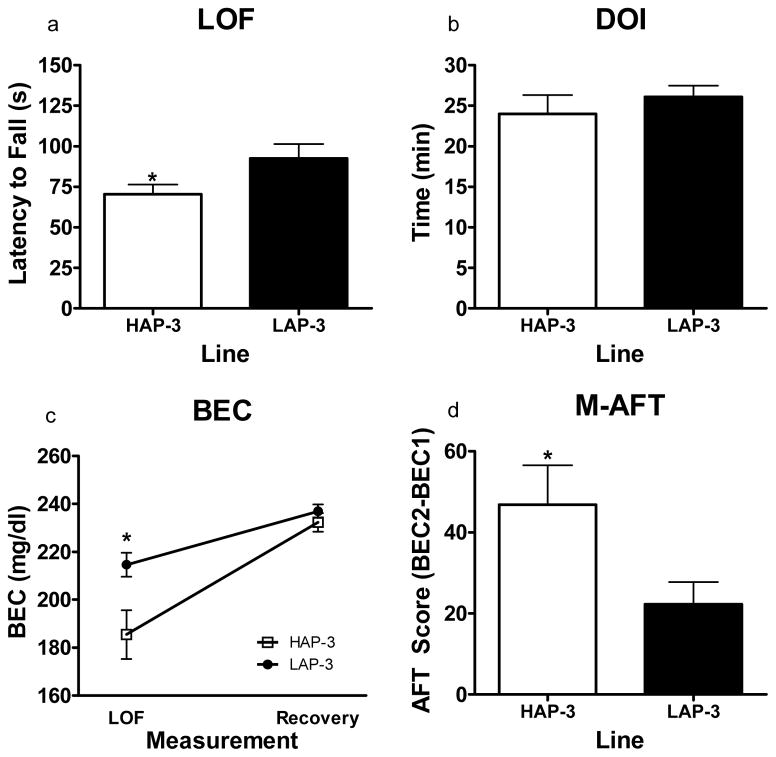
Figure 2.
Static dowel assessment of sensitivity and development of AFT to the ataxic effects of ethanol in HAP/LAP-3 mice (N = 24–26 per line) using the Mellanby approach. (a) Time taken to lose upright position on the dowel immediately following the 1.75 g/kg injection of ethanol. HAP-3 mice lost the ability to stay on the dowel significantly sooner than LAP-3 mice (P < .05) (b) DOI following the injection of ethanol (1.75 g/kg) to reach recovery criterion. No differences were detected for recovery time (c) BEC measurement at both fall and recovery. The BEC values for HAP-3 mice at fall were significantly lower than those for LAP-3 mice as detected by a significant Measurement × Line interaction (P < .05). (d) Development of AFT (BEC Recovery – BEC Fall). HAP-3 mice developed greater AFT than LAP-3 mice (P < .05). *P < .05
Table 1.
Mean Values (± SEM) for Each Measurement Using the ‘Mellanby’ Approach
| Line | Sex | n | LOF (sec) | DOI (min) | BEC1 (mg/dl) | BEC2 (mg/dl) | M-AFT (B2-B1) |
|---|---|---|---|---|---|---|---|
| HAP2 | M | 12 | 71.8 ± 8.7 | 30.9 ± 2.5 | 178.0 ± 12.3 | 245.8 ± 5.8 | 35.5 ± 11.4 |
| F | 12 | 62.1 ± 6.4 | 25.3 ± 2.2 | 202.0 ± 9.3 | 220.8 ± 3.6 | 18.8 ± 8.4 | |
| HAP3 | M | 10 | 69.6 ± 9.6 | 25.7 ± 3.3 | 181.4 ± 13.7 | 236.2 ± 3.7 | 54.8 ± 14.5 |
| F | 11 | 71.3 ± 7.6 | 22.5 ± 3.3 | 189.2 ± 15.4 | 228.7 ± 6.6 | 39.6 ± 13.5 | |
| LAP2 | M | 10 | 112.4 ± 5.3 | 20.0 ± 1.3 | 230.9 ± 7.9 | 239.2 ± 5.2 | 8.4 ± 8.9 |
| F | 12 | 126.0 ± 14.2 | 18.1 ± 1.7 | 245.8 ± 5.8 | 254.3 ± 3.7 | 8.5 ± 6.8 | |
| LAP3 | M | 11 | 88.8 ± 11.2 | 28.5 ± 2.0 | 216.4 ± 5.8 | 233.3 ± 2.6 | 27.7 ± 9.5 |
| F | 11 | 96.4 ± 14.2 | 23.6 ± 1.6 | 212.9 ± 8.4 | 240.6 ± 4.7 | 16.9 ± 5.6 |
Assessment of LOF found that HAP mice lost balance on the dowel significantly sooner than LAP mice following the injection of ethanol (F1,85 = 26.01 P < .001) (Figs. 1a & 2a). A significant Line × Replicate interaction was also detected (F1,85 = 4.25, P < .05), indicating that overall, LAP2 mice were able to maintain balance on the dowel the longest following injection (P < .05 compared to LAP3).
Following injection, both line and sex differences were observed in the time it took mice to recover the ability to remain upright on the dowel (Table 1; Figs. 1b & 2b). Main effects of Line (F1,81 = 4.57, P < .05) and Sex (F1,81 = 5.61, P < .05) were detected with HAP animals taking significantly longer to recover than LAP animals and male animals taking longer to recover than females. However, a significant Line × Replicate interaction was also found (F1,81 = 11.27, P = .001) with post-hoc testing indicating a significant difference between lines only in the replicate 2 mice (P < .05).
For the index of initial sensitivity, a main effect of Line was found with HAP mice losing function at significantly lower BEC values than their LAP counterparts (F1,81 = 28.24, P < .001), displaying significantly greater initial sensitivity to ethanol (Figs. 1c & 2c). All other main effects or interactions were non-significant (P > .05).
Following a single injection of ethanol, HAP mice recovered at significantly higher BEC values than which they fell compared to LAP mice (F1,85 = 9.3, P < .01), indicating significantly greater M-AFT development in HAP mice (Figs. 1c/1d & 2c/2d). Replicate 3 mice were also found to develop greater M-AFT in general than replicate 2 mice (F1,85 = 5.61, P < .05).
Sensitivity and AFT - Two-recovery Approach
LOF, Recovery Times, and BEC values at relevant behavioral endpoints using the Two-Recovery approach for HAP2/LAP2 and HAP3/LAP3 mice are depicted in Figures 3 and 4, respectively. All graphs are shown collapsed on Sex (for group means broken down by Line, Replicate, and Sex, see Table 2).
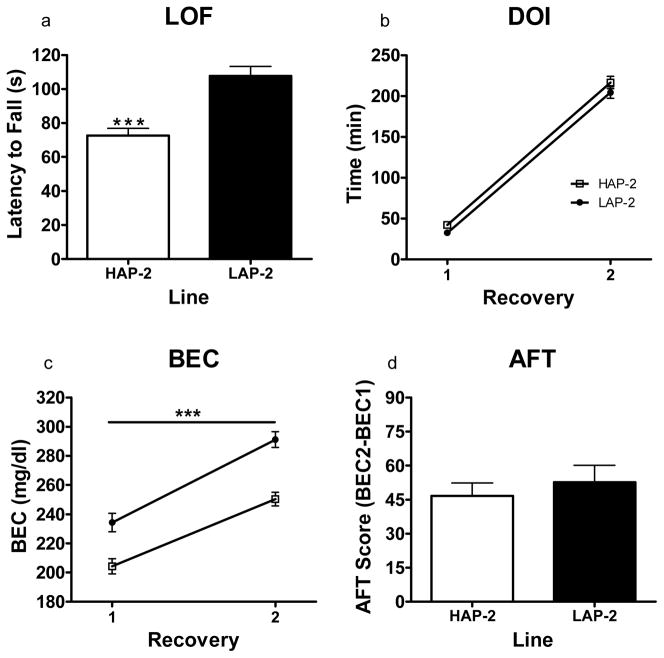
Figure 3.
Static dowel assessment of sensitivity and development of acute functional tolerance (AFT) to the ataxic effects of ethanol in HAP/LAP-2 mice (N = 24–26 per line) using the two-recovery approach. (a) Time taken to lose upright position on the dowel immediately following the 1.75 g/kg injection of ethanol. HAP-2 mice lost the ability to stay on the dowel significantly earlier than LAP-2 mice (P < .001) (b) DOI following the initial injection of ethanol for each recovery. The lines did not differ in time taken to recover. (c) Blood ethanol concentration (BEC) measurement at each recovery. BEC values for HAP-2 mice were significantly lower than those for LAP-2 mice at each recovery (P < .001). (d) Development of AFT. This was calculated by subtracting the BEC measurement at the first recovery from the BEC measurement at the second recovery. HAP-2 and LAP-2 lines did not differ in AFT development. *P < .05, **P < .01, ***P < .001
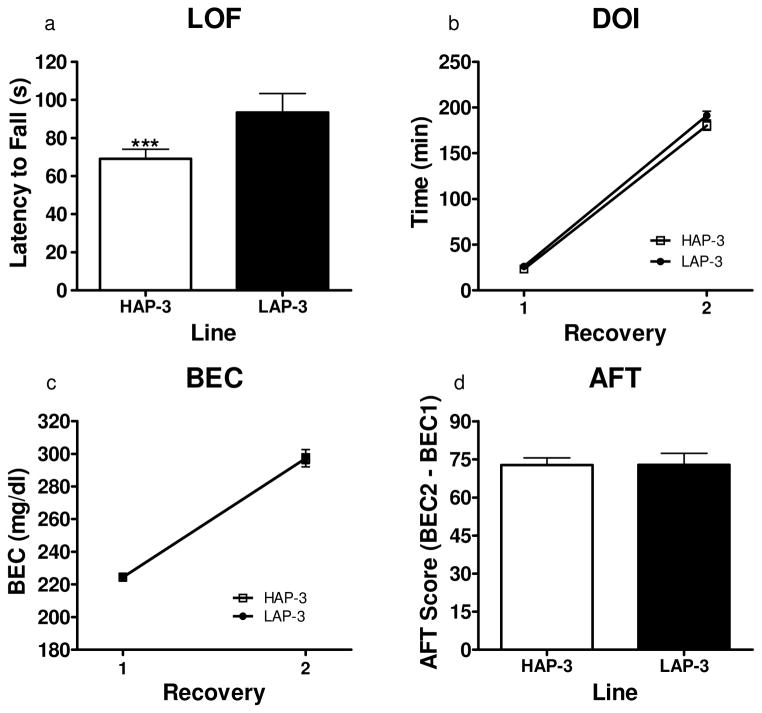
Figure 4.
Static dowel assessment of sensitivity and development of AFT to the ataxic effects of ethanol in HAP/LAP-3 mice (N = 24–26 per line) using the two-recovery approach. (a) Time taken to lose upright position on the dowel immediately following the 1.75 g/kg injection of ethanol. HAP-3 mice lost the ability to stay on the dowel significantly earlier than LAP-3 mice (P < .05) (b) DOI following the initial injection of ethanol for each recovery. HAP-3 and LAP-3 lines did not differ on recovery time. (c) BEC measurement at each recovery. No differences were detected for BEC values. (d) Development of AFT (BEC 2 – BEC 1). The lines did not differ in AFT development. *P < .05, **P < .01, ***P < .001
Table 2.
Mean Values (± SEM) for Each Measurement Using the Two-Recovery Approach
| Line | Sex | N | LOF (sec) | DOI-R1 (min) | DOI-R2 (min) | BEC1 (mg/dl) | BEC2 (mg/dl) | AFT (B2-B1) |
|---|---|---|---|---|---|---|---|---|
| HAP2 | M | 12 | 67.1 ± 4.2 | 55.1 ± 2.7 | 239.3 ± 13.1 | 203.4 ± 7.1 | 239.2 ± 5.5 | 58.2 ± 5.9 |
| F | 14 | 76.8 ± 6.8 | 31.1 ± 3.3 | 196.9 ± 4.4 | 205.2 ± 8.1 | 263.4 ± 6.0 | 35.9 ± 7.5 | |
| HAP3 | M | 12 | 68.7 ± 7.2 | 27.7 ± 2.7 | 193.9 ± 6.1 | 220.4 ± 3.9 | 298.5 ± 4.2 | 78.1 ± 3.7 |
| F | 14 | 69.3 ± 7.4 | 19.2 ± 1.2 | 166.6 ± 4.7 | 228.3 ± 2.2 | 295.8 ± 4.5 | 67.5 ± 3.6 | |
| LAP2 | M | 11 | 104.5 ± 8.1 | 36.4 ± 5.6 | 211.5 ± 7.4 | 218.3 ± 8.4 | 281.7 ± 7.5 | 63.3 ± 12.5 |
| F | 13 | 108.0 ± 6.8 | 29.0 ± 3.8 | 198.0 ± 11.4 | 249.7 ± 8.4 | 295.7 ± 7.7 | 46.0 ± 7.0 | |
| LAP3 | M | 11 | 94.5 ± 16.2 | 28.4 ± 2.9 | 201.0 ± 6.5 | 226.1 ± 4.2 | 304.8 ± 6.0 | 78.7 ± 4.2 |
| F | 13 | 92.5 ± 12.2 | 24.5 ± 1.2 | 181.6 ± 5.8 | 222.8 ± 2.5 | 289.9 ± 8.4 | 67.1 ± 7.8 |
Following the initial injection of 1.75 g/kg ethanol, HAP mice were again found to lose function significantly earlier than their LAP counterparts (F1,92 = 19.38, P < .001) (Figs. 3a & 4a). All other main effects and interactions were non-significant (P > .05).
The duration of impairment on the static dowel task was measured by recording the time it took for mice to recover the ability to remain upright on the dowel following each injection of ethanol and is shown graphically in Figures 3b and 4b. A repeated measures four-way ANOVA (with Line, Sex, Replicate, and Meaurement as factors) found main effects of Replicate (F1,88 = 30.58, P < .001) and Sex (F1,88 = 27.31, P < .001) with replicate 2 animals generally taking longer to recover than replicate 3 animals and males taking longer than females (Table 2; Figs. 3b & 4b), but did not detect a significant main effect of Line (P = 0.459). Significant interactions of Line × Replicate (F1,88 = 7.62, P < .01) and Line × Sex (F1,88 = 4.45, P < .05) were also detected with post-hoc tests revealing that HAP mice generally took longer to recover than LAP mice in replicate 2 (P < .05) and HAPs were impaired for a longer duration than LAPs among male mice (P < .05). Interactions of Measurement × Replicate (F1,88 = 6.06, P < .05) and Measurement × Sex (F1,46 = 7.86, P < .01) were also found with replicate 2 (P < .05) and male (P < .05) animals taking particularly longer to recover following the second injection of ethanol, respectively (Table 2).
The blood sample taken at Recovery 1 revealed that LAP mice regained function at higher BEC values than HAP mice overall (Figs. 3c & 4c). The three-way ANOVA of BEC value at Recovery 1 indicated main a main effect of Line (F1,88 = 11.09, P < .001) as well as a significant Line × Replicate × Sex interaction (F1,88 = 6.15, P < .05) with post-hoc testing revealing that LAP2 females recovered at particularly high BEC values relative to other groups (P < .05) (Table 1).
The development of AFT (difference between BEC values from Recovery 1 to Recovery 2) was not different between lines (Figs. 1d & 2d). A three-way ANOVA found no effect of Line, however, main effects of Sex (F1,88 = 9.51, P < .01) and Replicate (F1,88 = 19.19, P < .001) were detected with male and replicate 3 animals developing greater AFT than females and replicate 2 animals, respectively (Table 2; Figs. 3d & 4d).
Discussion
The main findings in this study were that selection for High Alcohol Preference drinking in mice associates with greater initial sensitivity to ethanol as well as greater tolerance development within 30 minutes of administration using the single injection, Mellanby approach. Consistent with previous work using these lines (Grahame et al. 2000), no genetic differences were seen in alcohol tolerance during later adaptation periods probed by using two injections. Together, these findings argue that predisposition to high drinking behavior in HAP mice is associated with both high initial sensitivity and high initial rapid tolerance, but not with differences in tolerance assessed at later (~30 – 240 min) time points. Importantly, these findings are unlikely due to genetic drift between High- and Low- Preference lines because both replicate lines showed consistent line differences in both initial sensitivity and subsequent tolerance.
Findings in humans have been mixed concerning whether blunted or enhanced sensitivity to alcohol-induced ataxia is a predictor of subsequent alcohol use disorder development (Newlin & Thomson 1990; Schuckit 1994). There is also divergence in the animal literature concerning the association between alcohol sensitivity and alcohol intake (Crabbe et al. 1994; Waller et al. 1983). One potential reason for this observed divergence is that a variety of procedures have been used to determine sensitivity and are therefore likely assessing different domains of sensitivity. For example, the highest drinking inbred mouse strain, C57BL/6J, was found to be less sensitive to the ataxic effects of ethanol as assessed by an accelerating rotarod procedure relative to lower alcohol-drinking strains (Crabbe et al. 1994). However, a later study in our lab utilizing the balance beam apparatus found no difference in initial sensitivity between adult mice of the high-drinking C57BL/6J stain and the low-drinking DBA/2J strain (Linsenbardt et al. 2009). Related to the current research question, no difference in initial sensitivity was found between HAFT and LAFT lines using the static dowel task (Deitrich et al. 2000). Based on this previous research, we expected that selection for high or low alcohol preference would produce no significant differences in initial sensitivity to alcohol’s ataxic effects between lines. Nonetheless, we found that HAP mice exhibited greater initial sensitivity to ethanol than their LAP counterparts (Figs. 1a&c, 2a&c). Not only did the HAP animals lose their upright position earlier than LAP animals, their BEC values were correspondingly lower at LOF as well (Figs 1c, 2c) showing that pharmacodynamics, rather than absorption/distribution, are responsible for this enhanced sensitivity. While pharmacokinetic differences were not explicitly addressed in the current study, slopes for HAP and LAP mice were computed for the rising phase of the alcohol curve using time post-injection for LOF and IS in the Mellanby approach study, probing absorption differences between lines. It was found that the slopes for HAP and LAP mice were not significantly different (P > 0.1). Slopes were also computed for the falling phase using Recovery time and BEC and again, slopes were not different between lines (P = 0.417). While this will be studied more directly in future work, this brief analysis suggests that there are no significant differences between lines in the pharmacokinetics of ethanol.
Based on the guidelines laid out by Crabbe and colleagues (1990) for assessing correlated responses, the observed main effect of Line in each experiment confers strong evidence for a correlated response between alcohol preference and initial sensitivity. One possible reason high initial sensitivity associates with high alcohol intake may be that the temporal relationship between alcohol drinking and perceived intoxication is tightened. Motor impairment is a salient cue for intoxication perception in humans (Brumback et al. 2007; Nicholson et al. 1992) and therefore, the more quickly one feels and perceives alcohol’s effects - ataxic and otherwise - the more reinforcing consumption may be during the establishment of drinking. Studies concerning drugs of abuse have found that the more quickly the effects of a drug are perceived after administration/consumption, the more addictive their potential (Samaha & Robinson 2005). Although these studies have been primarily concerned with differences in the route of administration, the principle still applies in that the more quickly certain effects are perceived, perhaps due to heightened pharmacodynamic sensitivity rather than rate of absorption into blood/tissue in this case, the more reinforcing a drug may be.
Acute tolerance has also been implicated in high alcohol consumption. It has been theorized that that the ability to develop tolerance over the course of a single alcohol exposure may enhance one’s ability to engage in sessions of sustained, high alcohol intake (Tabakoff & Hoffman 1988). Whether or not high alcohol consumption in various genetic mouse models associates with enhanced acute tolerance is not clear (Hu et al. 2008; Ponomarev & Crabbe 2004; Waller et al. 1983). An earlier study found no difference in AFT between the first replicate of HAP and LAP lines using the two-recovery approach (Grahame et al. 2000); a lack of difference replicated here in HAP/LAP2 and 3. Also, the selectively-bred HAFT and LAFT lines displayed significant differences in their ability to develop AFT on the static dowel task using the aforementioned two-recovery approach (Erwin & Deitrich 1996), the trait on which they were selected. However, whereas these lines were found to significantly differ in voluntary alcohol consumption with HAFT mice consuming more alcohol than LAFT mice (Erwin et al. 2000), both lines consumed relatively low amounts of alcohol in a continuous access paradigm (~0.5–1.5 g/kg/hr), making it difficult to draw conclusions concerning the association between AFT capacity and excessive alcohol consumption. Perhaps one reason understanding this relationship in these lines has been difficult may be that the random fixation of genes (i.e. genetic drift) related to gustatory sensitivity could have conflicted with intake.
Studies examining AFT using selectively-bred high-drinking genotypes, such as HAP mice, circumvent the above issue while still addressing baseline ability to develop AFT and its relation to alcohol consumption. Due to larger differences in alcohol consumption between HAP and LAP replicate 2 lines at the time of testing in the current study compared to the former study with replicate 1 lines (see Table 3 for means), we hypothesized that HAP mice would exhibit greater AFT to the ataxic effects of ethanol compared to LAP mice, a prediction not borne out by the AFT phenotype. Based on unpublished observations in our lab as well as some findings in the human literature (Morzorati et al. 2002), we also hypothesized that AFT may develop over a shorter period than the standard two-recovery approach can detect, which is why we added the M-AFT probe. Following the intriguing findings of the M-AFT experiment in replicate 2 animals, replicate 3 mice were tested for the existence of a correlated response. Indeed, the single-injection/recovery Mellanby approach found that both HAP lines developed greater M-AFT compared to their LAP counterparts (Figs 1d, 2d). Using this approach, mice from both lines and replicates met recovery criterion within 30 minutes of ethanol injection on average (Figs 1b, 2b), implying that important pharmacodynamic processes underlying M-AFT occur rapidly after alcohol enters the system, likely during the rising limb of the BEC curve. As BEC peaks ~5–10 minutes following intraperitoneal ethanol injection, the only behavioral measure we have of sensitivity to rising phase BEC is BEC1 from the M-AFT study. In contrast, in the AFT measure from Experiment Two, both blood samples were taken during the falling limb of the BEC curve in the Two-Recovery approach, and no differences in tolerance were found. Due to the enhanced ability to rapidly overcome alcohol-induced ataxia, these mice may consistently drink heavily in a continuous access paradigm in an attempt to achieve an earlier intoxication state that can quickly be masked by AFT. While there is somewhat more variability in the line 3 as compared to line 2 animals overall, this was expected as replicate 3 was not as far along in selection and the HAP line was not consuming as much alcohol at the time of testing (see Table 3). Although the replicate 3 lines differ less in alcohol drinking, animals from both lines displayed significantly greater AFT overall in each experiment as compared to those from replicate 2. Due to the larger difference between lines in replicate 2 in the selected phenotype (alcohol preference), it was reasonably expected that any observed difference in tolerance between lines would be greater than those observed in replicate 3. Although replicate 3 exhibited greater AFT capacity in general, which could potentially be attributable to genetic drift, it was observed that the difference in tolerance between lines was similar between replicates, further supporting the existence of a correlated response with alcohol preference. In addition, the BEC and LOF measurements were highly similar at the same behavioral endpoints in the Mellanby and Two-Recovery experiments (i.e. time post-injection for LOF; BEC at Recovery in Mellanby and Recovery 1 in Two-Recovery), showing that blood sampling at LOF using the Mellanby approach did not disrupt the experiment. Together, this pattern of data may suggest that selection for drinking may recruit sensitivity/tolerance differences quite rapidly. M-AFT was not addressed in the first replicate of these lines and unfortunately, LAP1 mice no longer exist due to fecundity issues. However, it would be interesting and useful to determine how consistent our finding of a genetic correlation between M-AFT and alcohol consumption is by extending the comparison to a large panel of inbred strains, although such work remains beyond the scope of this investigation.
Table 3.
Mean Daily Alcohol Intake (g/kg/24 hrs) of Each Line at the Time of Testing
| Line | Sex |
Grahame ET AL. (2000) Replicate 1 Generation 12 |
Current Study: Replicate 2 Generations 38–39 |
Current Study Replicate 3 Generation 16 |
|---|---|---|---|---|
| HAP | M | 10.9 ± 0.59 | 19.95 ± 0.58 | 9.08 ± 0.77 |
| F | 15.62 ± 0.67 | 22.23 ± 0.67 | 15.43 ± 1.09 | |
| LAP | M | 0.70 ± 0.04 | 0.89 ± 0.03 | 0.89 ± 0.12 |
| F | 1.18 ± 0.09 | 1.12 ± 0.07 | 1.68 ± 0.34 |
Collectively, these findings highlight that the timing of assessments of behavioral intoxication may end up being an important factor in understanding what appear to have been inconsistencies in outcomes between laboratories in both human and animal studies. Our observed pattern of line differences in sensitivity to alcohol early, but not after longer exposure, was observed by Morzorati et al. (2002), who showed that humans with a family history of alcohol (FHP) were more sensitive to the subjective effects of alcohol early (25 min after intravenous administration) but not later (105 min after administration), echoing our current findings of both higher initial sensitivity and greater tolerance in FHP subjects. Although Schuckit and colleagues have long argued that FHP creates lower alcohol sensitivity (often using an ataxia-like measure such as body sway), their initial assessments are typically about 60 min after administration (e.g., Schuckit 1985) to allow complete absorption following oral dosing. Therefore, they may miss events occurring quickly after alcohol dosing, on the rising phase of the alcohol curve. As argued above, the early effects of alcohol may be the most important when seeking to understand how the consequences of drinking come to act on subsequent drinking choices. Indeed, the current findings showing the largest genetic differences in alcohol sensitivity and tolerance just after administration suggest that early responses, missed even in animal studies focusing on 2-recovery AFT, may be the most important to study when seeking to understand the excessive drinking behavior seen in HAP lines (Matson & Grahame 2011) as well as in human alcoholics (Mello & Mendelson 1970).
Finally, the findings of the current study, as well as the study by Morzorati et al. (2002), resemble the Differentiator Model put forth by Newlin and Thomson (1990). The model proposes that FHP manifests in heightened sensitivity to alcohol on the ascending limb of the BEC curve coupled with greater acute tolerance on the descending limb. Numerous studies have found support for this model in humans (Conrod et al. 2001; Gianoulakis et al. 1996; Holdstock et al. 2000; King et al. 2002; Peterson et al. 1996). While the heightened sensitivity the Differentiator model refers to concerns the stimulating effects of alcohol rather than ataxia, these animals still fit the philosophy behind the model, although it is related to a different response. Therefore, we propose that HAP mice illustrate a Differentiator Model of ataxia. Future work will address how AFT and sensitivity may be altered by the duration of alcohol pre-exposure in HAP mice, which may provide valuable insight on the mechanisms governing the initiation, compared to the maintenance, of excessive alcohol intake in this animal model.
Acknowledgments
Funding was provided by NIAAA grants AA07611 to the Indiana Alcohol Research Center (David Crabb) and AA016789 (S. B.).
References
- Association, A.P. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Brumback T, Cao D, King A. Effects of alcohol on psychomotor performance and perceived impairment in heavy binge social drinkers. Drug and Alcohol Dependence. 2007;91:10–17. doi: 10.1016/j.drugalcdep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology. 2001;157:20. doi: 10.1007/s002130100741. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Gallaher ES, Phillips TJ, Belknap JK. Genetic determinants of sensitivity to ethanol in inbred mice. Behavioral Neuroscience. 1994;108:186–195. doi: 10.1037//0735-7044.108.1.186. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of Genetic Correlation: Interpretation of Experiments Using Selectively Bred and Inbred Animals. Alcoholism: Clinical and Experimental Research. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Deitrich RA, Bludeau P, Erwin VG. Phenotypic and Genotypic Relationships Between Ethanol Tolerance and Sensitivity in Mice Selectively Bred for Initial Sensitivity to Ethanol (SS and LS) or Development of Acute Tolerance (HAFT and LAFT) Alcoholism: Clinical and Experimental Research. 2000;24:595–604. [PubMed] [Google Scholar]
- Ducci F, Goldman D. Genetic approaches to addiction: genes and alcohol. Addiction. 2008;103:1414–1428. doi: 10.1111/j.1360-0443.2008.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M-A. Pharmacogenomics of Alcohol Response and Addiction. American Journal of PharmacoGenomics. 2003;3:217–232. doi: 10.2165/00129785-200303040-00001. [DOI] [PubMed] [Google Scholar]
- Erwin V, Deitrich R. Genetic selection and characterization of mouse lines for acute functional tolerance to ethanol. J Pharmacol Exp Ther. 1996;279:1310–1317. [PubMed] [Google Scholar]
- Erwin VG, Gehle VM, Deitrich RA. Selectively Bred Lines of Mice Show Response and Drug Specificity for Genetic Regulation of Acute Functional Tolerance to Ethanol and Pentobarbital. Journal of Pharmacology and Experimental Therapeutics. 2000;293:188–195. [PubMed] [Google Scholar]
- Gehle VM, Erwin VG. The Genetics of Acute Functional Tolerance and Initial Sensitivity to Ethanol for an Ataxia Test in the LSxSS RI Strains. Alcoholism: Clinical and Experimental Research. 2000;24:579–587. [PubMed] [Google Scholar]
- Gianoulakis C, Krishnan B, Thavundayil J. Enhanced Sensitivity of Pituitary {beta}Endorphin to Ethanol in Subjects at High Risk of Alcoholism. Arch Gen Psychiatry. 1996;53:250–257. doi: 10.1001/archpsyc.1996.01830030072011. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Li TK, Lumeng L. Selective Breeding for High and Low Alcohol Preference in Mice. Behavior Genetics. 1999;29:47–57. doi: 10.1023/a:1021489922751. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Rodd-Henricks K, Li TK, Lumeng L. Ethanol locomotor sensitization, but not tolerance correlates with selection for alcohol preference in high- and low-alcohol preferring mice. Psychopharmacology. 2000;151:252. doi: 10.1007/s002130000388. [DOI] [PubMed] [Google Scholar]
- Holdstock L, King AC, de Wit H. Subjective and Objective Responses to Ethanol in Moderate/Heavy and Light Social Drinkers. Alcoholism: Clinical and Experimental Research. 2000;24:789–794. [PubMed] [Google Scholar]
- Hu W, Saba L, Kechris K, Bhave SV, Hoffman PL, Tabakoff B. Genomic Insights into Acute Alcohol Tolerance. J Pharmacol Exp Ther. 2008;326:792–800. doi: 10.1124/jpet.108.137521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic Alcohol Response Differs in Heavy Versus Light Drinkers. Alcoholism: Clinical and Experimental Research. 2002;26:827–835. [PubMed] [Google Scholar]
- Kirstein SL, Davidson KL, Ehringer MA, Sikela JM, Erwin VG, Tabakoff B. Quantitative Trait Loci Affecting Initial Sensitivity and Acute Functional Tolerance to Ethanol-Induced Ataxia and Brain cAMP Signaling in BXD Recombinant Inbred Mice. J Pharmacol Exp Ther. 2002;302:1238–1245. doi: 10.1124/jpet.302.3.1238. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm SL., II Sensitivity and Tolerance to the Hypnotic and Ataxic Effects of Ethanol in Adolescent and Adult C57BL/6J and DBA/2J Mice. Alcoholism: Clinical and Experimental Research. 2009;33:464–476. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, Grahame NJ. Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addiction Biology. 2011 doi: 10.1111/j.1369-1600.2011.00412.x. no-no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield RD, Harris RA, Schuckit MA. Genetic factors influencing alcohol dependence. British Journal of Pharmacology. 2008;154:275–287. doi: 10.1038/bjp.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellanby E. Alcohol: Its absorption into and disappearance from the blood under different conditions. Med Res Commun. 1919;31:1–48. [Google Scholar]
- Mello NK, Mendelson JH. Experimentally induced intoxication in alcoholics: A comparison between programed and spontaneous drinking. Journal of Pharmacology and Experimental Therapeutics. 1970;173:101–116. [PubMed] [Google Scholar]
- Morzorati SL, Ramchandani VA, Flury L, Li TK, O’Connor S. Self-Reported Subjective Perception of Intoxication Reflects Family History of Alcoholism When Breath Alcohol Levels Are Constant. Alcoholism: Clinical and Experimental Research. 2002;26:1299–1306. doi: 10.1097/01.ALC.0000025886.41927.83. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: A critical review and analysis. Psychological Bulletin. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Nicholson ME, Wang M, Airhihenbuwa CO, Mahoney BS, Maney DW. Predicting Alcohol Impairment: Perceived Intoxication Versus BAC. Alcoholism: Clinical and Experimental Research. 1992;16:747–750. doi: 10.1111/j.1530-0277.1992.tb00671.x. [DOI] [PubMed] [Google Scholar]
- Oberlin B, Best C, Matson L, Henderson A, Grahame N. Derivation and Characterization of Replicate High- and Low-Alcohol Preferring Lines of Mice and a High-Drinking Crossed HAP Line. Behavior Genetics. 2011;41:288–302. doi: 10.1007/s10519-010-9394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JB, Pihl RO, Gianoulakis C, Conrod P, Finn PR, Stewart SH, LeMarquand DG, Bruce KR. Ethanol-Induced Change in Cardiac and Endogenous Opiate Function and Risk for Alcoholism. Alcoholism: Clinical and Experimental Research. 1996;20:1542–1552. doi: 10.1111/j.1530-0277.1996.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Ponomarev I, Crabbe JC. Characterization of Acute Functional Tolerance to the Hypnotic Effects of Ethanol in Mice. Alcoholism: Clinical and Experimental Research. 2004;28:991–997. doi: 10.1097/01.alc.0000131978.79857.5e. [DOI] [PubMed] [Google Scholar]
- Samaha A-N, Robinson TE. Why does the rapid delivery of drugs to the brain promote addiction? Trends in Pharmacological Sciences. 2005;26:82–87. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Schuckit M. An overview of genetic influences in alcoholism. Journal of Substance Abuse Treatment. 2009;36:S5–14. [PubMed] [Google Scholar]
- Schuckit MA. Ethanol-Induced Changes in Body Sway in Men at High Alcoholism Risk. Arch Gen Psychiatry. 1985;42:375–379. doi: 10.1001/archpsyc.1985.01790270065007. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Hesselbrock V, Bucholz KK, Bierut L, Edenberg H, Kramer J, Longacre E, Fukukura T, Kalmijn J, Danko GP, Trim R. Clinical Implications of Tolerance to Alcohol in Nondependent Young Drinkers. American Journal of Drug & Alcohol Abuse. 2008;34:133–149. doi: 10.1080/00952990701877003. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. Tolerance and the Etiology of Alcoholism: Hypothesis and Mechanism. Alcoholism: Clinical and Experimental Research. 1988;12:184–186. doi: 10.1111/j.1530-0277.1988.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Newton PM, Oyasu M, McMahon T, Chou W-H, Connolly J, Messing RO. Acute Functional Tolerance to Ethanol Mediated by Protein Kinase C[epsiv] Neuropsychopharmacology. 2006;32:127–136. doi: 10.1038/sj.npp.1301059. [DOI] [PubMed] [Google Scholar]
- Waller MB, McBride WJ, Lumeng L, Li TK. Initial sensitivity and acute tolerance to ethanol in the P and NP lines of rats. Pharmacology Biochemistry and Behavior. 1983;19:683–686. doi: 10.1016/0091-3057(83)90345-3. [DOI] [PubMed] [Google Scholar]
- Wu PH, Tabakoff B, Szabó G, Hoffman PL. Chronic ethanol exposure results in increased acute functional tolerance in selected lines of HAFT and LAFT mice. Psychopharmacology. 2001;155:405. doi: 10.1007/s002130100722. [DOI] [PubMed] [Google Scholar]