Abstract
Orthotopic liver transplantation (OLT) models in rats have been investigated in many studies, but detailed information on the impact of hepatic artery (HA) reconstruction on postoperative factors remains to be investigated. HA reconstruction also requires advanced skills. The effect of the reconstruction of the HA by a hand-suture technique in rats with a whole-liver syngeneic graft was investigated. Long-term survival, histopathological assessment, immunohistological evaluation, and blood biochemistry were investigated until postoperative day (POD) 28. From the early postoperative period, significant differences between OLTs with or without HA reconstruction were found in graft parenchymal damage, induction of apoptosis, and transaminase levels, though survival curves and the coagulation profile showed no differences. In OLT without HA reconstruction, biliary proliferation was decreased at POD 5–14, and total bilirubin level was increased at PODs 10 and 14. The study indicates that HA reconstruction is required for reliable OLT in rats.
Keywords: hepatic artery, microsurgery
INTRODUCTION
The surgical technique for orthotopic liver transplantation (OLT) in a rat model was first reported using hand-suture methods in 1973 [1]. A second study in 1975 described a modified rat OLT model without reconstruction of the hepatic artery (HA) and a temporal shunt with porto-jugular veno-venous bypass [2]. However, since these models required advanced surgical skills, they were not widely adopted. The cuff method for venous reconstruction was introduced in 1973 [3], and thereafter more modified models of rat OLT without HA reconstruction became the standard for research in OLT [4, 5].
Although the detailed effects of HA reconstruction on rodent OLT are still controversial, several researchers have suggested that transplanted grafts with HA reconstruction resulted in an improved function after OLT compared with grafts without HA reconstruction [6–10]. However, HA reconstruction is technically difficult and requires advanced skills [6–9, 11, 12]. End-to-end anastomosis of HA is the ideal method, but this requires ultra-microsurgery, including reconstruction of vessels or nerves with a diameter <0.5 mm in rodents [11–13]. HA reconstruction is very difficult in rats and may be impossible or unreliable in mice [11, 12]. In order to simplify the procedures of HA reconstruction, many researchers have focused on modification of the technical aspect of microsurgery [4, 6, 11, 12, 14–20].
The aims of this study were to determine the impact of HA reconstruction on the syngeneic graft in rat OLT, and we discuss the necessity for HA reconstruction.
MATERIALS AND METHODS
Animals
Lewis rats (RT-11) were purchased from Harlan Laboratories, Inc. (Indianapolis, IN, USA). Graft donors and recipients were 8- to 10-week-old rats. All rats were male. The experimental protocols were approved by the Ethical Committee of our institution (Mayo Clinic, Institutional Animal Care and Use Committee, No. A19609), and all operative procedures were performed aseptically under the accordance with approved protocol. Rats were cared for according to the institutional guidelines for Animal Welfare based on the Ethical Guidelines of the Declaration of Helsinki.
Surgical Procedures for OLT
Comprehensive details of the surgical procedures for rat OLT in our institution have previously been described [11, 12]. General anesthesia was performed using a commercial anesthesia system (VetEquip Inc., Pleasanton, CA), including an evacuation canister (f/air, Bickford Inc., New York, NY) and induction chamber (2 Liter Induction Chamber, VetEquip Inc.). All operative procedures were performed under general anesthesia using isoflurane accompanied with oxygen, and inhalational anesthesia was induced and maintained by isoflurane using an anesthesia system. In brief, the suprahepatic and intrahepatic inferior vena cavae were reconstructed by the hand-suture or cuff method, although only the cuff method was employed in portal venous reconstruction to shorten the anhepatic phase [12]. The common biliary duct was reconstructed using a stent tube. A surgical microscope of ×5–20 magnification (Surgical Scope M680, Type 10445496, Leica Microsystems Inc., IL, USA) was used. End-to-end anastomosis of the HA was performed under ×20 magnification. The sleeve technique was documented as an innovative technique for HA reconstruction [4, 15, 17], but we often found that the graft common HA and recipient’s proper HA had similar diameters. Thus, the common and proper HAs were skeletonized from the portal venous trunk, then the donor and recipient common HAs were anastomosed in an end-to-end fashion using the hand-suture technique. Interrupted sutures were made with monofilament nylon suture (10–0 Ethilon, BV130–3, 2820G, Ethicon Inc., Johnson & Johnson Company, NJ, USA).
The recipient was warmed on a hot pad immediately after surgery. A total of 2.0–2.5 ml of warm lactate Ringer’s solution was injected via the penile vein, at every 2 hr after surgery until 6 hr after surgery. As an analgesia, 0.1 mg/kg of analgesic agent (buprenor-phine, Buprenex Injectable; Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA) was routinely given intramuscularly every 8 hr for five days after surgery. As part of the aseptic technique, 30 mg/kg of antibiotics (Cephalexin Hydrate, MP Biomedicals, Cleveland, OH) was administered intravenously every 8 hr for two days after surgery. For the euthanasia, all rats were sacrificed by using a CO2 chamber.
In this study, the whole-liver graft had a cold ischemic time of 2 hr at 4°C in normal Ringer’s preservation solution. In rat OLT, we previously demonstrated the importance of a short anhepatic phase and the exclusion of unreliable samples based on autopsy and histopathological findings [11, 12]. In this study, the anhepatic phase was kept within 20 min in each surgery, and autopsy findings showing no critical complications and reasonable histopathological findings were confirmed in each case.
Impact of HA Reconstruction on Postoperative Factors
To confirm the effect of HA reconstruction on long-term survival after OLT, we performed laparotomy only (n = 10), OLT with HA reconstruction using the hand-suture technique (n = 10), and OLT without HA reconstruction (n = 10). Liver specimens immediately after induction of graft circulation were used for samples at postoperative day (POD) 0. Samples (n = 3) at each of nine time points at POD 1, 2, 3, 5, 7, 10, 14, 21, and 28 were obtained in the three groups.
Biochemical Assay and Coagulation Profile after OLTs with or without HA Reconstruction
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (T-Bil), and the international normalized ratio of prothrombin time (PT-INR) were measured at each time point. Serum and plasma were obtained by separator tubes (BD Microtainer, Becton, Dickson and Company, Franklin Lakes, NJ, USA). Serum AST, ALT, and T-Bil were assessed by commercial kits (SGOT, SGPT, and total bilirubin reagent, respectively, Biotron, Hemet, CA, USA). A wavelength of 540 nm was set in the microplate reader to measure absorbance (Spectra Max M5e, Molecular Devices, Sunnyvale, CA, USA). The PT-INR in the plasma was measured by i-STAT System (Abbott, Princeton, NJ 08540, USA).
Histopathological and Immunohistological Assessments after OLTs with or without HA Reconstruction
Histopathological and immunohistological parameters were assessed at each time point. Liver tissue was fixed in 10% neutral-buffered formalin (Protocol, 032–059, Fisher Scientific, Inc., Kalamazoo, MI, USA), embedded in paraffin, and sliced into 4-μm sections. The morphological characteristics and graft injury scores were assessed after hematoxylin-eosin (H-E) staining. The graft damage score (point) for the rat OLT model, which was slightly modified from a previous score [21, 22], has previously been described [11].
Induction of apoptosis was measured by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) (Apop-Tag Peroxidase In Situ Apoptosis Detection Kit, S7100, Chemicon International, Inc., Billerica, MA, USA), and cysteine aspartic acid protease (caspase)-3 immunostaining [Cleaved Caspase-3 (Asp175) Antibody, 9661S, Cell Signaling Technology, Inc., Danvers, MA, USA]. A TUNEL-positive nucleus was stained brown, and a negative nucleus was stained light blue. A caspase-3-positive nucleus was stained brown, and a negative nucleus was stained blue. Slides were scanned with an automated high-throughput scanning system (Scanscope XT, Aperio Technologies, Inc., Vista, CA, USA). To quantify the histological findings, positive-stained nuclei were counted by Aperio Imagescope software (Aperio Technologies, Inc.). All nuclei were classified into four-color intensity levels, and the higher two levels were considered as positive. The ratio of positive-stained nuclei to all nuclei was calculated and the mean ratio per mm2 was determined.
Cytokeratin-7 (CK7) is widely used for immunostaining of the biliary epithelium because the detection of the peripheral biliary duct is often difficult in H-E staining. HA flow has a large influence on biliary proliferation after OLT [8, 23]. Therefore, biliary proliferation after OLT was evaluated by CK7 immunostaining (cytokeratin-7 antibody, RCK105, ab9021, Abcam, Cambridge, MA, USA). Previous reports documented CK7 antibodies for immunostaining of the biliary epithelium in the rat [24, 25], but because we found unspecific immunostaining of red blood cells, steatosis, and Mallory bodies, blocking reagents for farnesoid X receptor (XR Factor Blocking Reagent, XRF964 CGH, Biocare Medical, LLC., Concord, CA 94520, USA) and red blood cells (Rodent Block R Blocking Reagent, RBR962 GHL, Biocare Medical, LLC) were used beforehand. To quantify the existence of the peripheral biliary duct, we manually counted CK7-positive peripheral biliary ducts in the field under ×100 magnification with no count of unspecific stains. During this procedure, the biliary duct was confirmed under ×600 magnification, if required.
Statistical Analysis
The results were presented as mean ± standard deviation. The Student t test was used for the comparison of unpaired continuous variables between groups. Survival curves were constructed by the Kaplan–Meier method. Statistical calculations were performed using SPSS Software Version 16.0 (SPSS Inc., Chicago, IL, USA). A p value < .05 was considered statistically significant.
RESULTS
Survival Curves after OLTs with or without HA Reconstruction
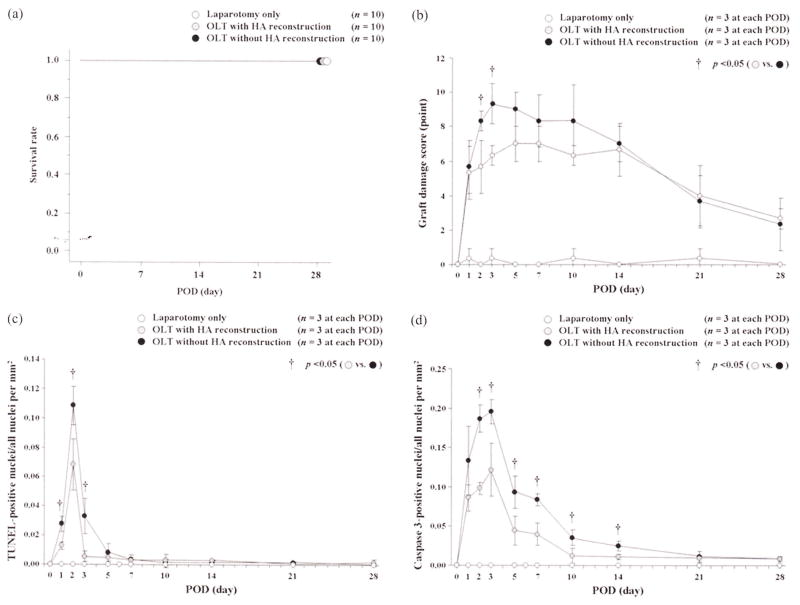
Recipients in all groups survived until POD 28 (Figure 1a). HA reconstruction itself had no significant effect on long-term survival after OLT with a whole-liver graft.
FIGURE 1.
Survival curves and histopathological/immunohistological findings after OLTs with or without HA reconstruction. (a) Survival curves: HA reconstruction itself had no significant effect on long-term survival after OLT with a whole-liver graft. (b) Changes in graft injury score: there were significant differences between OLTs with or without HA reconstruction at PODs 2 and 3. (c) Changes in TUNEL-positive nuclei and all nuclei per mm2: OLT without HA reconstruction showed a high ratio of TUNEL-positive cells compared with OLT with HA reconstruction at PODs 1, 2, and 3. (d) Changes in caspase-3-positive nuclei and all nuclei per mm2: OLT without HA reconstruction showed a high ratio of caspase-3-positive cells compared with OLT with HA reconstruction at PODs 2, 3, 5, 7, 10, and 14.
Biochemical and Coagulation Profiles after OLTs with or without HA Reconstruction
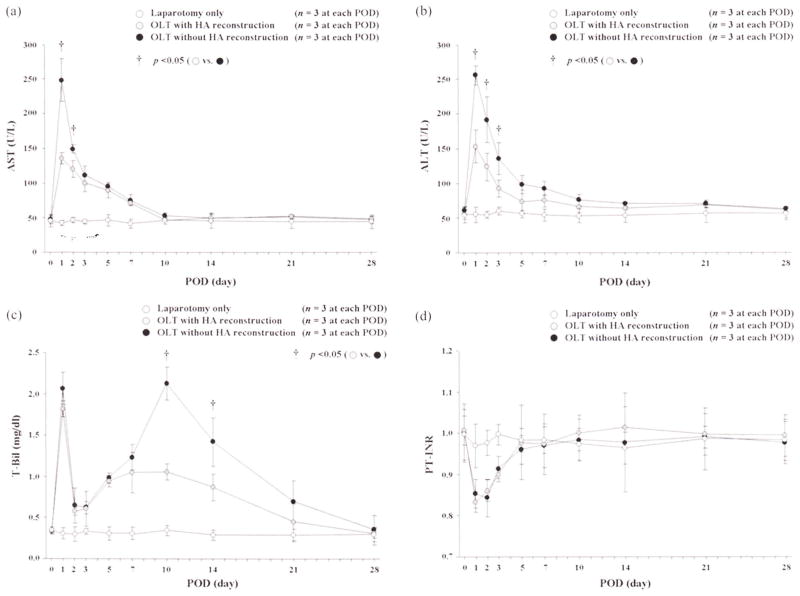
Actual changes in AST, ALT, T-Bil, and PT-INR are shown in Figure 2. There were significant differences in AST levels between OLTs with or without HA reconstruction at PODs 1 (135.2 ± 3.6 vs. 248.7 ± 8.2 U/L, p = .0038) and 2 (119.9 ± 12.0 vs. 148.8 ± 6.5 U/L, p = .0212). There were significant differences in ALTs between the two groups at PODs 1 (153.8 ± 23.6 vs. 256.5 ± 13.7 U/L, p = .0028), 2 (124.4 ± 19.5 vs. 191.9 ± 33.0 U/L, p = .0381), and 3 (93.3 ± 12.3 vs. 135.9 ± 23.0 U/L, p = .0470). T-Bil showed significant differences between the two groups at PODs 10 (1.06 ± 0.10 vs. 2.13 ± 0.20 mg/dL, p = .0011) and 14 (0.87 ± 0.17 vs. 1.42 ± 0.29 mg/dL, p = .0028). There were no significant differences in PT-INR between the two groups at any time point.
FIGURE 2.
Changes in AST, ALT, T-Bil, and PT-INR after OLTs with or without HA reconstruction. For AST, there were significant differences between OLTs with or without HA reconstruction at PODs 1 and 2. ALT showed significant differences between the two OLT groups at PODs 1, 2, and 3. There were significant differences in T-Bil between groups at PODs 10 and 14. There were no differences in PT-INR between the two groups at any time point.
Graft Parenchymal Injury after OLTs with or without HA Reconstruction
In H-E staining, actual changes in graft injury scores are shown in Figure 1b. Important findings, such as neutrophil aggregation, mononuclear cell infiltration, vacuolization, hepatocyte ballooning, and hepatocyte necrosis, were confirmed after OLTs with or without HA reconstruction, and the graft injury score showed significant differences between the two groups at PODs 2 (5.7 ± 1.5 vs. 8.3 ± 0.6 points, p = .0474) and 3 (6.3 ± 0.6 vs. 9.3 ± 1.2 points, p = .0158).
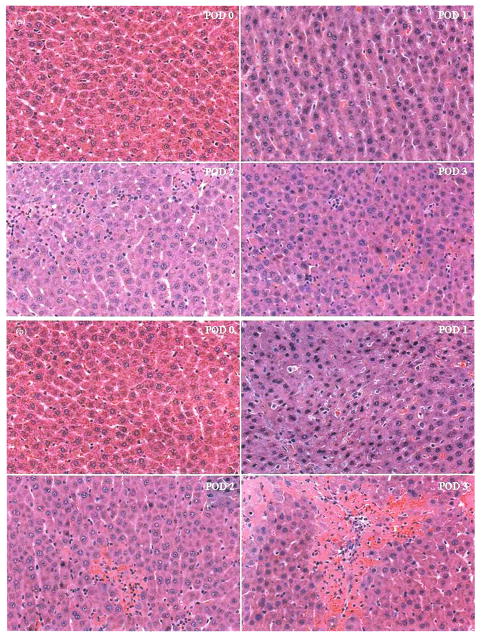
The severe parenchymal damage in OLT without HA reconstruction in the early postoperative period (POD 0–3) compared with OLT with HA reconstruction is shown in Figure 3. Although neutrophil aggregation and mononuclear cell infiltration appeared to be more obvious in OLT with HA reconstruction at POD 2, patchy and/or focal necrosis was clearly more severe in OLT without HA reconstruction at PODs 2 and 3. Histopathologically, OLT without HA reconstruction clearly showed more severe parenchymal damage than OLT with HA reconstruction.
FIGURE 3.
Histopathological findings in the early postoperative period after OLTs with or without HA reconstruction. Actual histopathological findings by H-E staining from POD 0 to POD 3 after OLT with HA reconstruction (a) and OLT without HA reconstruction (b) (×100).
Induction of Apoptosis after OLTs with or without HA Reconstruction
Patchy and/or focal necrosis was confirmed from the early postoperative period by TUNEL and caspase-3 immunostaining at each time point. Actual values in TUNEL immunostaining are shown in Figure 1c. OLT without HA reconstruction showed a high ratio of TUNEL-positive cells compared with OLT with HA reconstruction at PODs 1 (0.013 ± 0.003 vs. 0.028 ± 0.005, p = .0099), 2 (0.068 ± 0.017 vs. 0.108 ± 0.013, p = .0318), and 3 (0.005 ± 0.004 vs. 0.033 ± 0.012, p = .0192).
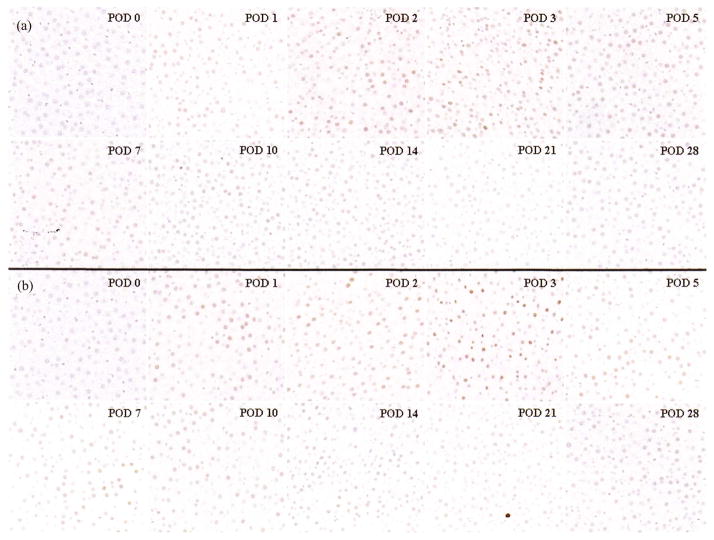
Actual values of caspase-3 immunostaining are shown in Figure 1d. OLT without HA reconstruction showed a high ratio of caspase 3-positive cells compared with OLT with HA reconstruction at PODs 2 (0.098 ± 0.008 vs. 0.186 ± 0.017, p = .0318), 3 (0.121 ± 0.034 vs. 0.195 ± 0.016, p = .0258), 5 (0.044 ± 0.018 vs. 0.093 ± 0.021, p = .0373), 7 (0.040 ± 0.014 vs. 0.084 ± 0.008, p = .0097), 10 (0.012 ± 0.010 vs. 0.035 ± 0.010, p = .0274), and 14 (0.011 ± 0.004 vs. 0.025 ± 0.006, p = .0245). Caspase-3 showed significant differences during the longer term to POD 14 compared with TUNEL. Typical immunohistological findings of caspase-3 in OLTs with or without HA reconstruction at each time point are shown in Figure 4. These findings showed that OLT without HA reconstruction had markedly increased induction of apoptosis compared with OLT with HA reconstruction.
FIGURE 4.
Immunohistological findings for caspase-3 after OLTs with or without HA reconstruction. OLT with HA reconstruction (a) and OLT without HA reconstruction (b) (×200). Caspase-3-positive nuclei are stained brown; normal nuclei are stained in blue.
Biliary Proliferation after OLTs with or without HA reconstruction
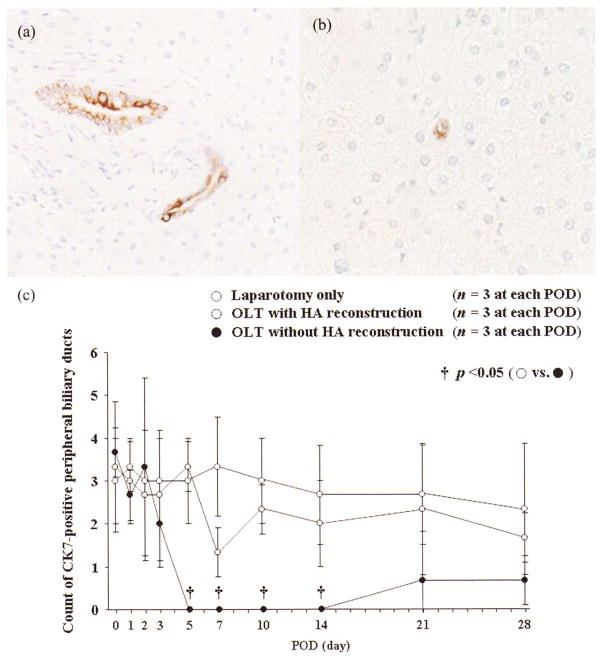
Although major biliary duct proliferation was easily confirmed around the Glisson capsule (Figure 5a), peripheral biliary ducts were also important in biliary proliferation (Figure 5b). The number of CK7-positive peripheral bile ducts was clearly decreased in OLT without HA reconstruction at PODs 5 (3.3 ± 0.6 vs. 0.0 ± 0.0, p = .0006), 7 (1.3 ± 0.6 vs. 0.0 ± 0.0, p = .0161), 10 (2.3 ± 0.6 vs. 0.0 ± 0.0, p = .0022), and 14 (2.0 ± 1.0 vs. 0.0 ± 0.0, p = .0257) (Figure 5c).
FIGURE 5.
Histopathological findings of major and peripheral biliary ducts with CK7-positive biliary epithelium after OLTs with or without HA reconstruction. (a) Typical immunohistological findings of major biliary duct with CK7-positive biliary epithelium (×400). (b) Typical immunohistological findings of the peripheral biliary duct with CK7-positive biliary epithelium (×600). (c)The number of peripheral biliary ducts with CK7-positive biliary epithelium was clearly decreased at PODs 5, 7, 10, and 14 in OLT without HA reconstruction.
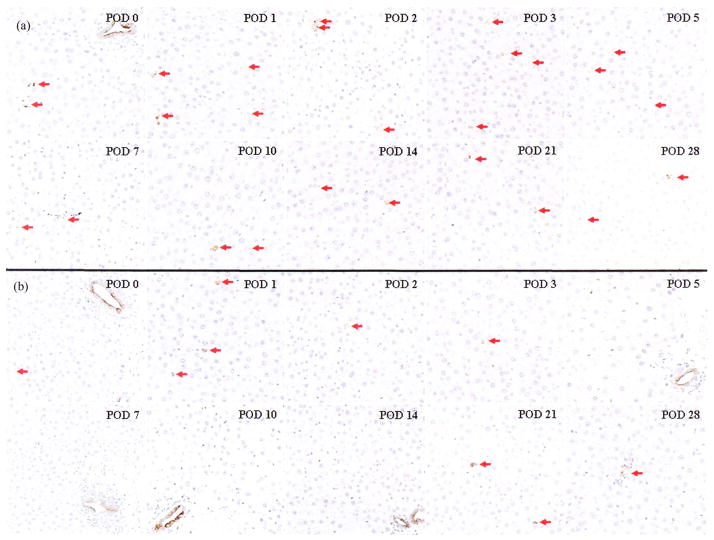
In OLTs with or without HA reconstruction, typical immunohistological findings of CK7 at each time point are shown in Figure 6. Major and peripheral biliary ducts were confirmed at each time point in OLT with HA reconstruction. Note that peripheral biliary ducts were never detected in OLT without HA reconstruction at PODs 5, 7, 10, and 14, although major biliary ducts were confirmed at each time point (Figure 6b).
FIGURE 6.
Immunohistological findings for CK7 after OLTs with or without HA reconstruction. OLT with HA reconstruction (a) and OLT without HA reconstruction (b) (×200). CK7-positive biliary epithelium is stained brown; normal nuclei are stained blue. Peripheral biliary ducts with CK7-positive biliary epithelium are marked by red arrows.
DISCUSSION
Valuable studies for liver transplantation focus into two fields, i.e., liver regeneration including reperfusion injury and transplant immunity including alloantigen-specific tolerance. Rat OLT is a demanding procedure, but has become a popular model to investigate these themes [4, 5]. In this study, from the viewpoint of liver regeneration, we focused on the necessity of HA reconstruction in a whole-liver syngeneic graft because the impact of HA reconstruction on the graft remains controversial [6–10]. Many investigators had different opinions about the necessity of HA reconstruction in long-term survival studies of rat OLT. Although several investigators documented that HA reconstruction was indispensable for a reliable model of long-term survival [4, 8, 26], other investigators suggested that the importance of HA reconstruction for experimental investigations was negligible [27–30]. In our study, HA reconstruction had no effect on survival after OLT with a whole-liver graft. However, significant differences were confirmed in the graft injury score at PODs 2 and 3, in TUNEL expression and ALT level at PODs 1–3, in AST level at PODs 1 and 2, and in caspase-3 expression at PODs 2–14. Our results clearly demonstrated that graft parenchymal damage and induction of apoptosis were more severe in the transplanted grafts that did not have HA reconstruction.
During long-term observation, arterialized grafts may develop better graft function after the early postoperative period. One of the current topics in the liver transplantation field is that an initial migration of inflammatory cells plays an important role for liver regeneration from the early postoperative period after OLT [31–33]. Our histopathological findings at POD 2 revealed differences in the infiltration of inflammatory cells between grafts with or without HA reconstruction. We suggest that HA reconstruction may also be required in research in the transplant immunity field and should be performed in any studies using samples after POD 1.
Some investigators have paid serious attention to HA reconstruction in rat OLT in order to prevent bile duct ischemia [9, 15, 34]. The expression of CK7 was clearly decreased in OLT without HA reconstruction at PODs 5–14 and was unexpectedly observed in the peripheral biliary ducts but not in the major biliary duct. In order to maintain biliary proliferation over the long term after OLT, we suggest that HA reconstruction is important for reliable grafting without injury. OLT with a whole-liver graft showed bimodal peaks of T-Bil levels at PODs 1 and 10. Although the T-Bil level at POD 1 showed no significant difference between OLT with or without HA reconstruction, OLTs without HA reconstruction clearly showed higher T-Bil levels at PODs 10 and 14, and thereafter seemed to recover slowly. These higher levels at PODs 10 and 14 in OLT may reflect the attenuation of biliary proliferation. Previous researchers documented that arterial collateral vessels from adjacent tissues were observed in the nonarterialized grafts at three weeks after OLT [27, 35]. A possible explanation for the slow recovery of biliary proliferation and T-Bil levels after POD 14 is the time taken for establishment of arterial flow from adjacent tissues and/or the graft volume we used. The reconstruction of the HA supply is crucial for rat liver regeneration after small-for-size grafting [36], and we speculate that the worst scenario in the split OLT model without HA reconstruction is prolonged jaundice after POD 10 and subsequent poor survival during the long-term postoperative course. Our results clearly showed that a deficiency in HA flow did not affect long-term survival in OLT with a whole-liver graft. However, we recognize that HA reconstruction is necessary for reliability in some studies, such as a survival study using the split OLT model.
Onset of graft injury related to reperfusion occurs at least several hours after resumption of portal flow [37–39]. Patchy and/or focal necrosis is a key histopathological finding in shear stress and reperfusion injury resulting from reduced liver volume and graft recirculation after cold ischemia, and this finding is also confirmed several hours after surgery [40, 41]. Paradoxically, many researchers actually ignored the effects of HA flow in studies using samples within 24 hr after OLT [42, 43]. OLT with HA reconstruction is more physiological than OLT without HA reconstruction [15, 34]. Because HA reconstruction is indispensable for reliable studies of liver regeneration, HA reconstruction by suturing has already been established, but it does involve technical difficulties [6–9, 11, 12]. Therefore, technical modifications that simplify the process of HA reconstruction have also been documented [4, 6, 14–20, 34]. Although Kashfi et al. [4] considered faster surgical techniques in short-term survival studies and Post et al. [26] documented that HA reconstruction is not necessary for study of leukocyte–endothelial interaction after rat OLT, we suggest that a good physiologic model with a simple but sure technique is currently needed even in short-term studies of reperfusion injury.
The highly accurate animal models in the research field are so important, and the reproducibility depends on the reliability of the surgical techniques [44, 45]. The frequency of surgical issues was reported as approximately 10%–40% in rat OLT models including split OLT [5, 11, 12, 27]. Many researchers recognized the surgical difficulties of this model. We also understood that the hand-suture method under ultra microsurgery for end-to-end anastomosis of the common HAs is always very stressful, even for experienced microsurgeons. However, HA reconstruction is important in the rat OLT model and can provide reliable and relevant data in the liver transplantation field.
Acknowledgments
Financial Support: This work was partially supported by grants to J.H. Nguyen from the Deason Foundation, Sandra and Eugene Davenport, Mayo Clinic CD CRT-II, and from the National Institutes of Health (R01NS051646–01A2); and grants to T. Hori from the Japan Society of the Promotion of Science (No. C20591523) and the Uehara Memorial Foundation (No. 200940051, Tokyo, 171–0033, Japan).
We are very grateful to Dickson W. Dennis, Monica Castanedes-Casey, Virginia R. Phillips, Linda G. Rousseau, and Melissa E. Murray (Department of Neuroscience, Mayo Clinic Florida, Jacksonville, FL, USA) for their diagnostic and technical support in the histopathological and immunohistological assessments; Xiangdong Zhao (Innovation Center for Immunoregulation Technologies and Drugs, Transplant Tolerance Unit, Kyoto University Graduate School of Medicine, Kyoto, Japan) and Naoko Kamo (Dumont-UCLA Transplantation Research Center, Department of Surgery, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA) for their surgical advice; Takayuki Kogure (Division of Gastroenterology, Department of Internal Medicine, Tohoku University Hospital, Sendai, Miyagi 980–8574, Japan) for his help with the statistical analyses; and Masafumi Ueno (Division of Cardiology, Department of Medicine, University of Florida College of Medicine, Jacksonville, FL, USA) for his help in the measurement of PT-INR.
ABBREVIATIONS
- OLT
orthotopic liver transplantation
- HA
hepatic artery
- POD
postoperative day
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- T-Bil
total bilirubin
- PT-INR
international normalized ratio of prothrombin time
- H-E
hematoxylin-eosin
- TUNEL
transferase-mediated deoxyuridine triphosphate nick-end labeling
- caspase
cysteine aspartic acid protease
- CK7
cytokeratin 7
Footnotes
There was no financial conflict of interest.
Declaration of interest: The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Lee S, Charters AC, Chandler JG, et al. A technique for orthotopic liver transplantation in the rat. Transplantation. 1973;16:664–669. doi: 10.1097/00007890-197312000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Lee S, Charters AC, III, Orloff MJ. Simplified technic for orthotopic liver transplantation in the rat. Am J Surg. 1975;130:38–40. doi: 10.1016/0002-9610(75)90453-5. [DOI] [PubMed] [Google Scholar]
- 3.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47–50. [PubMed] [Google Scholar]
- 4.Kashfi A, Mehrabi A, Pahlavan PS, et al. A review of various techniques of orthotopic liver transplantation in the rat. Transplant Proc. 2005;37:185–188. doi: 10.1016/j.transproceed.2004.12.257. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi E, Kamada N, Goto S, et al. Protocol for the technique of orthotopic liver transplantation in the rat. Microsurgery. 1993;14:541–546. doi: 10.1002/micr.1920140812. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann TG, Bunzendahl H, Langrehr JM, et al. Arterial reconstruction in rat liver transplantation—development of a new tubing technique of the common hepatic artery. Transpl Int. 2005;18:56–64. doi: 10.1111/j.1432-2277.2004.00004.x. [DOI] [PubMed] [Google Scholar]
- 7.Gao W, Lemasters JJ, Thurman RG. Development of a new method for hepatic rearterialization in rat orthotopic liver transplantation. Reduction of liver injury and improvement of surgical outcome by arterialization. Transplantation. 1993;56:19–24. doi: 10.1097/00007890-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Imamura H, Rocheleau B, Cote J, et al. Long-term consequence of rat orthotopic liver transplantation with and without hepatic arterial reconstruction: a clinical, pathological, and hemodynamic study. Hepatology. 1997;26:198–205. doi: 10.1002/hep.510260126. [DOI] [PubMed] [Google Scholar]
- 9.Howden B, Jablonski P, Grossman H, et al. The importance of the hepatic artery in rat liver transplantation. Transplantation. 1989;47:428–431. doi: 10.1097/00007890-198903000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Kern H, Bald C, Brill T, et al. The influence of retrograde reperfusion on the ischaemia-/reperfusion injury after liver transplantation in the rat. Int J Exp Pathol. 2008;89:433–437. doi: 10.1111/j.1365-2613.2008.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hori T, Uemoto S, Zhao X, et al. Surgical guide including innovative techniques for orthotopic liver transplantation in the rat: key techniques and pitfalls in whole and split liver grafts. Ann Gastroenterol. 2010;23:270–295. [Google Scholar]
- 12.Hori T, Nguyen JH, Zhao X, et al. Comprehensive and innovative techniques for liver transplantation in rats: a surgical guide. World J Gastroenterol. 2010;16:3120–3132. doi: 10.3748/wjg.v16.i25.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehdorn H, Muller G, editors. Microsurgical exercises (basic techniques, anastomoses, refertilization, transplantation) New York: Thieme Medical Publishers, Inc; 1989. [Google Scholar]
- 14.Inoue S, Tahara K, Shimizu H, et al. Rat liver transplantation for total vascular reconstruction, using a suture method. Microsurgery. 2003;23:470–475. doi: 10.1002/micr.10168. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Dahmen U, Dirsch O, et al. Modified sleeve anastomosis for reconstruction of the hepatic artery in rat liver transplantation. Microsurgery. 2002;22:62–68. doi: 10.1002/micr.21726. [DOI] [PubMed] [Google Scholar]
- 16.Spiegel HU, Palmes D. Surgical techniques of orthotopic rat liver transplantation. J Invest Surg. 1998;11:83–96. doi: 10.3109/08941939809032187. [DOI] [PubMed] [Google Scholar]
- 17.Sato Y, Farges O, Akpinar E, et al. An easy and physiologic arterial reconstruction method (sleeve technique) for orthotopic rat liver transplantation. Transplant Proc. 1996;28:3649–3651. [PubMed] [Google Scholar]
- 18.Dippe BE, Broelsch CE, Krueger SB, et al. An improved model for rat liver transplantation including arterial reconstruction and simplified microvascular suture techniques. J Invest Surg. 1992;5:361–373. doi: 10.3109/08941939209012452. [DOI] [PubMed] [Google Scholar]
- 19.Dippe B, Kreisel D, Petrowsky H, et al. Simplified microvascular suture techniques for rat liver transplantation as a microsurgical model with arterial blood supply. Transpl Int. 1992;5:S357–361. doi: 10.1007/978-3-642-77423-2_108. [DOI] [PubMed] [Google Scholar]
- 20.Hasuike Y, Monden M, Valdivia LA, et al. A simple method for orthotopic liver transplantation with arterial reconstruction in rats. Transplantation. 1988;45:830–832. doi: 10.1097/00007890-198804000-00039. [DOI] [PubMed] [Google Scholar]
- 21.Hori T, Iida T, Yagi S, et al. KICG value, a reliable real-time estimator of graft function, accurately predicts outcomes in adult living-donor liver transplantation. Liver Transpl. 2006;12:605–613. doi: 10.1002/lt.20713. [DOI] [PubMed] [Google Scholar]
- 22.Iida T, Yagi S, Taniguchi K, et al. Significance of CT attenuation value in liver grafts following right lobe living-donor liver transplantation. Am J Transpl. 2005;5:1076–1084. doi: 10.1111/j.1600-6143.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- 23.Chan FK, Zhang Y, Shaffer EA. Bile secretory function of the arterialized versus nonarterialized rat liver allograft. Transplantation. 1996;62:1657–1663. doi: 10.1097/00007890-199612150-00021. [DOI] [PubMed] [Google Scholar]
- 24.Paku S, Dezso K, Kopper L, et al. Immunohistochemical analysis of cytokeratin 7 expression in resting and proliferating biliary structures of rat liver. Hepatology. 2005;42:863–870. doi: 10.1002/hep.20858. [DOI] [PubMed] [Google Scholar]
- 25.Mitaka T, Sato F, Mizuguchi T, et al. Reconstruction of hepatic organoid by rat small hepatocytes and hepatic non-parenchymal cells. Hepatology. 1999;29:111–125. doi: 10.1002/hep.510290103. [DOI] [PubMed] [Google Scholar]
- 26.Post S, Menger MD, Rentsch M, et al. The impact of arterialization on hepatic microcirculation and leukocyte accumulation after liver transplantation in the rat. Transplantation. 1992;54:789–794. doi: 10.1097/00007890-199211000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Wong J, Zhang Y, Lee SS. Hemodynamic characterization of arterialized and nonarterialized liver transplants in the rat. Can J Gastroenterol. 2001;15:435–440. doi: 10.1155/2001/190508. [DOI] [PubMed] [Google Scholar]
- 28.Hossain MA, Hamamoto I, Wakabayashi H, et al. Long-term follow up of heterotopic liver allograft survival with or without hepatic arterial reconstruction. Transplant Proc. 2000;32:2254–2257. doi: 10.1016/s0041-1345(00)01654-7. [DOI] [PubMed] [Google Scholar]
- 29.Zhao D, Zimmermann A, Wheatley AM. Morphometry of the liver after liver transplantation in the rat: significance of an intact arterial supply. Hepatology. 1993;17:310–317. [PubMed] [Google Scholar]
- 30.Kamada N, Sumimoto R, Kaneda K. The value of hepatic artery reconstruction as a technique in rat liver transplantation. Surgery. 1992;111:195–200. [PubMed] [Google Scholar]
- 31.Man K, Lo CM, Xiao JW, et al. The significance of acute phase small-for-size graft injury on tumor growth and invasiveness after liver transplantation. Ann Surg. 2008;247:1049–1057. doi: 10.1097/SLA.0b013e31816ffab6XXX. [DOI] [PubMed] [Google Scholar]
- 32.Sumpter TL, Abe M, Tokita D, et al. Dendritic cells, the liver, and transplantation. Hepatology. 2007;46:2021–2031. doi: 10.1002/hep.21974. [DOI] [PubMed] [Google Scholar]
- 33.Fondevila C, Shen XD, Duarte S, et al. Cytoprotective effects of a cyclic RGD peptide in steatotic liver cold ischemia and reperfusion injury. Am J Transpl. 2009;9:2240–2250. doi: 10.1111/j.1600-6143.2009.02759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Y, Wang GD, Guo ZY, et al. Surgical techniques of arterialized orthotopic liver transplantation in rats. Chin Med J. 2007;120:1914–1917. [PubMed] [Google Scholar]
- 35.Svensson G, Naredi P, Hafstrom L, et al. Quantitative measurements of collateral arterial blood flow in nonarterialized rat liver grafts. Transpl Int. 1994;7:136–139. doi: 10.1007/BF00336476. [DOI] [PubMed] [Google Scholar]
- 36.Meng KW, Lv Y, Yu L, et al. Effects of emodin and double blood supplies on liver regeneration of reduced size graft liver in rat model. World J Gastroenterol. 2005;11:2941–2944. doi: 10.3748/wjg.v11.i19.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumamoto Y, Suematsu M, Shimazu M, et al. Kupffer cell-independent acute hepatocellular oxidative stress and decreased bile formation in post-cold-ischemic rat liver. Hepatology. 1999;30:1454–1463. doi: 10.1002/hep.510300601. [DOI] [PubMed] [Google Scholar]
- 38.Stolz DB, Ross MA, Ikeda A, et al. Sinusoidal endothelial cell repopulation following ischemia/reperfusion injury in rat liver transplantation. Hepatologi. 2007;46:1464–1475. doi: 10.1002/hep.21887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huet PM, Nagaoka MR, Desbiens G, et al. Sinusoidal endothelial cell and hepatocyte death following cold ischemia-warm reperfusion of the rat liver. Hepatology. 2004;39:1110–1119. doi: 10.1002/hep.20157. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3:1167–1170. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- 41.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43:S31–44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 42.Ma ZY, Qian JM, Rui XH, et al. Inhibition of matrix metalloproteinase-9 attenuates acute small-for-size liver graft injury in rats. Am J Transpl. 2010;10:784–795. doi: 10.1111/j.1600-6143.2009.02993.x. [DOI] [PubMed] [Google Scholar]
- 43.Padrissa-Altes S, Zaouali MA, Franco-Gou R, et al. Matrix metalloproteinase 2 in reduced-size liver transplantation: beyond the matrix. Am J Transpl. 2010;10:1167–1177. doi: 10.1111/j.1600-6143.2010.03092.x. [DOI] [PubMed] [Google Scholar]
- 44.Toledo AH, Flikkema R, Toledo-Pereyra LH. Developing the research hypothesis. J Invest Surg. 2011;24:191–194. doi: 10.3109/08941939.2011.609449. [DOI] [PubMed] [Google Scholar]
- 45.Zhang C, Zhang M, Xia L, et al. The benefits of ligating the lobar portal triads before partial hepatectomy in the mouse. J Invest Surg. 2010;23:224–227. doi: 10.3109/08941930903469433. [DOI] [PubMed] [Google Scholar]