Abstract
Accumulated soluble amyloid beta- (Aβ-) induced aberrant neuronal network activity may directly contribute to cognitive deficits, which are the most outstanding characteristics of Alzheimer's disease (AD). The entorhinal cortex (EC) is one of the earliest affected brain regions in AD. Impairments of EC neurons are responsible for the cognitive deficits in AD. However, little effort has been made to investigate the effects of soluble Aβ on the discharge properties of EC neurons in vivo. The present study was designed to examine the effects of soluble Aβ 1−42 on the discharge properties of EC neurons, using in vivo extracellular single unit recordings. The protective effects of gastrodin (GAS) were also investigated against Aβ 1−42-induced alterations in EC neuronal activities. The results showed that the spontaneous discharge of EC neurons was increased by local application of soluble Aβ 1−42 and that GAS can effectively reverse Aβ 1−42-induced facilitation of spontaneous discharge in a concentration-dependent manner. Moreover, whole-cell patch clamp results indicated that the protective function of GAS on abnormal hyperexcitability may be partially mediated by its inhibitory action on Aβ 1−42-elicited inward currents in EC neurons. Our study suggested that GAS may provide neuroprotective effects on Aβ 1−42-induced hyperactivity in EC neurons of rats.
1. Introduction
Alzheimer's disease (AD) is the most common but incurable neurodegenerative disorder and is known to contribute to dementia among the elderly. Amyloid beta (Aβ) peptide, derived from the amyloid precursor protein, is regarded as the pivotal toxicant of AD and causes a variety of neuropathological changes, such as synaptic loss, dysfunction of neuronal transmission, and neuronal death [1]. Recently, increasing evidence has suggested that Aβ-induced perturbation of neuronal network activity may be a major and early contributor to AD pathogenesis. Aberrant neuronal network activity is observed in AD patients and transgenic mouse models of AD, which is thought to induce dysfunction and memory deficits [2, 3].
The entorhinal-hippocampal network is a vital circuit in memory consolidation and recall [4], which is seriously affected during the progression of AD [3]. The entorhinal cortex (EC) has been proven to be one of the earliest brain regions impaired in AD [5]. Radiological studies have also provided evidence that early-stage AD patients exhibit structural and functional changes of the EC [6, 7]. Previous studies suggested that elevated levels of Aβ, especially Aβ 1–42, which has been described as the most toxic variant of Aβ, altered the excitability of neurons in the rat hippocampus [8, 9]. High levels of Aβ 1–42 promoted suprasynchronization between individual rat prefrontal cortical neurons by increasing their excitability [10]. In addition, it is reported that Aβ 1–42 could disturb the patterns of spontaneous discharge in the hippocampal CA1 region of rats [11]. However, there is little related electrophysiological data concerning EC neurons in the context of high level of Aβ 1–42.
Gastrodin (GAS) is a traditional Chinese medicine isolated from Gastrodia elata (Figure 1) and is regarded as one of the most important traditional medicines in Oriental countries. It is officially listed in the Chinese Pharmacopoeia and is used as an anticonvulsant, analgesic, and sedative against vertigo, general paralysis, epilepsy, and tetanus [12]. Modern clinical studies have demonstrated its efficiency as an antiepileptic drug [13] and its protective effects against cognitive decline in patients after cardiac surgery with cardiopulmonary bypass [14]. Furthermore, recent in vitro studies found that GAS had neuroprotective effects on the cellular model of AD induced by Aβ 25–35 [15] and could facilitate learning and memory [16]. A recent study also demonstrated that Aβ 1–42-related oxidative damage was decreased by GAS in primary cultured rat hippocampal neurons [17]. Despite these observations, whether GAS has neuroprotective effects against Aβ 1–42-induced perturbation of neuronal network activity in vivo is unknown. Therefore, whether Aβ 1–42 could disturb the spike discharge of EC neurons in vivo and whether the Aβ 1–42-disturbed spike discharge could be remolded by GAS are significant questions. In the present study, we used extracellular recording and whole-cell patch clamp recording to investigate the effects of GAS on EC aberrant firing patterns induced by the local application of soluble Aβ 1–42.
Figure 1.
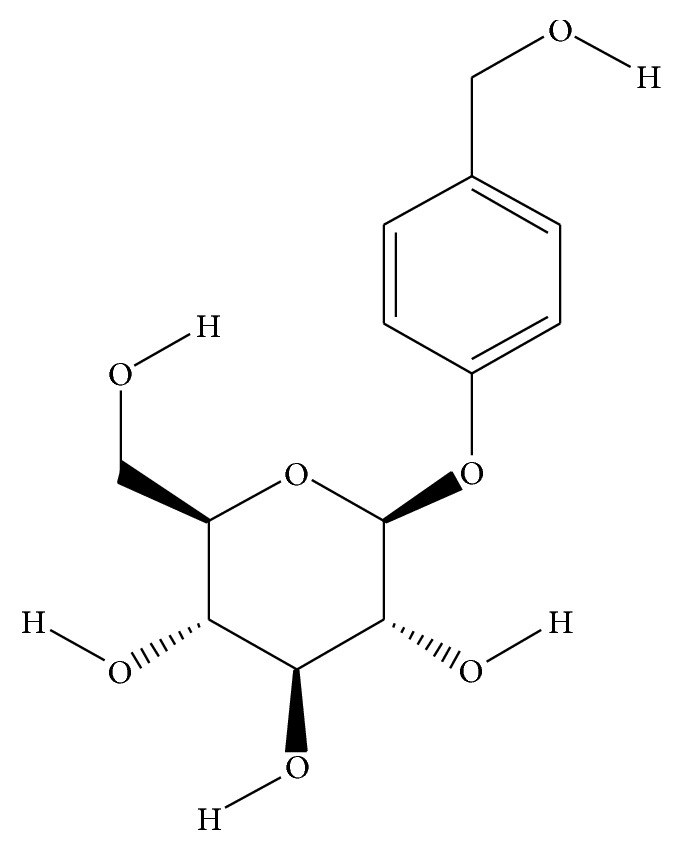
Structures of 4-hydroxybenzyl alcohol 4-O-beta-D-glucopyranoside (GAS).
2. Materials and Methods
2.1. Animals and Surgery
Male Sprague-Dawley rats (200–250 g) were obtained from the Laboratory Animal Center at the Third Military Medical University in China. All protocols and procedures were approved by the University Animal Care and Use Committee. Animals were deeply anesthetized using urethane (1.5 g/kg) before being placed in a stereotaxic apparatus. The skull was exposed, and a small hole was drilled to expose EC region (from bregma: AP −6.6 mm and ML 4.7 mm; depth from skull surface: 6.8 mm) [18].
2.2. Drugs
All reagents were obtained from (Sigma-Aldrich, USA) with the exception of scrambled Aβ 1–42 peptide (Anaspec, Fremont, CA, USA). Soluble Aβ 1–42 was prepared as described previously [19]. In brief, Aβ 1–42 was first dissolved in hexafluoro-2-propanol (HFIP) and aliquoted. HFIP was then removed by evaporation under vacuum, and the resulting clear peptide films were stored at −20°C. Prior to use, an aliquot of Aβ 1–42 peptide film was dissolved in anhydrous dimethyl sulfoxide (DMSO) and added to ice-cold artificial cerebral spinal fluid (ACSF) to obtain a working concentration of 200 μM. This solution was then incubated at 4°C for 24 h and centrifuged. The supernatant included the soluble Aβ 1–42 preparation; the major species was Aβ 1–42 monomer and also included the trimer, tetramer, and, to a lesser extent, the dimer [19]. Scrambled Aβ 1–42 peptide was prepared in the same manner as Aβ 1–42. GAS was dissolved in 0.9% sterile saline to deserved concentrations.
2.3. Single Unit Recordings
A five-barrel glass microelectrode (total tip diameter 3–10 μm, resistance 5–20 MΩ) was used for electrophysiological recording and micropressure injection. The recording glass microelectrode was filled with a 0.9% sodium chloride solution. The other four barrels connected with a 4-channel pressure injector (PM2000B, Micro Data Instrument, Inc., USA) and were filled with different drugs as needed. The drugs were ejected on the surface of the firing cells with gas pressure [20]. The intrabarrel drugs' concentrations were chosen based on previous established works [8, 21, 22] and their efficacy to reliably alter neuronal firing. 0.5 μL of drug was applied to the firing neurons during injection. The signals from the recording electrode were fed to an AM system amplifier (Carlsborg, WA, USA) and filtered with a band-pass of 0.3–10 kHz. A single unit was isolated and analyzed using Spike 2 (Cambridge Electronic Design Limited, London, UK). The signal was stored in a computer equipped with MATLAB analysis system for further offline analysis. The unit activities were subsequently analyzed per 500 s epoch for averaging discharge rates (bin width, 10 s), which were normalized relative to 500 s baseline values. To avoid the transient effects of the injection, windows of analysis were started 500 s after the injection of the drugs of interest.
2.4. Whole-Cell Patch Clamp Recordings
Sprague-Dawley rats (P12-21) were used in this study. After halothane anesthesia, each animal was decapitated and the brain was removed quickly. The brain was subsequently submerged in cold ACSF containing (in mM) 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 KH2PO4, 1.2 MgSO4, 2 CaCl2, and 10 dextrose, bubbled with 95% O2-5% CO2, with a pH of 7.4. The brain was blocked, and an oscillating tissue slicer (Leica, VT1000, Wetzlar, Germany) was used to cut 400 μm thick horizontal sections with a slicing angle of 20°. Slices were initially incubated for a minimum of 90 min at room temperature (22–24°C) in ACSF and were then transferred to and submerged in a recording chamber where they were perfused continuously with carbogen buffered ACSF at room temperature. Whole-cell patch clamp recordings were obtained from cell bodies of EC stellate neurons. Data acquisition was conducted with EPC10 amplifiers (HEKA Elektronik, Lambrecht/Pfalz, Germany). The signal was stored for offline analysis with Pulse/Pulse fit v.8.74 (HEKA Elektronik) and Igor Pro v.4.03 (WaveMatrics). Pipettes (4–8 MΩ) for whole-cell recordings were pulled on a horizontal micropipette puller (P-97, Sutter Instrument) from filamented capillary glass and were filled with a pipette solution containing (in mM) 145 K-gluconate, 0.5 EGTA, 2 MgCl2, 5 HEPES, 5 K-ATP, 0.4 Na-GTP, pH 7.4, and 290–295 mOsm. Liquid junction potential was calculated to be approximately −10 mV for the internal solutions, and membrane voltages were corrected offline. Series resistance was compensated by 80% and was continually monitored throughout the experiment. Neurons were discarded if the series resistance changed by more than 15%.
2.5. Data Analysis
Statistical analysis was made using statistical analysis software Origin 8.0 (Microcal, Inc, Northampton, MA, USA) and SPSS 18.0 (IBM, New York, NY). The values were presented as the mean ± S.E.M. Differences in the mean values among groups were analyzed using Student's t-test and one-way ANOVA. Values of P < 0.05 were considered significant.
3. Results
3.1. Soluble Aβ 1–42 Increased the Spontaneous Firing Activities of EC Neurons
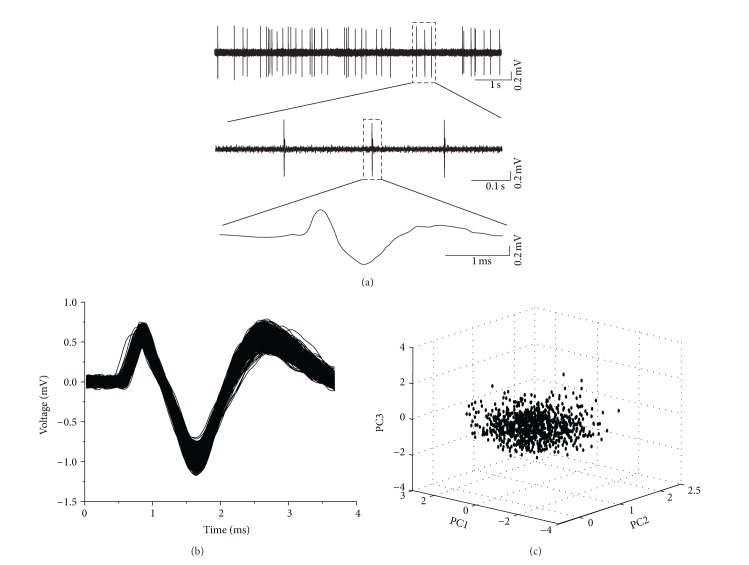
The changes in activity of EC neurons were determined by the spontaneous discharge before and after the application of soluble Aβ 1–42. Figure 2(a) shows a typical recording of unit activity from a single neuron. All of the spikes recorded in the present study showed a biphasic positive/negative waveform. A spike-sorting technique was used to separate single neuronal activity. Based on the different amplitudes and waveforms of spikes, recorded activities were sorted by principal components analysis (PCA). Figure 2(b) shows representative sampling of waveforms from one EC neuron over 1800 s. Under basal conditions, the waveforms were stable, indicating that all of the recorded spikes were indeed from a single neuron. Figure 2(c) presents offline classification of spikes with PCA. It is obvious that PCA analysis revealed one data cluster in the PCA-feature space, further indicating that all of the spikes recorded were from one neuron.
Figure 2.
Recording and sorting of spontaneous discharge of EC neurons. (a) Single unit spontaneous discharge recorded in EC neurons (upper). The lower two panels are the enlargements of the indicated regions in the upper panel. (b) The overdrawn waveforms of firing events during a 30 min period form one cluster, indicating that all of the spikes were from one neuron. (c) Offline classification of spikes with principle component analysis (PCA). Every spike shown in (b) is projected onto the PCA-feature space shown in (c), further indicating that all of the spikes were from one neuron.
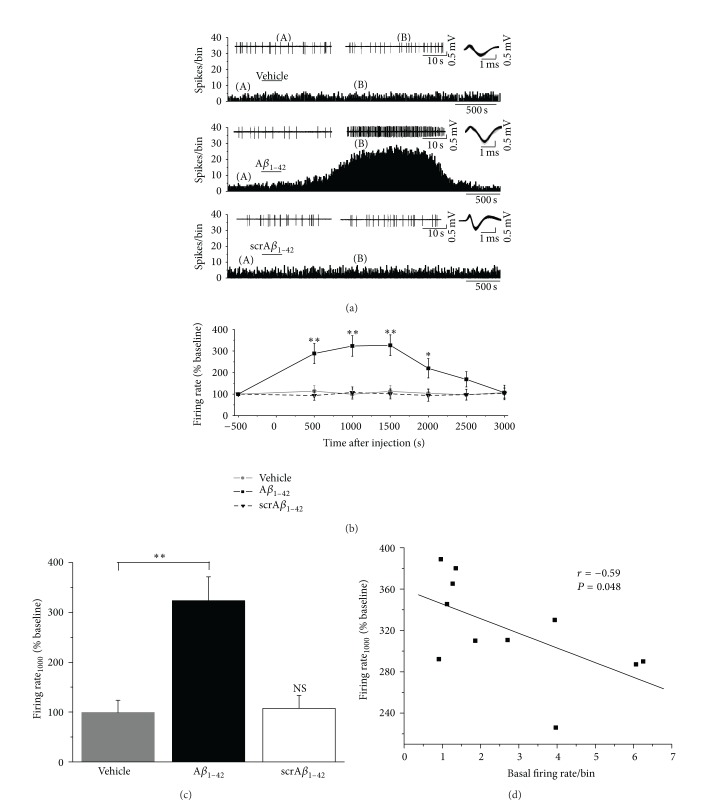
Previous electrophysiological results elucidated that Aβ 1–42 could excite hippocampal neurons in vitro [9]. In the following experiment, we further investigated the effects of soluble Aβ 1–42 on the activities of EC neurons in anesthetized rats in vivo. Representative illustrations of firing changes in EC neurons after the application of vehicle or soluble Aβ 1–42 are shown in Figure 3(a). The administration of 200 μM Aβ 1–42 increased the firing rates of EC neurons with an onset time about 300–900 s. As is shown in Figure 3(b), potentiation of the mean firing rate built up over the first 1000 s following the injection of Aβ 1–42, stable for 500 s. Then, the mean firing rate gradually returned to baseline values, indicating that the effects of soluble Aβ 1–42 on the discharge of EC neurons were reversible. At 1000 s after injection of Aβ 1–42, the mean firing rate was 323.4 ± 47.9% of the baseline values (Figure 3(b), n = 11, P < 0.01 versus baseline (500 s before injection)). In contrast, the vehicle group, as a control, had no obvious effect on the firing activities of EC neurons (Figure 3(b), n = 11, P > 0.05 versus baseline). The mean firing rate of vehicle group at 1000 s after injection was 99.0 ± 24.5% of baseline, which was significantly different from that of the Aβ 1–42 group (Figure 3(c), n = 11 for each group, P < 0.01). To ensure that the increase in discharge induced by Aβ 1–42 was not due to nonspecific peptide effects, we applied a scrambled Aβ 1–42 peptide to EC neurons. As shown in Figures 3(a)–3(c), the scrambled Aβ 1–42 peptide did not have significant effect on the mean firing rate of EC neurons. The average firing rate of scrambled Aβ 1–42 group at 1000 s after injection was 107.3 ± 26.0%, which was not significantly different from that of the vehicle group (Figure 3(c), n = 6 for scrambled Aβ 1–42 group, P > 0.05). Altogether, these findings suggest that soluble Aβ 1–42, but not its scrambled form, can significantly increase the spontaneous discharge of EC neurons.
Figure 3.
Effects of Aβ 1–42 on the spontaneous firing of EC neurons. (a) Representative traces show the changes of firing activity of EC neurons after application of vehicle (upper), Aβ 1–42 (middle), and scrambled Aβ 1–42 (lower). Drugs were applied during the bar. The two traces in upper panel show firing patterns before (A) and after (B) the application of drugs. The upper right panel of each group displays the waveform of the recorded neuron across each protocol. No obvious change in the spike shape parameters was observed. (b) Time-response curves of the firing rate changes in EC neurons after administration of vehicle, Aβ 1–42, and scrambled Aβ 1–42 (* P < 0.05, ** P < 0.01 versus baseline values (500 s before injection)). (c) Bar graph summarizing effects of vehicle, Aβ 1–42, and scrambled Aβ 1–42 on the firing rate of EC neurons at 1000 s after local injection (** P < 0.01, NS, no significant difference versus vehicle group). (d) Correlation between the firing rate at 1000 s after injection and the basal firing values in EC neurons. Aβ 1–42 tended to enhance the low-rate spontaneous discharge more strongly.
As shown in Figure 3(d), we further analyzed the correlation between Aβ 1–42-induced excitation at 1000 s after injection and the basal firing rate in the 11 neurons. Although modest, there was a negative correlation (r = −0.59, P < 0.05) between these two parameters, suggesting that the neurons with slower basal firing rate are more affected by Aβ 1–42 in the EC.
3.2. GAS Prevented Soluble Aβ 1–42-Induced Increase in Firing Activity
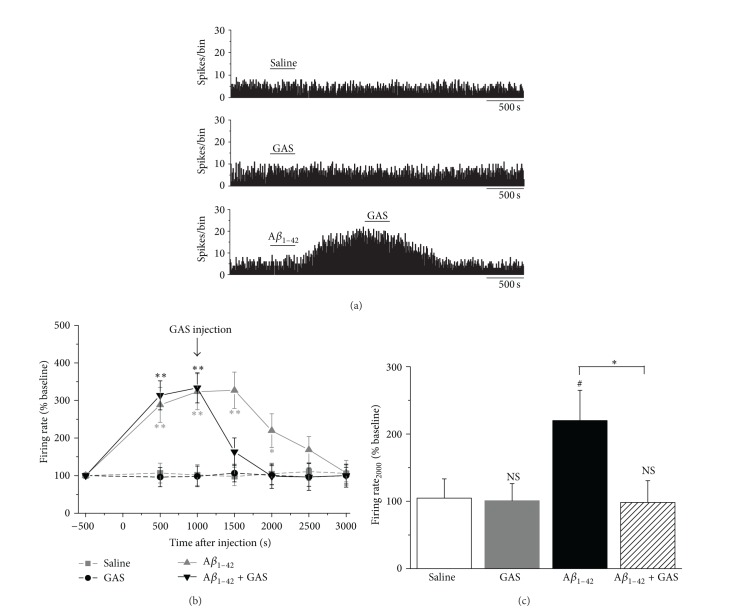
We then tested whether GAS can inhibit the soluble Aβ 1–42-induced perturbation of spontaneous discharge. Both saline (used as a control) and 10 mM GAS alone had no obvious effect on the firing activities of EC neurons during the experiment (Figure 4(a)). The mean firing rates at 1000 s after application of the saline or 10 mM GAS were 101.7 ± 27.6% (n = 6, P > 0.05 versus baseline) and 98.1 ± 27.4% (n = 6, P > 0.05 versus baseline), respectively (Figures 4(b) and 4(c)). The administration of 200 μM Aβ 1–42 increased the spontaneous discharge of EC neurons (Figure 4(a)). At 1000 s after injection of Aβ 1–42, the mean firing rate was 333.6 ± 39.9% of the baseline values (Figures 4(b) and 4(c), n = 10, P < 0.01 versus baseline). Applying 10 mM GAS to EC neurons significantly suppressed the Aβ 1–42-induced increase in firing activity (Figure 4(a)). The firing rate at 2000 s after Aβ 1–42 application (equal to that at 1000 s after GAS application) was 98.3 ± 32.3% of the baseline values, which was significantly less than that of Aβ 1–42 alone group at the time point (Figures 4(b) and 4(c), 219.9 ± 44.9%, n = 11 for Aβ 1–42 alone group, P < 0.05).
Figure 4.
Inhibitory effects of GAS on Aβ 1–42-induced increase in spontaneous discharge in EC region. (a) Representative traces of firing rate of EC neurons after the application of saline (upper), GAS alone (middle), or Aβ 1–42 plus GAS (lower). Saline or GAS alone did not exert any obvious effect on the firing activities. Application of the GAS decreased the spontaneous discharge of Aβ 1–42-induced hyperexcitation. (b) Time-response curves for saline, GAS alone, Aβ 1–42 alone, and Aβ 1–42 plus GAS groups on the firing rates of EC neurons (* P < 0.05, ** P < 0.01 versus baseline values). (c) Histograms showing the mean discharge rate of EC neurons in different groups at 2000 s after injection (NS, no significant difference versus vehicle group, # P < 0.05 versus saline group, * P < 0.05).
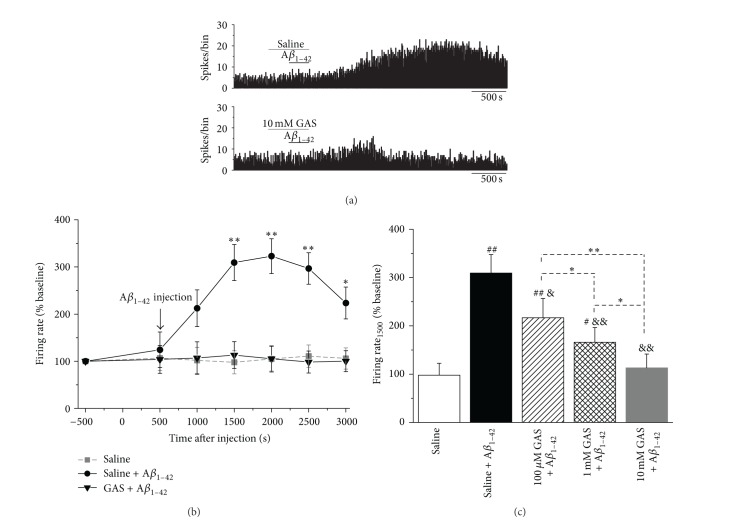
To further investigate the relationship between concentration and inhibitory effects of GAS, we pretreated the recorded EC neurons with different concentrations of GAS before Aβ 1–42 injection. A representative illustration of the firing changes in EC neurons pretreated with GAS alone is shown in Figure 5(a). Saline or 10 mM GAS was applied when the spontaneous discharge was stable for 500 s. As shown in Figures 5(a) and 5(b), the administration of 200 μM Aβ 1–42 increased the firing rate of EC neurons pretreated with saline. At 1000 s after injection of Aβ 1–42 (equal to that at 1500 s after saline application), the mean firing rate was 309.4 ± 38.3% of the baseline values (Figure 5(b), n = 6, P < 0.01 versus baseline). In contrast, following pretreatment with 10 mM GAS, no obvious increase in the firing activities in EC neurons was detected after the application of 200 μM Aβ 1–42 (Figures 5(a) and 5(b), 113.1 ± 28.4%, n = 6, P > 0.05 versus baseline). Analysis of the firing rates at 1000 s after application of Aβ 1–42 (equal to that at 1500 s after pretreatment with different concentrations of GAS) revealed that the inhibitory effects of GAS against Aβ 1–42 were concentration-dependent (Figure 5(c), saline alone group: 97.8 ± 24.5%, saline plus Aβ 1–42 group: 309.4 ± 38.3%, 100 μM GAS plus Aβ 1–42 group: 216.9 ± 36.3%, 1 mM GAS plus Aβ 1–42 group: 166.0 ± 30.5%, and 10 mM GAS plus Aβ 1–42 group: 113.1 ± 28.4%, n = 6 for each group, # P < 0.05, ## P < 0.01 versus saline alone group, & P < 0.05, && P < 0.01 versus saline plus Aβ 1–42 group, * P < 0.05, ** P < 0.01). These results indicated that GAS had protective effects against the abnormal, Aβ 1–42-induced activities of EC neurons.
Figure 5.
GAS pretreatment inhibited Aβ 1–42-induced increase in firing rate of EC neurons in a concentration-dependent manner. (a) Representative traces show that pretreatment with 10 mM GAS (lower) prevented the Aβ 1–42-induced increase in firing rate of EC neurons, whereas saline (upper) had no obvious effect. (b) Time-response curves of Aβ 1–42-induced changes of firing rate in EC neurons, pretreated with saline or 10 mM GAS (* P < 0.05, ** P < 0.01 versus baseline values (500 s before saline or GAS injection)). (c) Concentration-dependent inhibitory effects of GAS on Aβ 1–42-induced firing rate changes (# P < 0.05, ## P < 0.01 versus saline alone group, & P < 0.05, && P < 0.01 versus saline plus Aβ 1–42 group, * P < 0.05, ** P < 0.01).
3.3. GAS Blocked Aβ 1–42-Elicited Inward Currents in EC Neurons
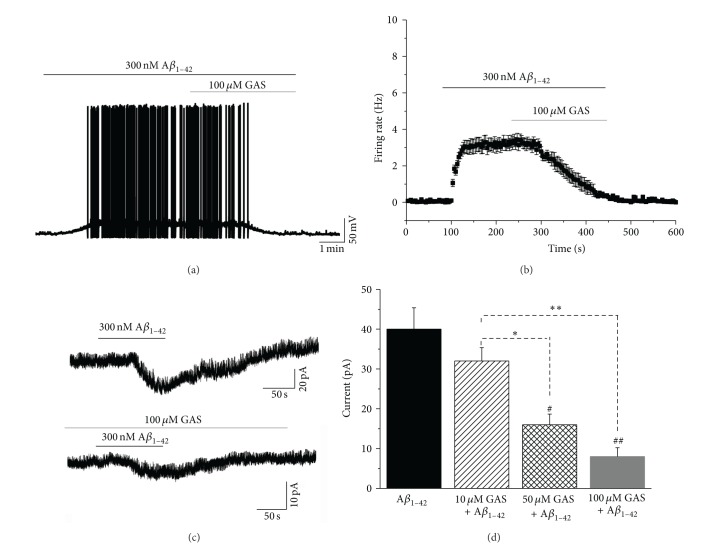
To explore the cellular mechanisms underlying the protective effects of GAS on Aβ 1–42-induced perturbation of neuronal activity, we analyzed the effects of GAS on the excitability of soluble Aβ 1–42-treated EC neurons in an in vitro slice preparation. As stellate neurons are the most abundant EC neurons [23], we took recordings from 8 randomly selected EC stellate neurons in current-clamp mode. Consistent with the in vivo results (Figure 3), bath application of 300 nM Aβ 1–42 significantly increased the excitability of all recorded EC neurons (Figure 6(a)). Meanwhile, the robust Aβ 1–42-induced firing of EC neurons was gradually diminished and completely abolished by the coapplication of 100 μM GAS (Figures 6(a) and 6(b)). We next examined the effects of Aβ 1–42 and GAS on whole-cell currents of EC neurons. In voltage-clamp mode, the recorded EC neurons were held at −70 mV. A brief (100 s) application of 300 nM Aβ 1–42 elicited stable inward currents in the majority of the neurons (6/8). Intriguingly, administration of GAS significantly blocked the Aβ 1–42-elicited inward currents in a dose-dependent manner (Figures 6(c) and 6(d)). The amplitude of Aβ 1–42-elicited inward currents gradually decreased as the dose of GAS increased from 10 μM to 100 μM (Figure 6(d), Aβ 1–42 alone group: 40.1 ± 5.4 pA, 10 μM GAS plus Aβ 1–42 group: 32.3 ± 3.4 pA, 50 μM GAS plus Aβ 1–42 group: 16.2 ± 2.6 pA, and 100 μM GAS plus Aβ 1–42 group: 8.0 ± 2.3 pA, n = 6 for each group, # P < 0.05, ## P < 0.01 versus Aβ 1–42 alone group, * P < 0.05, ** P < 0.01). Altogether, these results suggest that GAS inhibits Aβ 1–42-induced hyperactivity by blocking Aβ 1–42-elicited inward currents in EC neurons.
Figure 6.
Inhibitory effects of GAS on Aβ 1–42-elicited inward currents in EC neurons. (a) Aβ 1–42-induced neuronal hyperexcitation was significantly inhibited in the presence of GAS. (b) Group data of 8 tested EC neurons showing the Aβ 1–42-induced changes in the firing rate calculated at 5 s intervals. (c) 300 nM Aβ 1–42 elicits inward currents in an EC neuron. GAS blocked the Aβ 1–42-induced inward currents. (d) Group data of 6 tested EC neurons. The pretreatment of GAS decreased Aβ 1–42-elicited inward currents in a dose-dependent manner (# P < 0.05, ## P < 0.01 versus Aβ 1–42 alone group, * P < 0.05, ** P < 0.01).
4. Discussion
In the present study, we used extracellular single unit recording techniques to investigate the action of soluble Aβ 1–42 on EC neuronal discharge in vivo. Our results demonstrated that local application of soluble Aβ 1–42 significantly increased neuronal spontaneous discharge in the EC region (Figure 3). GAS prevented the alteration of the spontaneous discharge of EC neurons induced by Aβ 1–42 (Figures 4 and 5). Whole-cell patch clamp results showed GAS blocked Aβ 1–42-elicited inward currents of EC neurons (Figure 6).
4.1. GAS May Have Potential Therapeutic Value for Aβ-Induced Aberrant Activity
The EC occupies a central position in the limbic forebrain by providing bidirectional interconnections between the hippocampal formation and the rest of the cerebral cortex [4]. Animal experimentations and clinical observations in humans have demonstrated that the EC-hippocampal-neocortical circuit is fundamental in some forms of memory [4]. Some aspects of memory impairments in AD patients have been attributed to the damage of EC [24], which is one of the earliest and most severely damaged brain areas in this disease [5]. In the present in vivo study, we provided electrophysiological evidence that direct application of soluble Aβ 1–42 could increase the spontaneous discharge of EC neurons (Figure 3). The production of Aβ and its secretion into the extracellular space are tightly regulated by neuronal activity. Increased neuronal activity enhances Aβ production, whereas blocking neuronal activity has the opposite effect [25]. We suspect that Aβ-induced hyperactivity of EC neurons can increase the production of Aβ, raising the possibility of a vicious cycle in which Aβ promotes its own production through alterations of EC neuronal activity. This vicious cycle may further impair the integrity and functions of neurons in the EC-hippocampal circuit, as Aβ synthesized by EC neurons can be transported via the perforant pathway to the hippocampus [3]. Thus, early interference in the EC to break this vicious cycle might be of therapeutic benefits, perhaps halting disease progression.
The ancient Chinese herb Gastrodia elata is considered to have several beneficial effects in treating headaches, dizziness, tetanus, and epilepsy [12, 13, 26, 27]. Importantly, GAS, the main active component of Gastrodia elata, can penetrate through the blood-brain barrier into the brain [13]. A recent study suggests that GAS protects primary cultured rat hippocampal neurons against Aβ-induced neurotoxicity, alleviating memory deficits and reducing neuropathology in a mouse model of AD [17]. These findings suggested that GAS might be effective in AD. The present study showed that administration of GAS reversed the Aβ 1–42-induced spontaneous discharge alteration of EC neurons (Figure 4) and that this inhibitory effect of GAS was dose-dependent (Figure 5). In contrast, the same dose of GAS alone did not affect the firing activity of EC neurons (Figure 4). These results indicate that GAS may selectively act on spontaneous activity in abnormal Aβ states. We infer from the above results that GAS may be a beneficial agent in relieving the aberrant EC-hippocampal network activity in AD progression.
4.2. Potential Ionic Mechanisms Underlying the Protective Effects of GAS against Aβ-Induced Aberrant Activity
To explore the cellular mechanisms underlying the protective effects of GAS against Aβ 1–42-induced aberrant activity, we used an in vitro slice preparation to perform whole-cell patch clamp recordings in EC neurons. Soluble Aβ 1–42 caused a membrane depolarization and discharge in rat EC neurons (Figure 6(a)). These findings are consistent with the observation that direct application of soluble Aβ 1–42 can induce EC neuronal hyperactivity in vivo (Figure 3). Furthermore, we found that Aβ 1–42 elicited inward currents in EC neurons (Figure 6(b)). It has been reported that intracellularly or extracellularly applied Aβ or its fragments can modulate the function of ion channels, including potassium (K+), calcium (Ca2+), and sodium (Na+) channels [28]. In wild-type mice, Aβ 1–42 decreases a suite of K+ conductance, including the delayed rectifier, the transient A-type, and the Ca2+-activated K+ currents [28].
In the present study, we found that the effects of Aβ 1–42 were almost completely prevented by treatment with GAS (Figures 6(c) and 6(d)). A possible interpretation of the present findings is that GAS may exert its beneficial effects partially through the regulation of Na+ or K+ currents. Previous study has shown that GAS can dose-dependently reverse the pathologically altered Na+ and K+ currents in small dorsal root ganglion neurons in a model of diabetes [29]. In addition, GAS may also exert its beneficial effects through modulating Ca2+ currents. It has been shown that GAS can prevent glutamate-induced Ca2+ influx [30]. In contrast, Aβ enhances excitatory activity in glutamatergic synaptic networks and causes Ca2+ influx [31]. We infer that this opposing regulation of Ca2+ influx may contribute to the beneficial effects of GAS on the Aβ 1–42-evoked abnormal excitability of EC neurons. It should be noted that ionic currents may not be the only target for GAS, and the effects of GAS on other cellular targets and signaling pathways cannot be excluded.
In summary, the current study showed that GAS treatment ameliorated Aβ 1–42-induced perturbation of EC neuronal activity. These results suggested that GAS may be a potential candidate for AD therapy.
Acknowledgments
This work was supported by Grants from the National Natural Foundation of China (31100759), the Scientific Foundation of Chongqing (CSTC2011BB5039), and the High Education Teaching Reform Project of Chongqing (no. 132080) to Dr. Zhi-ru Zhu. The authors also acknowledge the support by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC discovery grant) to Dr. Fenglian Xu.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Lee C., Park G. H., Lee S.-R., Jang J.-H. Attenuation of β-amyloid-induced oxidative cell death by sulforaphane via activation of NF-E2-related factor 2. Oxidative Medicine and Cellular Longevity. 2013;2013 doi: 10.1155/2013/313510.313510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palop J. J., Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Archives of Neurology. 2009;66(4):435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris J. A., Devidze N., Verret L., Ho K., Halabisky B., Thwin M. T., Kim D., Hamto P., Lo I., Yu G. Q., Palop J. J., Masliah E., Mucke L. Transsynaptic progression of amyloid-β-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron. 2010;68(3):428–441. doi: 10.1016/j.neuron.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichenbaum H., Lipton P. A. Towards a functional organization of the medial temporal lobe memory system: role of the parahippocampal and medial entorhinal cortical areas. Hippocampus. 2008;18(12):1314–1324. doi: 10.1002/hipo.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gómez-Isla T., Price J. L., McKeel D. W., Jr., Morris J. C., Growdon J. H., Hyman B. T. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. Journal of Neuroscience. 1996;16(14):4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du A. T., Schuff N., Amend D., Laakso M. P., Hsu Y. Y., Jagust W. J., Yaffe K., Kramer J. H., Reed B., Norman D., Chui H. C., Weiner M. W. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. Journal of Neurology Neurosurgery and Psychiatry. 2001;71(4):441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan U. A., Liu L., Provenzano F. A., Berman D. E., Profaci C. P., Sloan R., Mayeux R., Duff K. E., Small S. A. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer's disease. Nature Neuroscience. 2014;17(2):304–311. doi: 10.1038/nn.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yun S. H., Gamkrelidze G., Stine W. B., Sullivan P. M., Pasternak J. F., LaDu M. J., Trommer B. L. Amyloid-beta1-42 reduces neuronal excitability in mouse dentate gyrus. Neuroscience Letters. 2006;403(1-2):162–165. doi: 10.1016/j.neulet.2006.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren S. C., Chen P. Z., Jiang H. H., et al. Persistent sodium currents contribute to Abeta-induced hyperexcitation of hippocampal CA1 pyramidal neurons. Neuroscience Letters. 2014;580C:62–67. doi: 10.1016/j.neulet.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Zhang G., Zhou H., Barakat A., Querfurth H. Opposite effects of low and high doses of Aβ42 on electrical network and neuronal excitability in the rat prefrontal cortex. PLoS ONE. 2009;4(12) doi: 10.1371/journal.pone.0008366.e8366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan Y.-F., Jia X.-T., Wang X.-H., Chen X.-R., Li Q.-S., Gao X.-P., Qi J.-S. Arginine vasopressin remolds the spontaneous discharges disturbed by amyloid β protein in hippocampal CA1 region of rats. Regulatory Peptides. 2013;183(1):7–12. doi: 10.1016/j.regpep.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Tang W., Eisenbrand G. Chinese Drugs of Plant Origin. Berlin, Germany: Springer; 1992. Gastrodia elata Bl; pp. 545–548. [DOI] [Google Scholar]
- 13.Ojemann L. M., Nelson W. L., Shin D. S., Rowe A. O., Buchanan R. A. Tian ma, an ancient Chinese herb, offers new options for the treatment of epilepsy and other conditions. Epilepsy and Behavior. 2006;8(2):376–383. doi: 10.1016/j.yebeh.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z., Ma P., Xu Y., Zhan M., Zhang Y., Yao S., Zhang S. Preventive effect of gastrodin on cognitive decline after cardiac surgery with cardiopulmonary bypass: a double-blind, randomized controlled study. Journal of Huazhong University of Science and Technology (Medical Science) 2011;31(1):120–127. doi: 10.1007/s11596-011-0162-4. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z.-H., Hu H.-T., Feng G.-F., Zhao Z.-Y., Mao N.-Y. Protective effects of gastrodin on the cellular model of Alzheimer's disease induced by Aβ25–35. Sichuan Da Xue Xue Bao Yi Xue Ban. 2005;36(4):537–540. [PubMed] [Google Scholar]
- 16.Hsieh M.-T., Wu C.-R., Chen C.-F. Gastrodin and p-hydroxybenzyl alcohol facilitate memory consolidation and retrieval, but not acquisition, on the passive avoidance task in rats. Journal of Ethnopharmacology. 1997;56(1):45–54. doi: 10.1016/S0378-8741(96)01501-2. [DOI] [PubMed] [Google Scholar]
- 17.Zhao X., Zou Y., Xu H., Fan L., Guo H., Li X., Li G., Zhang X., Dong M. Gastrodin protect primary cultured rat hippocampal neurons against amyloid-beta peptide-induced neurotoxicity via ERK1/2-Nrf2 pathway. Brain Research. 2012;1482:13–21. doi: 10.1016/j.brainres.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Chen X., Guo Y., Feng J., Liao Z., Li X., Wang H., Li X., He J. Encoding and retrieval of artificial visuoauditory memory traces in the auditory cortex requires the entorhinal cortex. Journal of Neuroscience. 2013;33(24):9963–9974. doi: 10.1523/JNEUROSCI.4078-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert M. P., Viola K. L., Chromy B. A., Chang L., Morgan T. E., Yu J., Venton D. L., Krafft G. A., Finch C. E., Klein W. L. Vaccination with soluble Aβ oligomers generates toxicity-neutralizing antibodies. Journal of Neurochemistry. 2001;79(3):595–605. doi: 10.1046/j.1471-4159.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- 20.Jhamandas J. H., Lind R. W., Renaud L. P. Angiotensin II may mediate excitatory neurotransmission from the subfornical organ to the hypothalamic supraoptic nucleus: an anatomical and electrophysiological study in the rat. Brain Research. 1989;487(1):52–61. doi: 10.1016/0006-8993(89)90939-6. [DOI] [PubMed] [Google Scholar]
- 21.Szegedi V., Juhász G., Budai D., Penke B. Divergent effects of Aβ1-42 on ionotropic glutamate receptor-mediated responses in CA1 neurons in vivo. Brain Research. 2005;1062(1-2):120–126. doi: 10.1016/j.brainres.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Orbán G., Völgyi K., Juhász G., Penke B., Kékesi K. A., Kardos J., Czurkó A. Different electrophysiological actions of 24- and 72-hour aggregated amyloid-beta oligomers on hippocampal field population spike in both anesthetized and awake rats. Brain Research. 2010;1354:227–235. doi: 10.1016/j.brainres.2010.07.061. [DOI] [PubMed] [Google Scholar]
- 23.Alonso A., Klink R. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. Journal of Neurophysiology. 1993;70(1):128–143. doi: 10.1152/jn.1993.70.1.128. [DOI] [PubMed] [Google Scholar]
- 24.Wu W., Small S. A. Imaging the earliest stages of Alzheimer's disease. Current Alzheimer Research. 2006;3(5):529–539. doi: 10.2174/156720506779025161. [DOI] [PubMed] [Google Scholar]
- 25.Palop J. J., Mucke L. Amyloid-Β-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nature Neuroscience. 2010;13(7):812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J., Guo S. Retrospect on the research of the cultivation of Gastrodia elata Bl, a rare traditional Chinese medicine. Chinese Medical Journal. 2000;113(8):686–692. [PubMed] [Google Scholar]
- 27.Spinella M. Herbal medicines and epilepsy: the potential for benefit and adverse effects. Epilepsy and Behavior. 2001;2(6):524–532. doi: 10.1006/ebeh.2001.0281. [DOI] [PubMed] [Google Scholar]
- 28.Alier K., Ma L., Yang J., Westaway D., Jhamandas J. H. Aβ inhibition of ionic conductance in mouse basal forebrain neurons is dependent upon the cellular prion protein PrP C. Journal of Neuroscience. 2011;31(45):16292–16297. doi: 10.1523/JNEUROSCI.4367-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun W., Miao B., Wang X.-C., Duan J.-H., Ye X., Han W.-J., Wang W.-T., Luo C., Hu S.-J. Gastrodin inhibits allodynia and hyperalgesia in painful diabetic neuropathy rats by decreasing excitability of nociceptive primary sensory neurons. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0039647.e39647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang G., Wu H., Hu Y., Li J., Li Q. Gastrodin inhibits glutamate-induced apoptosis of pc12 cells via inhibition of CaMKII/ASK-1/p38 MAPK/p53 signaling cascade. Cellular and Molecular Neurobiology. 2014;34(4):591–602. doi: 10.1007/s10571-014-0043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Texidó L., Martín-Satué M., Alberdi E., Solsona C., Matute C. Amyloid peptide oligomers directly activate NMDA receptors. Cell Calcium. 2011;49(3):184–190. doi: 10.1016/j.ceca.2011.02.001. [DOI] [PubMed] [Google Scholar]